Regulating the Polypyrrole Ion-Selective Membrane and Au Solid Contact Layer to Improve the Performance of Nitrate All-Solid Ion-Selective Electrodes
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Apparatus
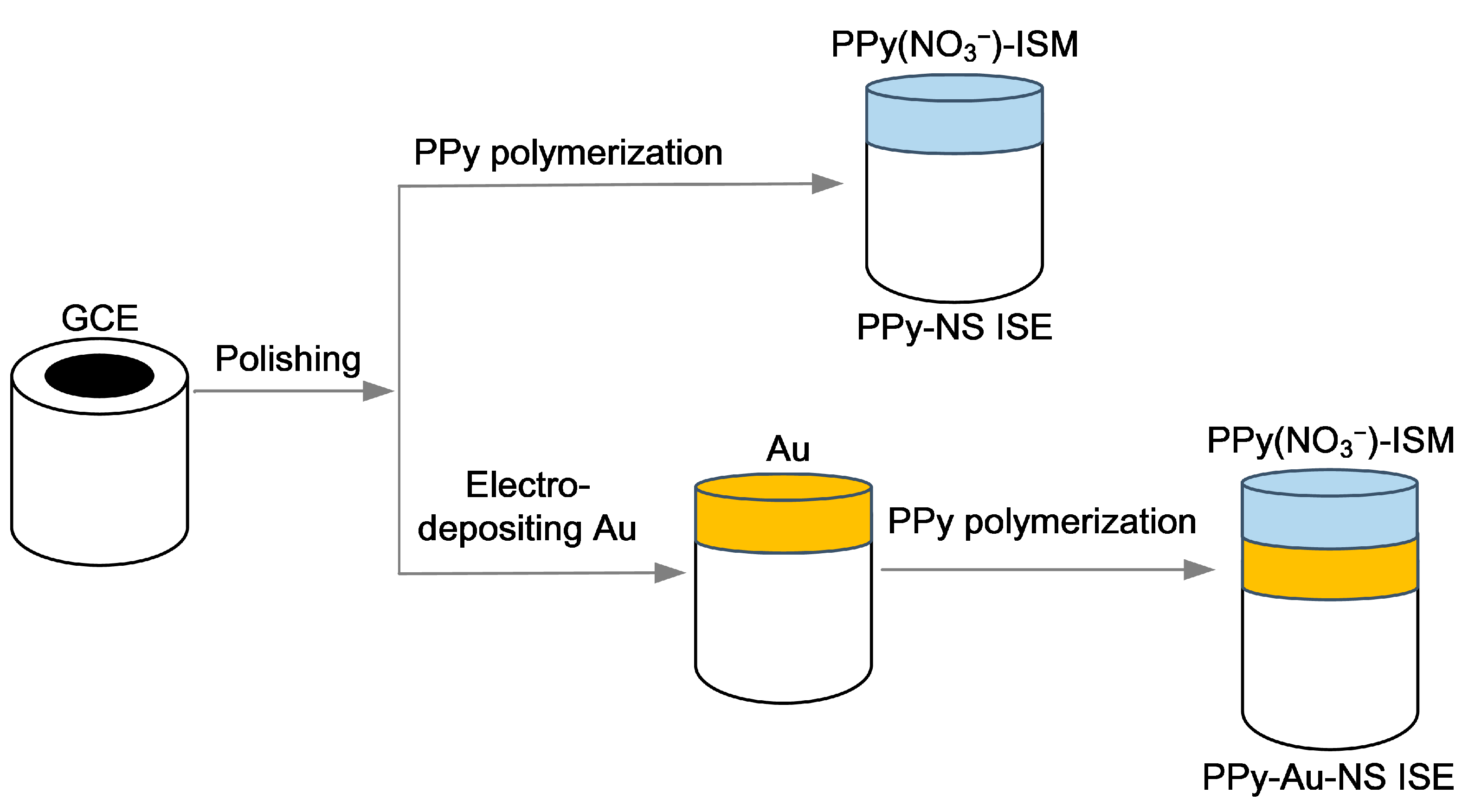
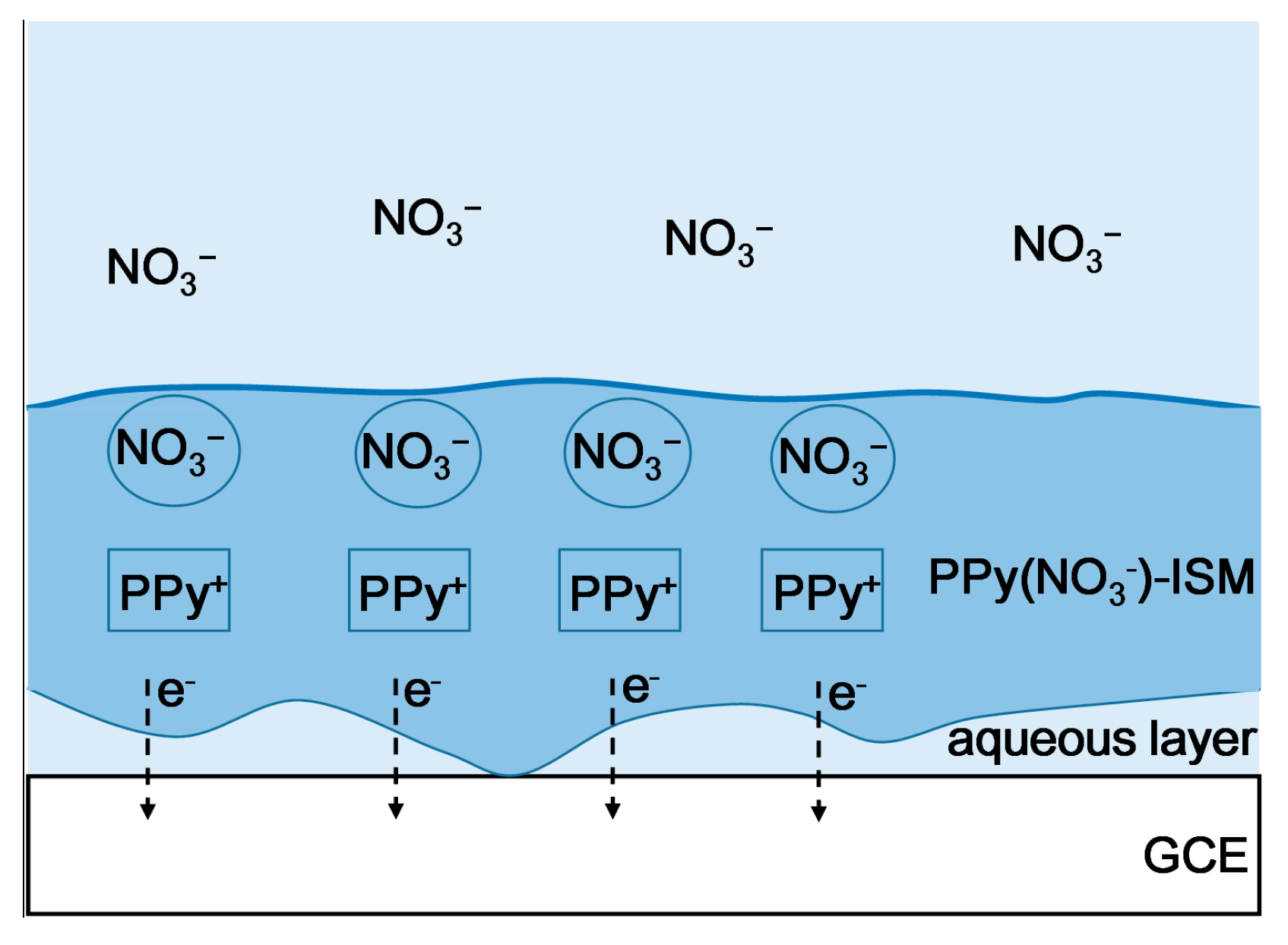
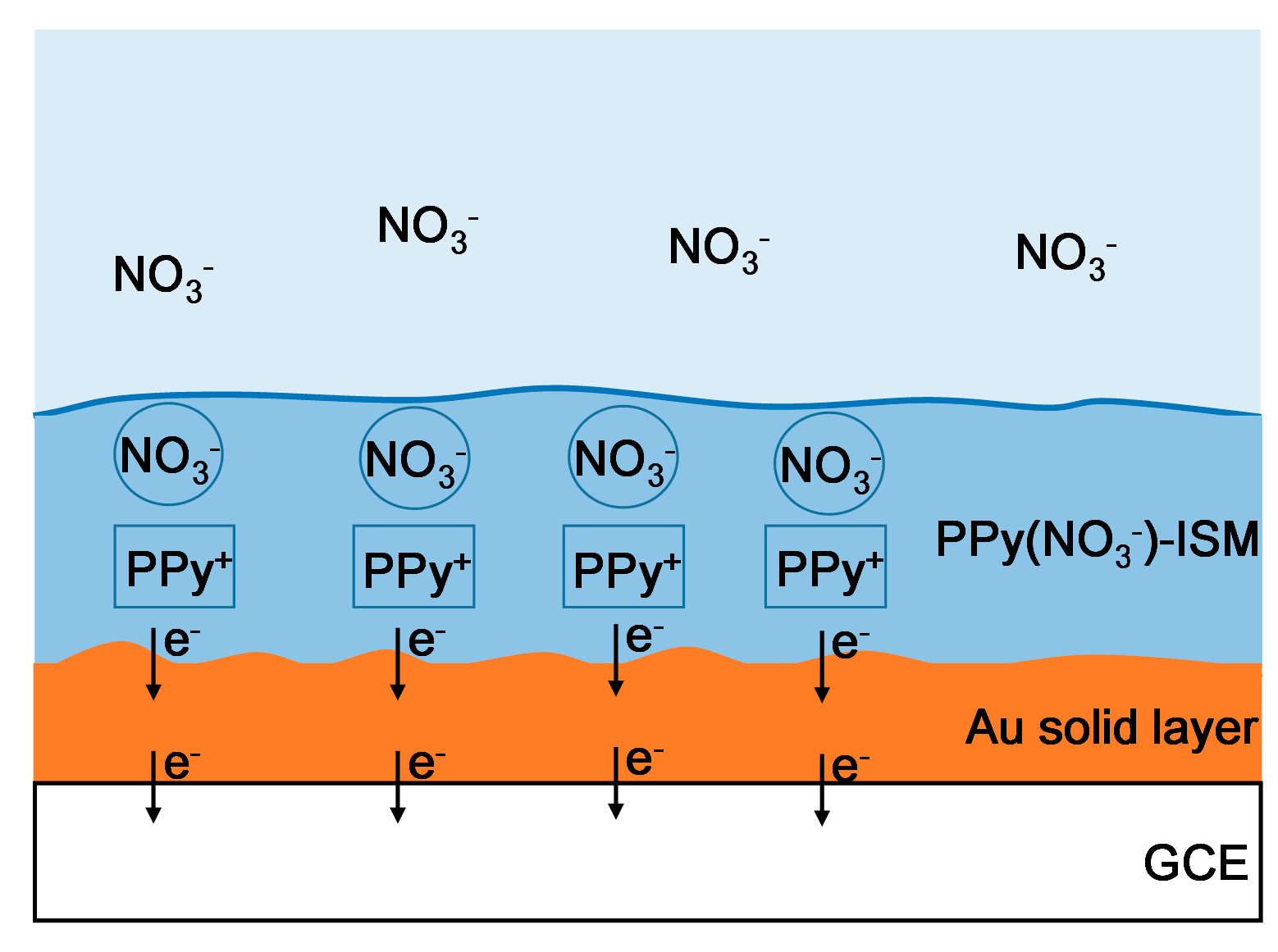
2.2. Construction of the PPy-Au-NS ISEs
2.3. Characterization of the As-Prepared PPy (NO3−)-ISMs and Au Solid Contact Layers
2.4. Electrochemical Characterization of the PPy-Au-NS ISEs
3. Results and Discussion
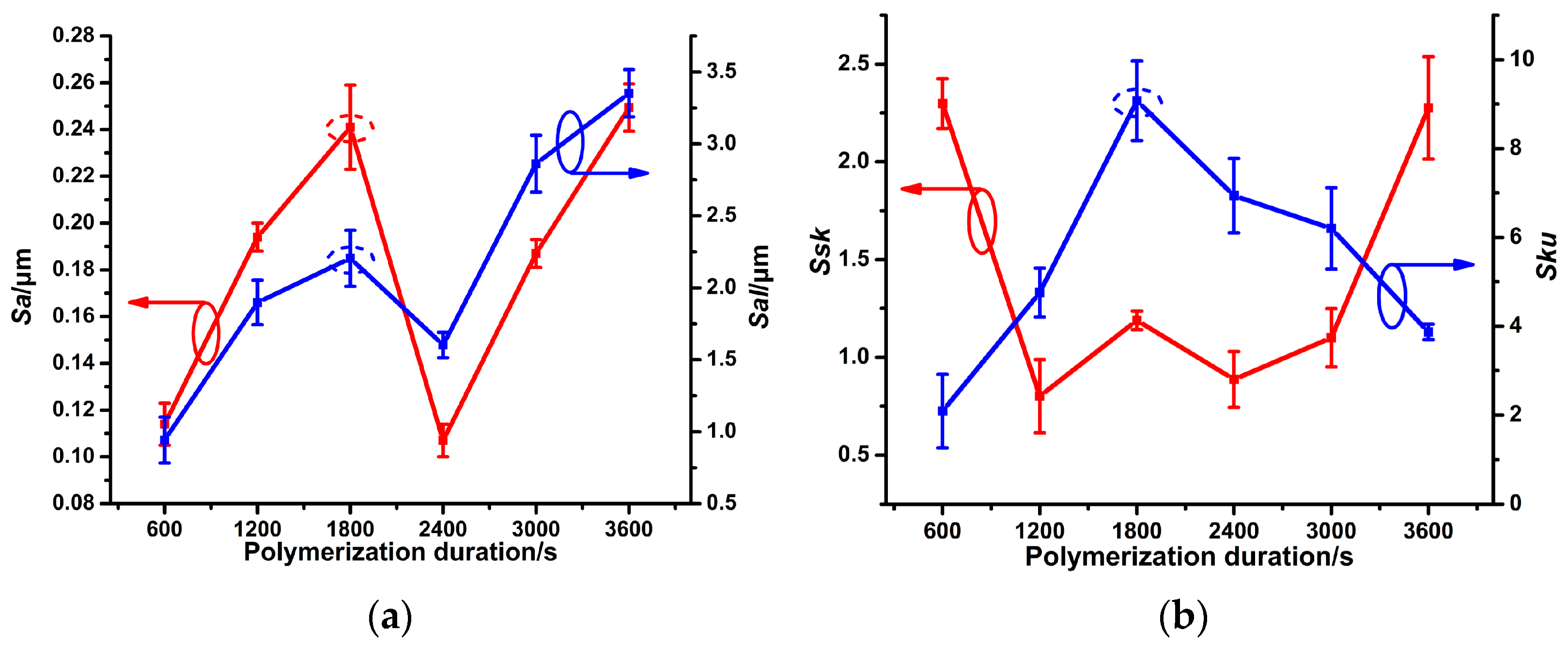
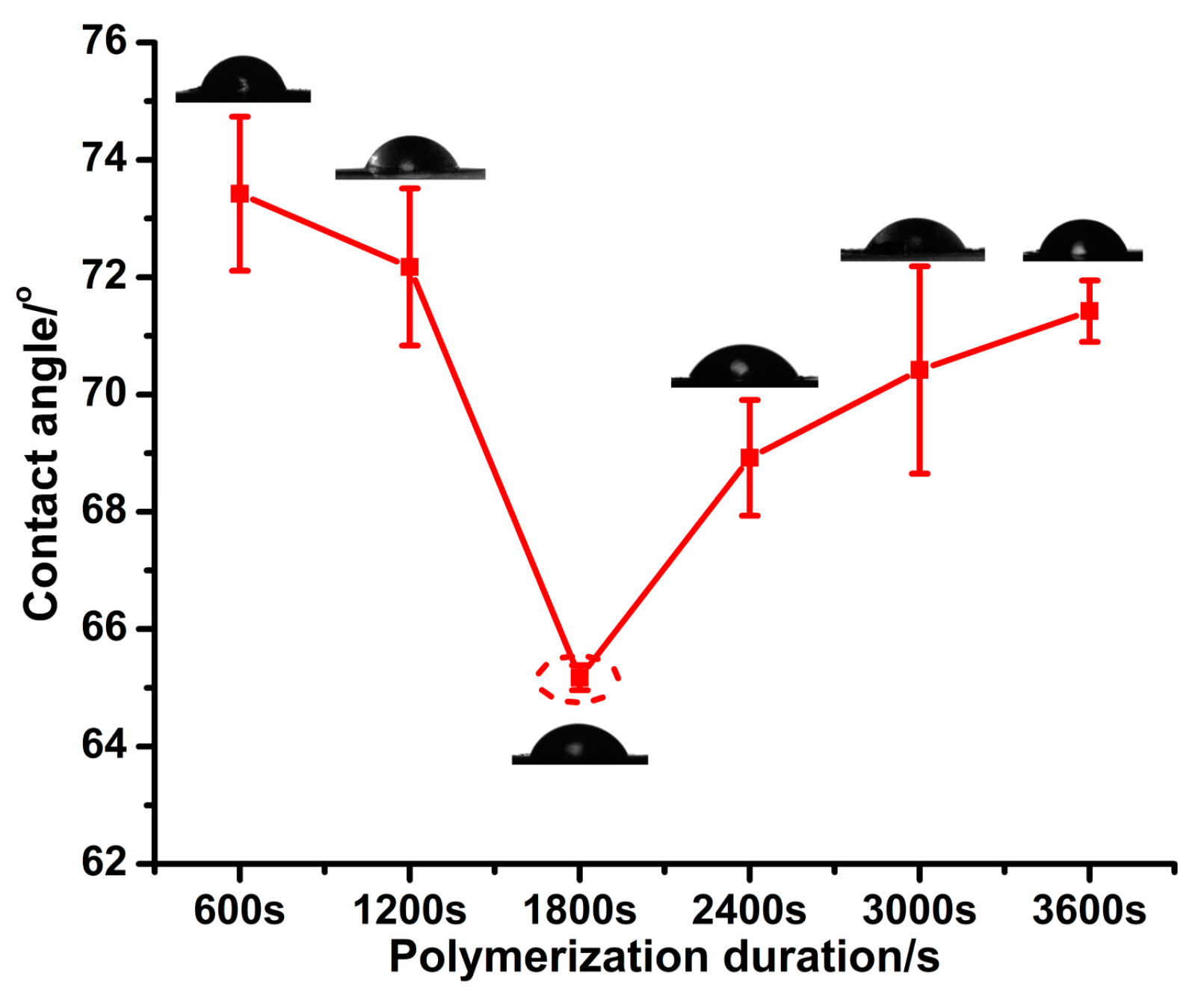
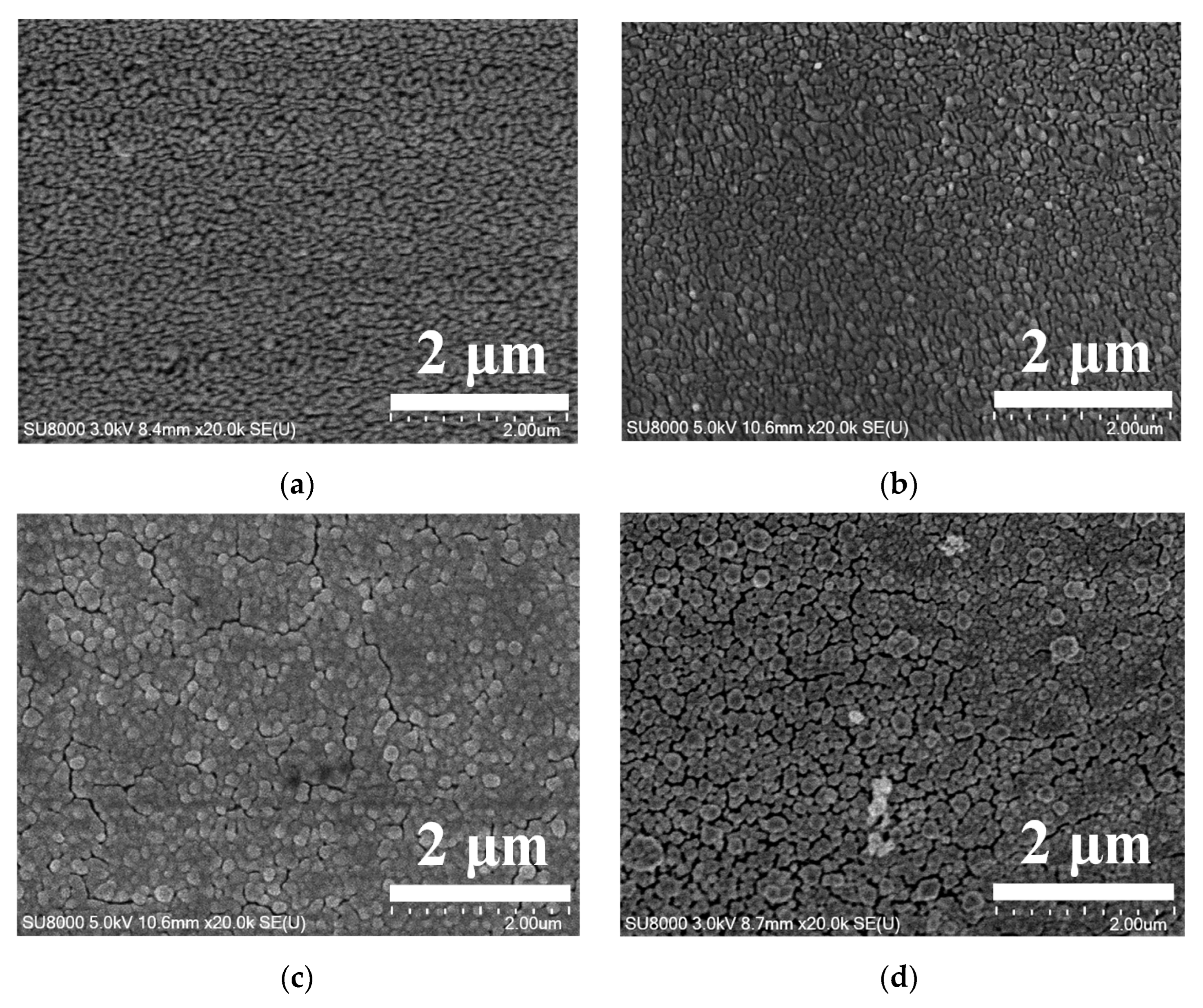
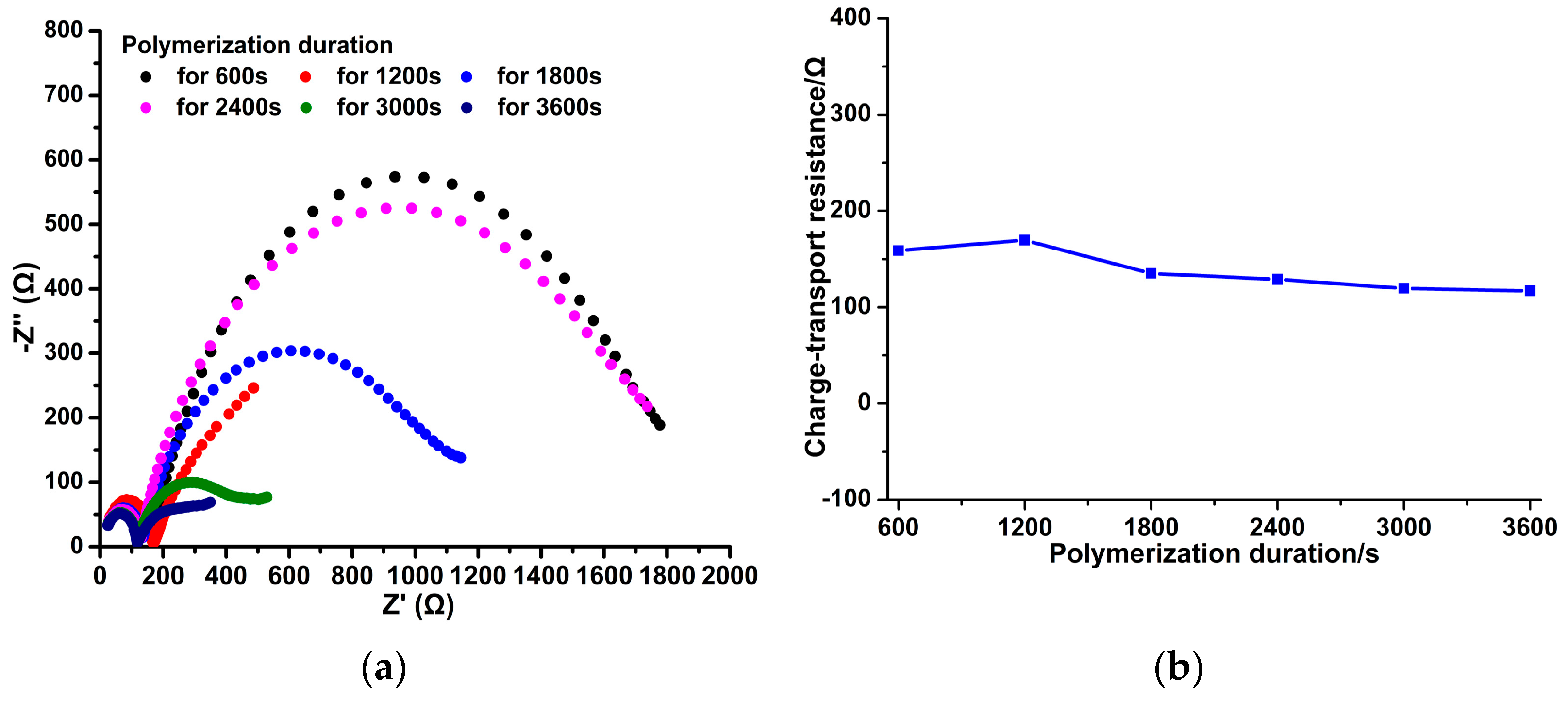
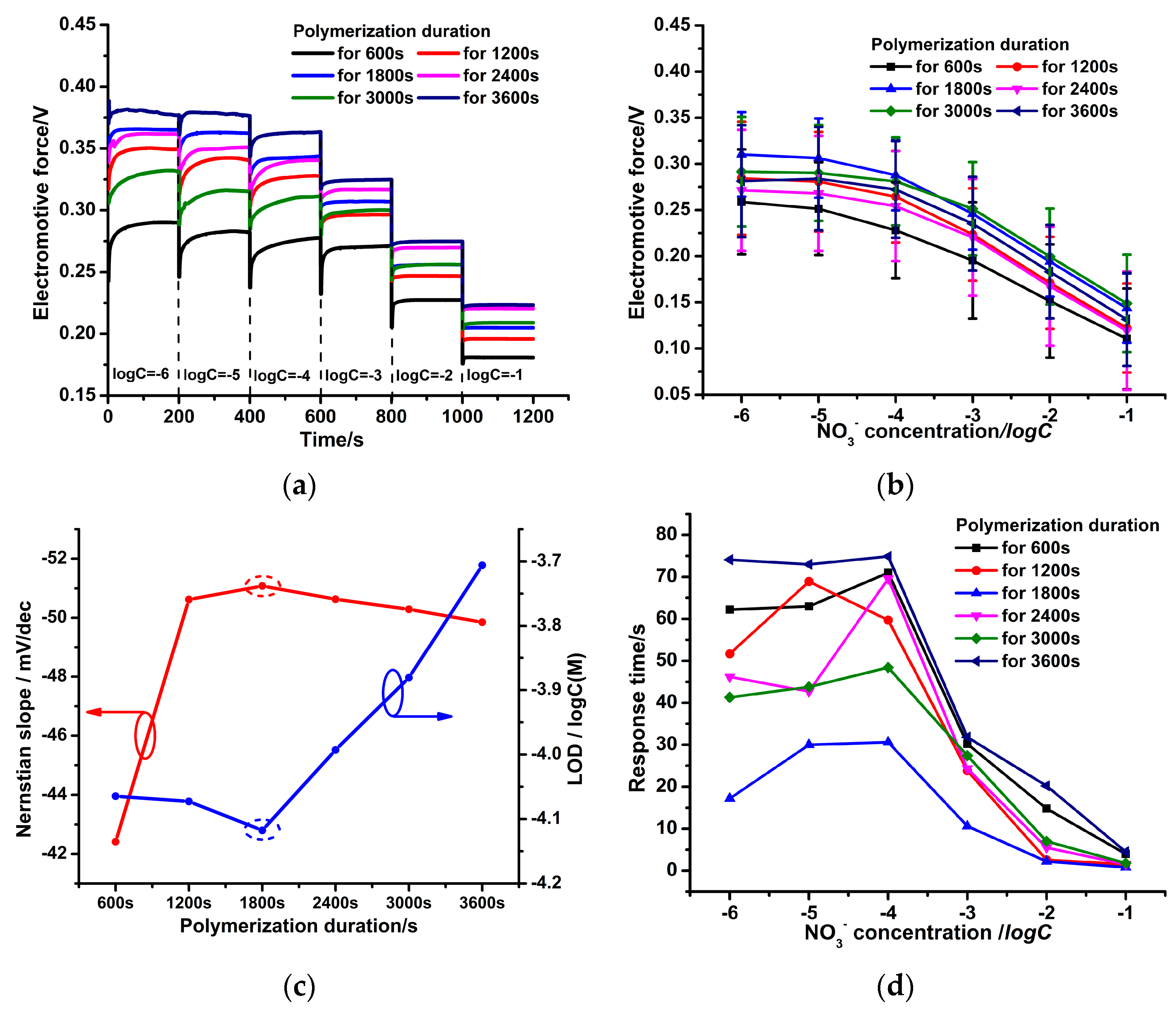
3.1. Different PPy(NO3−)-ISMs on the GCE for Various Polymerization Durations
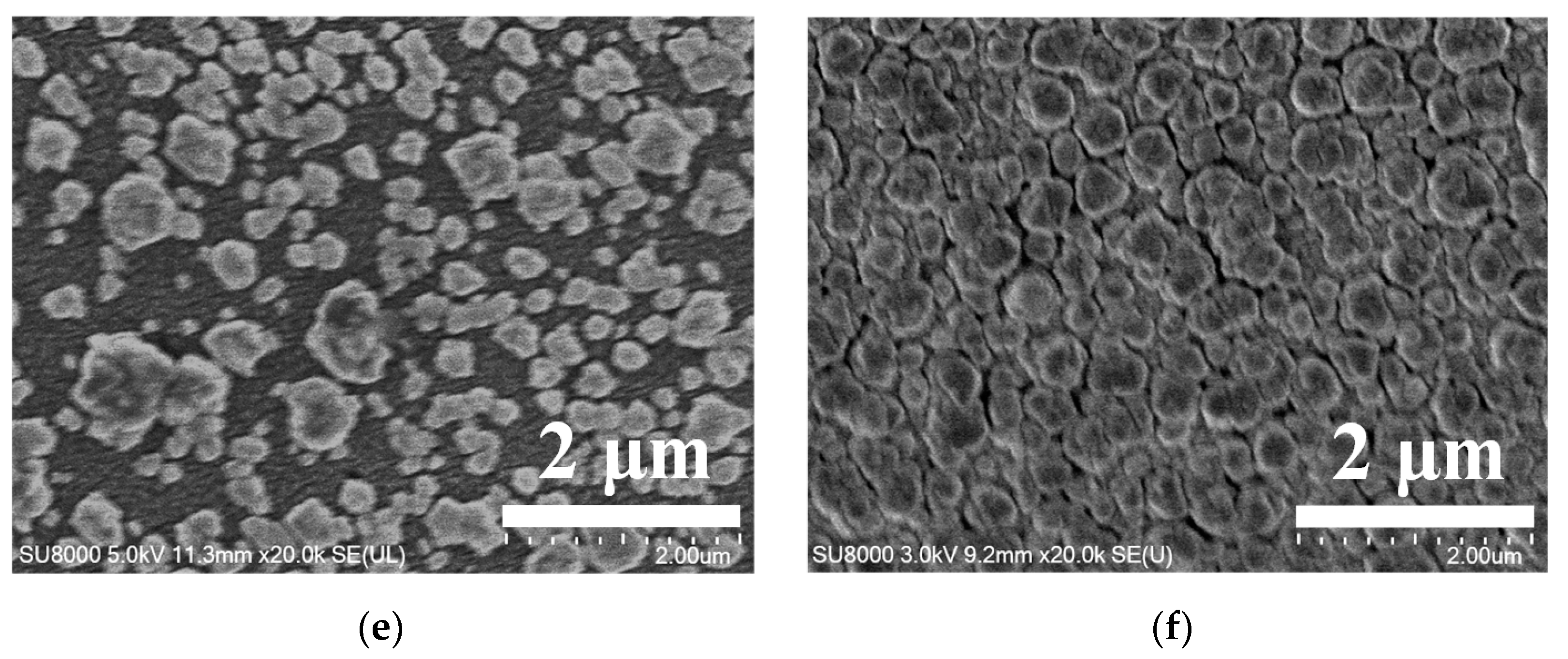
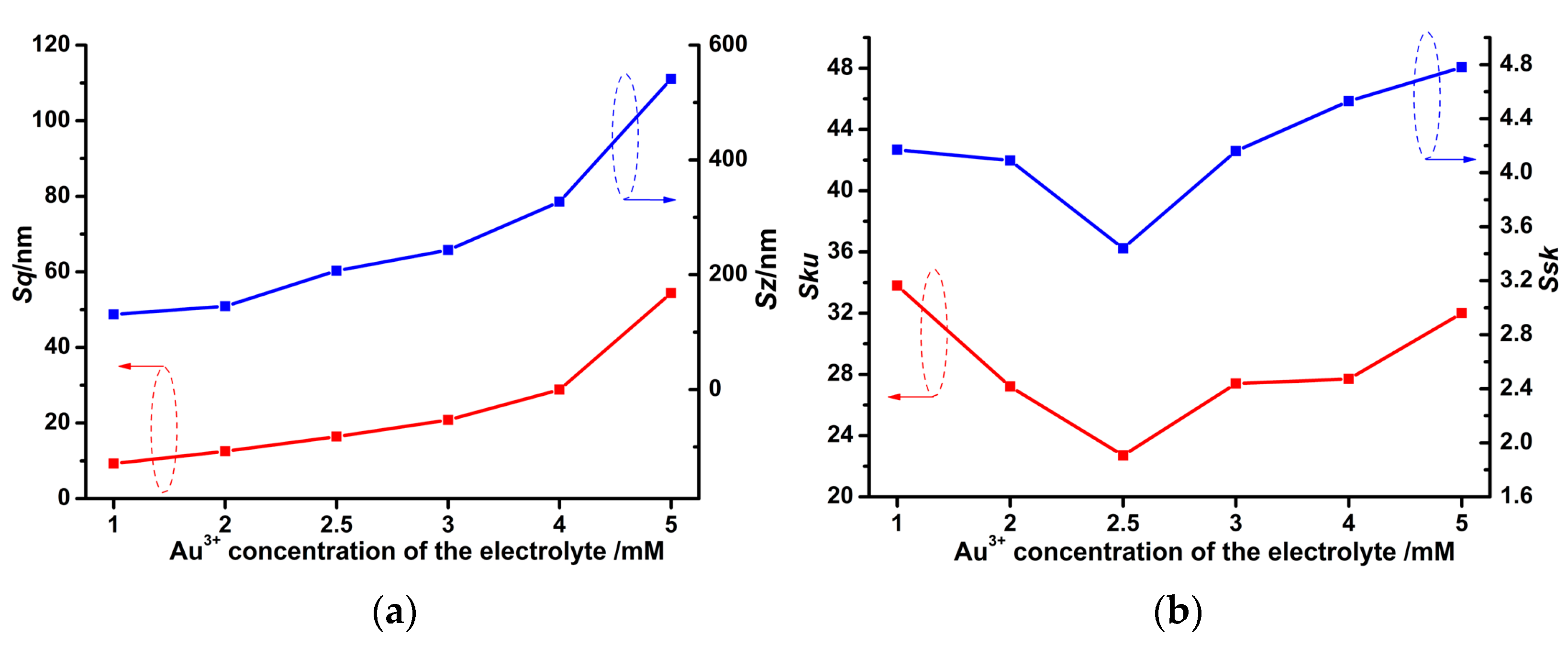
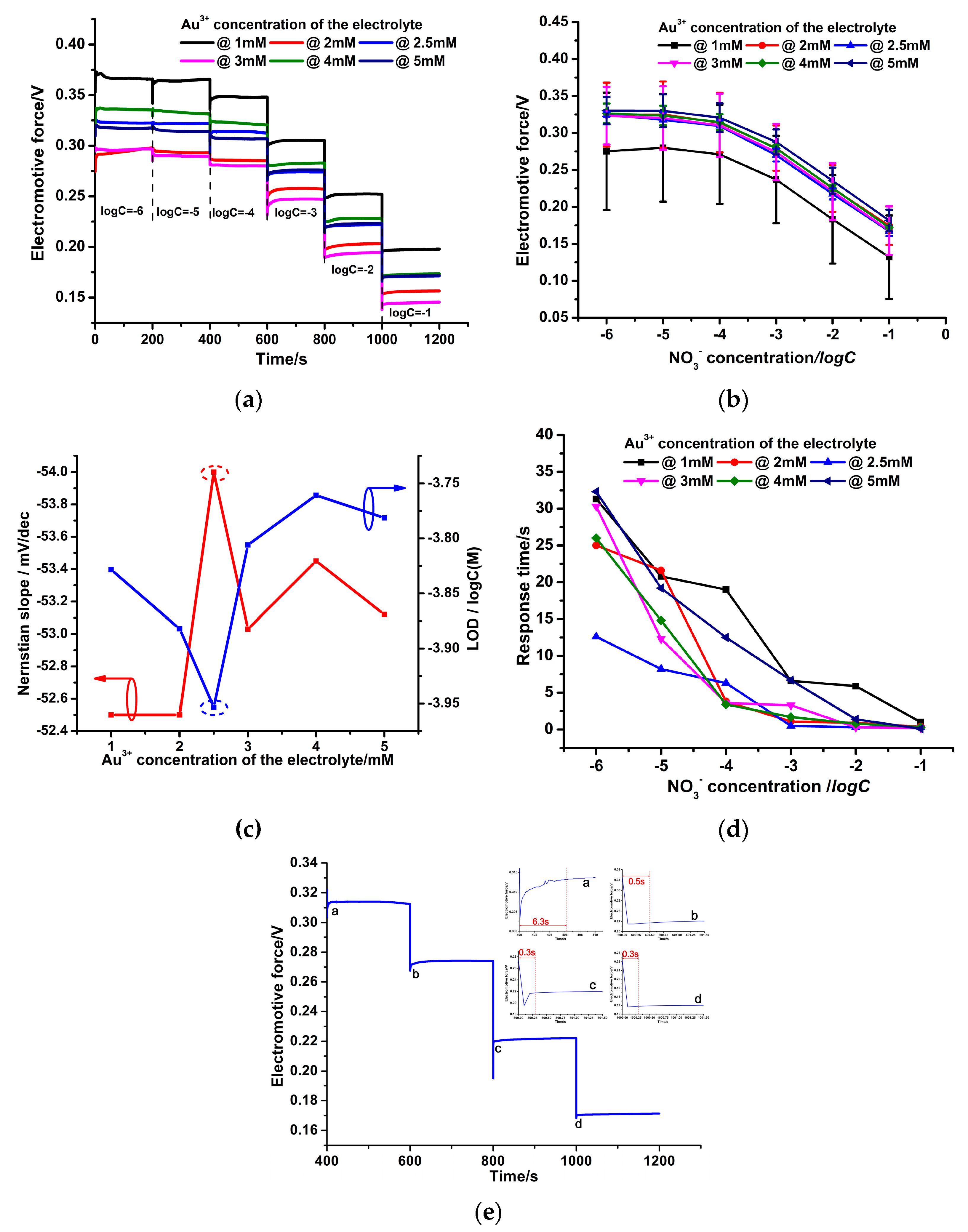
3.2. Various Au Solid Contact Layers at Different Au3+ Concentrations of the Electrolyte
3.3. Effect of Polymerization Durations on the NO3− Potential Response of the PPy(NO3−)-ISMs
3.4. Effect of Au3+ Concentrations of the Electrolyte on the Electrochemical Performance of the PPy-Au-NS ISEs
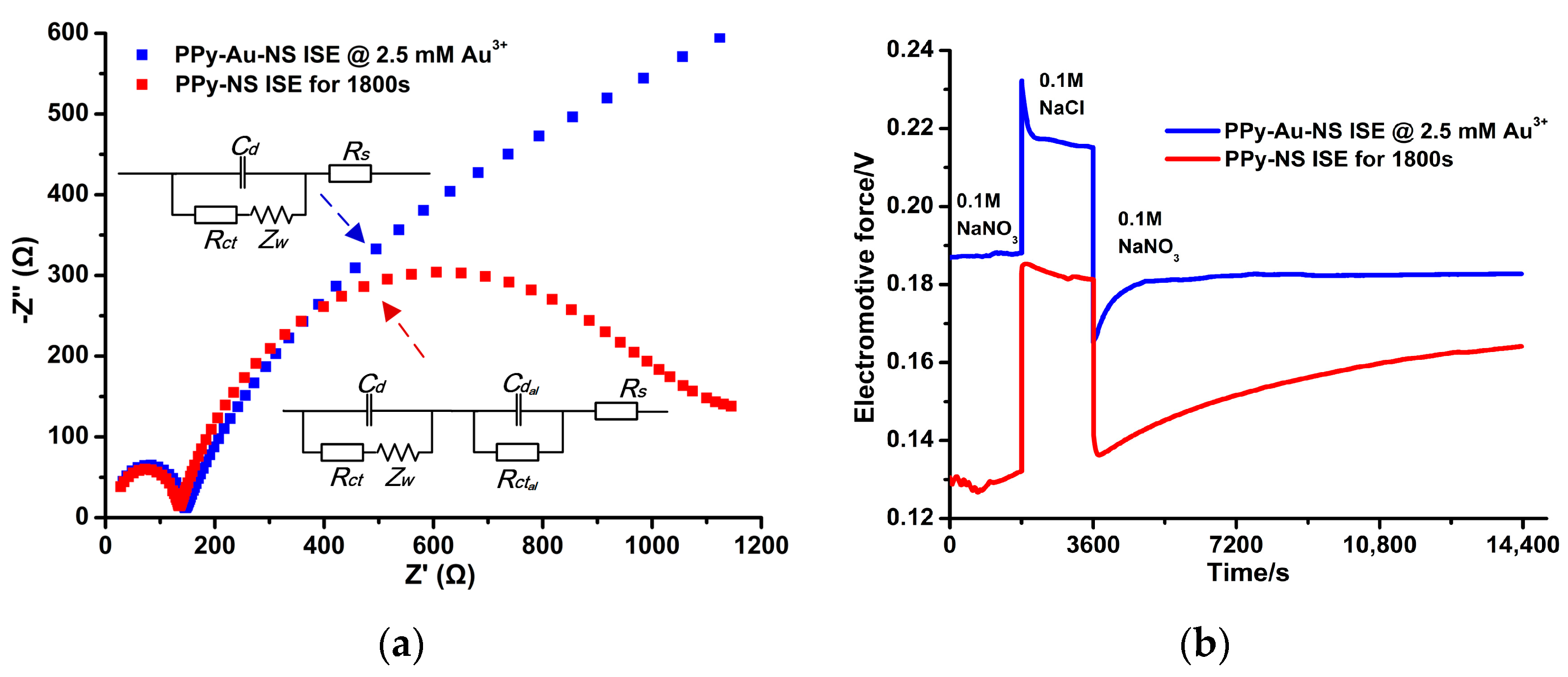
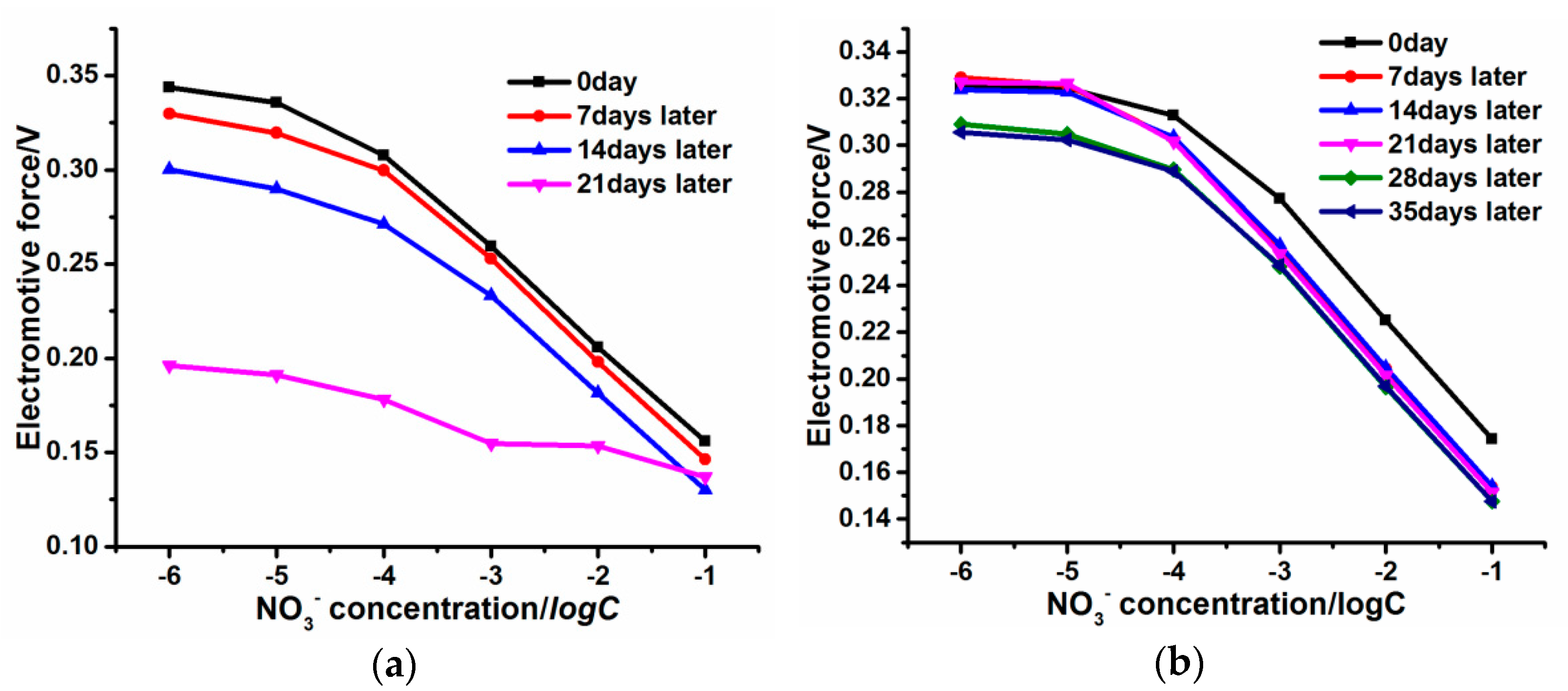
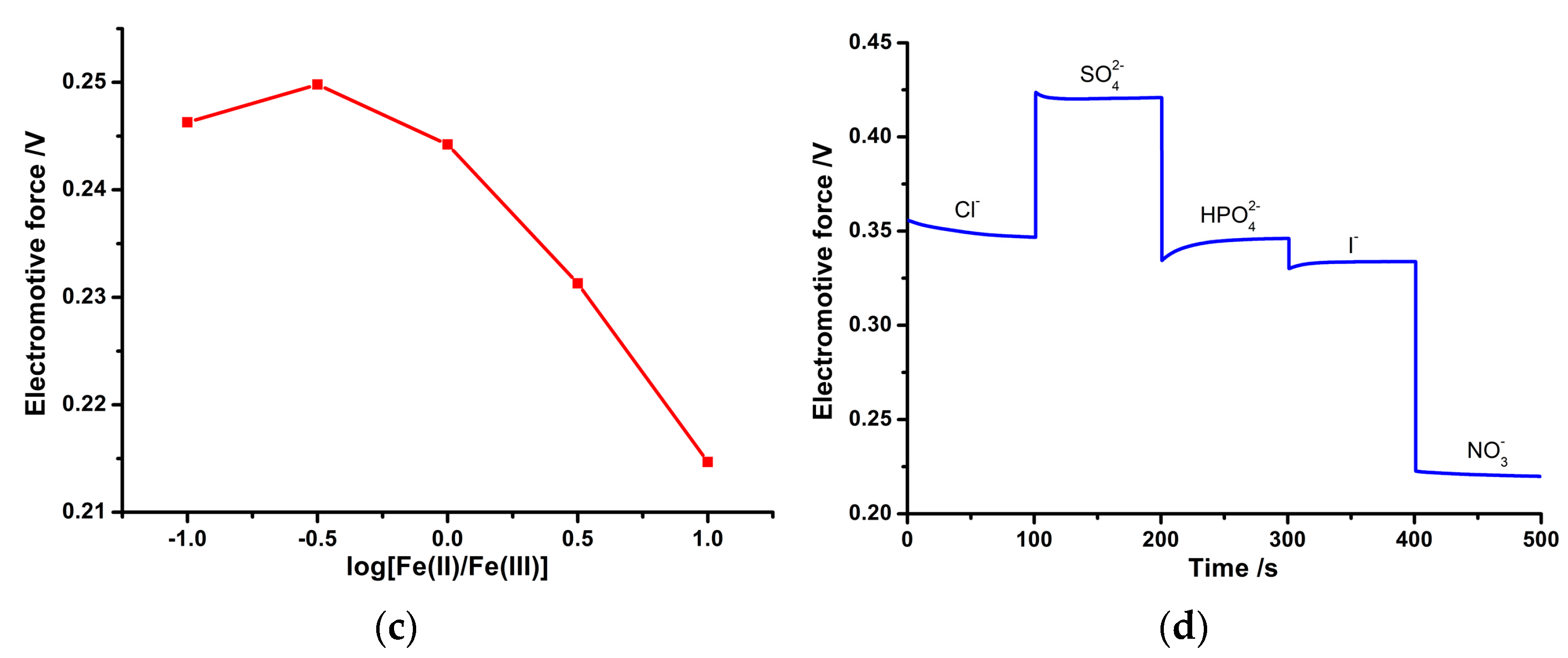
