Structural Features and Water Resistance of Glass–Matrix Composites in a System of RNO3-KHSO4-P2O5 Containing Different Additives
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Vitrification
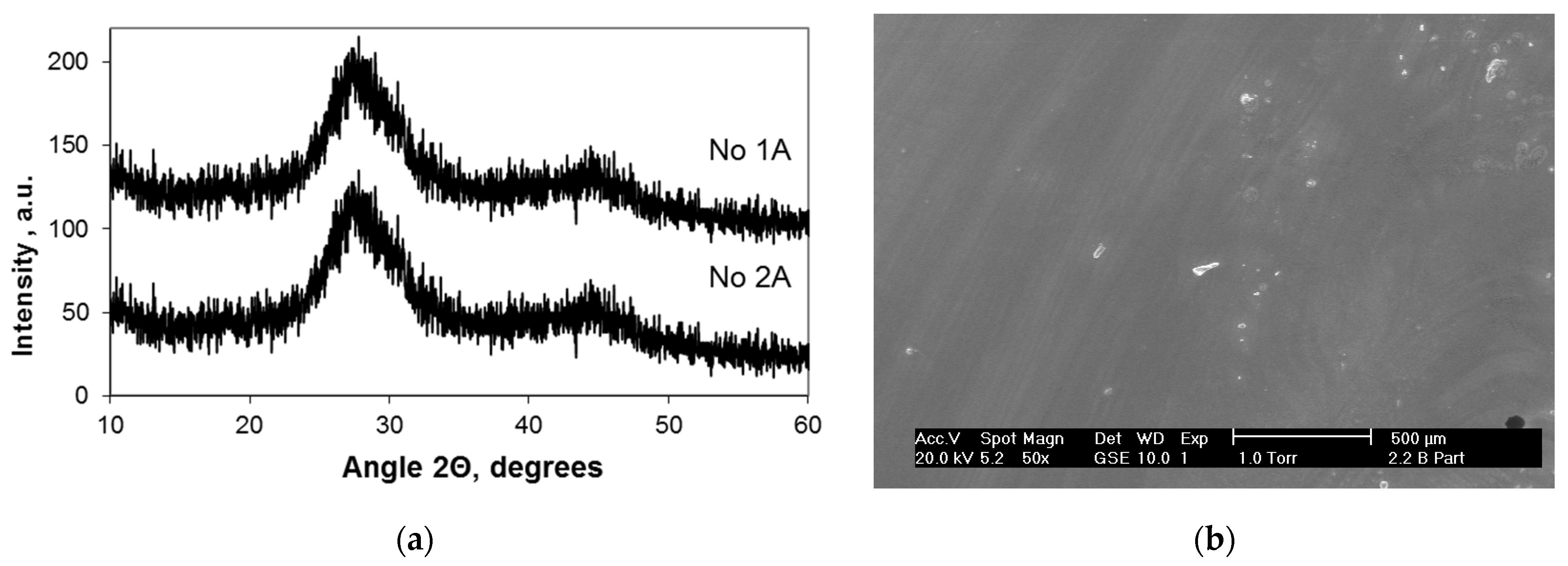
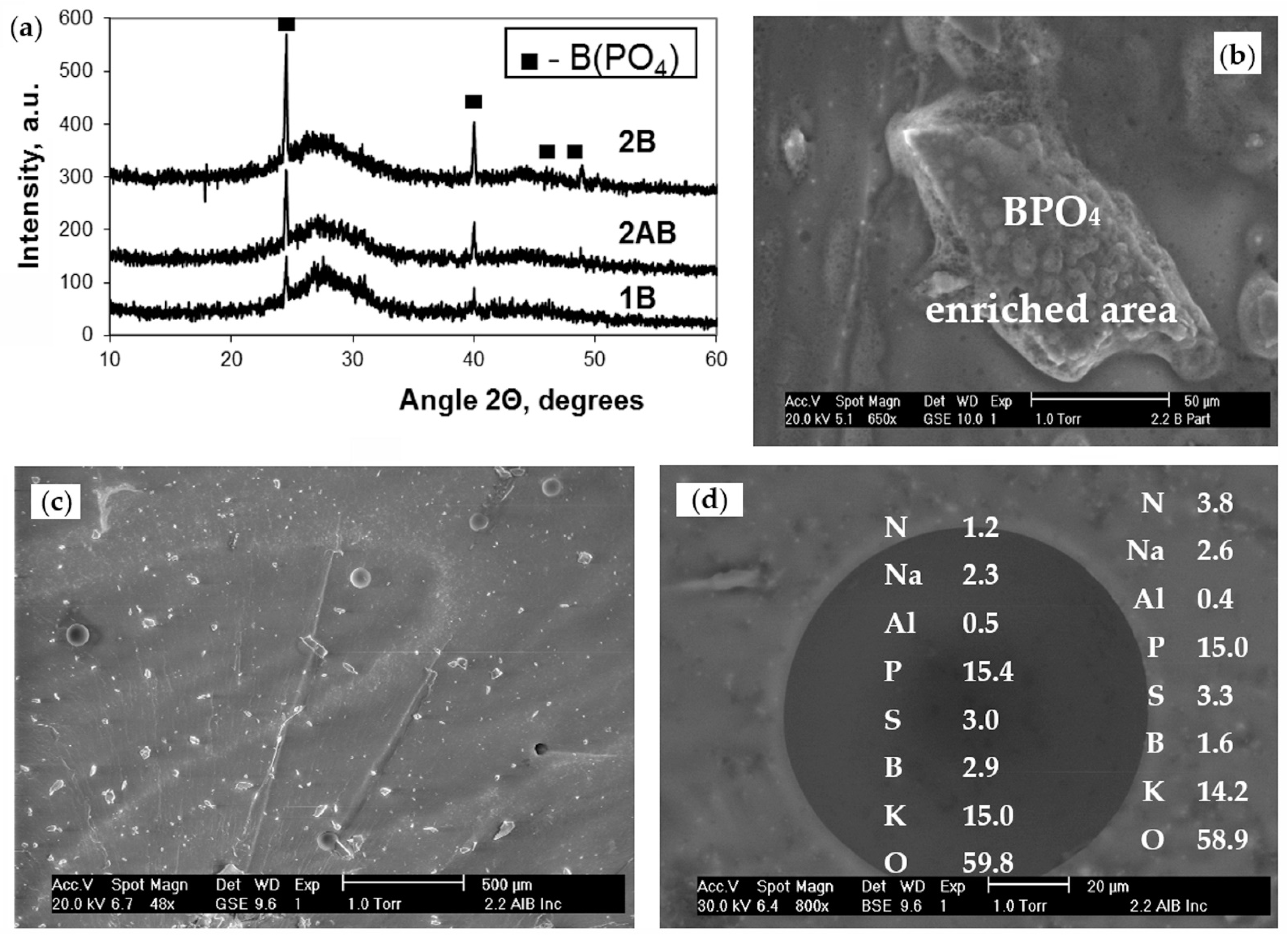
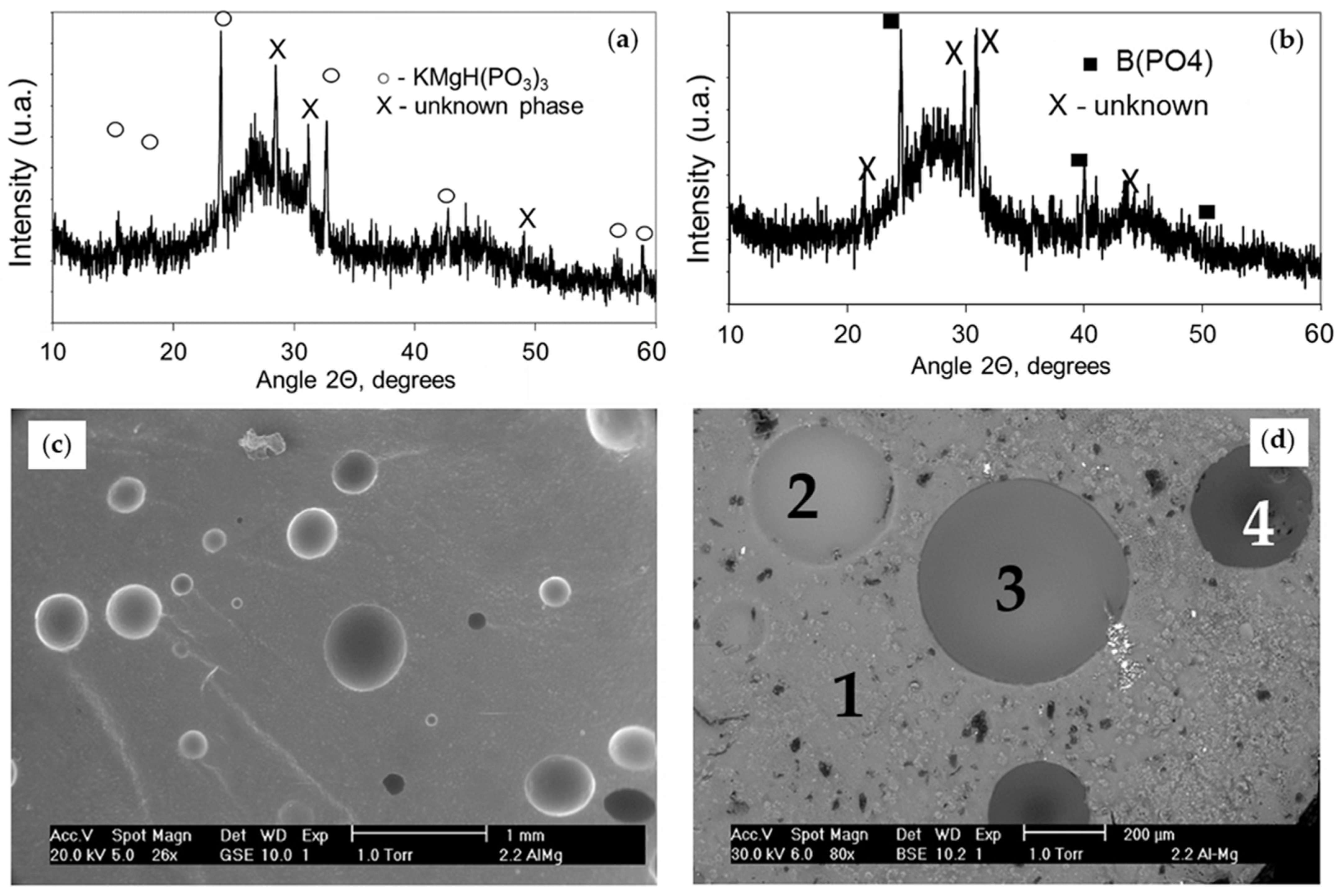
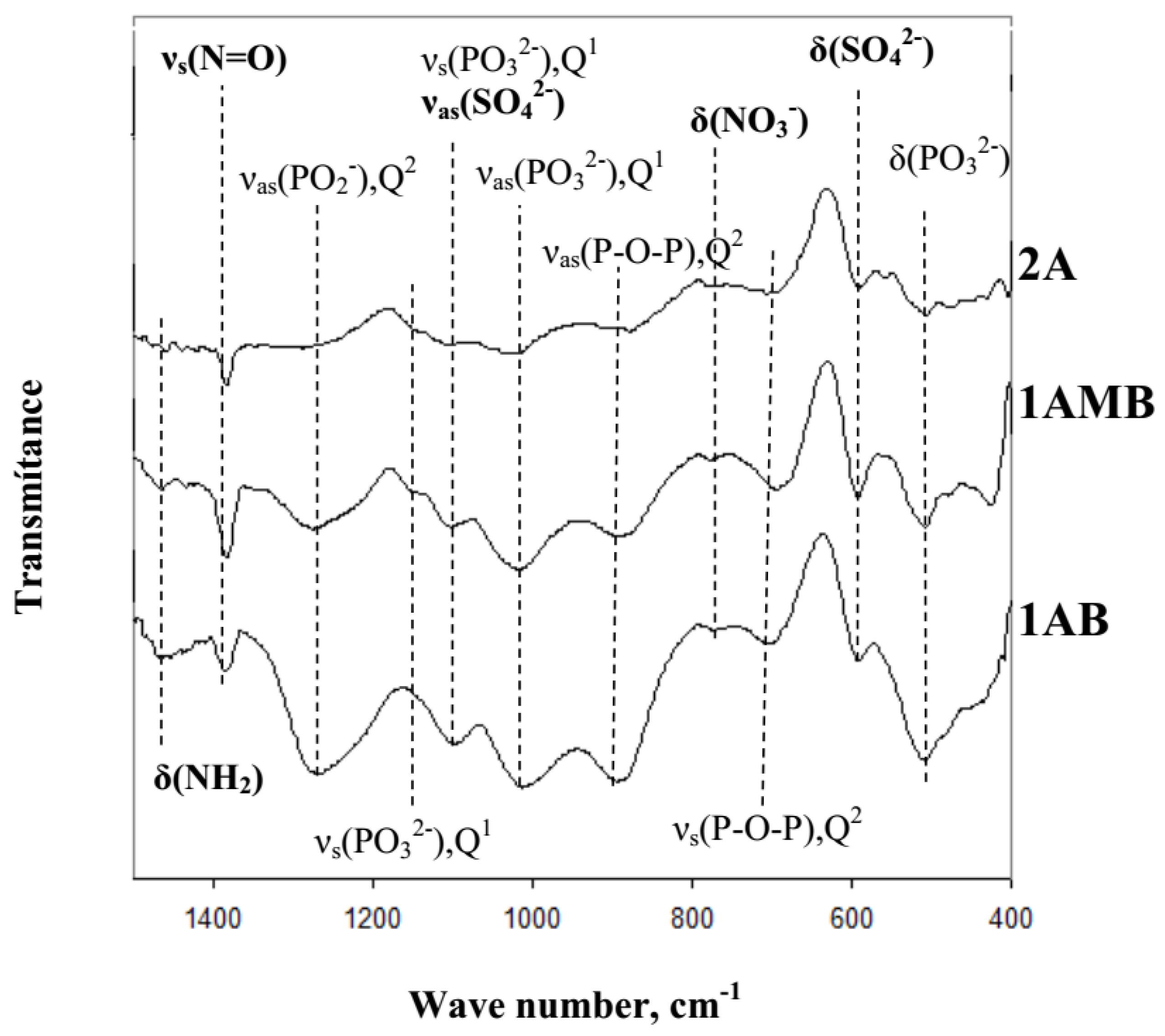
3.2. Structure
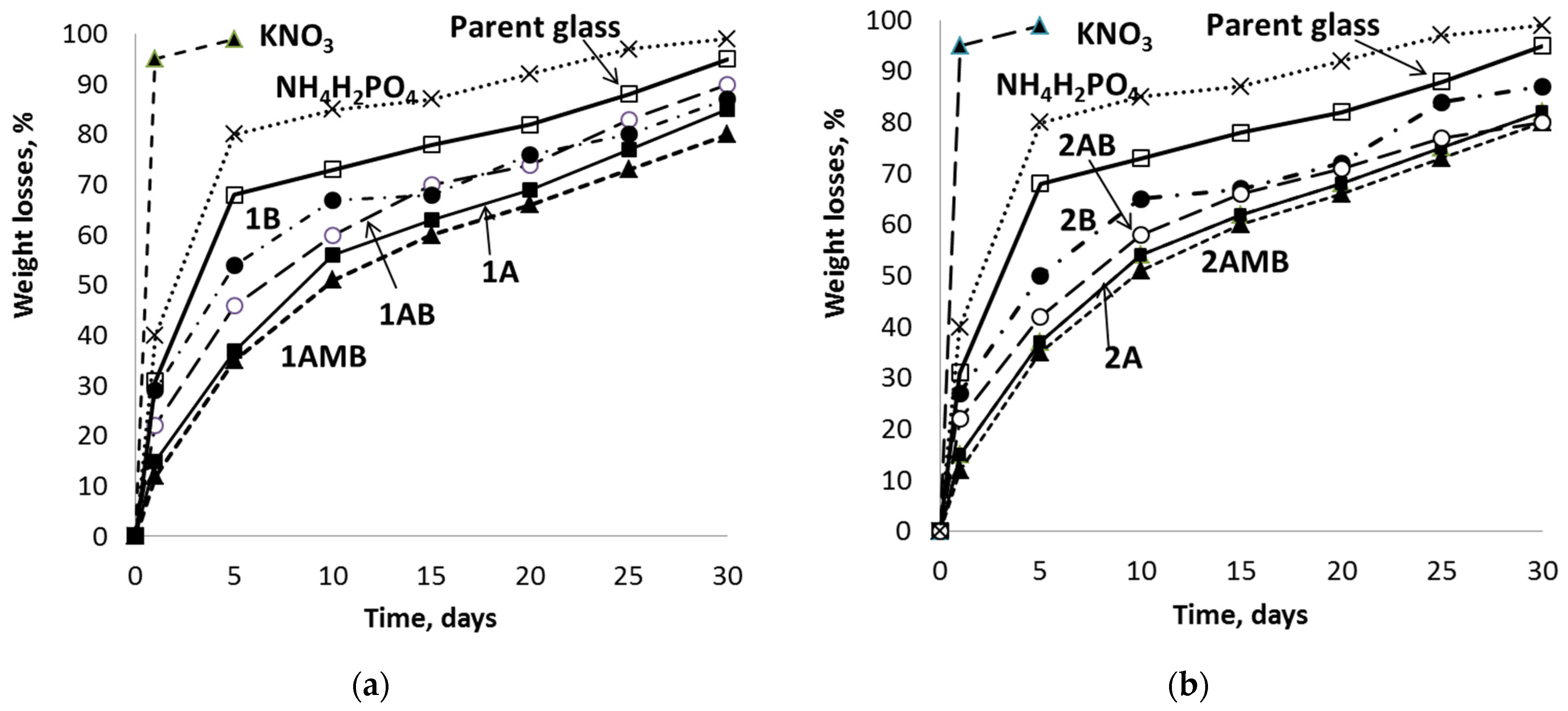
3.3. Chemical Durability
4. Discussion
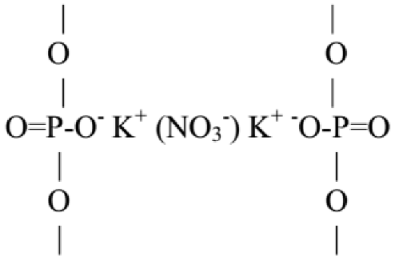
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hazra, G.; Das, T. A Review on Controlled Release Advanced Glassy Fertilizer. Glob. J. Sci. Front. Res. B Chem. 2014, 14, 33–44. [Google Scholar]
- Chen, J.; Lü, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally Friendly Fertilizers: A Review of Materials Used and Their Effects on the Environment. Sci. Total Environ. 2018, 613–614, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Sempeho, S.I.; Kim, H.T.; Egid, M.; Askwar, H. Meticulous Overview on the Controlled Release Fertilizers. Adv. Chem. 2014, 2014, 363071. [Google Scholar] [CrossRef] [Green Version]
- The International Organization for Standardization. ISO 18644 Fertilizers and Soil Conditioners, Controlled-Release Fertilizer, General Requirements, 1st ed.; ISO: Geneva, Switzerland, 2016; pp. 2–4. [Google Scholar]
