Two-Terminal Nonvolatile Write-Once-Read-Many-Times Memory Based on All-Inorganic Halide Perovskite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Test Equipment
2.3. Fabrication of the Memory Device
3. Results
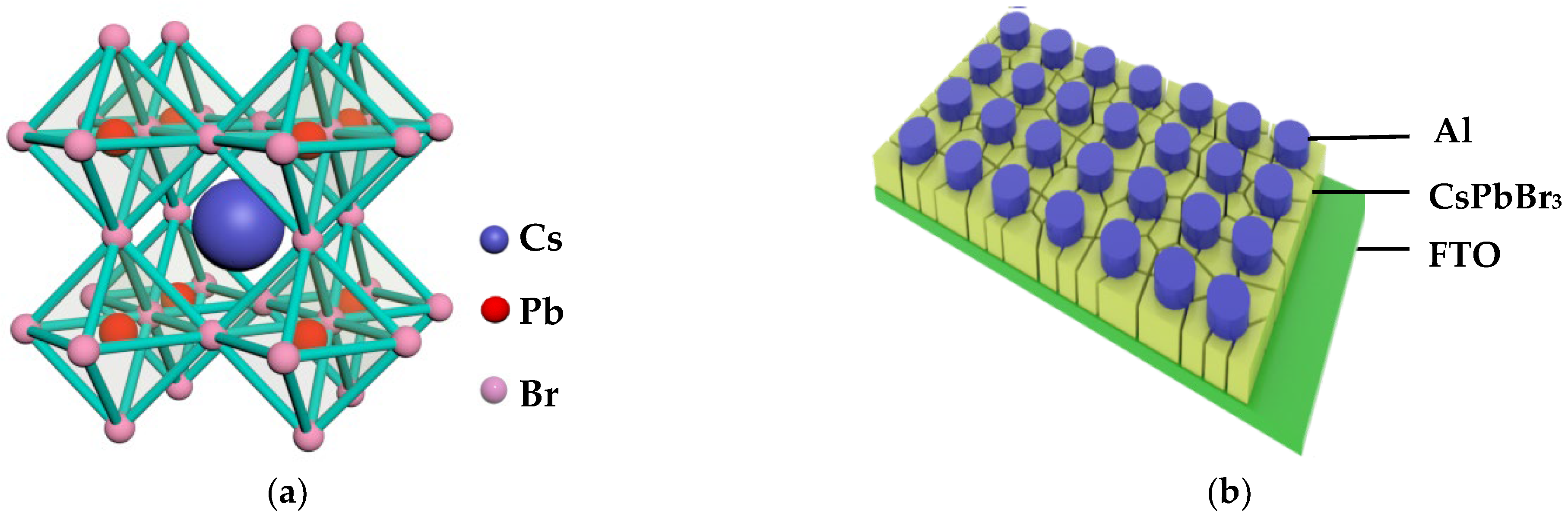
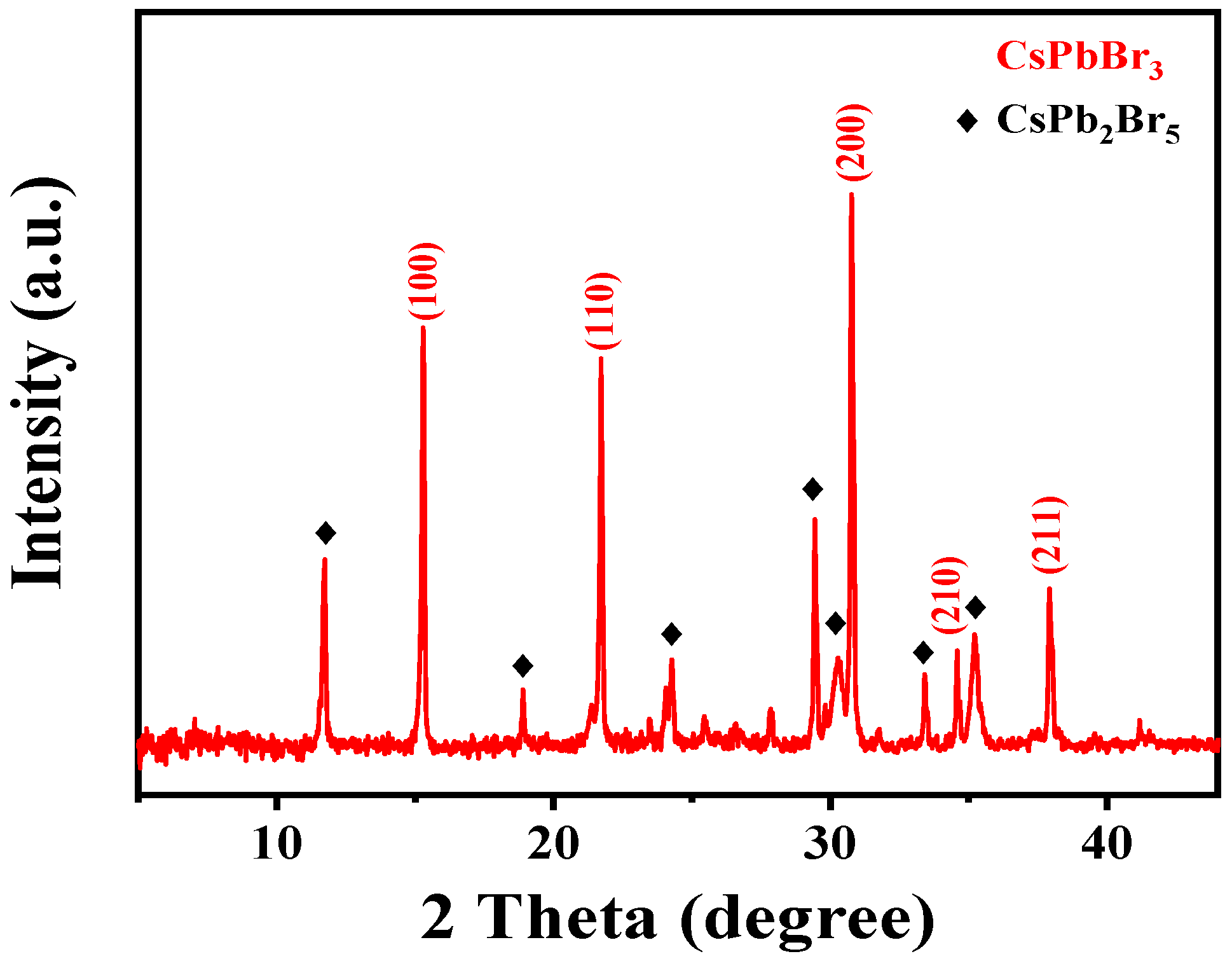
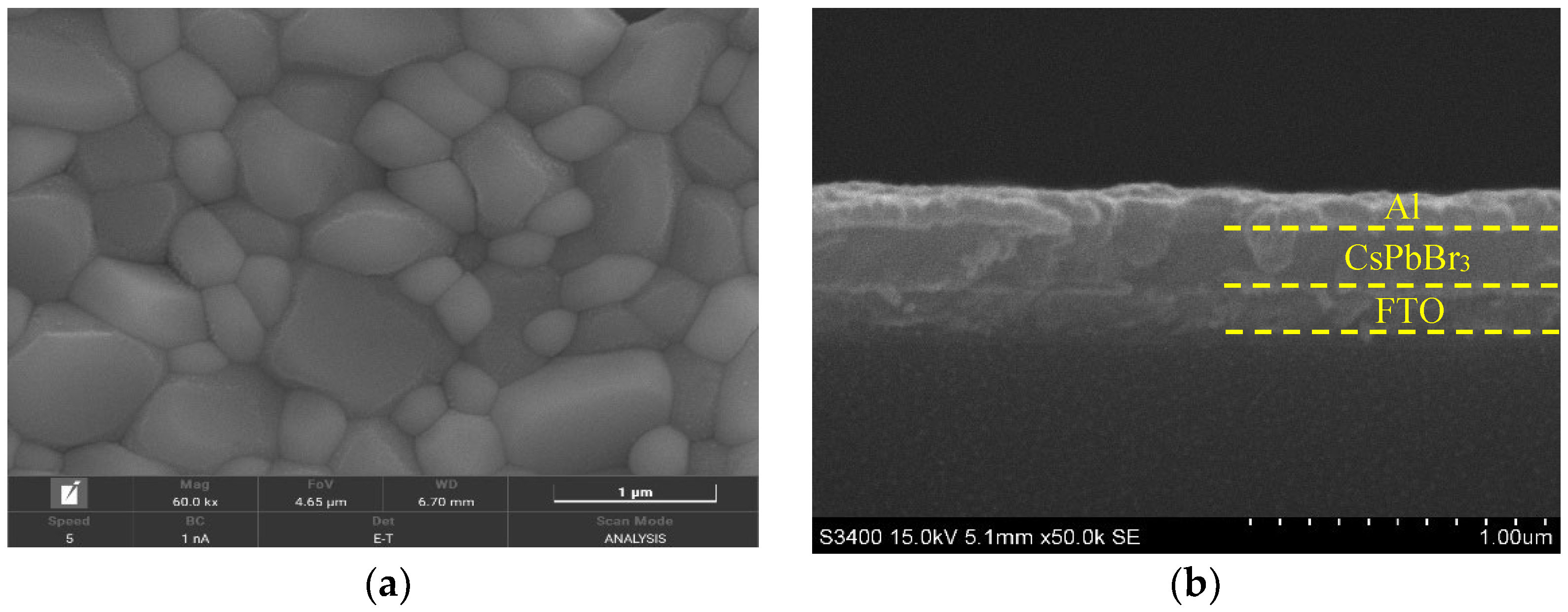
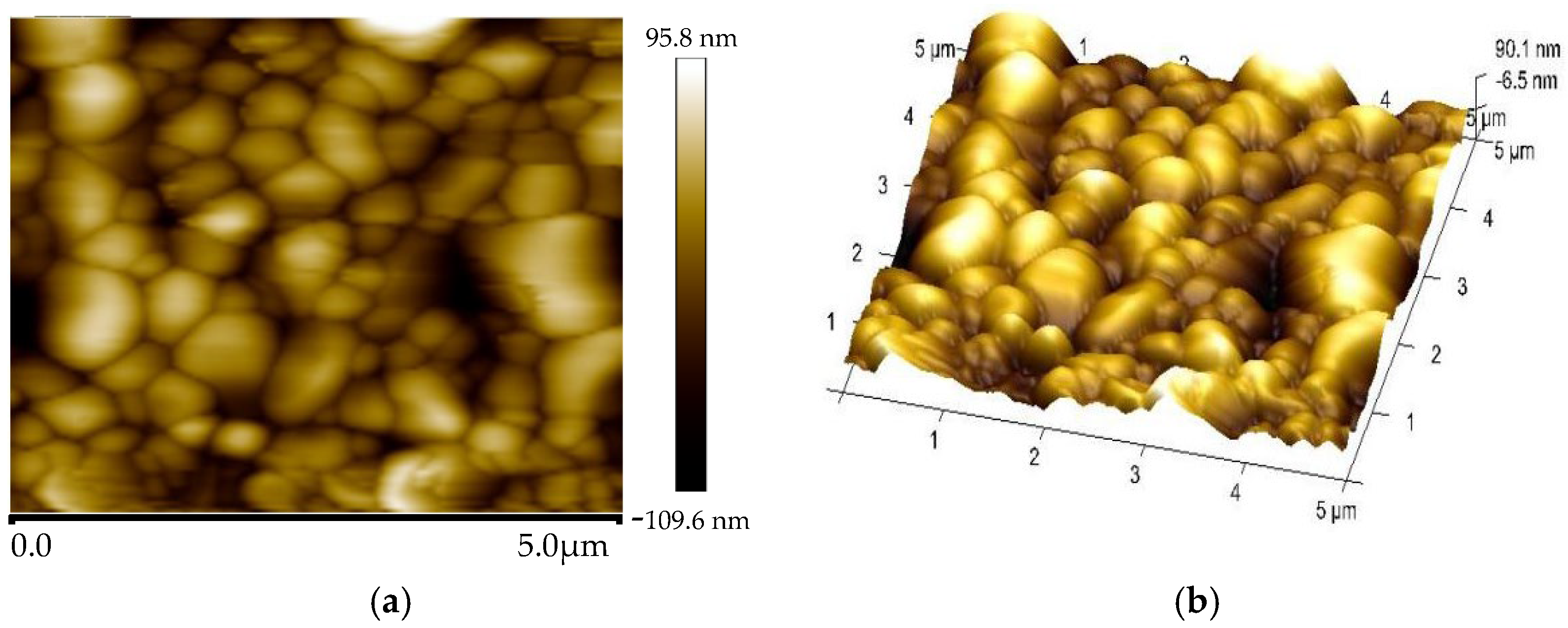
3.1. Material Characterization
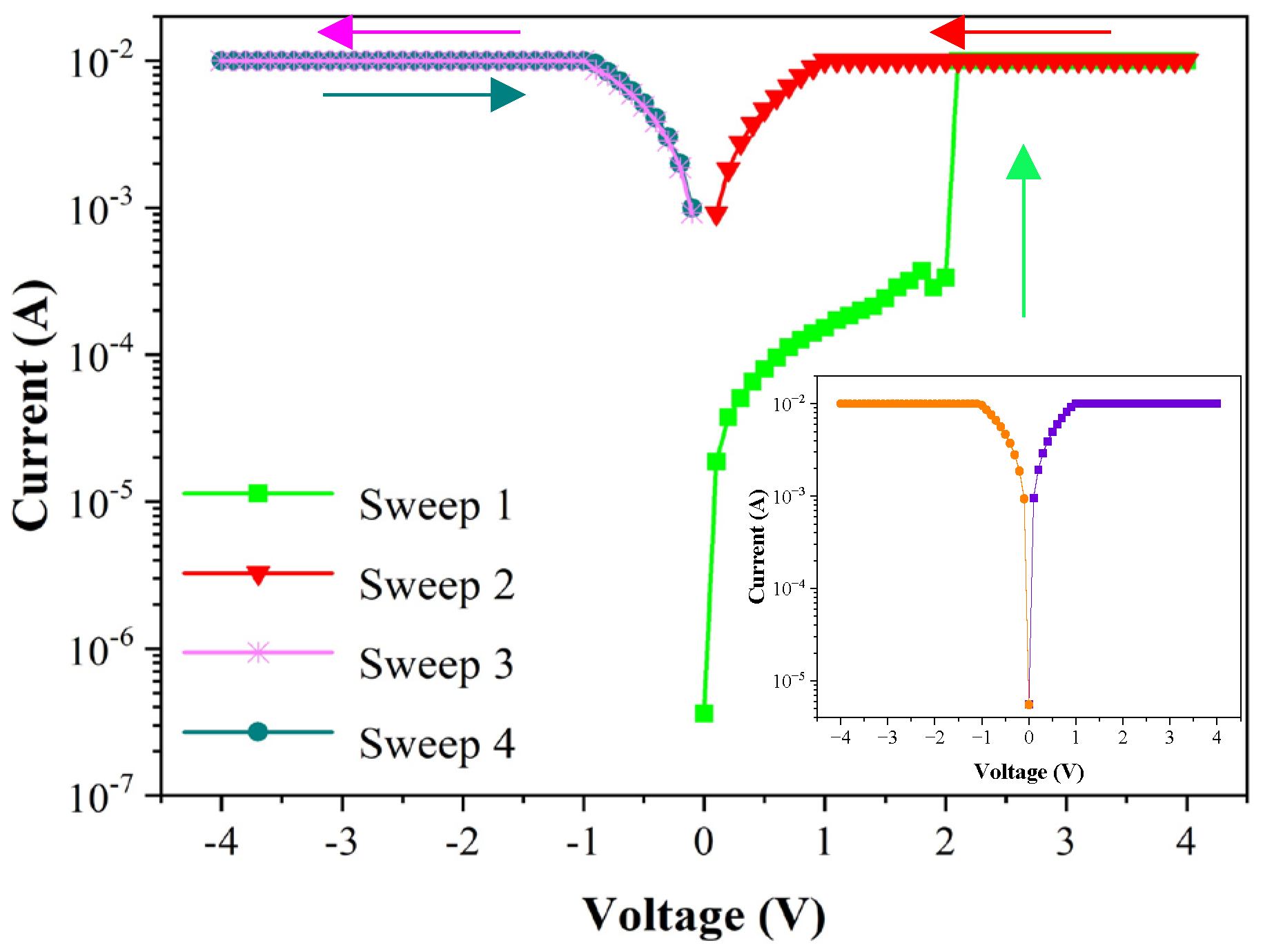
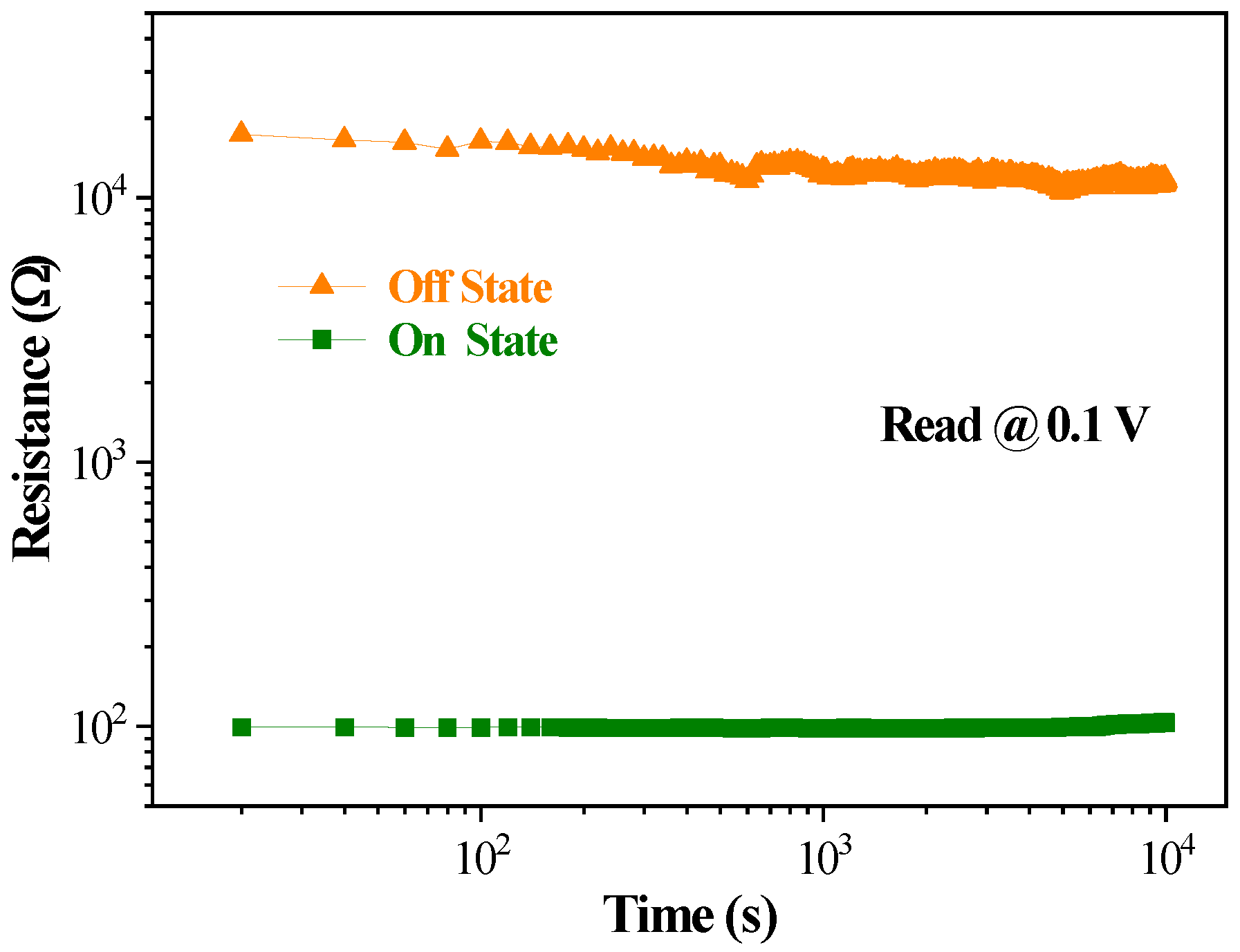
3.2. Current-Voltage Characteristic
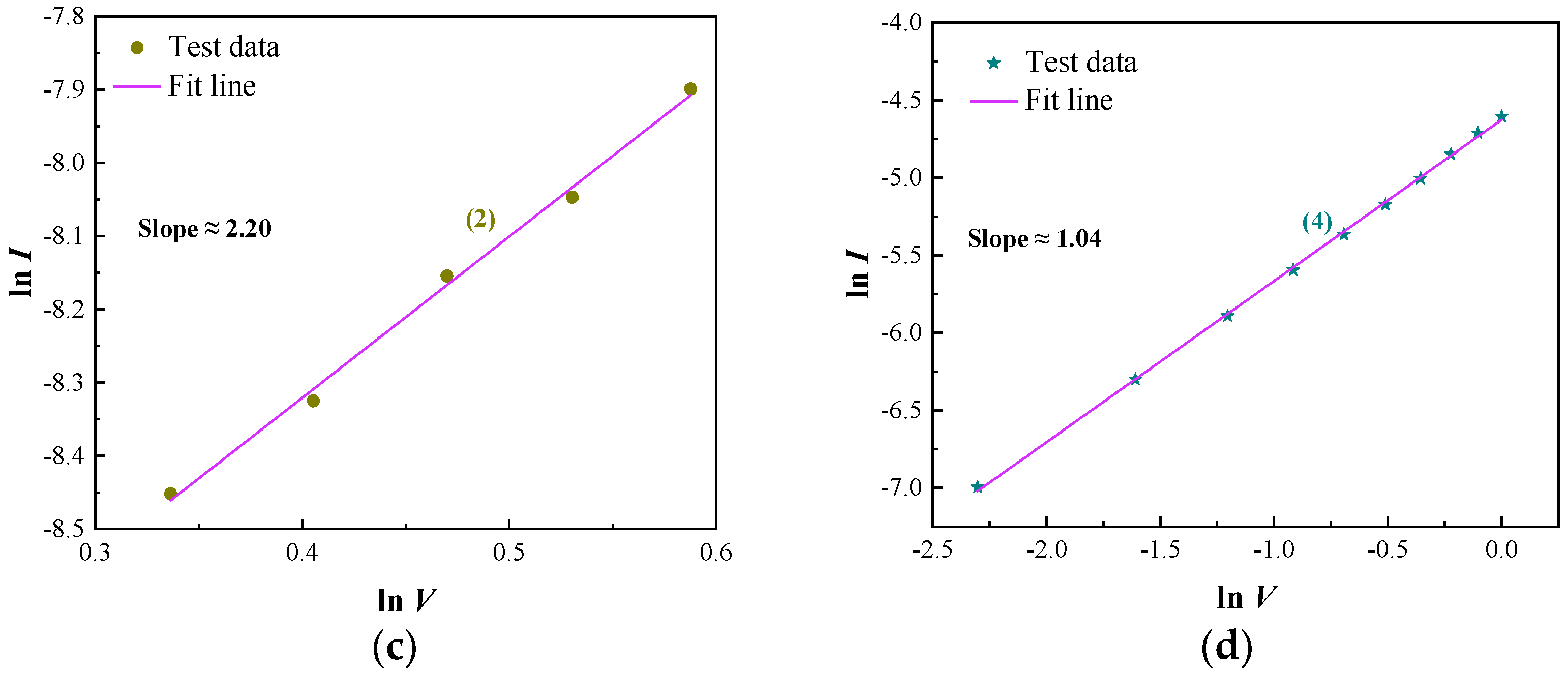
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guan, X.; Lei, Z.; Yu, X.; Lin, C.H.; Huang, J.K.; Huang, C.Y.; Hu, L.; Li, F.; Vinu, A.; Yi, J.; et al. Low-Dimensional Metal-Halide Perovskites as High-Performance Materials for Memory Applications. Small 2022, 18, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Hassan, M.; Khan, Q.; Wang, H.; Zhou, G.; Zubair, M.; Xu, X.; Peng, Z. A Low Power-consumption and Transient Nonvolatile Memory Based on Highly Dense All-Inorganic Perovskite Films. Adv. Electron. Mater. 2022, 8, 2101412. [Google Scholar] [CrossRef]
- Sawa, A. Resistive switching in transition metal oxides. Mater. Today 2008, 11, 28–36. [Google Scholar] [CrossRef]
