On reactive Ion Etching of Parylene-C with Simple Photoresist Mask for Fabrication of High Porosity Membranes to Capture Circulating and Exfoliated Tumor Cells
Abstract
1. Introduction
2. Materials and Methods
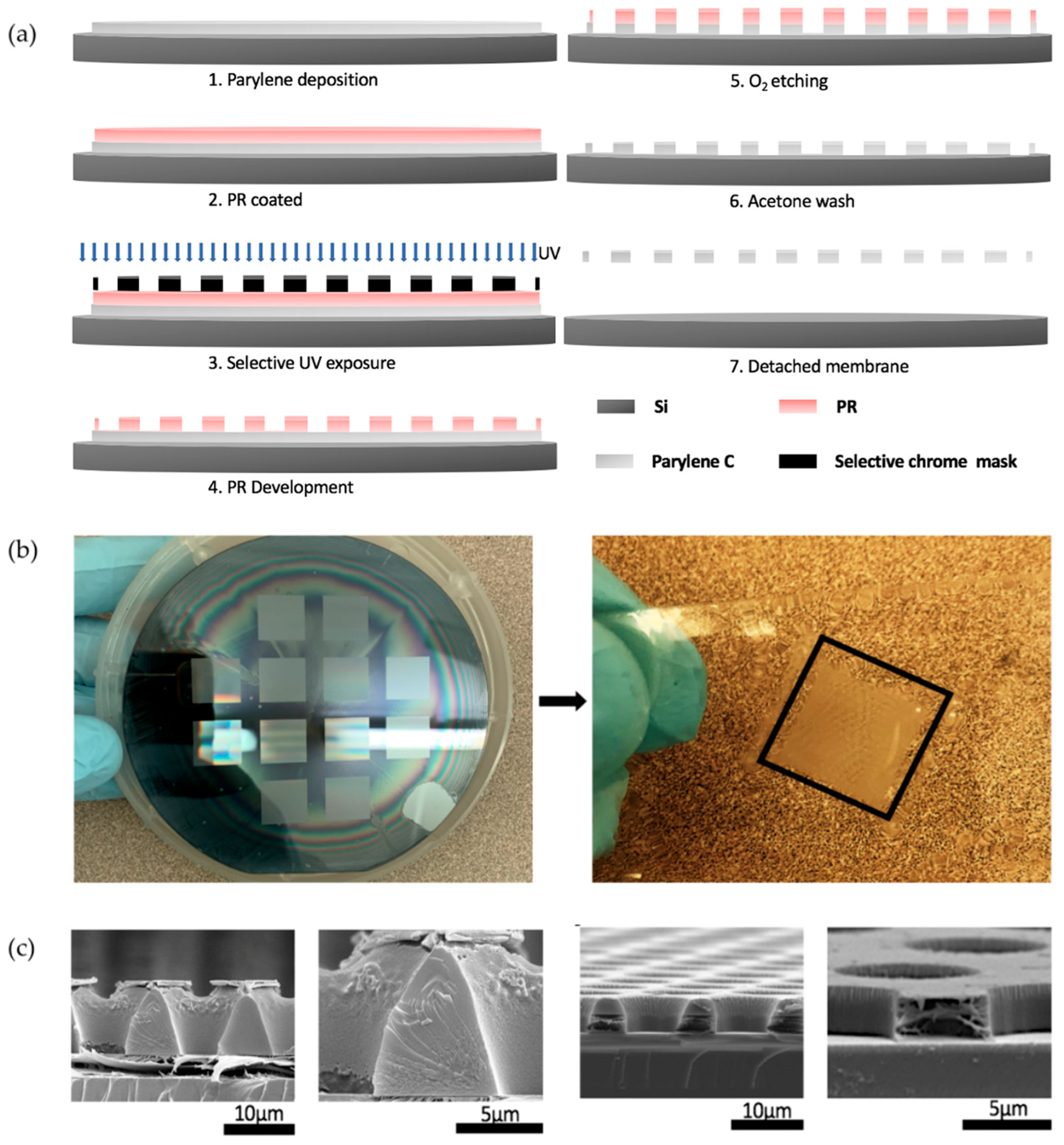
2.1. Pattern Design and Photomask Fabrication
2.2. Fabrication of Microarrayed Porous Parylene Membrane
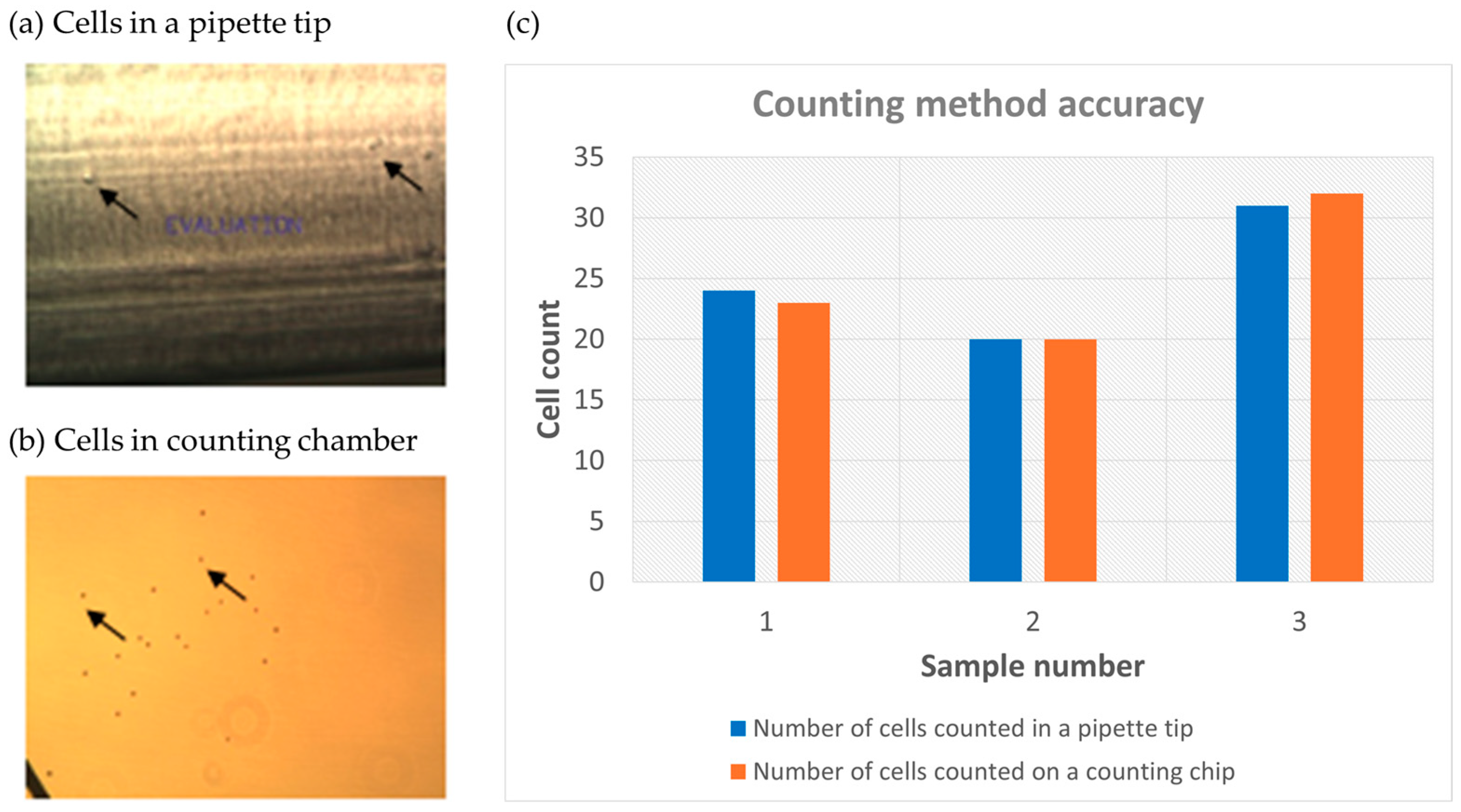
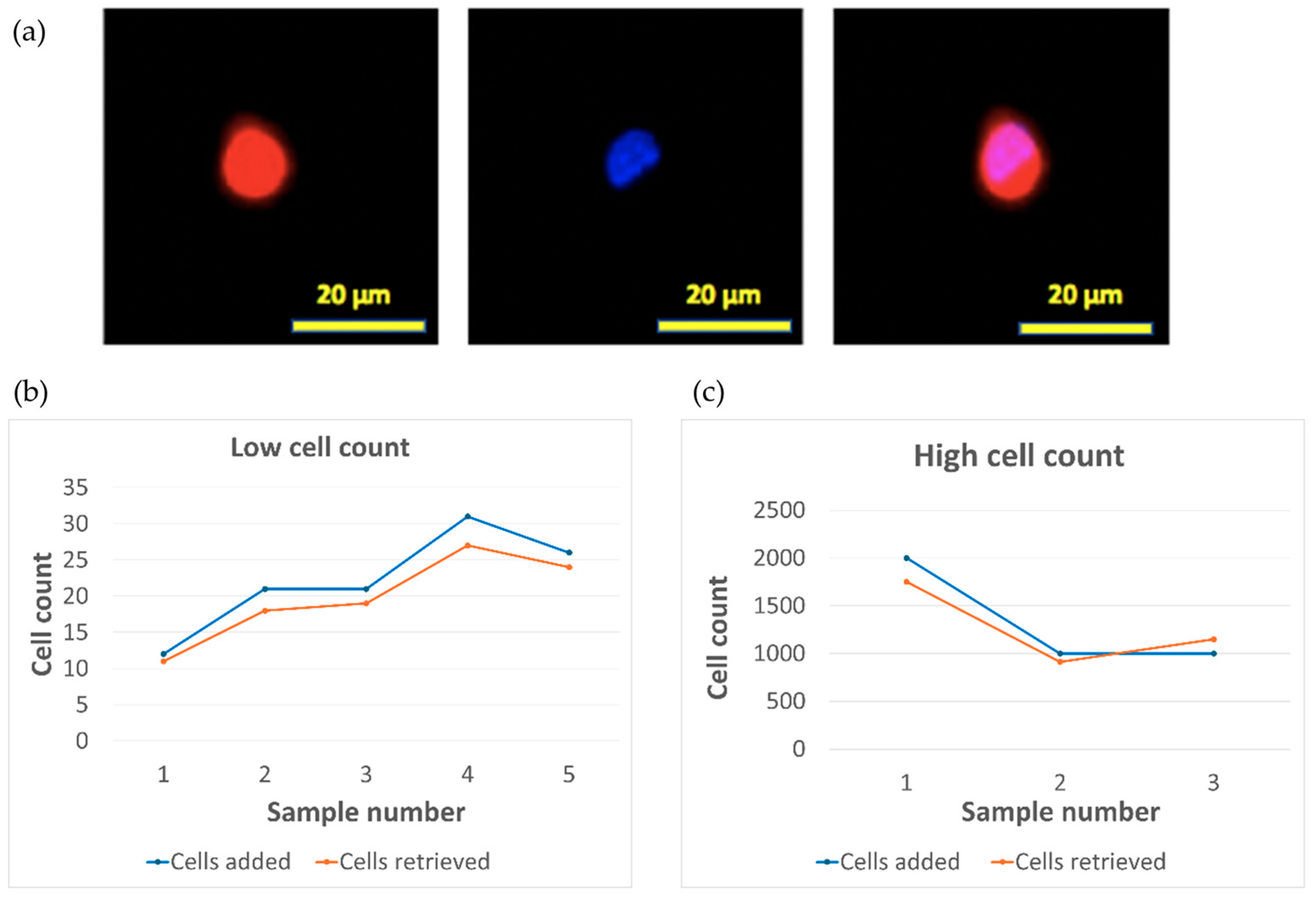
2.3. Spiked Tumor Cell Capture Using Fabricated Parylene-C Microporous Membrane
3. Results
3.1. RIE Fabrication of Micropore Arrayed Parylene Membrane
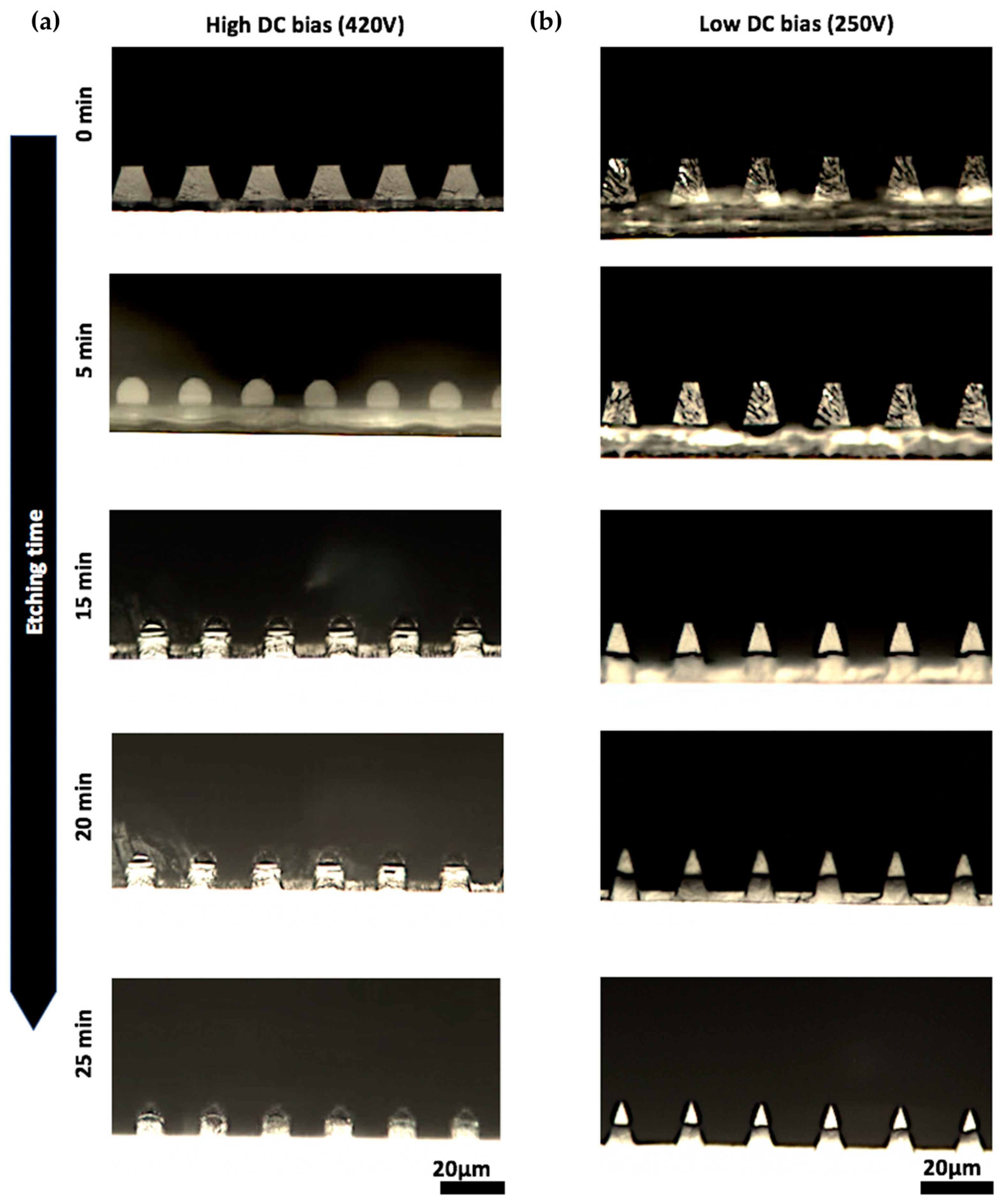
3.2. Condition for Vertical Sidewall Formation in Etched Parylene Pores
3.3. Dependance of Maximum Thickness of Porous Parylene Membrane on Pore-to-Pore Spacing by the Vertical Sidewall SPE Process
3.4. Capture of Spiked Tumor Cells Using SPE Fabricated Porous Membrane
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visal, T.H.; den Hollander, P.; Cristofanilli, M.; Mani, S.A. Circulating tumour cells in the -omics era: How far are we from achieving the ‘singularity’? Br. J. Cancer 2022, 127, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Lyu, T.; Lin, Y.; Wu, K.; Cao, Z.; Zhang, Q.; Zheng, J. Single-cell sequencing technologies in bladder cancer research: Applications and challenges. Front. Genet. 2022, 13, 1027909. [Google Scholar] [CrossRef] [PubMed]
