Glycerol Droplet Spreading on Growing Bacillus Subtilis Biofilms
Abstract
:1. Introduction
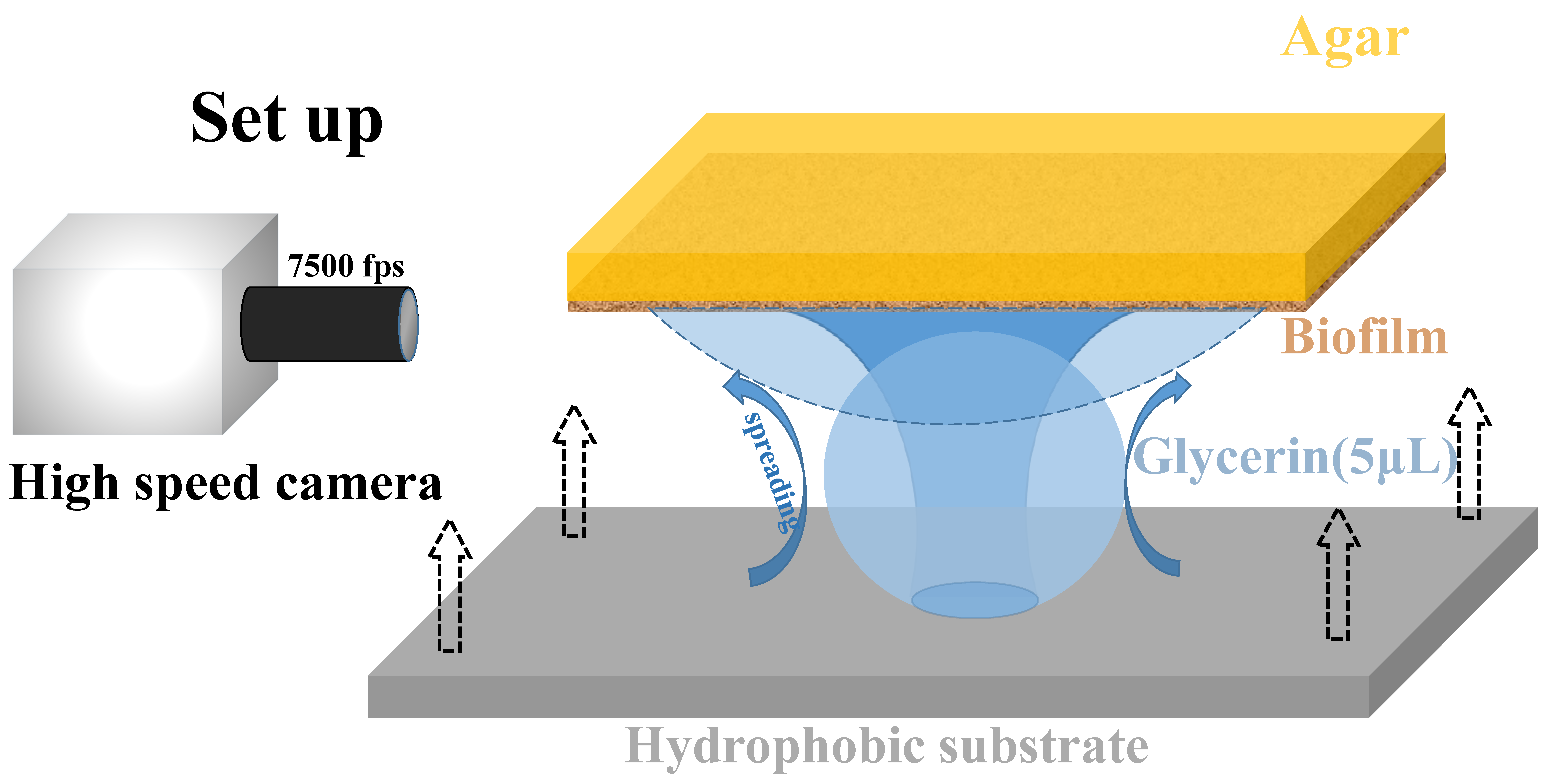
2. Materials and Methods
3. Results and Discussion
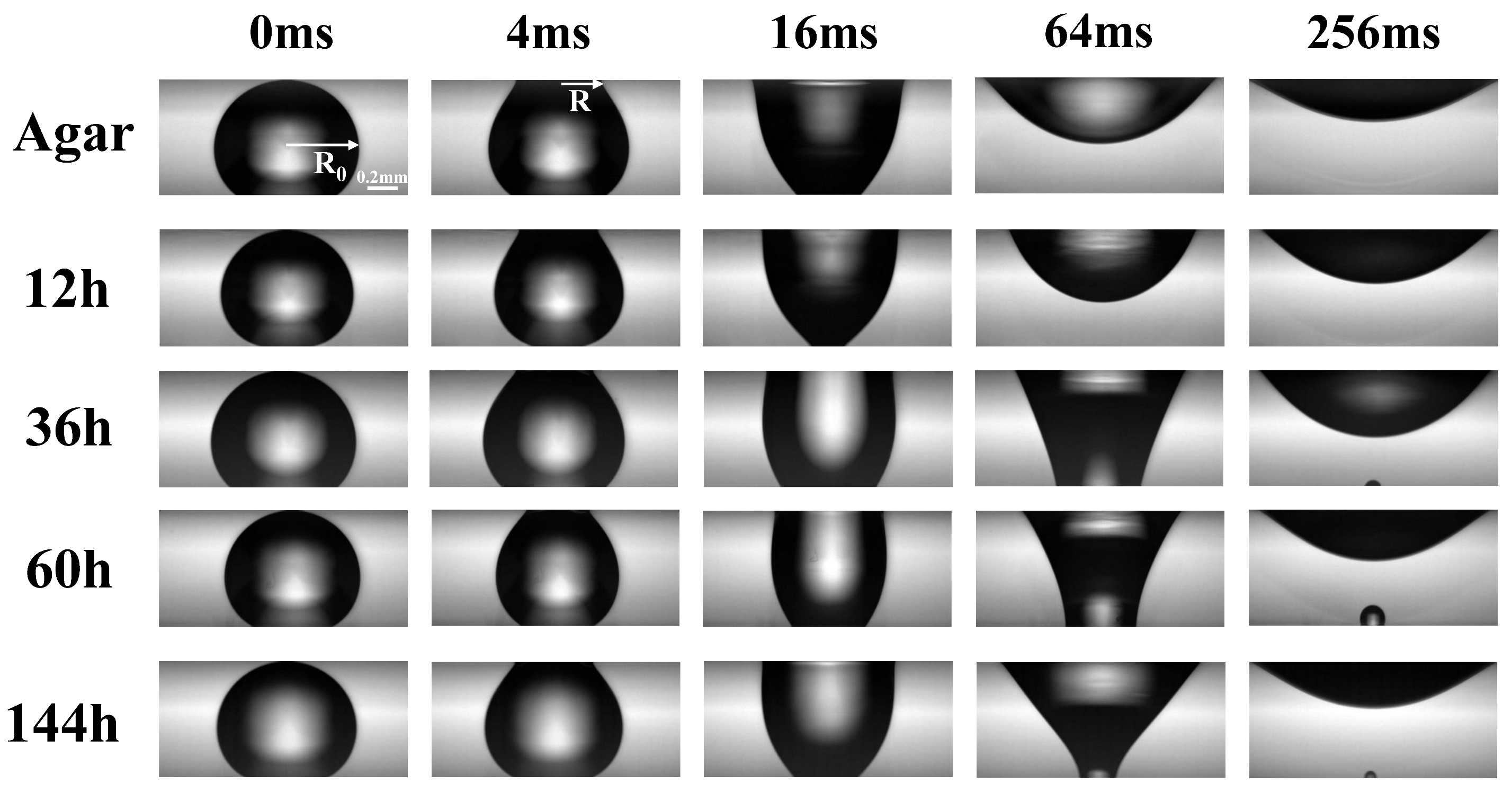
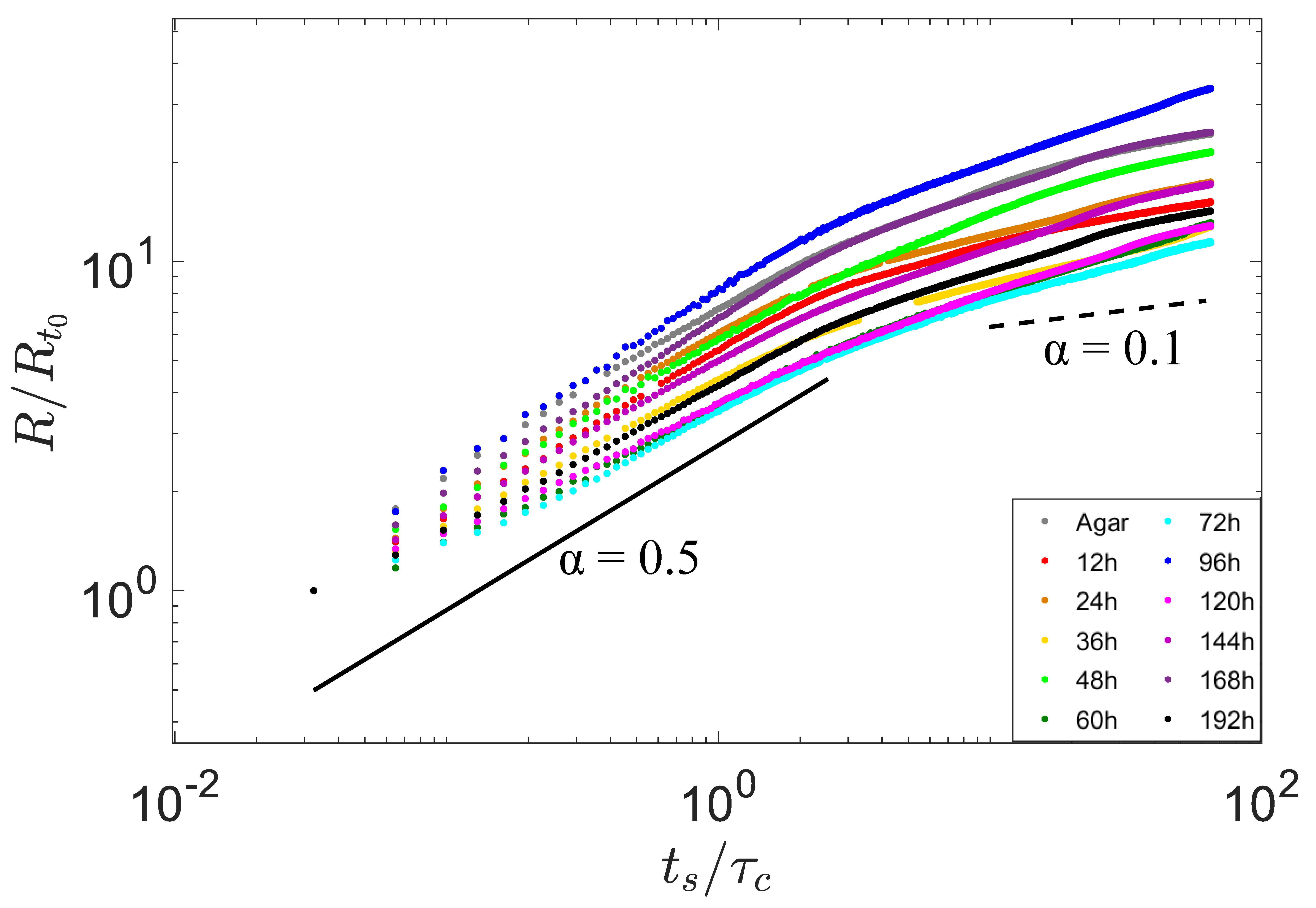
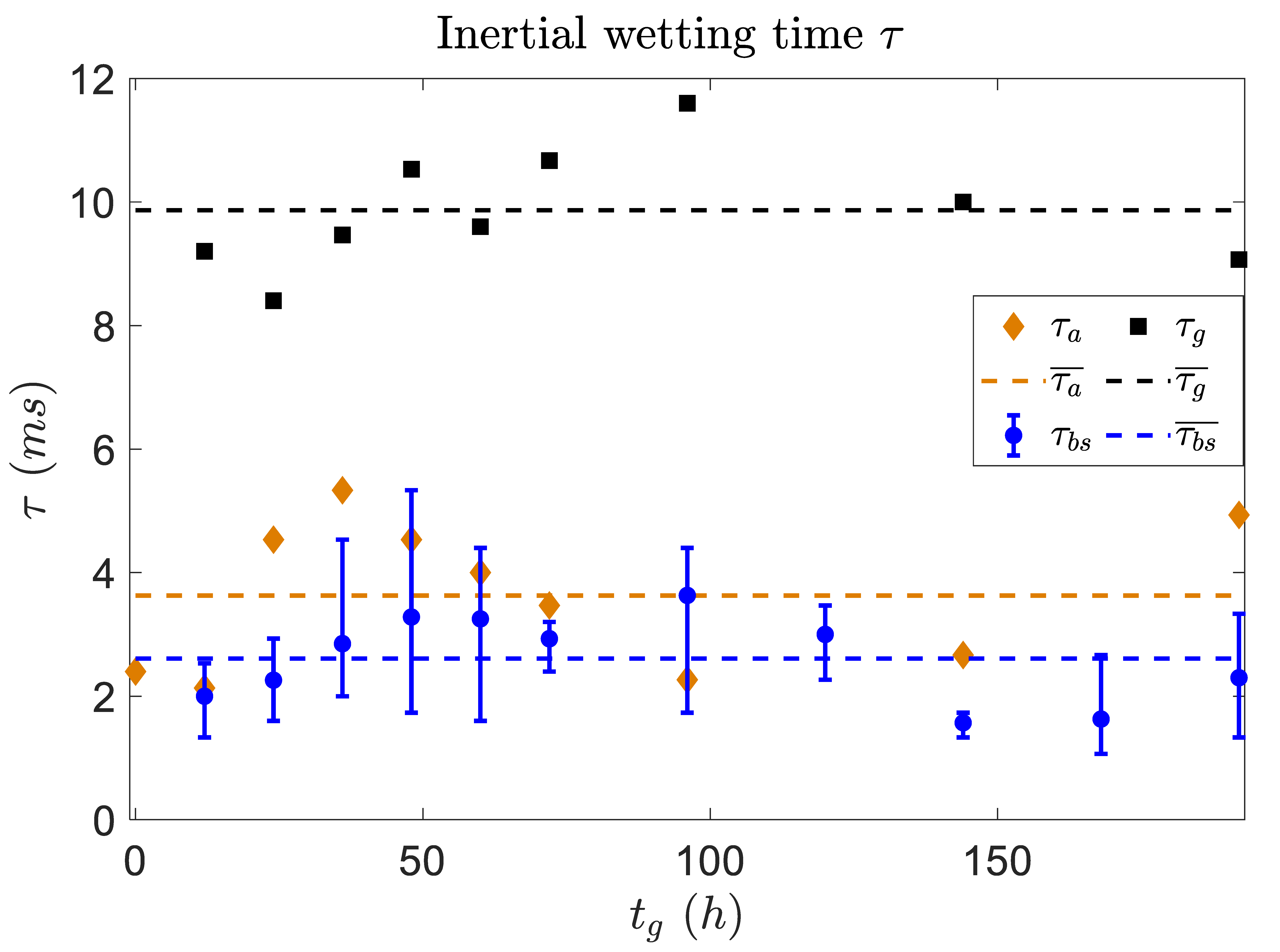
3.1. Fast Spreading of Droplet on Growing Biofilm
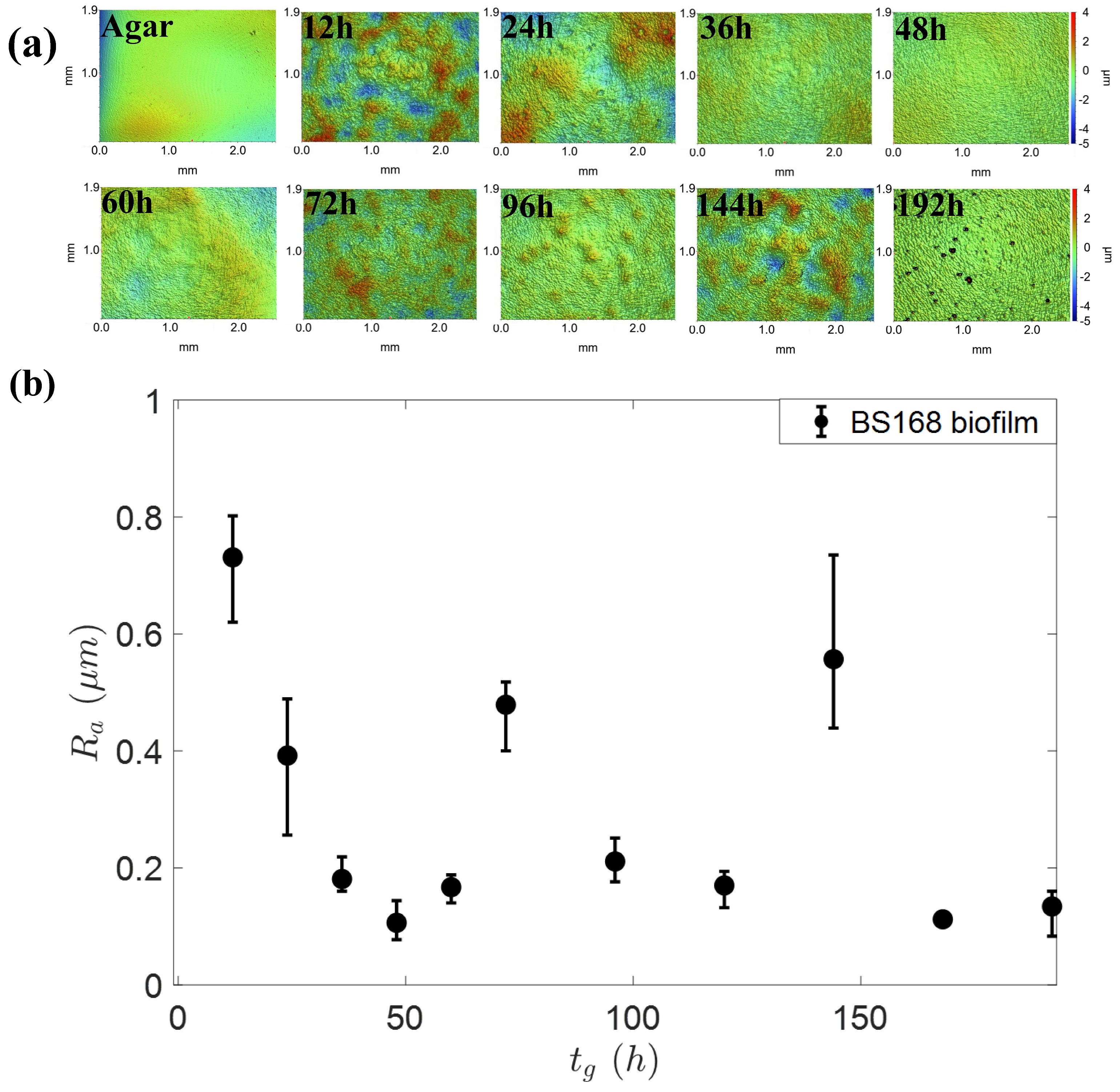
3.2. Viscoelastic Response of Biofilm to the Droplet Spreading
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De la Fuente-Núñez, C.; Reffuveille, F.; Fernández, L.; Hancock, R.E. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013, 16, 580–589. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [Green Version]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Schaumann, G.E.; Braun, B.; Kirchner, D.; Rotard, W.; Szewzyk, U.; Grohmann, E. Influence of biofilms on the water repellency of urban soil samples. Hydrol. Process. Int. J. 2007, 21, 2276–2284. [Google Scholar] [CrossRef]
- Hansen, S.K.; Rainey, P.B.; Haagensen, J.A.; Molin, S. Evolution of species interactions in a biofilm community. Nature 2007, 445, 533–536. [Google Scholar] [CrossRef]
- Motta, J.P.; Wallace, J.L.; Buret, A.G.; Deraison, C.; Vergnolle, N. Gastrointestinal biofilms in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 314–334. [Google Scholar] [CrossRef]