- Ling, Q.D.; Song, Y.; Lim, S.L.; Teo, E.Y.; Tan, Y.P.; Zhu, C.; Chan, D.S.; Kwong, D.L.; Kang, E.T.; Neoh, K.G. A dynamic random access memory based on a conjugated copolymer containing electron-donor and -acceptor moieties. Angew. Chem. Int. Ed. Engl. 2006, 45, 2947–2951. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Guan, X.; Wang, Y.; Lin, C.H.; Cui, Y.; Huang, Y.; Zhang, X.; Zhang, R.; Yang, X.; Wu, T. Low-Dimensional Lead-Free Inorganic Perovskites for Resistive Switching with Ultralow Bias. Adv. Funct. Mater. 2020, 30, 2002110. [Google Scholar] [CrossRef]
- Guan, X.; Wan, T.; Hu, L.; Lin, C.H.; Yang, J.; Huang, J.K.; Huang, C.Y.; Shahrokhi, S.; Younis, A.; Ramadass, K.; et al. A Solution-Processed All-Perovskite Memory with Dual-Band Light Response and Tri-Mode Operation. Adv. Funct. Mater. 2022, 32, 2110975. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Chien, Y.-S.; Jhang, W.-C. Nonvolatile and Voltage-Polarity-Independent Write-Once–Read-Many-Times Memory Feature of an Al/AlOₓ:N/n⁺-Si Device. IEEE Trans. Electron Devices 2022, 69, 2732–2737. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Yu, Y.; Che, Y.; Jin, L.; Li, Y.; Li, Q.; Li, T.; Dai, H.; Yao, J. Write once read many times resistance switching memory based on all-inorganic perovskite CsPbBr3 quantum dot. Opt. Mater. 2019, 90, 123–126. [Google Scholar] [CrossRef]
- Chen, F.; Tian, G.; Shi, L.; Qi, S.; Wu, D. Nonvolatile write-once read-many-times memory device based on an aromatic hyperbranched polyimide bearing triphenylamine moieties. RSC Adv. 2012, 2, 12879–12885. [Google Scholar] [CrossRef]
