Chitosan as an Alternative to Oil-Based Materials for the Fabrication of Lab-on-a-Chip
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chitosan Film Preparation
2.3. Chitosan Film Characterization
2.4. Channel Micropatterning
2.5. Microfluidic Device Preparation
3. Results and Discussion
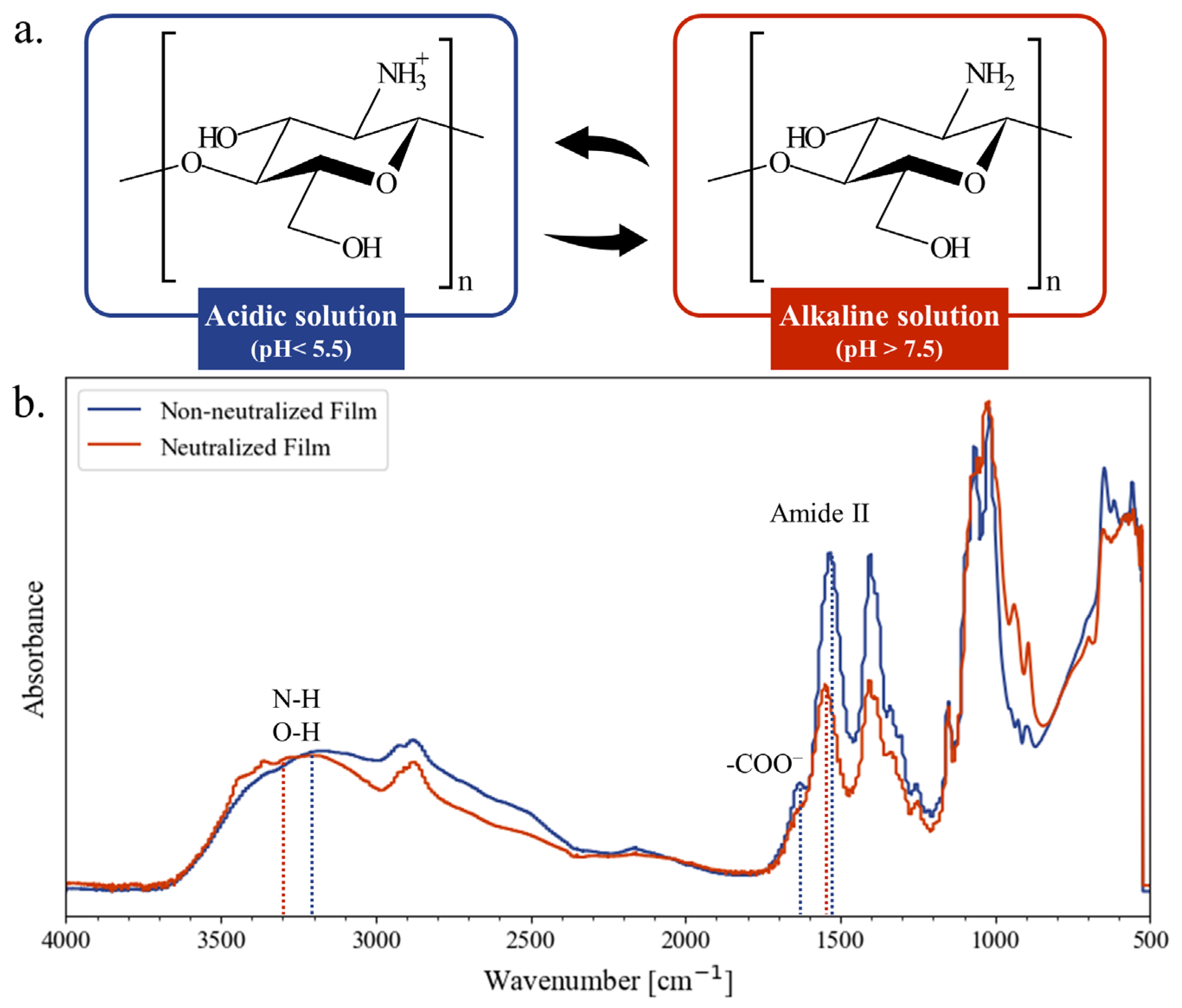
3.1. Chitosan Film Preparation and Characterization
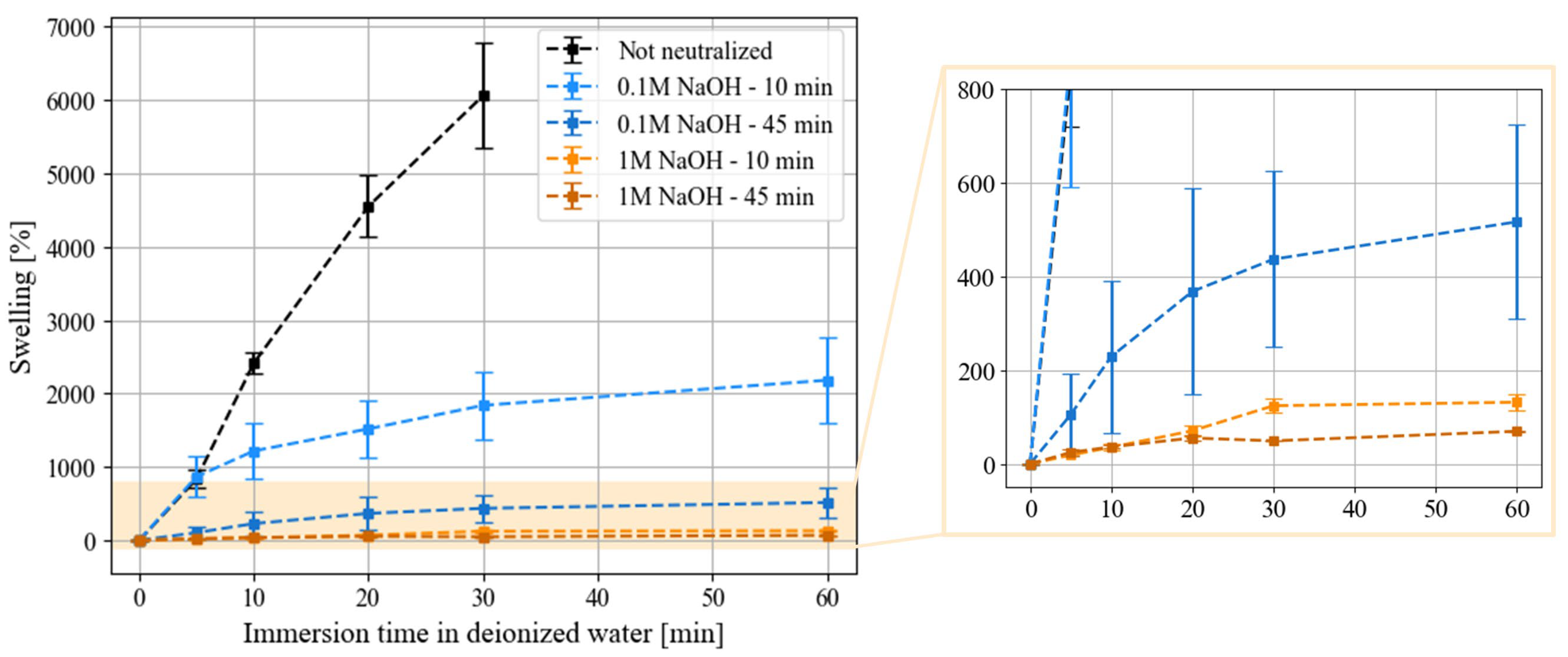
3.2. Properties of Neutralized Chitosan Films
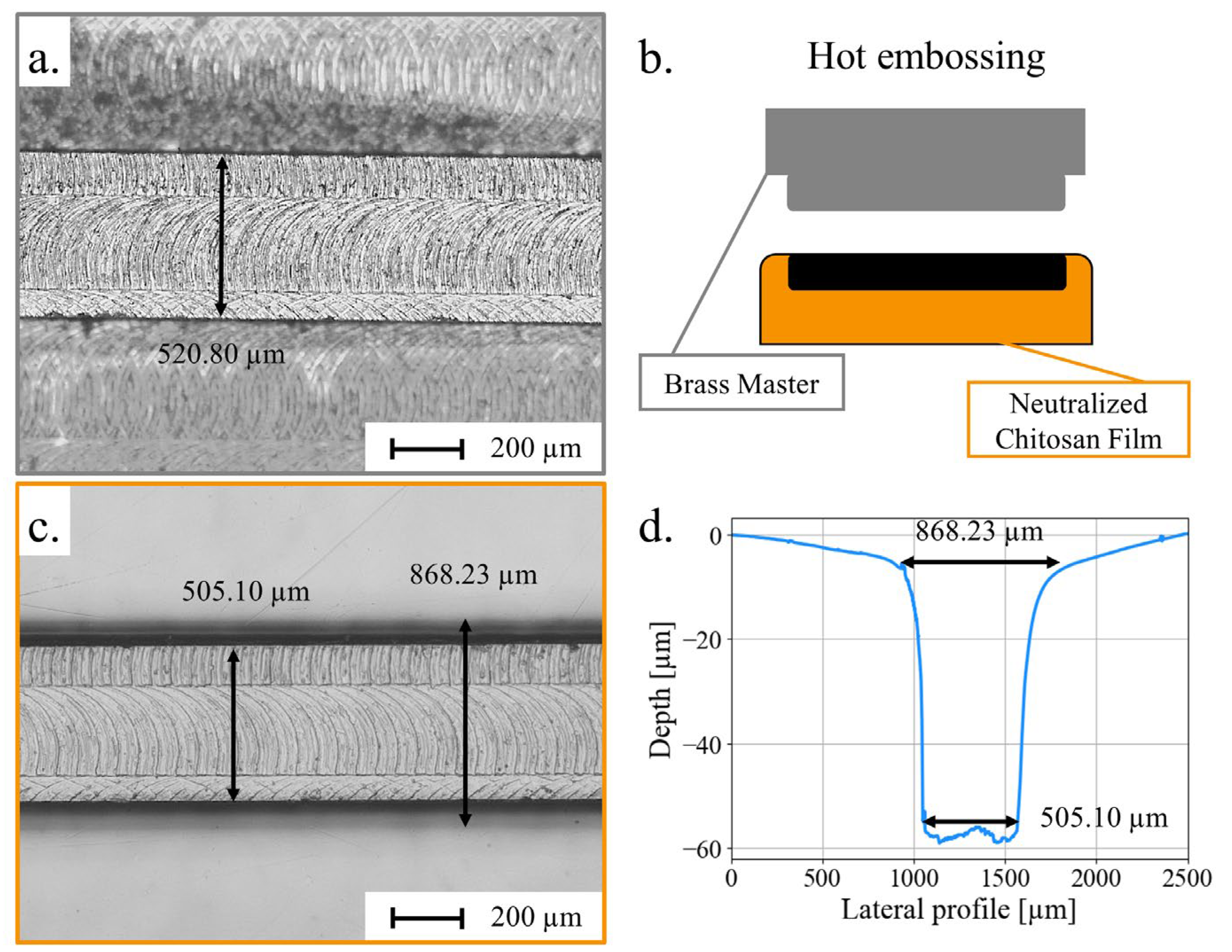
3.3. Micropatterning of Chitosan Films
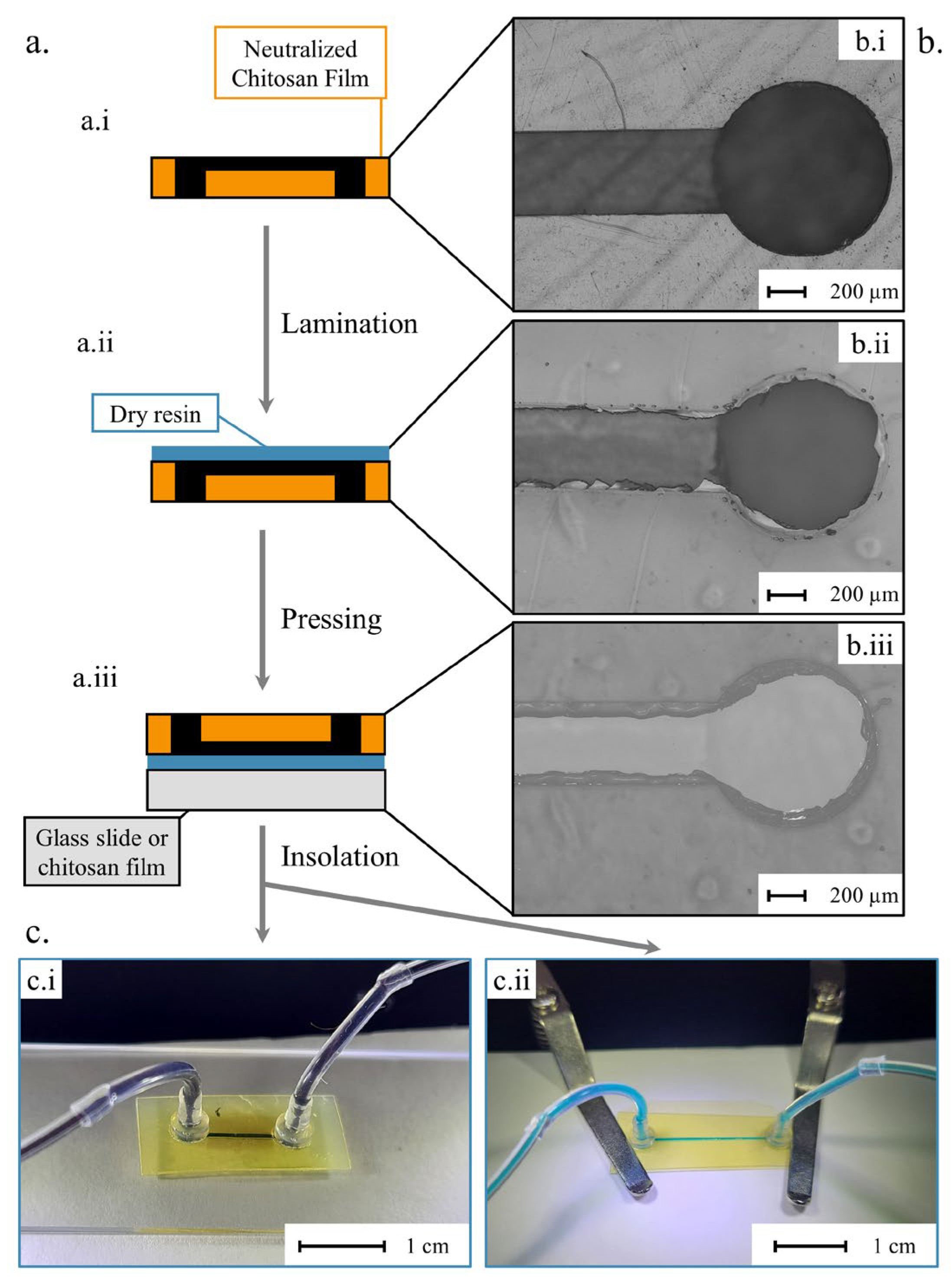
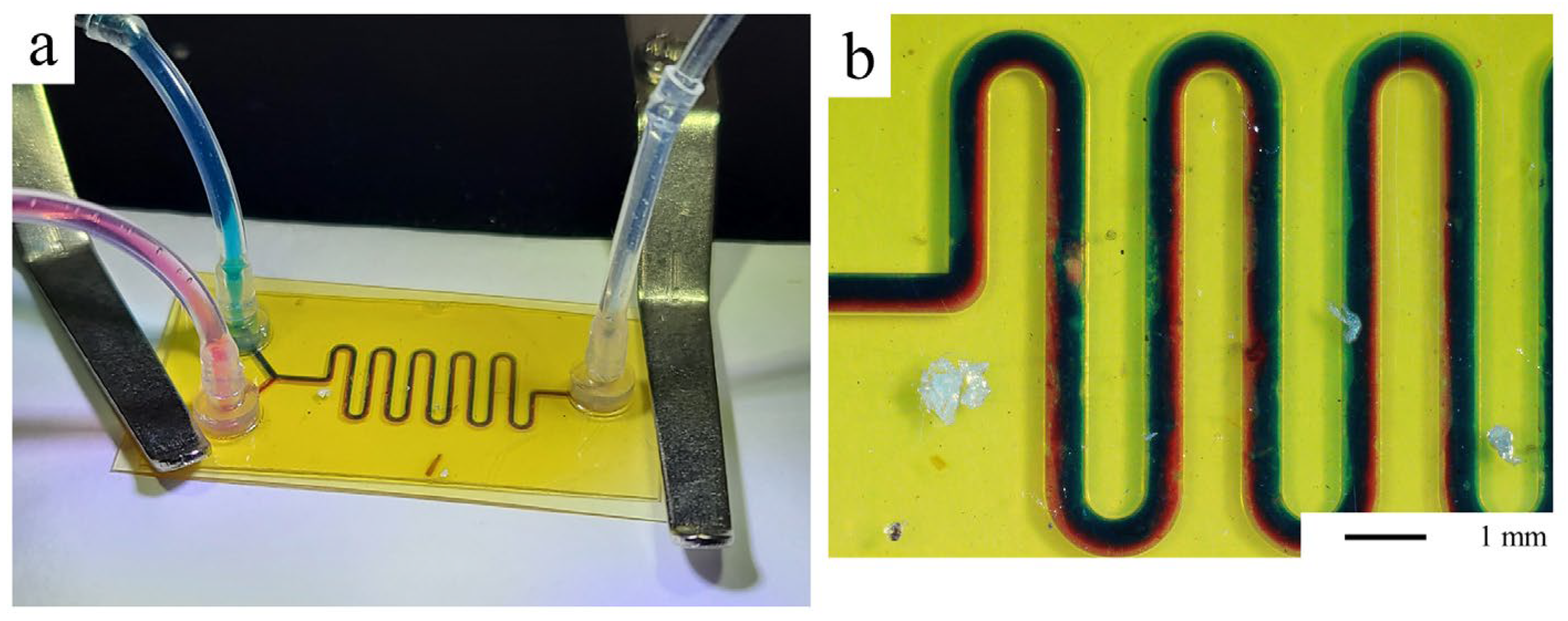
3.4. Microfluidic Protypes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitesides, G. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.-T.; Gao, H.; Lee, C.-Y.; Wang, Y.-N.; Dong, W.; Ko, C.-H.; Wang, G.; Fu, L.-M. Microfluidic synthesis control technology and its application in drug delivery, bioimaging, biosensing, environmental analysis and cell analysis. Chem. Eng. J. 2020, 399, 125748. [Google Scholar] [CrossRef]
- Huh, D.; Gu, W.; Kamotani, Y.; Grotberg, J.B.; Takayama, S. Microfluidics for flow cytometric analysis of cells and particles. Physiol. Meas. 2005, 26, R73. [Google Scholar] [CrossRef] [PubMed]
- Büyüktiryaki, S.; Sümbelli, Y.; Keçili, R.; Hussain, C.M. Lab-On-Chip Platforms for Environmental Analysis. In Encyclopedia of Analytical Science; Agon Elsevier: Amsterdam, The Netherlands, 2019; pp. 267–273. [Google Scholar]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in Poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef]
- Shi, H.; Nie, K.; Dong, B.; Long, M.; Xu, H.; Liu, Z. Recent progress of microfluidic reactors for biomedical applications. Chem. Eng. J. 2019, 361, 635–650. [Google Scholar] [CrossRef]
- Wheeler, A.R.; Throndset, W.R.; Whelan, R.J.; Leach, A.M. Microfluidic Device for Single-Cell Analysis. Anal. Chem. 2003, 75, 3581–3586. [Google Scholar] [CrossRef]
- Oosterbroek, R.E.; Berg, A. Lab-on-a-Chip; Miniaturized Systems for (BIO)Chemical Analysis and Synthesis; Elsevier: Amsterdam, The Netherland, 2003; pp. 37–64. [Google Scholar]
