Assessment of Early Glaucomatous Optic Neuropathy in the Dog by Spectral Domain Optical Coherence Tomography (SD-OCT)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ophthalmic Examination
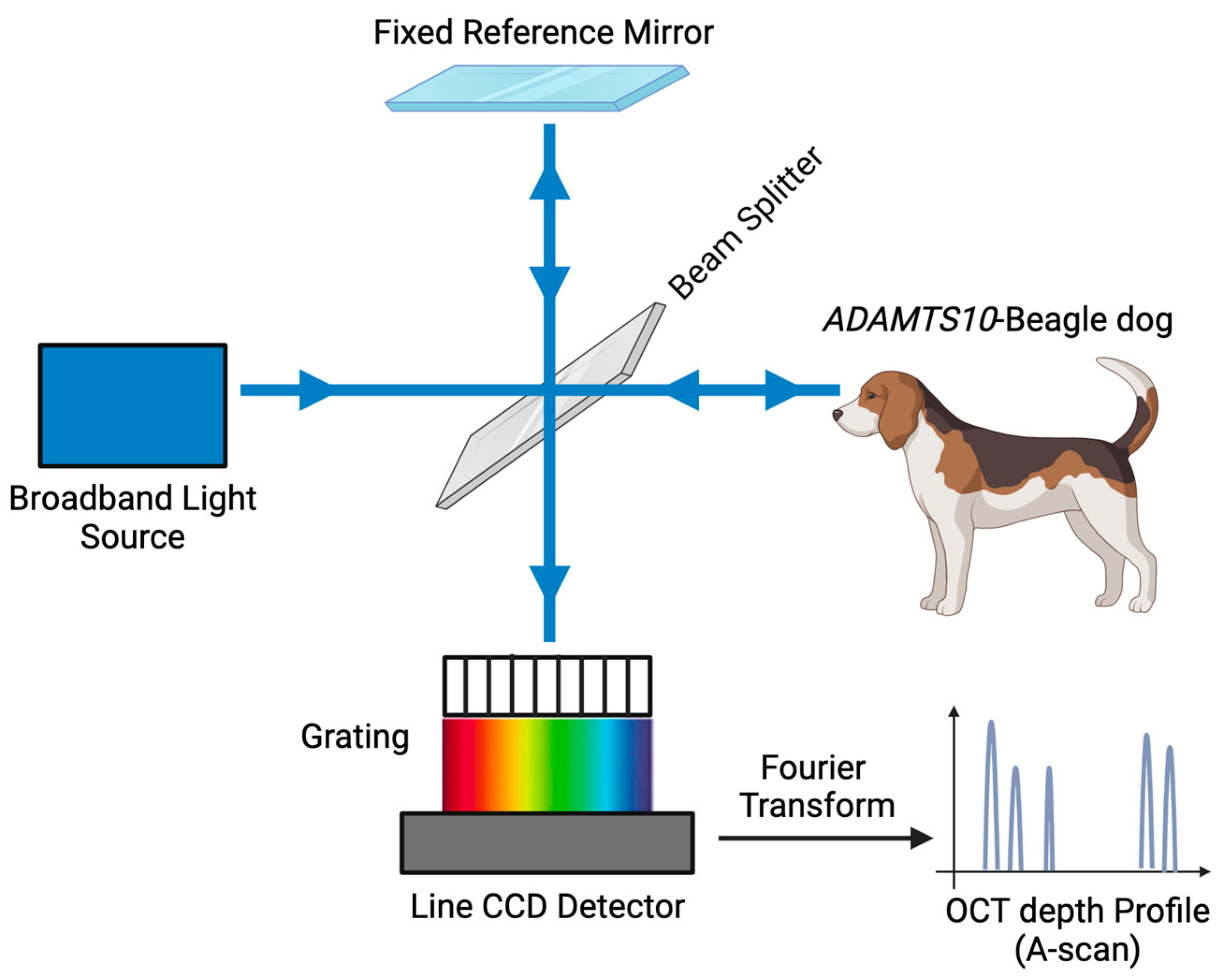
2.3. Spectral-Domain Optical Coherence Tomography (SD-OCT) Image Acquisition
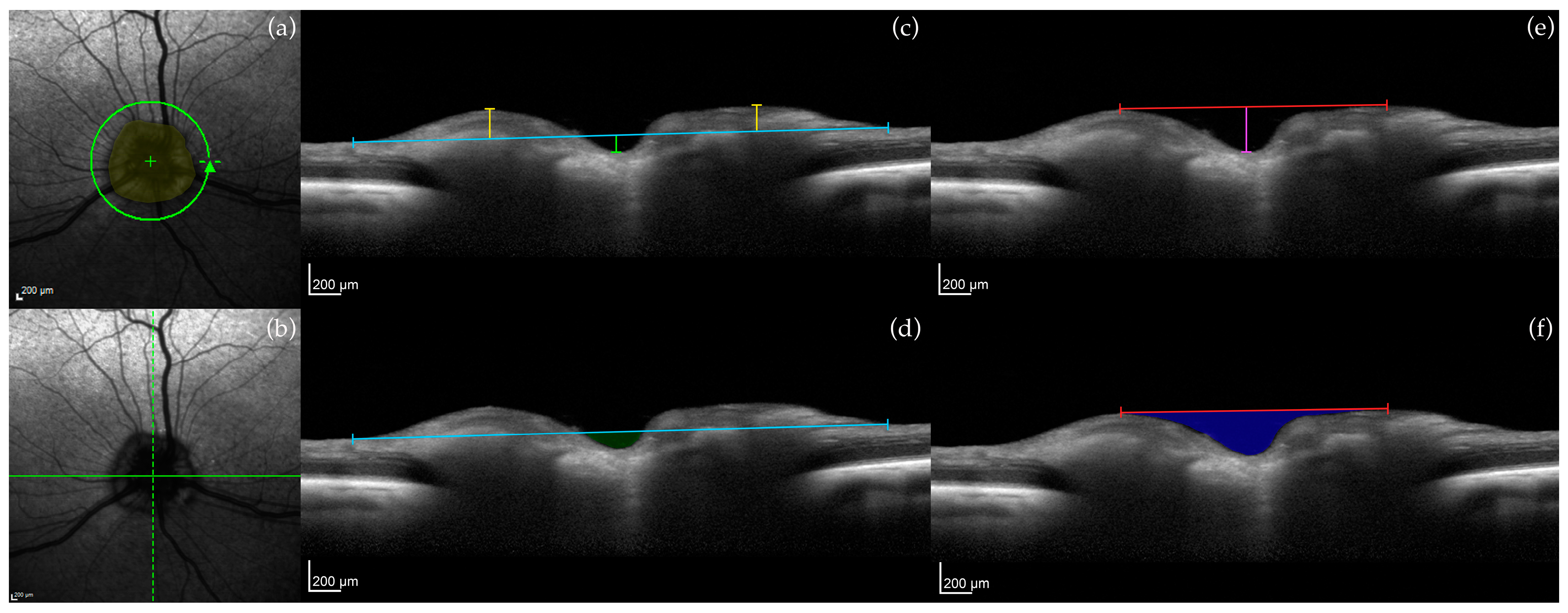
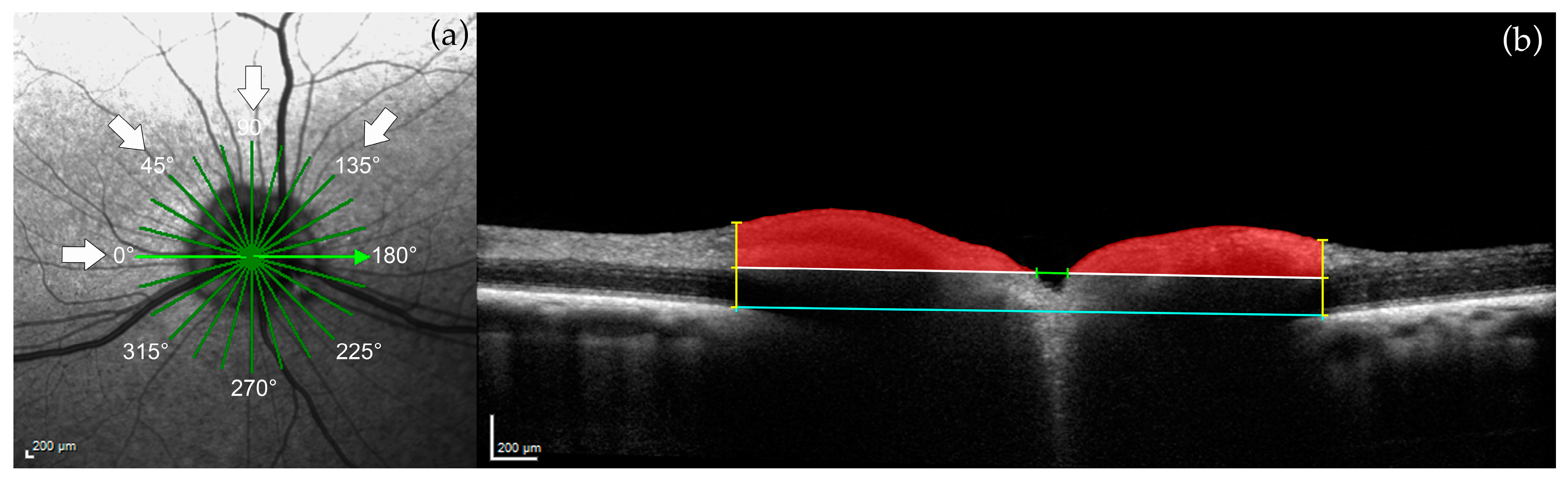
2.3.1. Optic Nerve Head (ONH) Measurements
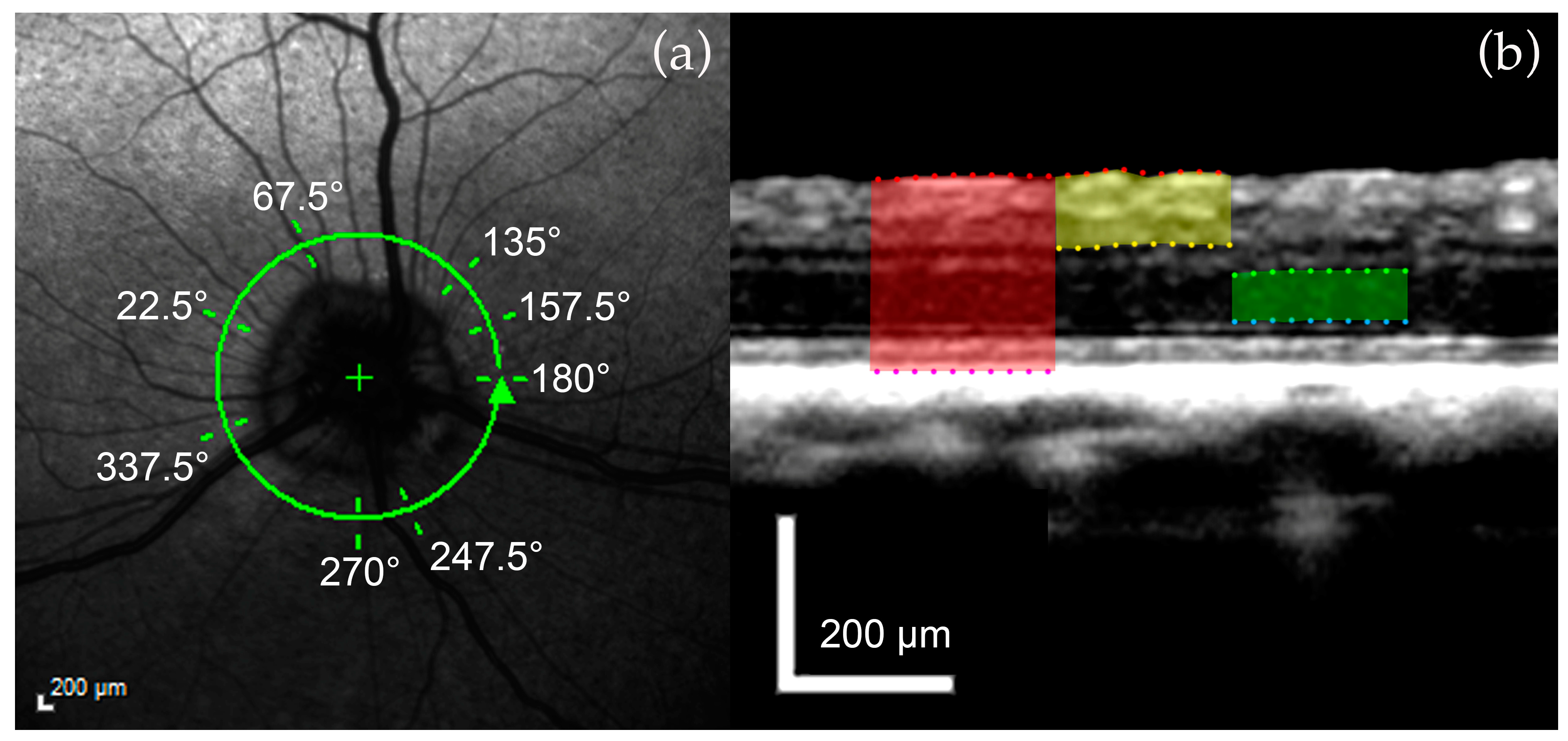
2.3.2. Retinal Layer Measurements
2.4. Statistical Analysis
2.5. Tissue Collection and Histology
3. Results
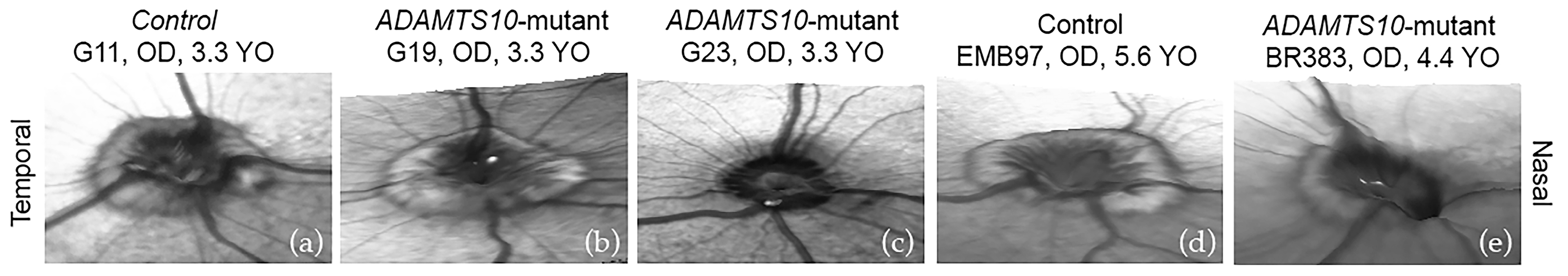
3.1. Optic Nerve Head
3.2. Retinal Layers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelatt, K.N.; MacKay, E.O. Prevalence of the breed-related glaucomas in pure-bred dogs in North America. Vet. Ophthalmol. 2004, 7, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Komáromy, A.M.; Petersen-Jones, S.M. Genetics of Canine Primary Glaucomas. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 1159–1182. [Google Scholar] [CrossRef] [PubMed]
- Plummer, C.E.; Komáromy, A.M.; Gelatt, K.N. The Canine Glaucomas. In Veterinary Ophthalmology, 6th ed.; Gelatt, K.N., Ben-Shlomo, G., Gilger, B.C., Hendrix, D.V.H., Kern, T.J., Plummer, C.E., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 1173–1256. [Google Scholar]
- Graham, K.L.; McCowan, C.I.; Caruso, K.; Billson, F.M.; Whittaker, C.J.G.; White, A. Optical coherence tomography of the retina, nerve fiber layer, and optic nerve head in dogs with glaucoma. Vet. Ophthalmol. 