- Shengwei, S.; Junbiao, P.; Jian, L.; Dongge, M. Write-Once Read-Many-Times Memory Based on a Single Layer of Pentacene. IEEE Electron Device Lett. 2009, 30, 343–345. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, Y.; Chen, T.P.; Liu, Z.; Yu, Y.F.; Lei, H.W.; Zhu, J.; Fung, S. Flexible Write-Once–Read-Many-Times Memory Device Based on a Nickel Oxide Thin Film. IEEE Trans. Electron Devices 2012, 59, 858–862. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Hu, C.; Ren, T.; Wang, B.; Qi, J.; Zhang, Q.; Zheng, J.-G.; Xin, Y.; Liu, J. Metal/ZnO/MgO/Si/Metal Write-Once-Read-Many-Times Memory. IEEE Trans. Electron Devices 2016, 63, 3508–3513. [Google Scholar] [CrossRef]
- Wu, S.; Chen, X.; Ren, L.; Hu, W.; Yu, F.; Yang, K.; Yang, M.; Wang, Y.; Meng, M.; Zhou, W.; et al. Write-once-read-many-times characteristics of Pt/Al2O3/ITO memory devices. J. Appl. Phys. 2014, 116, 07451. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, Q.; Huang, J.; Ren, J.; Olmedo, M.; Liu, J. Write-Once–Read-Many-Times Memory Based on ZnO on p-Si for Long-Time Archival Storage. IEEE Electron Device Lett. 2011, 32, 1445–1447. [Google Scholar] [CrossRef]
- Hung, Y.-C.; Hsu, W.-T.; Lin, T.-Y.; Fruk, L. Photoinduced write-once read-many-times memory device based on DNA biopolymer nanocomposite. Appl. Phys. Lett. 2011, 99, 277. [Google Scholar] [CrossRef]