- Flemming, H.C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2022, 21, 70–86. [Google Scholar] [CrossRef]
- Epstein, A.K.; Pokroy, B.; Seminara, A.; Aizenberg, J. Bacterial biofilm shows persistent resistance to liquid wetting and gas penetration. Proc. Natl. Acad. Sci. USA 2011, 108, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Kogo, T.; Hirai, N.; Ogawa, A.; Kanematsu, H.; Takahara, J.; Awazu, A.; Fujita, N.; Haruzono, Y.; Ichida, S.; et al. In-situ detection based on the biofilm hydrophilicity for environmental biofilm formation. Sci. Rep. 2019, 9, 8070. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Iwano, M. BslA (YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms. Mol. Microbiol. 2012, 85, 51–66. [Google Scholar] [CrossRef]
- Arnaouteli, S.; Ferreira, A.S.; Schor, M.; Morris, R.J.; Bromley, K.M.; Jo, J.; Cortez, K.L.; Sukhodub, T.; Prescott, A.R.; Dietrich, L.E.; et al. Bifunctionality of a biofilm matrix protein controlled by redox state. Proc. Natl. Acad. Sci. USA 2017, 114, E6184–E6191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werb, M.; García, C.F.; Bach, N.C.; Grumbein, S.; Sieber, S.A.; Opitz, M.; Lieleg, O. Surface topology affects