2020, 23, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Komáromy, A.M.; Bras, D.; Esson, D.W.; Fellman, R.L.; Grozdanic, S.D.; Kagemann, L.; Miller, P.E.; Moroi, S.E.; Plummer, C.E.; Sapienza, J.S.; et al. The future of canine glaucoma therapy. Vet. Ophthalmol. 2019, 22, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Hee, M.R.; Izatt, J.A.; Swanson, E.A.; Huang, D.; Schuman, J.S.; Lin, C.P.; Puliafito, C.A.; Fujimoto, J.G. Optical Coherence Tomography of the Human Retina. Arch. Ophthalmol. 1995, 113, 325–332. [Google Scholar] [CrossRef] [PubMed]
- A Townsend, K.; Wollstein, G.; Schuman, J.S. Imaging of the retinal nerve fibre layer for glaucoma. Br. J. Ophthalmol. 2009, 93, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Fujimoto, J.G. State-of-the-art retinal optical coherence tomography. Prog. Retin. Eye Res. 2008, 27, 45–88. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowski, M.; Bajraszewski, T.; Targowski, P.; Kowalczyk, A. Real-time in vivo imaging by high-speed spectral optical coherence tomography. Opt. Lett. 2003, 28, 1745–1747. [Google Scholar] [CrossRef] [PubMed]
- Mrejen, S.; Spaide, R.F. Optical coherence tomography: Imaging of the choroid and beyond. Surv. Ophthalmol. 2013, 58, 387–429. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Elgin, C.Y.; Chen, D.; Al-Aswad, L.A. Ophthalmic imaging for the diagnosis and monitoring of glaucoma: A review. Clin. Exp. Ophthalmol. 2022, 50, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A.; Zangwill, L.M.; Bowd, C.; Vessani, R.M.; Susanna, R.; Weinreb, R.N. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Arch. Ophthalmol. 