- Shim, J.; Lee, J.S.; Lee, J.H.; Yun, Y.J.; Park, S.K.; Angadi, B.; Son, D.I. Memory effect of vertically stacked hBN/QDs/hBN structures based on quantum-dot monolayers sandwiched between hexagonal boron nitride layer. Compos. Part B Eng. 2021, 225, 109307. [Google Scholar] [CrossRef]
- Li, B.; Yin, H.; Xia, F.; Sun, B.; Zhang, S.; Xia, Y.; Chen, Y.; Huang, W. Robust and Transient Write-Once-Read-Many-Times Memory Device Based on Hybrid Perovskite Film with Novel Room Temperature Molten Salt Solvent. Adv. Electron. Mater. 2020, 6, 2000109. [Google Scholar] [CrossRef]
- Younis, A.; Lin, C.H.; Guan, X.; Shahrokhi, S.; Huang, C.Y.; Wang, Y.; He, T.; Singh, S.; Hu, L.; Retamal, J.R.D.; et al. Halide Perovskites: A New Era of Solution-Processed Electronics. Adv. Mater. 2021, 33, e2005000. [Google Scholar] [CrossRef]
- Fang, Y.; Zhai, S.; Chu, L.; Zhong, J. Advances in Halide Perovskite Memristor from Lead-Based to Lead-Free Materials. ACS Appl. Mater. Interfaces 2021, 13, 17141–17157. [Google Scholar] [CrossRef]
- Liu, D.; Lin, Q.; Zang, Z.; Wang, M.; Wangyang, P.; Tang, X.; Zhou, M.; Hu, W. Flexible All-Inorganic Perovskite CsPbBr3 Nonvolatile Memory Device. ACS Appl. Mater. Interfaces 2017, 9, 6171–6176. [Google Scholar] [CrossRef] [PubMed]
- Tress, W. Metal Halide Perovskites as Mixed Electronic-Ionic Conductors: Challenges and Opportunities-From Hysteresis to Memristivity. J. Phys. Chem. Lett. 2017, 8, 3106–3114. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.N.; Rand, B.P.; Friend, R.H.; Mhaisalkar, S.G.; Lee, T.W.; Sargent, E.H. Perovskites for Next-Generation Optical Sources. Chem. Rev. 2019, 119, 7444–7477. [Google Scholar] [CrossRef] [PubMed]