3.5. Long-Term Stability, Redox Sensitivity, and Ion Selectivity of the PPy-Au-NS ISE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berends, J.E.; Berg, L.M.v.D.; Guggeis, M.A.; Henckens, N.F.; Hossein, I.J.; de Joode, M.E.; Zamani, H.; van Pelt, K.A.; Beelen, N.A.; Kuhnle, G.G.; et al. Consumption of Nitrate-Rich Beetroot Juice with or without Vitamin C Supplementation Increases the Excretion of Urinary Nitrate, Nitrite, and N-nitroso Compounds in Humans. Int. J. Mol. Sci. 2019, 20, 2277. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, A.; Sillanp, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Yang, R.Q.; Wang, Q.K.; Luo, X.Y.; Wei, D. High-efficient nitrate conversion to protein and chlorophylls from synthetic wastewater by mixotrophic Chlorella pyrenoidosa. Algal Res. 2023, 71, 103025. [Google Scholar] [CrossRef]
- Zatar, N.A.; Abu-Eid, M.A.; Eid, A.F. Spectrophotometric determination of nitrite and nitrate using phosphomolybdenum blue complex. Talanta 1999, 50, 819–826. [Google Scholar] [CrossRef]
- Li, Y.T.; Whitaker, J.S.; McCarty, C.L. Reversed-phase liquid chromatography/electrospray ionization/mass spectrometry with isotope dilution for the analysis of nitrate and nitrite in water. J. Chromatogr. A 2011, 1218, 476–483. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, J.W.; Park, D.J.; Lee, J.Y.; Myung, N.V.; Kwon, S.H.; Lee, K.H. Highly stable potentiometric sensor with reduced graphene oxide aerogel as a solid contact for detection of nitrate and calcium ions. J. Electroanal. Chem. 2021, 897, 115553. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, B.; Yin, T.; Qin, W. Alternative coulometric signal readout based on a solid-contact ion-selective electrode for detection of nitrate. Anal. Chim. Acta 2020, 1129, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Dębosz, M.; Wieczorek, M.; Paluch, J.; Migdalski, J.; Baś, B.; Kościelniak, P. 3D-printed flow manifold based on potentiometric measurements with solid-state ion-selective electrodes and dedicated to multicomponent water analysis. Talanta 2020, 217, 121092. [Google Scholar] [CrossRef]
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric sensing. Anal. Chem. 2018, 91, 2–26. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Stein, A.; Bühlmann, P. A Disposable Planar Paper-Based Potentiometric Ion-Sensing Platform. Angew. Chem. 2016, 128, 7670–7673. [Google Scholar] [CrossRef]
- Li, X.-G.; Ma, X.-L.; Huang, M.-R. Lead(II) ion-selective electrode based on polyaminoanthraquinone particles with intrinsic conductivity. Talanta 2009, 78, 498–505. [Google Scholar] [CrossRef]
- Tang, W.; Ping, J.; Fan, K.; Wang, Y.; Luo, X.; Ying, Y.; Wu, J.; Zhou, Q. All-solid-state nitrate-selective electrode and its application in drinking water. Electrochim. Acta 2012, 81, 186–190. [Google Scholar] [CrossRef]