- Riethdorf, S.; Fritsche, H.; Müller, V.; Rau, T.; Schindibeck, C.; Rack, B.; Janni, W.; Coith, C.; Beck, K.; Jänicke, F.; et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer:: A validation study of the CellSearch system. Clin. Cancer Res. 2007, 13, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Wang, Y.; Oliver, C.R.; Thamm, D.H.; Cooling, L.; Paoletti, C.; Smith, K.J.; Nagrath, S.; Hayes, D.F. A temporary indwelling intravascular aphaeretic system for in vivo enrichment of circulating tumor cells. Nat. Commun. 2019, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Chen, Q.; Liu, X.; Shen, S.; Pan, Z.; Shi, C. Detection of circulating stage III-IV gastric cancer tumor cells based on isolation by size of epithelial tumor: Using the circulating tumor cell biopsy technology. Transl. Cancer Res. 2019, 8, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mao, X.; Imrali, A.; Syed, F.; Mutsvangwa, K.; Berney, D.; Cathcart, P.; Hines, J.; Shamash, J.; Lu, Y.J. Optimization and Evaluation of a Novel Size Based Circulating Tumor Cell Isolation System. PLoS ONE 2015, 10, e0138032. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mao, Z.; Peng, Z.; Zhou, L.; Chen, Y.; Huang, P.H.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M.; et al. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef] [PubMed]
