Ultramicro Interdigitated Array Electrode Chip with Optimized Construction for Detection of Ammonia Nitrogen in Water
Abstract
:1. Introduction
2. Simulation and Experiment
2.1. Instruments and Reagents
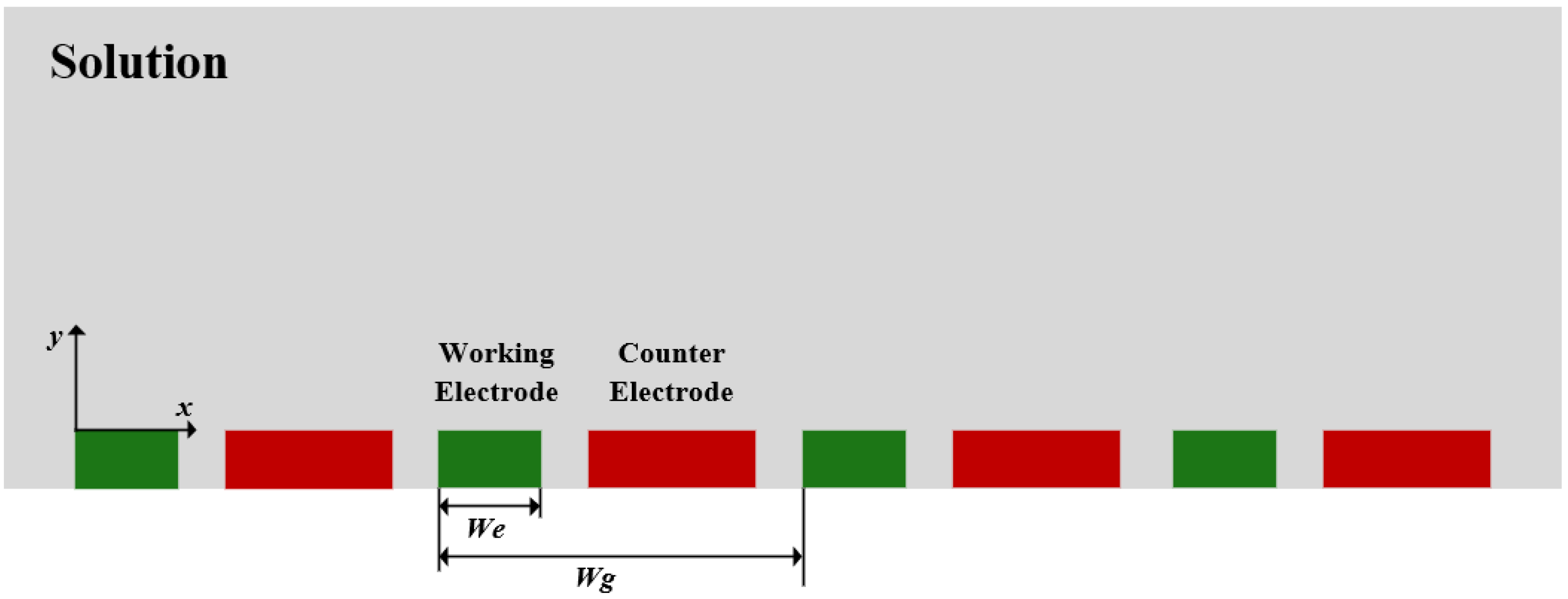
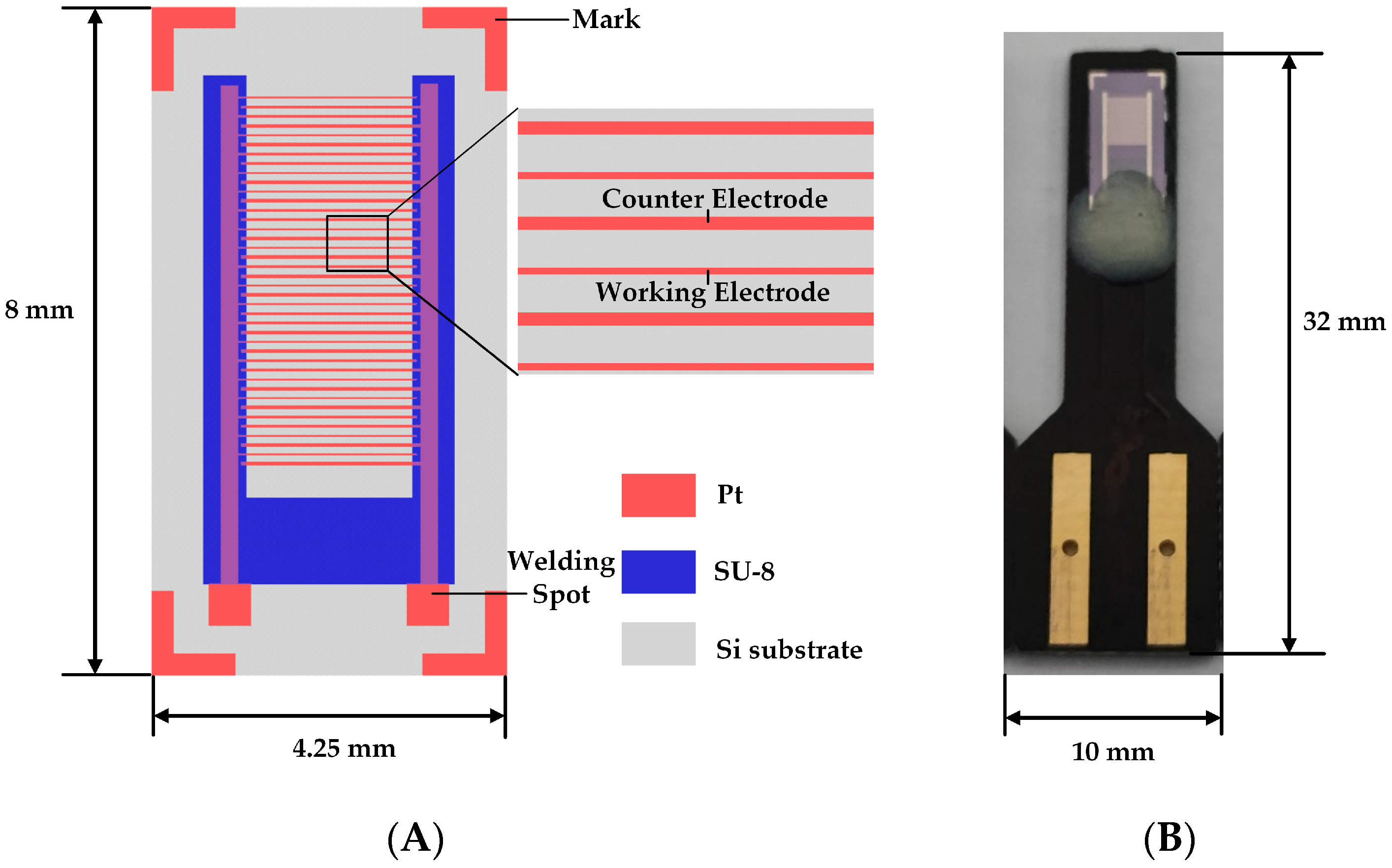
2.2. Ultramicro Array Electrode Chip Design and Simulation
2.3. Ultramicro Interdigitated Array Electrode Chip Fabrication
2.4. Electrochemical Characterization and Ammonia Nitrogen Determination
3. Results and Discussion
3.1. Simulation Results and Analysis of Ultramicro Array Electrodes
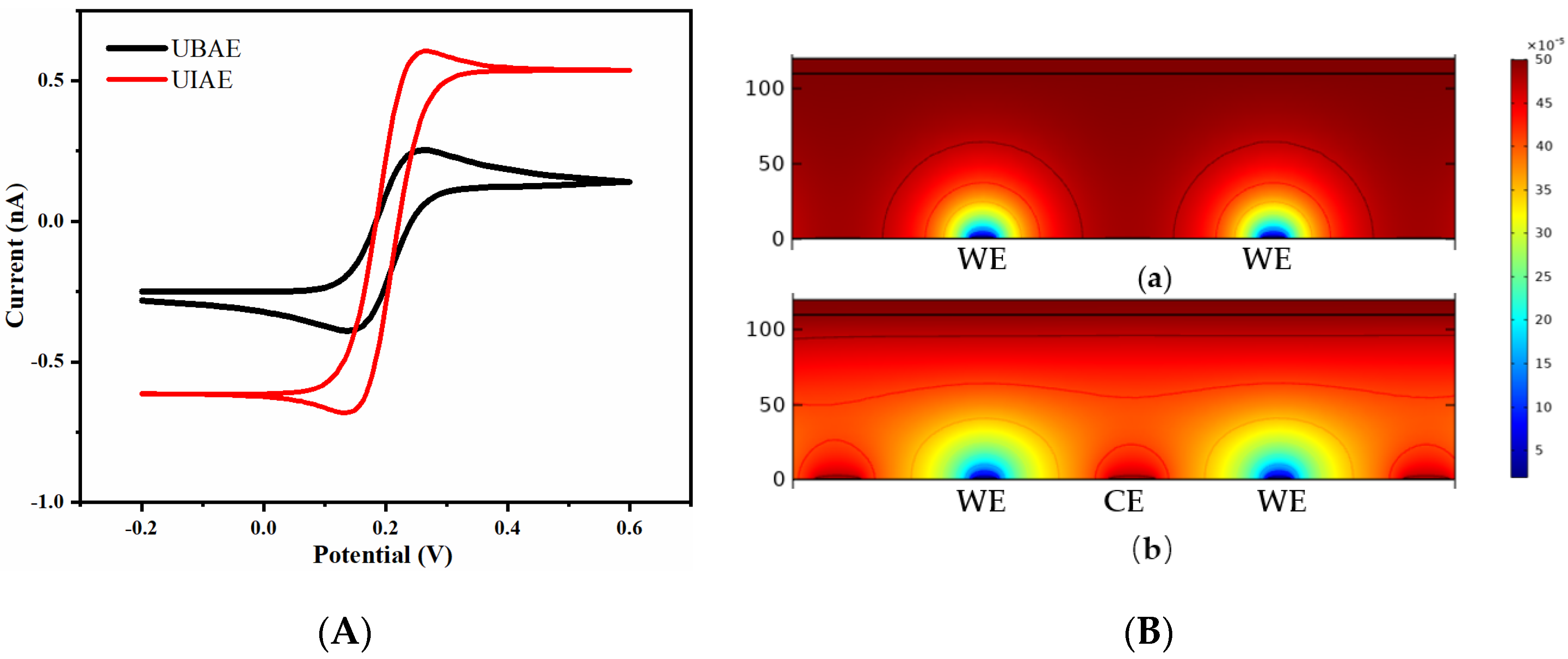
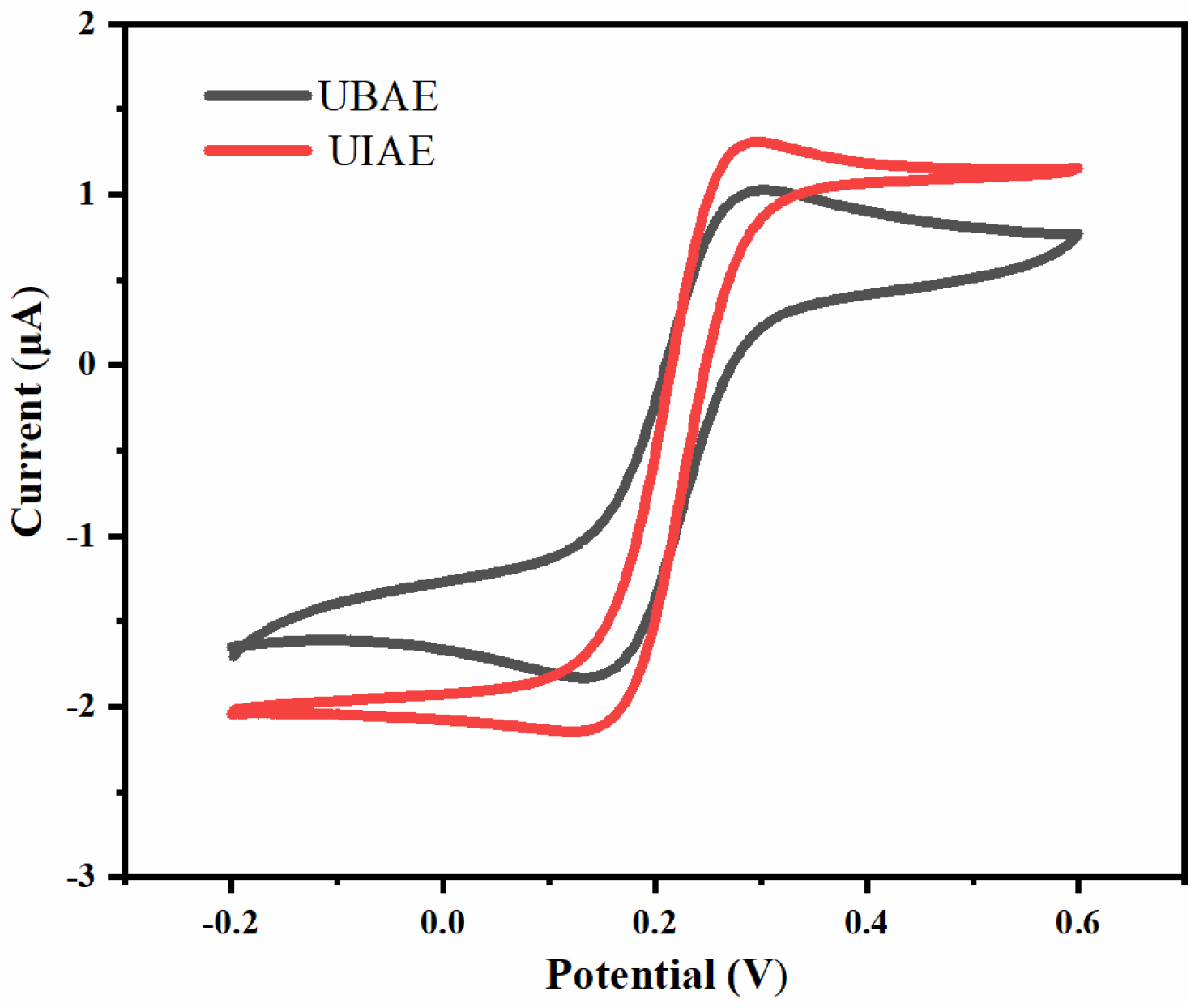
3.1.1. Simulation Comparison between UIAE and UBAE
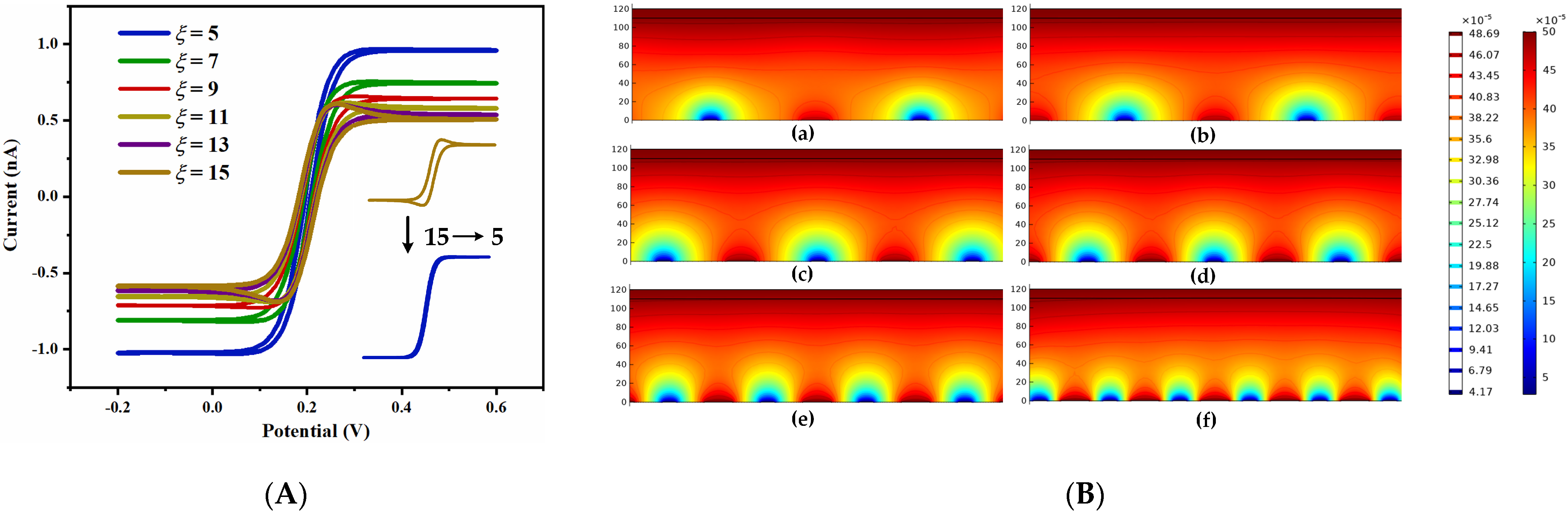
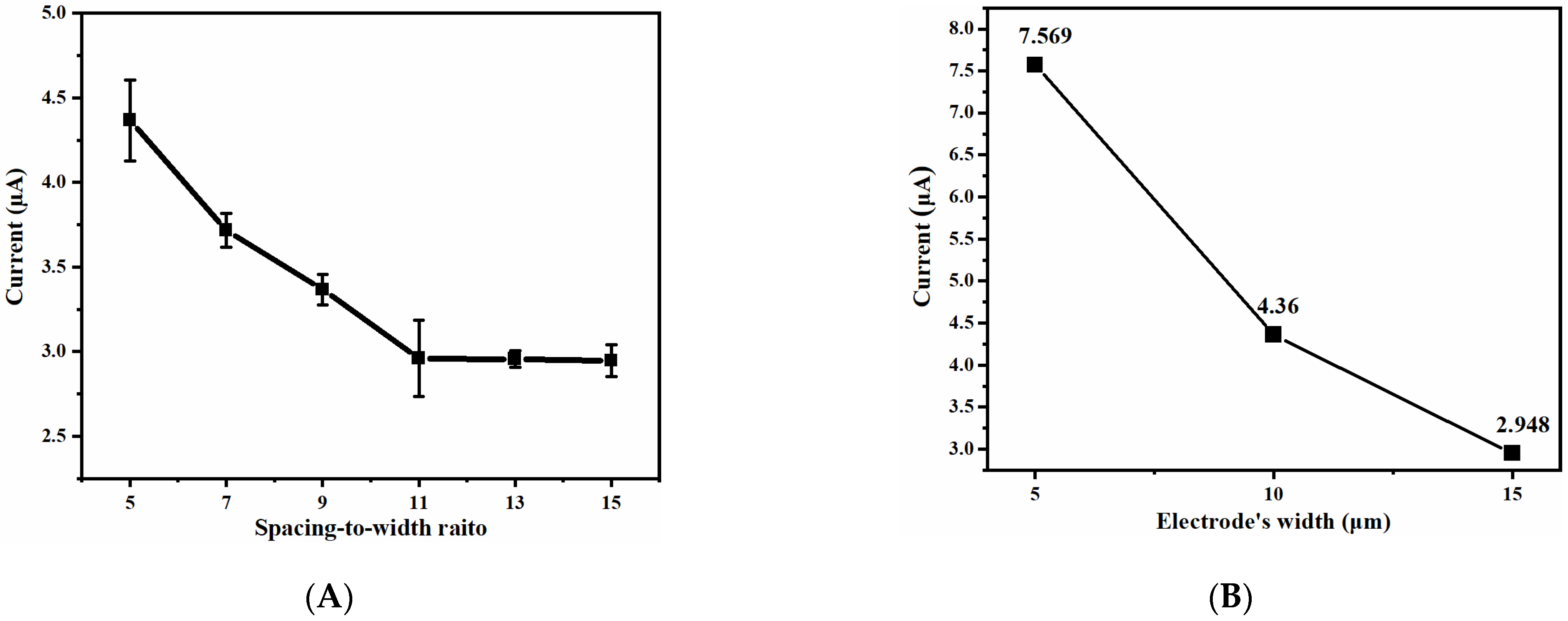
3.1.2. Simulation Results and Analysis of UIAE with Different Spacing-to-Width Ratios
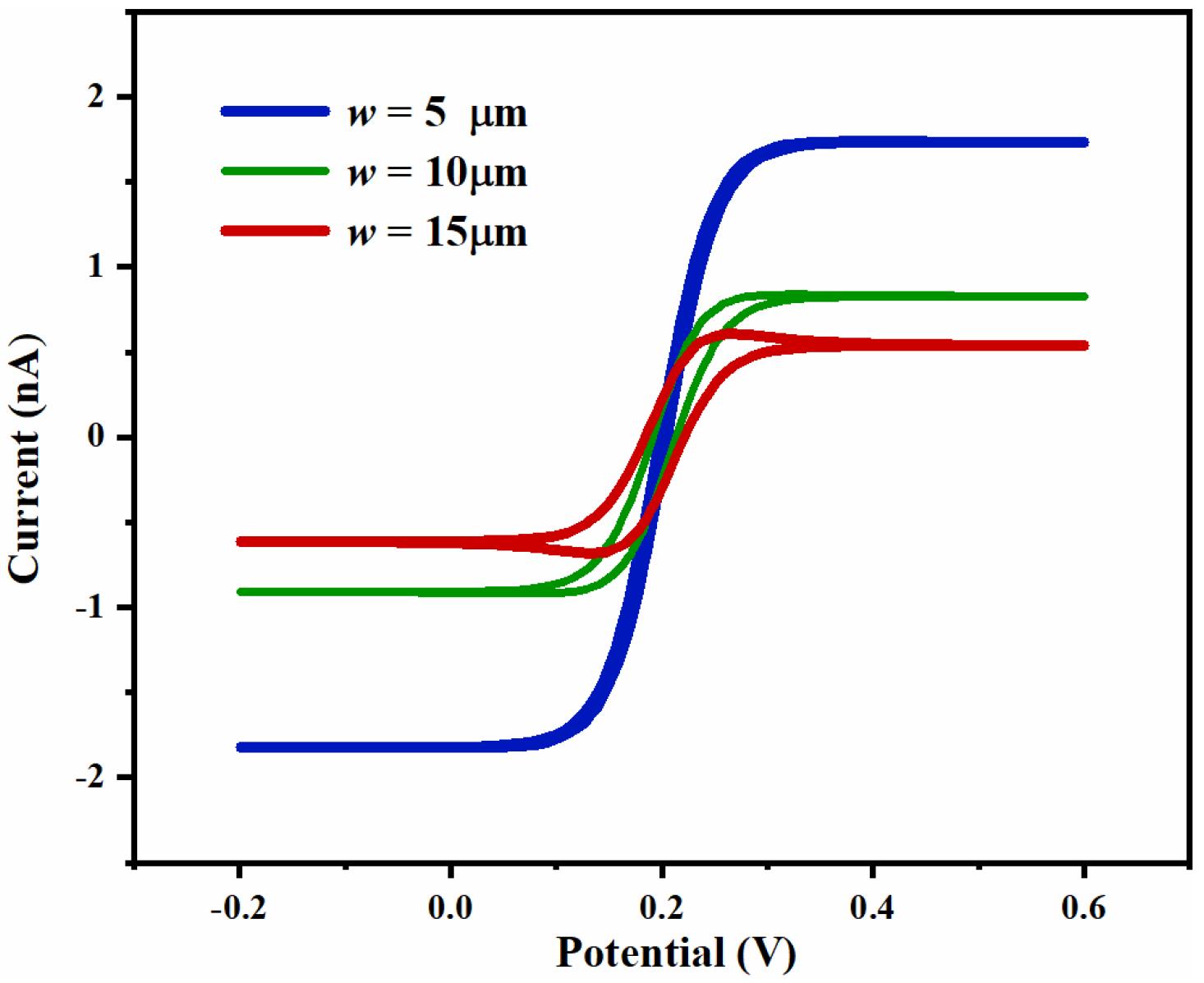
3.1.3. Simulation Results and Analysis of UIAE with Different Widths of Electrode
3.2. Electrochemical Characterization of Array Electrodes
3.2.1. Comparison between UIAE and UBAE
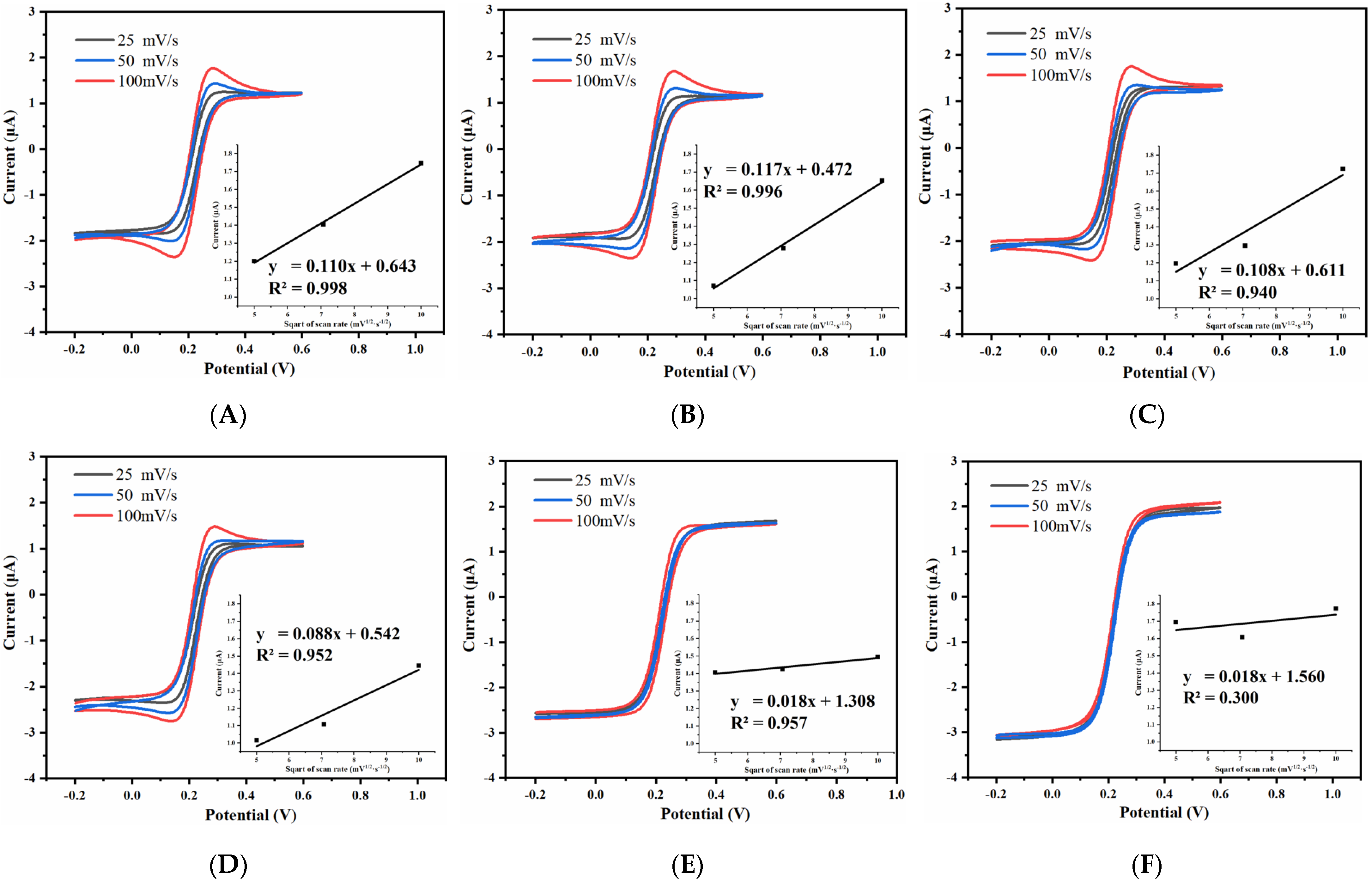
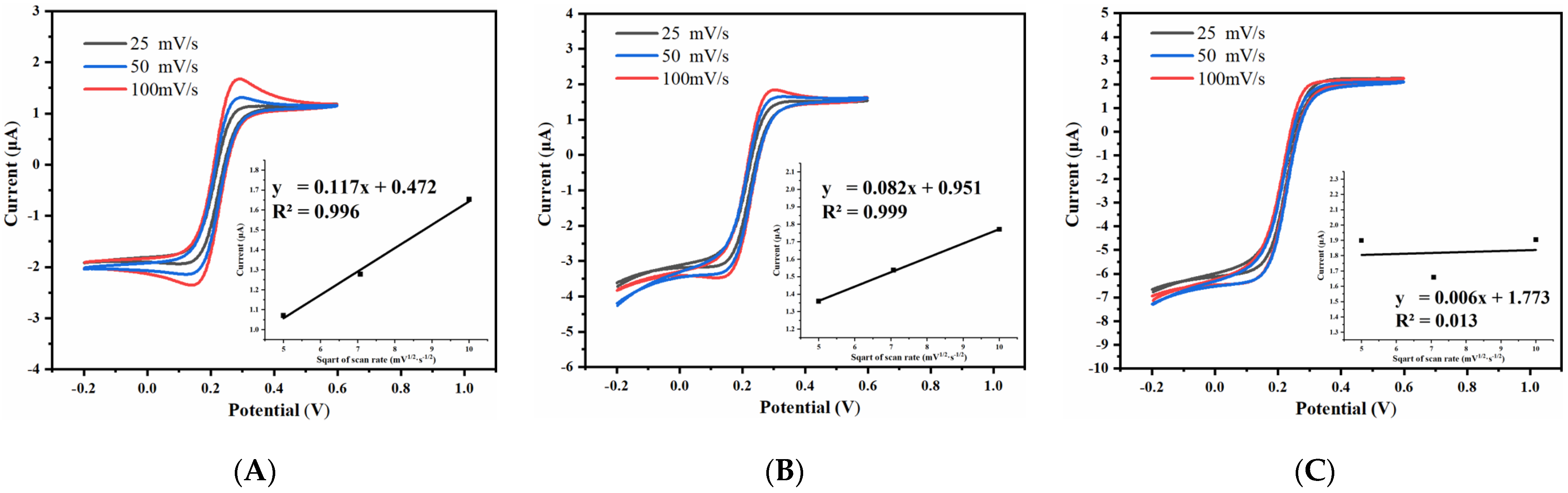