- Barba, M.F.; Callejas, P.; Arzabe, J.O.; Ajò, D. Characterization of two frit ceramic materials in low cost fertilizers. J. Eur. Ceram. Soc. 1998, 18, 1313–1317. [Google Scholar] [CrossRef]
- Ouis, M.A.; Abd-Eladl, M.; Abou-Baker, N.H. Evaluation of agriglass as an environment friendly slow release fertilizer. Silicon 2018, 10, 293–299. [Google Scholar] [CrossRef]
- D’Amato, R.; De Feudis, M.; Troni, E.; Gualtieri, S.; Soldati, R.; Famiani, F.; Businelli, D. Agronomic potential of two different glass-based materials as novel inorganic slow-release iron fertilizers. J. Sci. Food Agric. 2022, 102, 1660–1664. [Google Scholar] [CrossRef]
- Karapetyan, G.; Karapetyan, K.; Maksimov, L. Glassy Environmentally Friendly Fertilizers of Prolonged Action. Phosphorus Res. Bull. 2004, 15, 60–67. [Google Scholar] [CrossRef]
- Labbilta, T.; Ait-El-Mokhtar, M.; Abouliatim, Y.; Khouloud, M.; Meddich, A.; Mesnaoui, M. Innovative Formulations of Phosphate Glasses as Controlled-Release Fertilizers to Improve Tomato Crop Growth, Yield and Fruit Quality. Molecules 2021, 26, 3928. [Google Scholar] [CrossRef]
- Militaru, B.A.; Vancea, C.; Pode, R. Glass Fertilizers Obtained Using Sewage Sludge Ash Wastes. Rev. Chim. 2019, 70, 3824–3829. [Google Scholar] [CrossRef]