- Alizadeh, N.; Nabavi, S. Synthesis and characterization of novel tetra cyclo[4]pyrrole ether as an anion recognition element for nanocomposite nitrate ion selective carbon paste electrode. Sens. Actuators B 2014, 205, 127–135. [Google Scholar] [CrossRef]
- Pie, M.; Piech, R.; Paczosa-Bator, B. All-solid-state nitrate selective electrode with graphene/tetrathiafulvalene nanocomposite as high redox and double layer capacitance solid contact. Electrochim. Acta 2016, 210, 407–414. [Google Scholar]
- Pia, S.; Johan, B.; Andrzej, L.; Ari, I. All-solid-state chloride-selective electrode based on poly(3-octylthiophene) and tridodecylmethylammonium chloride. Electroanalysis 1999, 11, 821–824. [Google Scholar]
- Lindfors, T.; Ivaska, A. All-solid-state calcium-selective electrode prepared of soluble electrically conducting polyaniline and di(2-ethylhexyl)phosphate with tetraoctylammonium chloride as cationic additive. Anal. Chim. Acta 2000, 404, 111–119. [Google Scholar] [CrossRef]
- Pu, P.; Zhang, M.; Li, Y.H.; Zhang, L.N.; Ren, H.Y.; Kong, P.; Pan, L.P. Preparation and Evaluation of a Stable Solid State Ion Selective Electrode of Polypyrrole/Electrochemically Reduced Graphene/Glassy Carbon Substrate for Soil Nitrate Sensing. Int. J. Electrochem. Sci. 2016, 11, 4779–4793. [Google Scholar]