wetting behavior of Bacillus subtilis biofilms. npj Biofilms Microbiomes 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kretschmer, M.; Schüßler, C.A.; Lieleg, O. Biofilm adhesion to surfaces is modulated by biofilm wettability and stiffness. Adv. Mater. Interfaces 2021, 8, 2001658. [Google Scholar] [CrossRef]
- Stoodley, P.; Lewandowski, Z.; Boyle, J.D.; Lappin-Scott, H.M. Structural deformation of bacterial biofilms caused by short-term fluctuations in fluid shear: An in situ investigation of biofilm rheology. Biotechnol. Bioeng. 1999, 65, 83–92. [Google Scholar] [CrossRef]
- Peterson, B.W.; He, Y.; Ren, Y.; Zerdoum, A.; Libera, M.R.; Sharma, P.K.; Van Winkelhoff, A.J.; Neut, D.; Stoodley, P.; Van Der Mei, H.C.; et al. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol. Rev. 2015, 39, 234–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonn, D.; Eggers, J.; Indekeu, J.; Meunier, J.; Rolley, E. Wetting and spreading. Rev. Mod. Phys. 2009, 81, 739. [Google Scholar] [CrossRef]
- Snoeijer, J.H.; Andreotti, B. Moving contact lines: Scales, regimes, and dynamical transitions. Annu. Rev. Fluid Mech. 2013, 45, 269–292. [Google Scholar] [CrossRef] [Green Version]
- Shanahan, M.; Carre, A. Viscoelastic dissipation in wetting and adhesion phenomena. Langmuir 1995, 11, 1396–1402. [Google Scholar] [CrossRef]