- Perez-Medina, J.C.; Gorokhovsky, A.; Escalante-Garcia, J.I.; Peña-Cabriales, J.J. Synthesis and characterization of nitrate sulfate phosphate glasses. Glass Technol. 2005, 46, 183–186. [Google Scholar]
- Brow, R.K. Review: The structure of simple phosphate glasses. J. Non-Cryst. Solids 2000, 263–264, 1–28. [Google Scholar] [CrossRef]
- Downs, A.J. Chemistry of Aluminium, Gallium, Indium, and Thallium, 1st ed.; Chapman & Hall: London, UK, 1993; pp. 153–156. [Google Scholar]
- Donald, I.W.; Metcalfe, B.L.; Fong, S.K.; Gerrard, L.A. The influence of Fe2O3 and B2O3 additions on the thermal properties, crystallization kinetics and durability of a sodium aluminum phosphate glass. J. Non-Cryst. Solids 2006, 352, 2993–3001. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Chapter 13: The Group 13 Elements/Inorganic Chemistry, 3rd ed.; Pearson: London, UK, 2008; p. 340. [Google Scholar]
- Paulik, F.; Paulik, J.; Arnold, M.; Naumann, R. Investigation on the thermal behaviour of Mg(NO3)2∙6H2O. The decomposition behavior. J. Therm. Anal. Calorim. 1988, 34, 627–635. [Google Scholar] [CrossRef]
- Jlassi, I.; Elhouichet, H.; Ferida, M. Influence of MgO on structure and optical properties of alumino-lithium-phosphate glasses. Phys. E 2016, 81, 219–225. [Google Scholar] [CrossRef]