- Manz, A.; Harrison, D.; Verpoorte, E.M.; Fettinger, J.; Paulus, A.; Lüdi, H.; Widmer, H. Planar chips technology for miniaturization and integration of separation techniques into monitoring systems: Capillary electrophoresis on a chip. J. Chromatogr. A 1992, 593, 253–258. [Google Scholar] [CrossRef]
- Jensen, K.F. Silicon-Based Microchemical Systems: Characteristics and Applications. MRS Bull. 2006, 31, 101–107. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Bhat, S.; Mahato, K.K. Design and Fabrication of Low-cost Microfluidic Channel for Biomedical Application. Sci. Rep. 2020, 10, 9215. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.; Gärtner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Berthier, E.; Young, E.W.K.; Beebe, D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab Chip 2012, 12, 1224–1237. [Google Scholar] [CrossRef]
- Wan, A.M.; Devadas, D.; Young, E.W. Recycled polymethylmethacrylate (PMMA) microfluidic devices. Sens. Actuators B Chem. 2017, 253, 738–744. [Google Scholar] [CrossRef]
- Mela, P.; van den Berg, A.; Fintschenko, Y.; Cummings, E.B.; Simmons, B.A.; Kirby, B.J. The zeta potential of cyclo-olefin polymer microchannels and its effects on insulative (electrodeless) dielectrophoresis particle trapping devices. Electrophoresis 2005, 26, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Haswell, S.; Gibson, I. Lab-on-a-Chip or Chip-in-a-Lab: Challenges of Commercialization Lost in Translation. Proceida Technol. 2015, 20, 54–59. [Google Scholar]
- Becker, H.; Locascio, L.E. Polymer Microfluidic Devices. Talanta 2002, 56, 267–287. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef]
- Markets and Markets, Point of Care Diagnostics Market by Product (Glucose, HIV, Hepatitis C, Pregnancy), Platform (Microfluidics, Dipstick, Immunoassay), Purchase (OTC, Prescription), Sample (Blood, Urine), End User (Pharmacy, Hospital, Homecare) & Region—Global Forecast to 2027. Available online: https://www.marketsandmarkets.com/Market-Reports/point-of-care-diagnostic-market-106829185.html?gclid=Cj0KCQjwxtSSBhDYARIsAEn0thSq_WeoIJSYBUDc_RasH1oIbxcHQBjkcZlRi_96XlBe3oziTCKIYMQaApjCEALw_wcB (accessed on 12 January 2022).
- World Health Organization. Global Analysis of Health Care Waste in the Context of COVID-19—Status, Impacts and Recommendations. Available online: https://www.who.int/publications/i/item/9789240039612 (accessed on 2 January 2022).