- Fachin, F.; Spuhler, P.; Martel-Foley, J.M.; Edd, J.F.; Barber, T.A.; Walsh, J.; Karabacak, M.; Pai, V.; Yu, M.; Smith, K.; et al. Monolithic Chip for High-throughput Blood Cell Depletion to Sort Rare Circulating Tumor Cells. Sci. Rep. 2017, 7, 10936. [Google Scholar] [CrossRef]
- Aghaamoo, M.; Aghilinejad, A.; Chen, X.; Xu, J. On the design of deterministic dielectrophoresis for continuous separation of circulating tumor cells from peripheral blood cells. Electrophoresis 2019, 40, 1486–1493. [Google Scholar] [CrossRef]
- Gupta, V.; Jafferji, I.; Garza, M.; Melnikova, V.O.; Hasegawa, D.K.; Pethig, R.; Davis, D.W. ApoStream(), a new dielectrophoretic device for antibody independent isolation and recovery of viable cancer cells from blood. Biomicrofluidics 2012, 6, 24133. [Google Scholar] [CrossRef]
- Lee, Y.; Guan, G.F.; Bhagat, A.A. ClearCell® FX, a label-free microfluidics technology for enrichment of viable circulating tumor cells. Cytom. Part A 2018, 93A, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Shin, J.H.; Bae, C.Y.; Choi, S.Y.; Park, J.K. Label-Free Cancer Cell Separation from Human Whole Blood Using Inertial Microfluidics at Low Shear Stress. Anal. Chem. 2013, 85, 6213–6218. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.J.; Wan, Y.; Xia, Y.Q.; Zou, X.; Zheng, S.Y. Size-based separation methods of circulating tumor cells. Adv. Drug Deliv. Rev. 2018, 125, 3–20. [Google Scholar] [CrossRef]