- Paryab, A.H.; Abdollahi, S.; Khalilifard, R.; Hosseini, H.R.M. Porous Slow Release Silicate-Phosphate Glasses Synthesized by Polymer Derived Ceramics Method Appropriate for Plants Nourishment. Iran. J. Mater. Sci. Eng. 2021, 18, 81–90. [Google Scholar]
- Calahoo, C.; Wondraczek, L. Ionic glasses: Structure, properties and classification. J. Non-Cryst. Solids X 2020, 8, 100054. [Google Scholar] [CrossRef]
- Łączka, M.; Ciecinska, M. Preparation, structure and properties of silicate-phosphate glasses obtained by means of sol-gel method. J. Sol-Gel Sci. Technol. 1994, 3, 219–227. [Google Scholar] [CrossRef]
- de Jager, H.J.; Prinsloo, L.C. The dehydration of the phosphates monitored by DSC/TGA and in situ Raman spectroscopy. Thermochim. Acta 2001, 376, 187–196. [Google Scholar] [CrossRef]
- Achary, S.N.; Tyagi, A.K. Strong anisotropic thermal expansion in cristobalite-type BPO4. J. Solid State Chem. 2004, 177, 3918–3926. [Google Scholar] [CrossRef]
- Moustafa, Y.M.; El-Eligi, K. Infrared spectra of sodium phosphate glasses. J. Non-Cryst. Solids 1998, 340, 144–153. [Google Scholar] [CrossRef]
- Peak, D.R.; Ford, G.D.; Sparks, L. An In Situ ATR-FTIR Investigation of Sulfate Bonding Mechanisms on Goethite. J. Colloid Interface Sci. 1999, 218, 289–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de los Arada Perez, M.A.; Perez Marin, L.; Calvo Quintana, J.; Yazdani-Pedram, M. Influence of different plasticizers on the response of chemical sensors based on polymeric membranes for nitrate ion determination. Sens. Actuators B 2003, 89, 262–268. [Google Scholar] [CrossRef]