3.2.2. Effect of Spacing-to-Width Ratio of UIAE on Cyclic Voltammetry Characteristics
3.2.3. Effect of Width of UIAE on Cyclic Voltammetry Characteristics
3.3. Detection of Ammonia Nitrogen with UIAE
3.3.1. Effect of Spacing-to-Width Ratio on Ammonia Nitrogen Detection Performance
3.3.2. Effect of Width on Ammonia Nitrogen Detection Performance
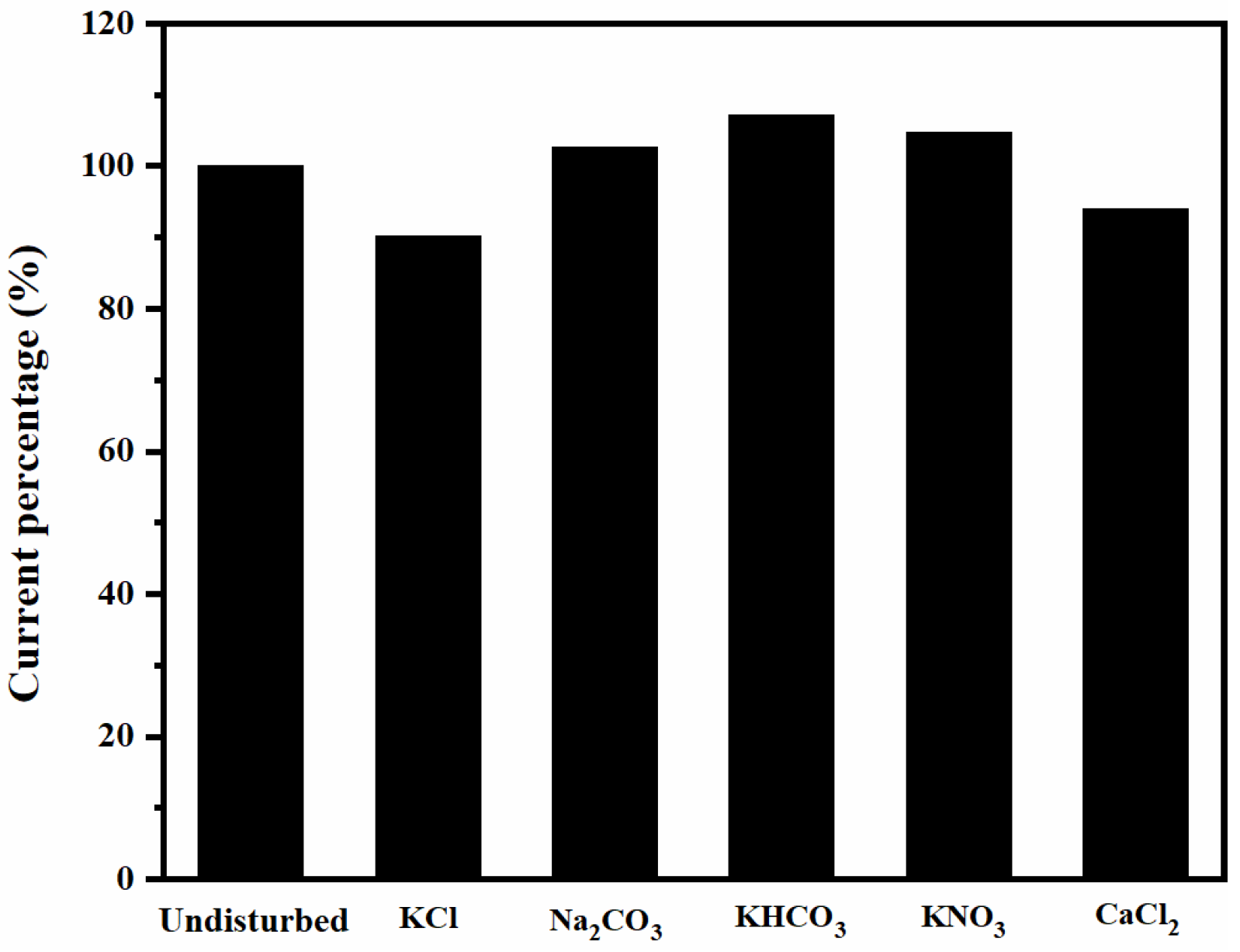
3.3.3. Other Performance Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, Y.; Mao, Y.; Kang, Z.; Luo, Q. Application of an ammonium ion-selective electrode for the real-time measurement of ammonia nitrogen based on pH and temperature compensation. Measurement 2019, 137, 98–101. [Google Scholar] [CrossRef]
- Zhu, R. The Harm and Controlling measures of Ammonia Nitrogen in Intensive Aquaculture Water. Henan Fish. 2018, 121, 5–7. [Google Scholar]
- Cong, M.; Li, Y.; Xu, H.; Lv, J.; Wu, H.; Zhao, Y. Ammonia nitrogen exposure caused structural damages to gill mitochondria of clam Ruditapes philippinarum. Ecotoxicol. Environ. Saf. 2021, 222, 112528. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, X.; Li, Z.; Wang, T.; Wang, C. Detection methods of ammonia nitrogen in water: A review. TrAC Trends Anal. Chem. 2020, 127, 115890. [Google Scholar] [CrossRef]