- Adams, D.L.; Zhu, P.; Makarova, O.V.; Martin, S.S.; Charpentier, M.; Chumsri, S.; Li, S.; Amstutz, P.; Tang, C.M. The systematic study of circulating tumor cell isolation using lithographic microfilters. RSC Adv. 2014, 9, 4334–4342. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Xu, M.; Zhang, W.; Xiong, Y.; Nie, L.; Wang, Q.; Li, H.; Wang, W. A high-throughput liquid biopsy for rapid rare cell separation from large-volume samples. Lab. A Chip 2019, 19, 68–78. [Google Scholar] [CrossRef]
- Zheng, S.; Lin, H.; Liu, J.Q.; Balic, M.; Datar, R.; Cote, R.J.; Tai, Y.C. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J. Chromatogr. A 2007, 1162, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.; Tai, Y.C. Parylene etching techniques for microfluidics and biomems. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Miami Beach, FL, USA, 30 January–3 February 2005. [Google Scholar]
- Loeb, G.E.; Bak, M.J.; Schmidt, E.M.; Salcman, M. Parylene as a Chronically Stable, Reproducible Microelectrode Insulator. IEEE Trans. Biomed. Eng. 1977, BME-24, 121–128. [Google Scholar]
- Herrault, F.; Ji, C.H.; Rajaraman, S.; Shafer, R.H.; Allen, M.G. Electrodeposited metal structures in high aspect ratio cavities using vapor deposited polymer molds and laser micromachining. In Proceedings of the TRANSDUCERS and EUROSENSORS ‘07—14th International Conference on Solid-State Sensors, Actuators and Microsystems, Lyon, France, 10–14 June 2007. [Google Scholar]