2005, 139, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Telle, M.R.; Snyder, K.C.; Oikawa, K.; Nilles, J.P.; Gehrke, S.; Teixeira, L.B.C.; Kiland, J.A.; Huang, A.; McLellan, G.J. Development and validation of methods to visualize conventional aqueous outflow pathways in canine primary angle closure glaucoma. Vet. Ophthalmol. 2022, 25, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Sledge, D.; Monahan, C.F.; Teixeira, L.; Boyd, R.; Freeman, K.; Koehl, K.; Harman, C.; Munoz, K.; Occelli, L.M.; et al. Atypical chorioretinal lesions in Siberian Husky dogs with primary angle-closure glaucoma: A case series. BMC Vet. Res. 2022, 18, 182. [Google Scholar] [CrossRef] [PubMed]
- Grozdanic, S.D.; Matic, M.; Betts, D.M.; Sakaguchi, D.S.; Kardon, R.H. Recovery of canine retina and optic nerve function after acute elevation of intraocular pressure: Implications for canine glaucoma treatment. Vet. Ophthalmol. 2007, 10, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Gelatt, K.N. Familial glaucoma in the Beagle dog. J. Am. Anim. Hosp. Assoc. 1972, 8, 23–28. [Google Scholar]
- Kuchtey, J.; Olson, L.M.; Rinkoski, T.; MacKay, E.O.; Iverson, T.M.; Gelatt, K.N.; Haines, J.L.; Kuchtey, R.W. Mapping of the disease locus and identification of ADAMTS10 as a candidate gene in a canine model of primary open angle Glaucoma. PLoS Genet. 2011, 7, e1001306. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.E.; Strubbe, D.T.; Kubilis, P.S.; MacKay, E.O.; Samuelson, D.A.; Gelatt, K.N. Histomorphometry of the optic nerves of normal dogs and dogs with hereditary glaucoma. Exp. Eye Res. 1995, 60, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.E.; A Samuelson, D.; Gelatt, K.N.; Smith, P.J. Morphologic changes in the lamina cribrosa of beagles with primary open-angle glaucoma. Am. J. Vet. Res. 1989, 50, 936–941. [Google Scholar]