- Mahmud, M.P.; Ejeian, F.; Azadi, S.; Myers, M.; Pejcic, B.; Abbassi, R.; Razmjou, A.; Asadnia, M. Recent progress in sensing nitrate, nitrite, phosphate, and ammonium in aquatic environment. Chemosphere 2020, 259, 127492. [Google Scholar] [CrossRef]
- Insausti, M.; Timmis, R.; Kinnersley, R.; Rufino, M.C. Advances in sensing ammonia from agricultural sources. Sci. Total Environ. 2020, 706, 135124. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhu, C.; Wang, Z.; Wang, Y.; Li, C.; Hu, Q.; Xu, R.; Du, T.; Xu, M.; Feng, L. A full-set and self-powered ammonia leakage monitor system based on CNTs-PPy and triboelectric nanogenerator for zero-carbon vessels. Nano Energy 2022, 98, 107271. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, H.; Huang, P.; Xiang, C.; Zou, Y.; Xu, F.; Sun, L. Inducement of nanoscale Cu–BTC on nanocomposite of PPy–rGO and its performance in ammonia sensing. Mater. Res. Bull. 2018, 99, 152–160. [Google Scholar] [CrossRef]
- Beniwal, A.; Sunny. Electrospun SnO2/PPy nanocomposite for ultra-low ammonia concentration detection at room temperature. Sens. Actuators B Chem. 2019, 296, 126660. [Google Scholar] [CrossRef]