- Schmidt, E.M.; Bak, M.J.; Christensen, P. Laser exposure of Parylene-C insulated microelectrodes. J. Neurosci. Methods 1995, 62, 89–92. [Google Scholar] [CrossRef]
- Youn, S.W.; Goto, H.; Takahashi, M.; Oyama, S.; Oshinomi, Y.; Matsutani, K.; Maeda, R. A replication process of metallic micro-mold by using parylene embossing and electroplating. Microelectron. Eng. 2008, 85, 161–167. [Google Scholar] [CrossRef]
- Noh, H.S.; Huang, Y.; Hesketh, P.J. Parylene micromolding, a rapid and low-cost fabrication method for parylene microchannel. Sens. Actuators B Chem. 2004, 102, 78–85. [Google Scholar] [CrossRef]
- Meng, E.; Li, P.Y.; Tai, Y.C. Plasma removal of Parylene C. J. Micromech. Microeng. 2008, 18, 045004. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Dai, W.; Li, H.; Wang, W. 2.5-Dimensional Parylene C micropore array with a large area and a high porosity for high-throughput particle and cell separation. Microsyst. Nanoeng. 2018, 4, 13. [Google Scholar]
- Zhang, L.; Liu, Y.; Li, Z.; Wang, W. SF6 optimized O2 plasma etching of parylene C. Micromachines 2018, 9, 162. [Google Scholar] [CrossRef]