- Wittenauer, R.; Nowak, S.; Luter, N. Price, quality, and market dynamics of malaria rapid diagnostic tests: Analysis of Global Fund 2009–2018 data. Malar. J. 2022, 21, 12. [Google Scholar] [CrossRef]
- Pai, M.; Furin, J. Point of View: Tuberculosis innovations mean little if they cannot save lives. eLife 2017, 6, e25956. [Google Scholar] [CrossRef]
- Ongaro, A.E.; Ndlovu, Z.; Sollier, E.; Otieno, C.; Ondoa, P.; Street, A.; Kersaudy-Kerhoas, M. Engineering a sustainable future for point-of-care diagnostics and single-use microfluidic devices. Lab Chip 2022, 22, 3122–3137. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M.; Pogue, B.W. Recent advances in microfluidics-based paper analytical devices (µPADs) for biochemical sensors: From fabrication to detection techniques. Biochem. Eng. J. 2023, 198, 109027. [Google Scholar] [CrossRef]
- Silva-Neto, H.A.; Arantes, I.V.S.; Ferreira, A.L.; do Nascimento, G.H.; Meloni, G.N.; de Araujo, W.R.; Paixão, T.R.; Coltro, W.K. Recent advances on paper-based microfluidic devices for bioanalysis. TrAC Trends Anal. Chem. 2023, 158, 116893. [Google Scholar] [CrossRef]
- Luecha, J.; Hsiao, A.; Brodsky, S.; Liu, G.L.; Kokini, J.L. Green microfluidic devices made of corn proteins. Lab Chip 2011, 11, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.L.; Xu, Q.; Lu, Z.Q.; Wang, J.Y. Preparation of transparent zein films for cell culture applications. Colloids Surf. B Biointerfaces 2014, 120, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Yajima, Y.; Yamada, M.; Yamada, E.; Iwase, M.; Seki, M. Facile fabrication processes for hydrogel-based microfluidic devices made of natural biopolymers. Biomicrofluidics 2014, 8, 024115. [Google Scholar] [CrossRef]
- Lee, C.; Shin, J.; Lee, J.S.; Byun, E.; Ryu, J.H.; Um, S.H.; Kim, D.I.; Lee, H.; Cho, S.W. Bioinspired, calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Y.; Partlow, B.; Golding, A.; Tseng, A.; Coburn, J.; Applegate, M.; Moreau, J.; Omenetto, F.; Kaplan, C. Bio-functionalized silk hydrogel microfluidic systems. Biomaterials 2016, 93, 60–70. [Google Scholar] [CrossRef]