- Brooks, D.E.; A Samuelson, D.; Gelatt, K.N. Ultrastructural changes in laminar optic nerve capillaries of beagles with primary open-angle glaucoma. Am. J. Vet. Res. 1989, 50, 929–935. [Google Scholar] [PubMed]
- Gelatt, K.N.; Gum, G.G.; Barrie, K.P.; Williams, L.W. Diurnal variation in intraocular pressure in normotensive and glaucomatous Beagles. Glaucoma 1981, 3, 21–24. [Google Scholar]
- Yang, H.; Qi, J.; Hardin, C.; Gardiner, S.K.; Strouthidis, N.G.; Fortune, B.; Burgoyne, C.F. Spectral-domain optical coherence tomography enhanced depth imaging of the normal and glaucomatous nonhuman primate optic nerve head. Investig. Opthalmol. Vis. Sci. 2012, 53, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Leary, K.A.; Steibel, J.P.; Harman, C.D.; Anderson, A.L.; Komáromy, A.M. Safety and efficacy of topically administered netarsudil-latanoprost fixed dose combination (FDC; RocklatanTM) in normal and glaucomatous dogs with ADAMTS10-open-angle glaucoma (ADAMTS10-OAG). Vet. Ophthalmol. 2021, 24, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bai, Y.; Xu, Z.; Liu, P.; Ni, G. Optical Coherence Tomography for Three-Dimensional Imaging in the Biomedical Field: A Review. Front. Phys. 2021, 9, 744346. [Google Scholar] [CrossRef]
- Brooks, D.; Komàromy, A.; Källberg, M. Comparative retinal ganglion cell and optic nerve morphology. Vet. Ophthalmol. 1999, 2, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.K.-S.; Chan, W.-M.; Hui, Y.-L.; Yung, W.-H.; Woo, J.; Tsang, M.-K.; Tse, K.