- Zhang, L.; Zhang, M.; Ren, H.; Pu, P.; Kong, P.; Zhao, H. Comparative investigation on soil nitrate-nitrogen and available potassium measurement capability by using solid-state and PVC ISE. Comput. Electron. Agric. 2015, 112, 83–91. [Google Scholar] [CrossRef]
- Álvarez-Romero, G.A.; Palomar-Pardavé, M.E.; Ramírez-Silva, M.T. Development of a novel nitrate-selective composite sensor based on doped polypyrrole. Anal. Bioanal. Chem. 2007, 387, 1533–1541. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, M.; Wang, X.; Yang, Q.; Wang, M.; Liu, G.; Yao, L. An All-Solid-State Nitrate Ion-Selective Electrode with Nanohybrids Composite Films for In-Situ Soil Nutrient Monitoring. Sensors 2020, 20, 2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khripoun, G.A.; Volkova, E.A.; Liseenkov, A.V.; Mikhelson, K.N. Nitrate-Selective Solid Contact Electrodes with Poly(3-octylthiophene) and Poly(aniline) as Ion-to-Electron Transducers Buffered with Electron-Ion-Exchanging Resin. Electroanalysis 2006, 18, 1322–1328. [Google Scholar] [CrossRef]
- Xu, J.; Jia, F.; Li, F.; An, Q.; Gan, S.; Zhang, Q.; Ivaska, A.; Niu, L. Simple and Efficient Synthesis of Gold Nanoclusters and Their Performance as Solid Contact of Ion Selective Electrode. Electrochimica Acta 2016, 222, 1007–1012. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.; Gu, Y.; Li, T.; Luo, H.; Li, L.