- Pericet-Camara, R.; Auernhammer, G.K.; Koynov, K.; Lorenzoni, S.; Raiteri, R.; Bonaccurso, E. Solid-supported thin elastomer films deformed by microdrops. Soft Matter 2009, 5, 3611–3617. [Google Scholar] [CrossRef] [Green Version]
- Pericet-Cámara, R.; Best, A.; Butt, H.J.; Bonaccurso, E. Effect of capillary pressure and surface tension on the deformation of elastic surfaces by sessile liquid microdrops: An experimental investigation. Langmuir 2008, 24, 10565–10568. [Google Scholar] [CrossRef]
- Jerison, E.R.; Xu, Y.; Wilen, L.A.; Dufresne, E.R. Deformation of an elastic substrate by a three-phase contact line. Phys. Rev. Lett. 2011, 106, 186103. [Google Scholar] [CrossRef] [Green Version]
- Biance, A.L.; Clanet, C.; Quéré, D. First steps in the spreading of a liquid droplet. Phys. Rev. E 2004, 69, 016301. [Google Scholar] [CrossRef] [Green Version]
- Tanner, L. The spreading of silicone oil drops on horizontal surfaces. J. Phys. D Appl. Phys. 1979, 12, 1473. [Google Scholar] [CrossRef]
- De Gennes, P.G. Wetting: Statics and dynamics. Rev. Mod. Phys. 1985, 57, 827. [Google Scholar] [CrossRef]
- Nakajima, A.; Hashimoto, K.; Watanabe, T. Recent studies on super-hydrophobic films. Mol. Mater. Funct. Polym. 2001, 132, 31–41. [Google Scholar]