- Singh, S.K.; Shukla, R.K.; Kumar, R.; Tripathi, U.K.; Mishra, S.K. Investigation on PANI/CuO nanocomposites for ammonia gas sensing applications. Mater. Lett. 2022, 309, 131325. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, Y.; Lang, M.; Lv, Z.; Ma, W.; Gu, N.; Liu, H.; Fang, J.; Zhang, H.; Cheng, Y. Core-shell Au@SiO2 nanocrystals doped PANI for highly sensitive, reproducible and flexible ammonia sensor at room temperature. Appl. Surf. Sci. 2022, 598, 153821. [Google Scholar] [CrossRef]
- Heming, B.; Chao, B.; Jianhua, T.; Jizhou, S.; Shanhong, X.; Jinghong, H. Ammonia nitrogen measurement system based on miniaturized ammonia sensing unit. Chin. J. Sci. Instrum. 2011, 32, 1936–1940. [Google Scholar]
- Ning, Y.-F.; Yan, P.; Chen, Y.-P.; Guo, J.-S.; Shen, Y.; Fang, F.; Tang, Y.; Gao, X. Development of a Pt modified microelectrode aimed for the monitoring of ammonium in solution. Int. J. Environ. Anal. Chem. 2017, 97, 85–98. [Google Scholar] [CrossRef]
- Wang, J.; Diao, P. Simultaneous detection of ammonia and nitrate using a modified electrode with two regions. Microchem. J. 2020, 154, 104649. [Google Scholar] [CrossRef]