- Dunlap-Shohl, W.A.; Zhou, Y.; Padture, N.P.; Mitzi, D.B. Synthetic Approaches for Halide Perovskite Thin Films. Chem. Rev. 2019, 119, 3193–3295. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Qian, W.H.; Cheng, X.F.; Zhou, J.; He, J.H.; Li, H.; Xu, Q.F.; Li, N.J.; Chen, D.Y.; Yao, Z.G.; Lu, J.M. Lead-free perovskite MASnBr3-based memristor for quaternary information storage. InfoMat 2019, 2, 743–751. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Kim, D.H.; Wu, C.; Kim, T.W. Memristive devices with a large memory margin based on nanocrystalline organic-inorganic hybrid CH3NH3PbBr3 perovskite active layer. Org. Electron. 2018, 62, 412–418. [Google Scholar] [CrossRef]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Tian, J.; Xue, Q.; Yao, Q.; Li, N.; Brabec, C.J.; Yip, H.L. Inorganic Halide Perovskite Solar Cells: Progress and Challenges. Adv. Energy Mater. 2020, 10, 2000183. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, T.; Xie, Q.; Gao, L.; Lu, L.; Tang, Y. First-principles study on the electronic and optical properties of the orthorhombic CsPbBr3 and CsPbI3 with Cmcm space group. New J. Chem. 2021, 45, 15857–15862. [Google Scholar] [CrossRef]