- Cazabat, A.; Stuart, M.C. Dynamics of wetting: Effects of surface roughness. J. Phys. Chem. 1986, 90, 5845–5849. [Google Scholar] [CrossRef]
- Bird, J.C.; Mandre, S.; Stone, H.A. Short-time dynamics of partial wetting. Phys. Rev. Lett. 2008, 100, 234501. [Google Scholar] [CrossRef] [Green Version]
- Koh, K.S.; Lam, K.W.; Alhede, M.; Queck, S.Y.; Labbate, M.; Kjelleberg, S.; Rice, S.A. Phenotypic diversification and adaptation of Serratia marcescens MG1 biofilm-derived morphotypes. J. Bacteriol. 2007, 189, 119–130. [Google Scholar] [CrossRef] [Green Version]
- De Gennes, P.G.; Brochard-Wyart, F.; Quéré, D. Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves; Springer: New York, NY, USA, 2004; Volume 315. [Google Scholar]
- Nita, S.; Do-Quang, M.; Wang, J.; Chen, Y.C.; Suzuki, Y.; Amberg, G.; Shiomi, J. Electrostatic cloaking of surface structure for dynamic wetting. Sci. Adv. 2017, 3, e1602202. [Google Scholar] [CrossRef] [Green Version]
- Kampouraki, Z.C.; Petala, M.; Boumpakis, A.; Skordaris, G.; Michailidis, N.; Deliyanni, E.; Kostoglou, M.; Karapantsios, T.D. Wetting and Imbibition Characteristics of Pseudomonas fluorescens Biofilms Grown on Stainless Steel. Langmuir 2022, 38, 9810–9821. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.C.; Tsai, S.S.; Stone, H.A. Inclined to splash: Triggering and inhibiting a splash with tangential velocity. New J. Phys. 2009, 11, 063017. [Google Scholar] [CrossRef]
- Seminara, A.; Angelini, T.E.; Wilking, J.N.; Vlamakis, H.; Ebrahim, S.; Kolter, R.; Weitz, D.A.; Brenner, M.P. Osmotic spreading of Bacillus subtilis biofilms driven by an extracellular matrix. Proc. Natl. Acad. Sci. USA 2012, 109, 1116–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Auernhammer, G.K.; Bonaccurso, E. Short time wetting dynamics on soft surfaces. Soft Matter 2011, 7, 9084–9089. [Google Scholar] [CrossRef]
- Chen, L.; Bonaccurso, E.; Shanahan, M.E. Inertial to viscoelastic transition in early drop spreading on soft surfaces. Langmuir 2013, 29, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Sheely, M.L. Glycerol viscosity tables. Ind. Eng. Chem. 1932, 24, 1060–1064. [Google Scholar] [CrossRef]
- Nayar, V.; Weiland, J.; Nelson, C.; Hodge, A. Elastic and viscoelastic characterization of agar. J. Mech. Behav. Biomed. Mater. 2012, 7, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Yang, Y.; El Haj, A.J.; Then, K.Y.; Liu, K.K. Characterizing the viscoelastic properties of thin hydrogel-based constructs for tissue engineering applications. J. R. Soc. Interface 2005, 2, 455–463. [Google Scholar] [CrossRef]