- Peng, X. Preparation of Carbon Cloth-Supported Polypyrrole/Platinum Nickel Alloy Composite Electrode and Its Ammonia-Nitrogen Sensitivity; Me, D., Ed.; JiLin University: Jilin, China, 2020. [Google Scholar]
- Wang, G.; Gao, J.; Sun, B.; He, D.; Zhao, C.; Suo, H. Enhanced Ammonia Sensitivity Electrochemical Sensors Based on PtCu Alloy Nanoparticles In-Situ Synthesized on Carbon Cloth Electrode. J. Electroanal. Chem. 2022, 922, 116721. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Peng, X.; Cui, Q.; He, D.; Zhao, C.; Suo, H. Fabrication of a Ni foam-supported platinum nanoparticles-silver/polypyrrole electrode for aqueous ammonia sensing. Synth. Met. 2020, 259, 116257. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, J.; Cui, G.; Zhao, C.; Suo, H.; He, D. A novel electrochemical ammonia–nitrogen sensor based on carbon cloth-supported hierarchical Pt nanosheets-Ni(OH)2 nanosheets nanocomposites. Chem. Eng. Sci. 2021, 239, 116634. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, T.; Ren, R.; Zhang, J.; He, D.; Zhao, C.; Suo, H. In situ synthesis of hierarchical platinum nanosheets-polyaniline array on carbon cloth for electrochemical detection of ammonia. J. Hazard. Mater. 2020, 392, 122342. [Google Scholar] [CrossRef]