- Huayashi, S.; Hayamizu, K. High-resolution solid state 31PNMR of alkali phosphates. Bull. Chem. Soc. Jpn 1989, 62, 3061–3068. [Google Scholar] [CrossRef] [Green Version]
- Brow, R.K.; Kirkpatrick, R.J.; Turner, G.L. Nature of alumina in phosphate glass: II Structure of sodium aluminophosphate glass. J. Am. Ceram. Soc. 1993, 76, 919–928. [Google Scholar] [CrossRef]
- Thilo, E.; Blumenthal, G. Zur chemie der kondensierten phosphate und arsenate über sulfatophosphate. Z. Anorg. Allg. Chem. 1966, 348, 77–88. [Google Scholar] [CrossRef]
- Nepomiluev, A.M.; Pletnev, R.N.; Lapina, O.B.; Kozlova, S.G.; Bamburov, V.G. Structure of Glasses in the Na2SO4–P2O5–H2O System. Glass Phys. Chem. 2002, 28, 1–4. [Google Scholar] [CrossRef]
- Greaves, G.N.; Sen, S. Inorganic glasses, glass-forming liquids and amorphizing solids. Adv. Phys. 2007, 56, 1–166. [Google Scholar] [CrossRef]
- Sirotkin, S.; Meszaros, R.; Wondraczek, L. Chemical Stability of ZnO-Na2O-SO3-P2O5 Glasses. Int. J. Appl. Glass Sci. 2012, 3, 44–52. [Google Scholar] [CrossRef]
- Le Sauze, A.; Montagne, L.; Palavit, G.; Fayon, F.; Marchand, R. X-ray photoelectron spectroscopy and nuclear magnetic resonance structural study of phosphorus oxynitride glasses, LiNaPON. J. Non-Cryst. Solids 2000, 263–264, 139–145. [Google Scholar] [CrossRef]