- Tacito, R.D.; Steinbrüchel, C. Fine-line patterning of parylene-n by reactive ion etching for application as an interlayer dielectric. J. Electrochem. Soc. 1996, 143, 1974–1977. [Google Scholar] [CrossRef]
- Wolf, S.; Tauber, R.N. Silicon Processing for the VLSI Era. Volume 1—Process Technology; Lattice Press: Sunset Beach, CA, USA, 1986. [Google Scholar]
- Yeh, J.T.C.; Grebe, K.R. Patterning of poly-para-xylylenes by reactive ion etching. J. Vac. Sci. Technol. A Vac. Surf. Film. 1983, 1, 604–608. [Google Scholar] [CrossRef]
- Hou, T.; Zheng, C.; Bai, S.; Ma, Q.; Bridges, D.; Hu, A.; Duley, W.W. Fabrication, characterization, and applications of microlenses. Appl. Opt. 2015, 54, 7366–7376. [Google Scholar] [CrossRef]
- Daly, D.; Stevens, R.F.; Hutley, M.C.; Davies, N. The manufacture of microlenses by melting photoresist. Meas. Sci. Technol. 1990, 1, 759–766. [Google Scholar] [CrossRef]
- Cho, S.I.; Park, H.K.; An, S.; Hong, S.J. Plasma Ion Bombardment Induced Heat Flux on the Wafer Surface in Inductively Coupled Plasma Reactive Ion Etch. Appl. Sci. 2023, 13, 9533. [Google Scholar] [CrossRef]
- Durandet, A.; Joubert, O.; Pelletier, J.; Pichot, M. Effects of ion bombardment and chemical reaction on wafer temperature during plasma etching. J. Appl. Phys. 1990, 67, 3862–3866. [Google Scholar] [CrossRef]
- SJSA-1 CRL-2098. Available online: https://www.atcc.org/products/crl-2098 (accessed on 7 February 2024).