- de Vooys, A.C.A.; Koper, M.T.M.; van Santen, R.A.; Veen, J.A.R.v. The role of adsorbates in the electrochemical oxidation of ammonia on noble and transition metal electrodes. J. Electroanal. Chem. 2001, 506, 127–137. [Google Scholar] [CrossRef]
- Elnabawy, A.O.; Herron, J.A.; Karraker, S.; Mavrikakis, M. Structure sensitivity of ammonia electro-oxidation on transition metal surfaces: A first-principles study. J. Catal. 2021, 397, 137–147. [Google Scholar] [CrossRef]
- Rosca, V.; Koper, M.T. Electrocatalytic oxidation of ammonia on Pt(111) and Pt(100) surfaces. Phys. Chem. Chem. Phys. 2006, 8, 2513–2524. [Google Scholar] [CrossRef]
- Gerischer, H.J.J.E.C. Untersuchungen Zur anodischen Oxidation von Ammoniak an Platin-Elektroden. J. Electroanal. Chem. 1970, 25, 421–433. [Google Scholar] [CrossRef]
- Jiyan Gu, X.S. Research Progress of Interdigitated Microelectrodes. Adv. Anal. Chem. 2021, 11, 28–37. [Google Scholar]
- Kosri, E.; Ibrahim, F.; Thiha, A.; Madou, M. Micro and Nano Interdigitated Electrode Array (IDEA)-Based MEMS/NEMS as Electrochemical Transducers: A Review. Nanomaterials 2022, 12, 4171. [Google Scholar] [CrossRef]