- Wang, Y.B.; Ryan, D.H.; Altounian, Z. Structural and thermal studies of nitrate glasses. J. Non-Cryst. Solids 1996, 205–207, 221–224. [Google Scholar] [CrossRef]
- Malugani, J.P.; Mercier, R.; Fahys, B.; Robert, G. Ionic conductivity of and Raman spectroscopy investigation binary oxosalts (1 − x)AgPO3−xAg2SO4 glasses. J. Solid State Chem. 1982, 45, 309–316. [Google Scholar] [CrossRef]
- Reibstein, S.; Da, N.; Simon, J.P.; Spiecker, E.; Wondraczek, L. Phase separation and crystal precipitation in supercooled sulphophosphate ionic melts. Phys. Chem. Glass-Eur. J. Glass Sci. Technol. Part B 2012, 53, 61–67. [Google Scholar]

| No | Content of the Component (wt.%) | ||||
|---|---|---|---|---|---|
| NH4H2PO4 | KNO3 | NaNO3 | KHSO4 | {H3BO3 + Al(NO3)3 + Mg(NO3)2} | |
| 0 | 67.7 | 21.3 | 7.4 | 4.9 | - |
| 1 | 64.8 | 20.4 | 7.1 | 3.5 | 4.2 |
| 2 | 62.1 | 19.4 | 6.7 | 3.4 | 8.4 |
| Group | Admixtures (Glass Composition Number) | ||||||
|---|---|---|---|---|---|---|---|
| Al | Mg | B | Al + Mg | Al + B | Mg + B | Al + Mg + B | |
| 1 | Transparent glass (1A) | Non (1M) | White glass (1B) | White glass (1AM) | White glass (1AB) | Non (1BM) | White glass (1AMB) |
| 2 | Transparent glass (2A) | Non (2M) | White glass (2B) | Non (2AM) | White glass (2AB) | Non (2MB) | White glass (2AMB) |