- Tellez-Gabriel, M.; Brown, H.K.; Young, R.; Heymann, M.F.; Heymann, D. The Challenges of Detecting Circulating Tumor Cells in Sarcoma. Front. Oncol. 2016, 6, 202. [Google Scholar] [CrossRef]
- Hosokawa, M.; Hayata, T.; Fukuda, Y.; Arakaki, A.; Yoshino, T.; Tanaka, T.; Matsunaga, T. Size-Selective Microcavity Array for Rapid and Efficient Detection of Circulating Tumor Cells. Anal. Chem. 2010, 82, 6629–6635. [Google Scholar] [CrossRef]
- Chen, H.M.; Cao, B.S.; Sun, B.; Cao, Y.P.; Yang, K.; Lin, Y.S.; Chen, H.D. Highly-sensitive capture of circulating tumor cells using microellipse filters. Sci. Rep. 2017, 7, 610. [Google Scholar] [CrossRef] [PubMed]
- Coumans, F.A.W.; van Dalum, G.; Beck, M.; Terstappen, L.W.M.M. Filter Characteristics Influencing Circulating Tumor Cell Enrichment from Whole Blood. PLoS ONE 2013, 8, e61770. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.D.; Shi, J.; Li, S.S.; Wang, L.; Cayre, Y.E.; Chen, Y. Microfluidic device with integrated microfilter of conical-shaped holes for high efficiency and high purity capture of circulating tumor cells. Sci. Rep. 2014, 4, 6052. [Google Scholar] [CrossRef] [PubMed]



| Recipe | O2 Flow (Sccm) | Pressure (mTorr) | Power (W) | DC Bias (V) | Rv,pa 1 (μm/min) | Rv,pr 1 (μm/min) | Se 1 = Rv,pa/Rv,pr | RL,pa 1 (μm/min) |
|---|---|---|---|---|---|---|---|---|
| Vertical sidewall | 25 | 200 | 300 | 420 | 0.23 | 0.25 | 0.92 | 0.052 |
| Sloped sidewall | 62 | 500 | 300 | 250 | 0.19 | 0.205 | 0.93 | 0.148 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabadi, I.; Carpentieri, D.; Wang, J.; Zenhausern, F.; Gu, J. On reactive Ion Etching of Parylene-C with Simple Photoresist Mask for Fabrication of High Porosity Membranes to Capture Circulating and Exfoliated Tumor Cells. Micromachines 2024, 15, 521. https://doi.org/10.3390/mi15040521
Rabadi I, Carpentieri D, Wang J, Zenhausern F, Gu J. On reactive Ion Etching of Parylene-C with Simple Photoresist Mask for Fabrication of High Porosity Membranes to Capture Circulating and Exfoliated Tumor Cells. Micromachines. 2024; 15(4):521. https://doi.org/10.3390/mi15040521
Chicago/Turabian StyleRabadi, Inad, David Carpentieri, Jue Wang, Frederic Zenhausern, and Jian Gu. 2024. "On reactive Ion Etching of Parylene-C with Simple Photoresist Mask for Fabrication of High Porosity Membranes to Capture Circulating and Exfoliated Tumor Cells" Micromachines 15, no. 4: 521. https://doi.org/10.3390/mi15040521
APA StyleRabadi, I., Carpentieri, D., Wang, J., Zenhausern, F., & Gu, J. (2024). On reactive Ion Etching of Parylene-C with Simple Photoresist Mask for Fabrication of High Porosity Membranes to Capture Circulating and Exfoliated Tumor Cells. Micromachines, 15(4), 521. https://doi.org/10.3390/mi15040521