- Ongaro, A.E.; Howarth, N.M.; La Carrubba, V.; Kersaudy-Kerhoas, M. Fast and Green: Sustainable rapid-prototyping of Microfluidic Chips on Polylactiv Acid Substrates. In Proceedings of the MicroTAS 2018, Kaohsiung, Taiwan, 11–15 November 2018. [Google Scholar]
- Ongaro, A.E.; Di Giuseppe, D.; Kermanizadeh, A.; Miguelez Crespo, A.; Mencattini, A.; Ghibelli, L.; Mancini, V.; Wlodarczyk, K.L.; Hand, D.P.; Martinelli, E.; et al. Polylactic is a Sustainable, Low Absorption, Low Autofluorescence Alternative to Other Plastics for Microfluidic and Organ-on-Chip Applications. Anal. Chem. 2020, 92, 6693–6701. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of So-Called Biodegradable Polymers in Seawater and Freshwater. Glob. Chall. 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Sinha, R.; Pandey, J.; Rutot, D.; Degeé, P.; Dubois, P. Biodegradation of poly (3-caprolactone)/starch blends and composites in composting and culture environment: The effect of compatibilization on the inherent biodegradability of the host polymer. Carbohydr. Res. 2003, 338, 1759–1769. [Google Scholar]
- Mattotti, M.; Alvarez, Z.; Delgado, L.; Mateos-Timoneda, M.A.; Aparicio, C.; Planell, J.A.; Alcántara, S.; Engel, E. Differential neuronal and glial behavior on flat and micro patterned chitosan films. Colloids Surf. B Biointerfaces 2017, 158, 569–577. [Google Scholar] [CrossRef]
- Fukuda, J.; Khademhosseini, A.; Yeo, Y.; Yang, X.; Yeh, J.; Eng, G.; Blumling, J.; Wang, C.F.; Kohane, D.S.; Langer, R. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials 2006, 27, 5259–5267. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, K.; Won, S.W.; Cha, C.; Gaharwar, A.K.; Selimović, Š.; Bae, H.; Lee, K.H.; Lee, D.H.; Lee, S.H.; et al. Microfluidic fabrication of cell adhesive chitosan microtubes. Biomed. Microdevices 2013, 15, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Koev, S.T.; Dykstra, P.H.; Luo, X.; Rubloff, G.W.; Bentley, W.E.; Payne, G.F.; Ghodssi, R. Chitosan: An integrative biomaterial for lab-on-a-chip devices. Lab Chip 2010, 10, 3026–3042. [Google Scholar] [CrossRef] [PubMed]
- Eulalio, H.Y.C.; Rodriges, J.F.B.; Santos, K.O.; Peniche, C.; LiaFook, M.V. Characterization and thermal properties of chitosan films prepared with different acid solvents. Rev. Cuba. Quím. 2019, 31, 309–323. [Google Scholar]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef] [PubMed]