- Xu, J.H.; Chen, H.Y.; Gao, H. Investigation on Microelectrodes ⅩⅤ. Diffusion on Microband and Microband-Array Electrodes. Acta Chim. Sin. 1992, 279, 20729–20741. [Google Scholar]
- Nengqin, J.; Chenyun, T.; Dajun, C.; Zongrang, Z.; Jianzhong, Z.; Guoxiong, Z. Electrochemical characterization of Planar microband array electrode. J. Shanghai Teach. Univ. Nat. Sci. 1997, 26, 6. [Google Scholar]
- Daniel, D.; Gutz, I.G.R. Microfluidic cells with interdigitated array gold electrodes: Fabrication and electrochemical characterization. Talanta 2005, 68, 429–436. [Google Scholar] [CrossRef]
- SAITO, Y.J.R.o.P. A theoretical study on the diffusion current at the stationary electrodes of circular and narrow band types. Rev. Polarogr. 1968, 15, 177–187. [Google Scholar] [CrossRef]
- Li, Y.; Roy, W.V.; Lagae, L.; Vereecken, P.M. Effects of Counter Electrode Induced Redox Cycling on Fe(III) Reduction within Microfluidic Electrochemical Cells. J. Electrochem. Soc. 2014, 161, E128. [Google Scholar] [CrossRef]

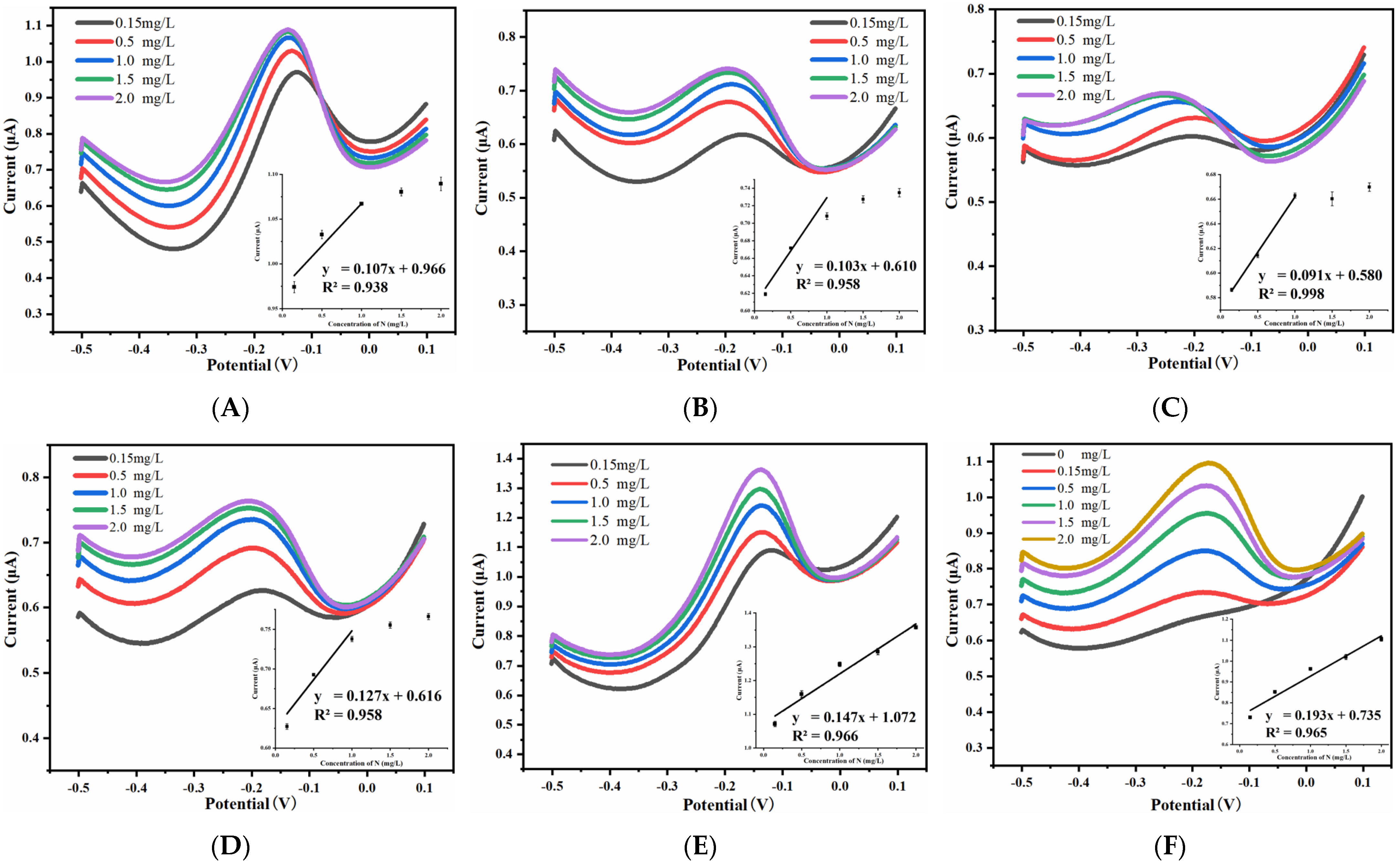