| No | Composition (Chemical Elements, at.%) | |||||||||
| H | N | S | K | Na | P | Al | B | Mg | O | |
| 0 | 8.2 | 8.1 | 4.4 | 14.0 | 2.8 | 14.7 | - | - | 47.7 | |
| 1AMB | 6.9 | 7.8 | 4.9 | 14.1 | 3.1 | 15.5 | 2.1 | 1.8 | 0.2 | 44.6 |
| 2AMB | 6.4 | 7.3 | 4.6 | 14.6 | 2.7 | 14.6 | 2.7 | 1.9 | 0.4 | 44.8 |
| Composition (oxides, mol.%) | ||||||||||
| H2O | N2O5 | SO3 | K2O | Na2O | P2O5 | Al2O3 | B2O3 | MgO | ||
| 0 | 12.2 | 14.1 | 15.4 | 24.3 | 4.9 | 29.1 | - | - | - | |
| 1AMB | 11.5 | 12.8 | 15.5 | 23.0 | 4.8 | 25.1 | 3.6 | 3.0 | 0.7 | |
| 2AMB | 10.5 | 12.2 | 15.0 | 23.6 | 4.6 | 25.6 | 4.3 | 3.3 | 0.9 | |
| Chemical Elements | Relative Contents, at.% | |||
|---|---|---|---|---|
| Point 1 | Point 2 | Point 3 | Point 4 | |
| N | 4.1 | 2 | 1.6 | 2.5 |
| B | 0.8 | 1.4 | 0.3 | 0.2 |
| Na | 2.6 | 2.8 | 2.6 | 3 |
| Al | 0.2 | 7.9 | 4.6 | 0.4 |
| P | 14.6 | 11.7 | 12.7 | 14.9 |
| S | 4.4 | 4.1 | 5.4 | 6 |
| K | 14.3 | 10.4 | 13 | 14 |
| Mg | 0.2 | 0.6 | 0.3 | - |
| O | 58.8 | 59.1 | 59.5 | 59 |
| Configuration | Content, mol.% | |
|---|---|---|
| 2AMB | 1AMB | |
| Orthophosphate | 9.4 | 4.5 |
| Pyrophosphate | 48.2 | 41.7 |