-H.; Bai, Y.; Li, L.; Liu, L.; Cao, Y.; et al. Wearable Sweatband Sensor Platform Based on Gold Nanodendrite Array as Efficient Solid Contact of Ion-Selective Electrode. Anal. Chem. 2017, 89, 10224–10231. [Google Scholar] [CrossRef] [PubMed]
- Woz, E.; Wo, M.M.; Mieczkowski, J.; Maksymiuk, K.; Michalska, A. Dithizone Modified Gold Nanoparticles Films as Solid Contact for Cu2+ Ion-Selective Electrodes. Electroanalysis 2013, 25, 141–146. [Google Scholar]
- Jing, W.; Cheng, Y.; Gao, W.; Jiang, Z.; Jiang, K.; Shi, J.; Zhou, F. Different photo-catalytic degradation of methylene blue by varied ZnO nanorods on dissimilar stainless steel wire sieves. Mater. Res. Bull. 2017, 86, 313–321. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 3. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Gao, W.; Jing, W.; Han, D.; Zhou, F.; Jiang, Z.; Wei, Z. Modulation of Stainless-Steel Wire Sieve-Supported ZnO Nanorods for Optimal De-Ionized Water/Diesel Oil Separation. J. Nanosci. Nanotechnol. 2019, 19, 5608–5618. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, R.J. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005; pp. 205–264. [Google Scholar]
- Pietrzak, K.; Wardak, C. Comparative study of nitrate all solid state ion-selective electrode based on multiwalled carbon nanotubes-ionic liquid nanocomposite. Sens. Actuators B Chem. 2021, 348, 130720. [Google Scholar] [CrossRef]