- Wu, W.; Han, S.-T.; Venkatesh, S.; Sun, Q.; Peng, H.; Zhou, Y.; Yeung, C.; Li, R.K.Y.; Roy, V.A.L. Biodegradable skin-inspired nonvolatile resistive switching memory based on gold nanoparticles embedded alkali lignin. Org. Electron. 2018, 59, 382–388. [Google Scholar] [CrossRef]
- Younis, A. Solution processed laterally grown Zinc Oxide microstructures for next generation computing devices. Phys. E Low-Dimens. Syst. Nanostructures 2021, 126, 114475. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Zhang, X.-Z.; Jhang, W.-C.; Cheng, C.-W.; Wu, Y.-M.; Tsai, J.-E.; Joodaki, M. Write-once-read-many-times characteristics of CuO layer with Ag conductive bridges. Semicond. Sci. Technol. 2021, 36, 095016. [Google Scholar] [CrossRef]
- Cao, Q.; Xiong, L.; Yuan, X.; Li, P.; Wu, J.; Bi, H.; Zhang, J. Resistive switching behavior of the memristor based on WS2 nanosheets and polyvinylpyrrolidone nanocomposites. Appl. Phys. Lett. 2022, 120, 232105. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Y.; Lin, C.-H.; Hu, L.; Ge, S.; Wan, T.; Younis, A.; Li, F.; Cui, Y.; Qi, D.-C.; et al. A monolithic artificial iconic memory based on highly stable perovskite-metal multilayers. Appl. Phys. Rev. 2020, 7, 031401. [Google Scholar] [CrossRef]
- Guan, X.; Hu, W.; Haque, M.A.; Wei, N.; Liu, Z.; Chen, A.; Wu, T. Light-Responsive Ion-Redistribution-Induced Resistive Switching in Hybrid Perovskite Schottky Junctions. Adv. Funct. Mater. 2018, 28, 1704665. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, X.; Zhou, G.; Li, P.; Zhang, Y.; Wang, H.; Xia, Y.; Zhao, Y. An organic nonvolatile resistive switching memory device fabricated with natural pectin from fruit peel. Org. Electron. 2017, 42, 181–186. [Google Scholar] [CrossRef]