- Tapan Kumar, G.; Bijaya, G. Polysaccharide Based Nano-Biocarrier in Drug Delivery; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Kaisler, M.; Van Den Broek, L.A.M.; Boeriu, C.G. Chitin and Chitosan as Sources of Bio-Based Building Blocks and Chemicals. In Chitin and Chitosan: Properties and Applications, 1st ed.; van der Broek, L.A., Boeriu, C.G., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 229–244. [Google Scholar]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Chitin and chitosan-based support materials for enzyme immobilization and biotechnological applications. Environ. Chem. Lett. 2020, 18, 315–323. [Google Scholar] [CrossRef]
- Jiang, T.; Deng, M.; James, R.; Nair, L.S.; Laurencin, C.T. Micro- and nanofabrication of chitosan structures for regenerative engineering. Acta Biomater. 2014, 10, 1632–1645. [Google Scholar] [CrossRef]
- Moura, J.M.; Farias, B.S.; Cadaval, T.R.S.; Pinto, L.A.A. Chitin/Chitosan Based Films for Packaging Applications. In Bio-Based Packaging; Sapuan, S.M., Ilyas, R.A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 69–83. [Google Scholar]
- Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Garcia, F.P.; Nakamura, C.V.; Pineda, E.A.G.; Rubira, A.F. Water transport properties through starch-based hydrogel nanocomposites responding to both pH and a remote magnetic field. Chem. Eng. J. 2015, 259, 620–629. [Google Scholar] [CrossRef]
- Fernandez, J.G.; Mills, C.A.; Martinez, E.; Lopez-Bosque, M.J.; Sisquella, X.; Errachid, A.; Samitier, J. Micro- and nanostructuring of freestanding, biodegradable, thin sheets of chitosan via soft lithography. J. Biomed. Mater. Res.-Part A 2007, 85, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Caillau, M.; Crémillieu, P.; Laurenceau, E.; Chevolot, Y.; Leclercq, J.-L. Fifty nanometer lines patterned into silica using water developable chitosan bioresist and electron beam lithography. J. Vac. Sci. Technol. B 2017, 35, 06GE01. [Google Scholar] [CrossRef]
- Cheng, J.C.; Cauley, T.H.; Pisano, A.P. Lithographic patterning of immobilized enzymes in chitosan thin films for multi-layer, chemical/biological sensors. In Proceedings of the 7th IEEE International Conference on Nanotechnology, Hong-Kong, China, 2–5 August 2007. [Google Scholar]