- Jing, W.X.; Gao, W.Z.; Li, Z.H.; Peng, M.L.; Han, F.; Wei, Z.Y.; Yang, Z.C.; Jiang, Z.D. Regulation of the volume flow rate of aqueous methyl blue solution and the wettability of CuO/ZnO nanorods to improve the photo-degradation performance of related microfluidic reactors. Langmuir 2021, 37, 7890–7906. [Google Scholar] [CrossRef] [PubMed]
- Bendikov, T.A.; Miserendino, S.; Tai, Y.-C.; Harmon, T.C. A parylene-protected nitrate selective microsensor on a carbon fiber cross section. Sens. Actuators B 2007, 123, 127–134. [Google Scholar] [CrossRef]
- Gupta, V.K.; Singh, L.P.; Chandra, S.; Kumar, S.; Singh, R.; Sethi, B. Anion recognition through amide-based dendritic molecule: A poly(vinyl chloride) based sensor for nitrate ion. Talanta 2011, 85, 970–974. [Google Scholar] [CrossRef]
- Sjöberg-Eerola, P.; Nylund, J.; Bobacka, J.; Lewenstam, A.; Ivaska, A. Soluble semiconducting poly(3-octylthiophene) as a solid-contact material in all-solid-state chloride sensors. Sens. Actuators B 2008, 134, 878–886. [Google Scholar] [CrossRef]
- Bobacka, J.; Lindfors, T.; McCarrick, M.; Ivaska, A.; Lewenstam, A. Single-piece all-solid-state ion-selective electrode. Anal. Chem. 1995, 67, 3819–3823. [Google Scholar] [CrossRef]


| ISM | Solid Contact Layer | Nernstian Slope (mV/dec) | LOD (M) | Average Response Time (s) | Ref. |