-K. Analysis of retinal nerve fiber layer and optic nerve head in glaucoma with different reference plane offsets, using optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2005, 46, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Schuman, J.S.; Wollstein, G.; Farra, T.; Hertzmark, E.; Aydin, A.; Fujimoto, J.G.; Paunescu, L.A. Comparison of optic nerve head measurements obtained by optical coherence tomography and confocal scanning laser ophthalmoscopy. Arch. Ophthalmol. 2003, 135, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.C.; Fortune, B.O.; Arthur, S.N.; Xing, D.B.; Salant, J.A.; Ritch, R.; Liebmann, J.M. Blood vessel contributions to retinal nerve fiber layer thickness profiles measured with optical coherence tomography. Eur. J. Gastroenterol. Hepatol. 2008, 17, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Naumann, G.O.H. The optic disc nerve: Its embryology, histology, and morphology. Opt. Nerve Glaucoma 1993, 3–26. [Google Scholar]
- Gelatt, K.N.; Gum, G.G.; Gwin, R.M.; Bromberg, N.M.; E Merideth, R.; Samuelson, D.A. Primary open angle glaucoma: Inherited primary open angle glaucoma in the beagle. Am. J. Pathol. 1981, 102, 292–295. [Google Scholar] [PubMed]
- Gelatt, K.; Peiffer, R.; Gwin, R.; Gum, G.; Williams, L. Clinical manifestations of inherited glaucoma in the beagle. Investig. Ophthalmol. Vis. Sci. 1977, 16, 1135–1142. [Google Scholar]
- DSamuelson, D.A.; Williams, L.W.; Gelatt, K.N.; Gum, G.; Meredith, R. Orthograde rapid axoplasmic transport and ultrastructural changes of the optic nerve. Part II. Beagles with primary open-angle glaucoma. Glaucoma 1983, 5, 174–184. [Google Scholar]