- Cheng, J.C.; Pisano, A.P. Photolithographic process for integration of the biopolymer chitosan into micro/nanostructures. J. Microelectromech. Syst. 2008, 17, 402–409. [Google Scholar] [CrossRef]
- Yi, H.; Wu, L.Q.; Bentley, W.E.; Ghodssi, R.; Rubloff, G.W.; Culver, J.N.; Payne, G.F. Biofabrication with chitosan. Biomacromolecules 2005, 6, 2881–2894. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, P.; Hao, J.; Koev, S.T.; Payne, G.F.; Yu, L.; Ghodssi, R. An optical MEMS sensor utilizing a chitosan film for catechol detection. Sens. Actuators B Chem. 2009, 138, 64–70. [Google Scholar] [CrossRef]
- Fu, J.; Yang, F.; Guo, Z. The chitosan hydrogels: From structure to function. New J. Chem. 2018, 42, 17162–17180. [Google Scholar] [CrossRef]
- Chang, W.; Liu, F.; Sharif, H.R.; Huang, Z.; Goff, H.D.; Zhong, F. Preparation of chitosan films by neutralization for improving their preservation effects on chilled meat. Food Hydrocoll. 2019, 90, 50–61. [Google Scholar] [CrossRef]
- Mirzaei, E.B.; Ramazani, A.; Shafiee, M.; Danaei, M. Studies on glutaraldehyde crosslinked chitosan hydrogel properties for drug delivery systems. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 605–611. [Google Scholar] [CrossRef]
- Nomoto, R.; Imai, M. Dominant Role of Acid–Base Neutralization Process in Forming Chitosan Membranes for Regulating Mechanical Strength and Mass Transfer Characteristics. J. Chitin Chitosan Sci. 2014, 2, 197–204. [Google Scholar] [CrossRef]
- Gabriele, F.; Donnadio, A.; Casciola, M.; Germani, R.; Spreti, N. Ionic and covalent crosslinking in chitosan-succinic acid membranes: Effect on physicochemical properties. Carbohydr. Polym. 2021, 251, 117106. [Google Scholar] [CrossRef] [PubMed]
- Ismillayli, N.; Hadi, S.; Dharmayani, N.K.T.; Sanjaya, R.K.; Hermanto, D. Characterization of alginate-chitosan membraneas potential edible film. IOP Conf. Ser. Mater. Sci. Eng. 2020, 833, 012073. [Google Scholar] [CrossRef]
- Toepke, M.W.; Beebe, D.J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef] [PubMed]