| Metaphisphate | 42.4 | 53.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorokhovsky, A.; Burmistrov, I.; Kuznetsov, D.; Gusev, A.; Khaydarov, B.; Kiselev, N.; Boychenko, E.; Kolesnikov, E.; Prokopovich, K. Structural Features and Water Resistance of Glass–Matrix Composites in a System of RNO3-KHSO4-P2O5 Containing Different Additives. Micromachines 2023, 14, 851. https://doi.org/10.3390/mi14040851
Gorokhovsky A, Burmistrov I, Kuznetsov D, Gusev A, Khaydarov B, Kiselev N, Boychenko E, Kolesnikov E, Prokopovich K. Structural Features and Water Resistance of Glass–Matrix Composites in a System of RNO3-KHSO4-P2O5 Containing Different Additives. Micromachines. 2023; 14(4):851. https://doi.org/10.3390/mi14040851
Chicago/Turabian StyleGorokhovsky, Alexander, Igor Burmistrov, Denis Kuznetsov, Alexander Gusev, Bekzod Khaydarov, Nikolay Kiselev, Elena Boychenko, Evgeny Kolesnikov, and Ksenia Prokopovich. 2023. "Structural Features and Water Resistance of Glass–Matrix Composites in a System of RNO3-KHSO4-P2O5 Containing Different Additives" Micromachines 14, no. 4: 851. https://doi.org/10.3390/mi14040851
APA StyleGorokhovsky, A., Burmistrov, I., Kuznetsov, D., Gusev, A., Khaydarov, B., Kiselev, N., Boychenko, E., Kolesnikov, E., & Prokopovich, K. (2023). Structural Features and Water Resistance of Glass–Matrix Composites in a System of RNO3-KHSO4-P2O5 Containing Different Additives. Micromachines, 14(4), 851. https://doi.org/10.3390/mi14040851