- Williams, L.W.; Gelatt, K.N.; Gum, G.G.; Samuelson, D.A.; Merideth, R.E. Orthograde rapid axoplasmic transport and ultrastructural changes of the optic nerve. Part I. Normotensive and acute ocular hypertensive beagles. Glaucoma 1983, 5, 117–128. [Google Scholar]
- Fan, N.; Huang, N.; Lam, D.S.C.; Leung, C.K.-S. Measurement of photoreceptor layer in glaucoma: A spectral-domain optical coherence tomography study. J. Ophthalmol. 2011, 2011, 264803. [Google Scholar] [CrossRef]
- Wygnanski, T.; Desatnik, H.; Quigley, H.A.; Glovinsky, Y. Comparison of ganglion cell loss and cone loss in experimental glaucoma. Arch. Ophthalmol. 1995, 120, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Schuman, J.S.; Hee, M.R.; Puliafito, C.A.; Wong, C.; Pedut-Kloizman, T.; Lin, C.P.; Hertzmark, E.; Izatt, J.A.; Swanson, E.A.; Fujimoto, J.G. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch. Ophthalmol. 1995, 113, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Addicks, E.M.; Green, W.R. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch. Ophthalmol. 1982, 100, 135–146. [Google Scholar] [CrossRef]
- Park, S.A.; Komáromy, A.M. Biomechanics of the optic nerve head and sclera in canine glaucoma: A brief review. Vet. Ophthalmol. 2021, 24, 316–325. [Google Scholar] [CrossRef] [PubMed]



| Canine ID | Sex | Age (Years) | POAG Status | IOP (mmHg) | Axial Length (mm) | |
|---|---|---|---|---|---|---|
| M685 | M | 2.6 | − | OD/OS | 14.7/16.6 | 20.9/20.5 |
| G11 | M | 3.3 | − | OD/OS | 20.9/18.9 | 20.9/20.9 |
| EMB97 | F | 5.6 | − | OD/OS | 19.9/17.2 | 20.1/20.1 |
| BLSU076 | M | 2.4 | + | OD/OS | 32.4/29.1 | 21.1/22.1 |
| G19 | M | 3.3 | + | OD/OS | 27.2/26.5 | 21.9/21.2 |
| G23 | F | 3.3 | + | OD/OS | 31.9/33.3 | 20.4/21.3 |