- Kardas, I.; Struszczyk, M.H.; Kucharska, M.; Broek, L.A.; van Dam, J.E.; Ciechańska, D. Chitin and chitosan as functional biopolymers for industrial applications. In The European Polysaccharide Network of Excellence (EPNOE): Research Initiatives and Results; Navard, P., Ed.; Springer: Vienna, Austria, 2012; pp. 329–373. [Google Scholar]
- Rush, M.N.; Brambilla, S.; Speckart, S.; Montaño, G.A.; Brown, M.J. Glass-particle adhesion-force-distribution on clean (laboratory) and contaminated (outdoor) surfaces. J. Aerosol Sci. 2018, 123, 231–244. [Google Scholar] [CrossRef]
- Rasmuson, A.; Pazmino, E.; Assemi, S.; Johnson, W.P. Contribution of Nano- to Microscale Roughness to Heterogeneity. Environ. Sci. Technol. 2017, 51, 2151–2160. [Google Scholar] [CrossRef]
- Ogilvie, I.R.; Sieben, V.J.; Floquet, C.F.; Zmijan, R.; Mowlem, M.C.; Morgan, H. Reduction of surface roughness for optical quality microfluidic devices in PMMA and COC. J. Micromech. Microeng. 2010, 20, 065016. [Google Scholar] [CrossRef]
- Sangsanoh, P.; Supaphol, P. Stability improvement of electrospun chitosan nanofibrous membranes in neutral or weak basic aqueous solutions. Biomacromolecules 2006, 7, 2710–2714. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.A.; Ataya, S.; Silberschmidt, V.V. Temperature-dependent mechanical behaviour of PMMA: Experimental analysis and modelling. Polym. Test. 2017, 58, 86–95. [Google Scholar] [CrossRef]
- Brydson, J.A. Plastics Materials; Butterworth-Heinemann: London, UK, 1999. [Google Scholar]
- Kim, M.; Moon, B.-U.; Hidrovo, C.H. Enhancement of the thermo-mechanical. J. Micromech. Microeng. 2013, 23, 095024. [Google Scholar] [CrossRef]
- Deshmukh, S.S.; Goswami, A. Hot Embossing of polymers—A review. Mater. Today Proc. 2020, 26, 405–414. [Google Scholar] [CrossRef]
- Hasan, M.; Zhao, J.; Jiang, Z. A review of modern advancements in micro drilling techniques. J. Manuf. Process. 2017, 29, 343–375. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of Degree of Deacetylation of Chitosan by 1H NMR Spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Schatz, C.; Viton, C.; Delair, T.; Pichot, C.; Domard, A. Typical Physicochemical Behaviors of Chitosan in Aqueous Solution. Biomacromolecules 2003, 4, 641–648. [Google Scholar] [CrossRef] [PubMed]

| Master Width [µm] | 123 ± 6 | 219 ± 4 | 270 ± 3 | 521 ± 3 | 1016 ± 2 |