- Lim, E.; Ismail, R. Conduction Mechanism of Valence Change Resistive Switching Memory: A Survey. Electronics 2015, 4, 586–613. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Hu, Y. Reproducible switching effect of an all-inorganic halide perovskite CsPbBr 3 for memory applications. Ceram. Int. 2017, 43, 7020–7025. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Wang, F.; Zhang, H.; Xiao, B.; Sun, Q.; Guo, J.; Hafsia, A.B.; Shao, A.; Xu, Y.; Zhou, J. Defect proliferation in CsPbBr3 crystal induced by ion migration. Appl. Phys. Lett. 2020, 116, 063505. [Google Scholar] [CrossRef]
- Shang, D.S.; Wang, Q.; Chen, L.D.; Dong, R.; Li, X.M.; Zhang, W.Q. Effect of carrier trapping on the hysteretic current-voltage characteristics inAg∕La0.7Ca0.3MnO3∕Ptheterostructures. Phys. Rev. B 2006, 73, 245427. [Google Scholar] [CrossRef]
- Ashery, A.; Elnasharty, M.M.M.; Hameed, T.A. Investigation of electrical and dielectric properties of epitaxially grown Au/n-GaAs/p-Si/Al heterojunction. Opt. Quantum Electron. 2020, 52, 1–19. [Google Scholar] [CrossRef]
- Murgatroyd, P.N. Theory of space-charge-limited current enhanced by Frenkel effect. J. Phys. D Appl. Phys. 1970, 3, 151–156. [Google Scholar] [CrossRef]