| G1 | M | 3.8 | + | OD/OS | 31.3/32.4 | 21.1/22.1 |
| G3 | M | 3.8 | + | OD/OS | 29.7/26.9 | 21.8/22.8 |
| G4 | M | 3.8 | + | OD/OS | 36.1/31.9 | 21.7/21.3 |
| G18 | M | 3.8 | + | OD/OS | 27.9/27.3 | 20.5/21.0 |
| BR383 | F | 4.4 | + | OD/OS | 31.5/33.1 | 23.4/24.0 |
| BR376 | F | 4.4 | + | OD/OS | 24.2/24.7 | 21.0/21.2 |
| RNFL Scan | Line Scan | Radial Scan | Volume Scan | |
|---|---|---|---|---|
| Scan Type | Circle | Single | Volume | Volume |
| Resolution Mode | HR or HS | HR or HS | HR or HS | HR or HS |
| Scan Angle | 30° | 30° | 15° or 30° | 20° or 30° |
| Scan Width | 30° | 15° or 30° | ||
| Pattern Size | 20° × 20° or 30° × 25° | |||
| Circle Diameter | 12° | |||
| ART | up to 100 images averaged | up to 100 images averaged | up to 16 images averaged | up to 16 images averaged |
| EDI Mode | Off | On | On | Off |
| Number of B-Scans | 12 | 49 or 61 | ||
| Angle between B-Scans | 15° | |||
| Distance between B-scans | 122 µm |
| ONH Measurements | Retinal Layer Measurements |
|---|---|
| ONH area | FRT |
| Vitreoretinal reference plane ONH cup depth, myelin peak height, cross-sectional area of the ONH cup | IRT |
| Neuroretinal rim reference plane | ONL |
| ONH cup depth, myelin peak distance, cross-sectional area of the ONH cup | |
| 150 μm reference plane | |
| Optic cup diameter, neuroretinal rim area |
| SD-OCT Parameter | Line Scans | Control (n = 3) | Mutant (n = 9) | p-Value | Correlation with IOP | p-Value |
|---|---|---|---|---|---|---|
| Myelin peak height | 0° | 154 +/− 38.4 μm | 9.3 +/− 22.1 μm | 0.010 | −0.78 | 0.003 |
| 90° | 119 +/− 30.9 μm | 24.0 +/− 17.7 μm | 0.026 | −0.57 | 0.05 |