| Sample width at the bottom of the channel [µm] | 120 ± 5 | 211 ± 5 | 262 ± 3 | 508 ± 4 | 1009 ± 13 |
| 122 ± 4 | 210 ± 5 | 273 ±10 | 506 ± 1 | 1013 ± 12 | |
| 110 ± 7 | 215 ± 5 | 262 ± 5 | 512 ± 6 | 1009 ± 5 | |
| p = 0.014 | p = 0.29 | p = 0.044 | p = 0.13 | p = 0.73 | |
| Sample width at the top of the channel [µm] | 183 ± 30 | 609 ± 92 | 611 ± 208 | 883 ± 156 | 1240 ± 33 |
| 443 ± 31 | 457 ± 62 | 602 ± 97 | 883 ± 32 | 1230 ± 54 | |
| 233 ± 51 | 391 ± 34 | 473 ± 30 | 712 ± 20 | 1278 ± 48 | |
| Master height [µm] | 88 ± 1 | 88 ± 2 | 88 ± 1 | 88 ± 1 | 88.5 ± 0.1 |
| Sample depth [µm] | 83 ± 8 | 79 ± 5 | 41 ± 42 | 83 ± 8 | 87 ± 2 |
| 89 ± 4 | 44 ± 9 | 77 ± 5 | 61 ± 1 | 54 ± 9 | |
| 29 ± 5 | 53 ± 5 | 68 ± 21 | 68 ± 21 | 67 ± 14 |
| Width Instruction [µm] | 105 | 200 | 250 | 500 | 1000 |
| Measured width [µm] | 114 ± 1 | 203 ± 2 | 245 ± 2 | 499 ± 2 | 988 ± 7 |
| 119 ± 2 | 206 ± 2 | 245 ± 2 | 500 ± 5 | 1003 ± 4 | |
| 117 ± 2 | 205.5 ± 0.8 | 249 ± 2 | 497 ± 4 | 985 ± 13 | |
| p = 0.0056 | p = 0.028 | p = 0.0055 | p = 0.54 | p = 0.018 | |
| Depth instruction [µm] | 50 | 100 | 150 | 200 | |
| Measured depth [µm] | 45.7 ± 0.3 | 105.8 ± 0.2 | 138.0 ± 1 | 174.6 ± 0.2 | |
| 44.6 ± 0.4 | 96.7 ± 0.3 | 136.3 ± 0.5 | 175.5 ± 0.2 | ||
| 47.9 ± 0.4 | 92.9 ± 0.2 | 138.9 ± 0.6 | 173.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmer, M.; Trombotto, S.; Laurenceau, E.; Deman, A.-L. Chitosan as an Alternative to Oil-Based Materials for the Fabrication of Lab-on-a-Chip. Micromachines 2024, 15, 379. https://doi.org/10.3390/mi15030379
Zimmer M, Trombotto S, Laurenceau E, Deman A-L. Chitosan as an Alternative to Oil-Based Materials for the Fabrication of Lab-on-a-Chip. Micromachines. 2024; 15(3):379. https://doi.org/10.3390/mi15030379
Chicago/Turabian StyleZimmer, Morgane, Stéphane Trombotto, Emmanuelle Laurenceau, and Anne-Laure Deman. 2024. "Chitosan as an Alternative to Oil-Based Materials for the Fabrication of Lab-on-a-Chip" Micromachines 15, no. 3: 379. https://doi.org/10.3390/mi15030379
APA StyleZimmer, M., Trombotto, S., Laurenceau, E., & Deman, A.-L. (2024). Chitosan as an Alternative to Oil-Based Materials for the Fabrication of Lab-on-a-Chip. Micromachines, 15(3), 379. https://doi.org/10.3390/mi15030379