|---|---|---|---|---|---|
| PPy(NO3−) | Au | 54 | 10−4 | <1.9 | This work |
| MTDDANO3 | CRGNO | 57.9 | 10−4 | <10 | [13] |
| PPy(NO3−) | GR | 56 | 10−5 | <10 | [18] |
| TDANO3 | POT | 53 | 10−5 | \ | [23] |
| TDANO3 | Poly(aniline) | 56 | 10−5 | \ | [23] |
| PPy(NO3−) | \ | 58 | 3 × 10−5 | 60 | [35] |
| I–PVC–TBAB–DBP | \ | 57 | 4 × 10−5 | <20 | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Jing, W.; Du, Y.; Li, Z.; Liu, P.; Han, F.; Zhao, L.; Yang, Z.; Jiang, Z. Regulating the Polypyrrole Ion-Selective Membrane and Au Solid Contact Layer to Improve the Performance of Nitrate All-Solid Ion-Selective Electrodes. Micromachines 2023, 14, 855. https://doi.org/10.3390/mi14040855
Gao W, Jing W, Du Y, Li Z, Liu P, Han F, Zhao L, Yang Z, Jiang Z. Regulating the Polypyrrole Ion-Selective Membrane and Au Solid Contact Layer to Improve the Performance of Nitrate All-Solid Ion-Selective Electrodes. Micromachines. 2023; 14(4):855. https://doi.org/10.3390/mi14040855
Chicago/Turabian StyleGao, Weizhuo, Weixuan Jing, Yanrui Du, Zehao Li, Pengcheng Liu, Feng Han, Libo Zhao, Zhaochu Yang, and Zhuangde Jiang. 2023. "Regulating the Polypyrrole Ion-Selective Membrane and Au Solid Contact Layer to Improve the Performance of Nitrate All-Solid Ion-Selective Electrodes" Micromachines 14, no. 4: 855. https://doi.org/10.3390/mi14040855