| SD-OCT Parameter | Location | Control (n = 3) | Mutant (n = 9) | p-Value |
|---|---|---|---|---|
| Average FRT | Peripapillary | 239 +/− 6.0 μm | 243 +/− 3.2 μm | 0.510 |
| Average IRT | Peripapillary | 89.4 +/− 4.4 μm | 90.8 +/− 2.7 μm | 0.790 |
| Average ONL | Peripapillary | 56.4 +/− 2.9 μm | 52.9 +/− 3.9 μm | 0.640 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, A.; Harman, C.D.; Koehl, K.L.; Huang, J.; Teixeira, L.B.C.; Occelli, L.M.; Storey, E.S.; Ying, G.-S.; Komáromy, A.M. Assessment of Early Glaucomatous Optic Neuropathy in the Dog by Spectral Domain Optical Coherence Tomography (SD-OCT). Micromachines 2024, 15, 780. https://doi.org/10.3390/mi15060780
Oh A, Harman CD, Koehl KL, Huang J, Teixeira LBC, Occelli LM, Storey ES, Ying G-S, Komáromy AM. Assessment of Early Glaucomatous Optic Neuropathy in the Dog by Spectral Domain Optical Coherence Tomography (SD-OCT). Micromachines. 2024; 15(6):780. https://doi.org/10.3390/mi15060780
Chicago/Turabian StyleOh, Annie, Christine D. Harman, Kristin L. Koehl, Jiayan Huang, Leandro B. C. Teixeira, Laurence M. Occelli, Eric S. Storey, Gui-Shuang Ying, and András M. Komáromy. 2024. "Assessment of Early Glaucomatous Optic Neuropathy in the Dog by Spectral Domain Optical Coherence Tomography (SD-OCT)" Micromachines 15, no. 6: 780. https://doi.org/10.3390/mi15060780
APA StyleOh, A., Harman, C. D., Koehl, K. L., Huang, J., Teixeira, L. B. C., Occelli, L. M., Storey, E. S., Ying, G.-S., & Komáromy, A. M. (2024). Assessment of Early Glaucomatous Optic Neuropathy in the Dog by Spectral Domain Optical Coherence Tomography (SD-OCT). Micromachines, 15(6), 780. https://doi.org/10.3390/mi15060780