- Ashley, K.L.; Milnes, A.G. Double Injection in Deep-Lying Impurity Semiconductors. J. Appl. Phys. 1964, 35, 369–374. [Google Scholar] [CrossRef]
- Lampert, M.A. Double Injection in Insulators. Phys. Rev. 1962, 125, 126–141. [Google Scholar] [CrossRef]

| Device Structure | Memory Type | Operation Voltage (v) | Reading Voltage (v) | Retention Time (s) | Ref. |
|---|---|---|---|---|---|
| Al/Au NPs: lignin/Al | WORM | 4.7 | 0.6 | >103 | [30] |
| ITO/CsPbBr3 QDs/Au | WORM | −1.1 | −0.5 | >103 | [8] |
| Au/ZnO MBs/Au | WORM | ≈6.5 | 0.35 | ≈104 | [31] |
| n+-Si/CuO/Ag | WORM | ≈3 | 1 | 105 | [32] |
| Ag/WS2-PVP/Cu | WORM | <1 | 0.02 | >2 × 102 | [33] |
| FTO/CsPbBr3/Al | WORM | 2.1 | 0.1 | 104 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Zhao, X.; Ai, C.; Fang, X.; Zhao, X.; Wang, Y.; Zhang, H. Two-Terminal Nonvolatile Write-Once-Read-Many-Times Memory Based on All-Inorganic Halide Perovskite. Micromachines 2023, 14, 93. https://doi.org/10.3390/mi14010093
Yu Z, Zhao X, Ai C, Fang X, Zhao X, Wang Y, Zhang H. Two-Terminal Nonvolatile Write-Once-Read-Many-Times Memory Based on All-Inorganic Halide Perovskite. Micromachines. 2023; 14(1):93. https://doi.org/10.3390/mi14010093
Chicago/Turabian StyleYu, Zhipeng, Xiaofeng Zhao, Chunpeng Ai, Xin Fang, Xiaohan Zhao, Yanchao Wang, and Hongquan Zhang. 2023. "Two-Terminal Nonvolatile Write-Once-Read-Many-Times Memory Based on All-Inorganic Halide Perovskite" Micromachines 14, no. 1: 93. https://doi.org/10.3390/mi14010093