- Kandemir, N.; Vollmer, W.; Jakubovics, N.S.; Chen, J. Mechanical interactions between bacteria and hydrogels. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Carré, A.; Shanahan, M.E. Direct evidence for viscosity-independent spreading on a soft solid. Langmuir 1995, 11, 24–26. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, P.C.; Huang, C.; Sun, P.; Monroy, G.L.; Wu, W.; Lin, J.; Espinosa-Marzal, R.M.; Boppart, S.A.; Liu, W.T.; et al. Effect of divalent ions and a polyphosphate on composition, structure, and stiffness of simulated drinking water biofilms. npj Biofilms Microbiomes 2018, 4, 15. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Liu, Y.; Luo, H.; Jing, G. Glycerol Droplet Spreading on Growing Bacillus Subtilis Biofilms. Micromachines 2023, 14, 599. https://doi.org/10.3390/mi14030599
Luo S, Liu Y, Luo H, Jing G. Glycerol Droplet Spreading on Growing Bacillus Subtilis Biofilms. Micromachines. 2023; 14(3):599. https://doi.org/10.3390/mi14030599
Chicago/Turabian StyleLuo, Siyang, Yanan Liu, Hao Luo, and Guangyin Jing. 2023. "Glycerol Droplet Spreading on Growing Bacillus Subtilis Biofilms" Micromachines 14, no. 3: 599. https://doi.org/10.3390/mi14030599