| Width (μm) | Spacing-To-Width Ratio | Detection Range (mg/L) | Sensitivity (μA·L·mg−1) | Linearity |
|---|---|---|---|---|
| 15 | 15 | 0.15~1.0 | 0.107 | 0.938 |
| 13 | 0.15~1.0 | 0.103 | 0.958 | |
| 11 | 0.15~1.0 | 0.091 | 0.998 | |
| 9 | 0.15~1.0 | 0.127 | 0.958 | |
| 7 | 0.15~2.0 | 0.147 | 0.966 | |
| 5 | 0.15~2.0 | 0.193 | 0.965 | |
| 10 | 13 | 0.15~2.0 | 0.170 | 0.990 |
| 5 | 13 | 0.15~2.0 | 0.418 | 0.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Li, Y.; Cong, A.; Tong, J.; Bian, C. Ultramicro Interdigitated Array Electrode Chip with Optimized Construction for Detection of Ammonia Nitrogen in Water. Micromachines 2023, 14, 629. https://doi.org/10.3390/mi14030629
Zhao H, Li Y, Cong A, Tong J, Bian C. Ultramicro Interdigitated Array Electrode Chip with Optimized Construction for Detection of Ammonia Nitrogen in Water. Micromachines. 2023; 14(3):629. https://doi.org/10.3390/mi14030629
Chicago/Turabian StyleZhao, Haifei, Yang Li, Aobo Cong, Jianhua Tong, and Chao Bian. 2023. "Ultramicro Interdigitated Array Electrode Chip with Optimized Construction for Detection of Ammonia Nitrogen in Water" Micromachines 14, no. 3: 629. https://doi.org/10.3390/mi14030629





