Computational and Experimental Characterization of Aligned Collagen across Varied Crosslinking Degrees
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrochemical Aligned Collagen Threads
2.3. Preparation of Random Collagen Threads
2.4. Quantification of Crosslinking Degree of Random and Electrochemical Aligned Collagen Threads
2.5. Assessment of Surface Microstructure of Random and ELAC Threads
2.6. Mechanical Assessment of Random and Electrochemical Aligned Collagen Threads using Uniaxial Tensile Testing
2.7. Statistical Analysis
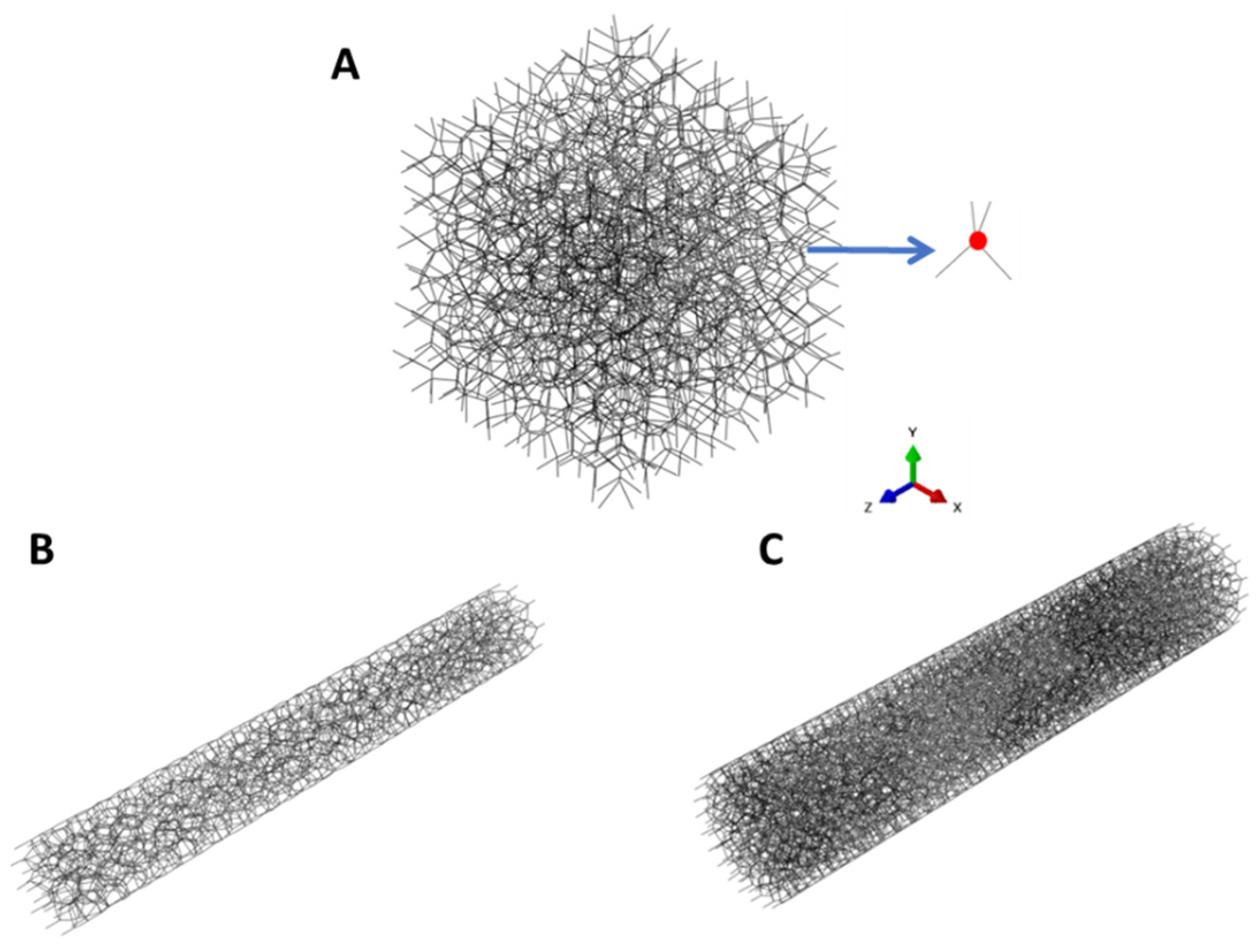
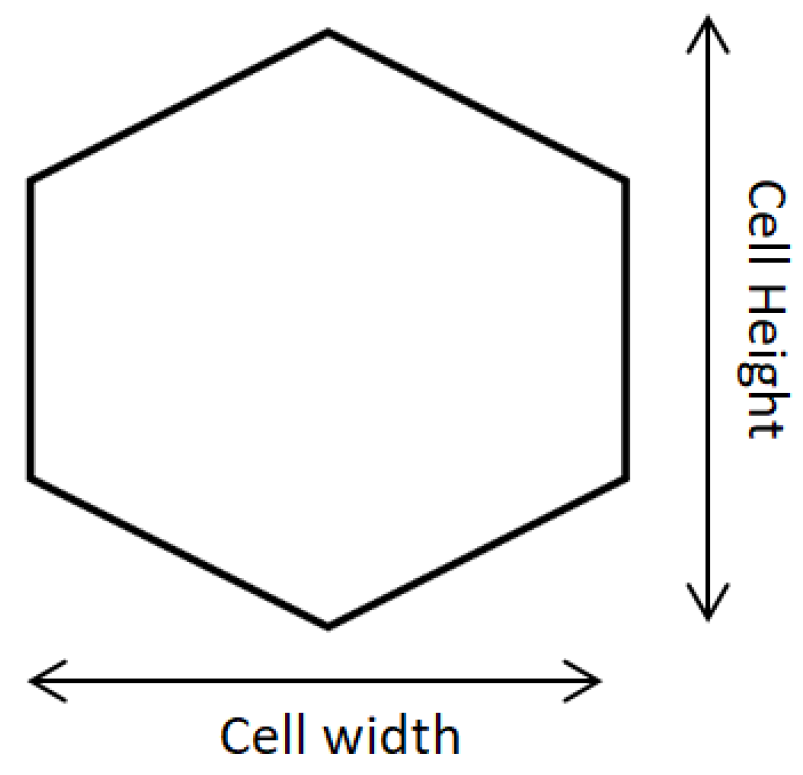
2.8. Computational Modeling
3. Experimental Results
3.1. Quantification of Crosslinking Degree of Random and Electrochemical Aligned Collagen Threads
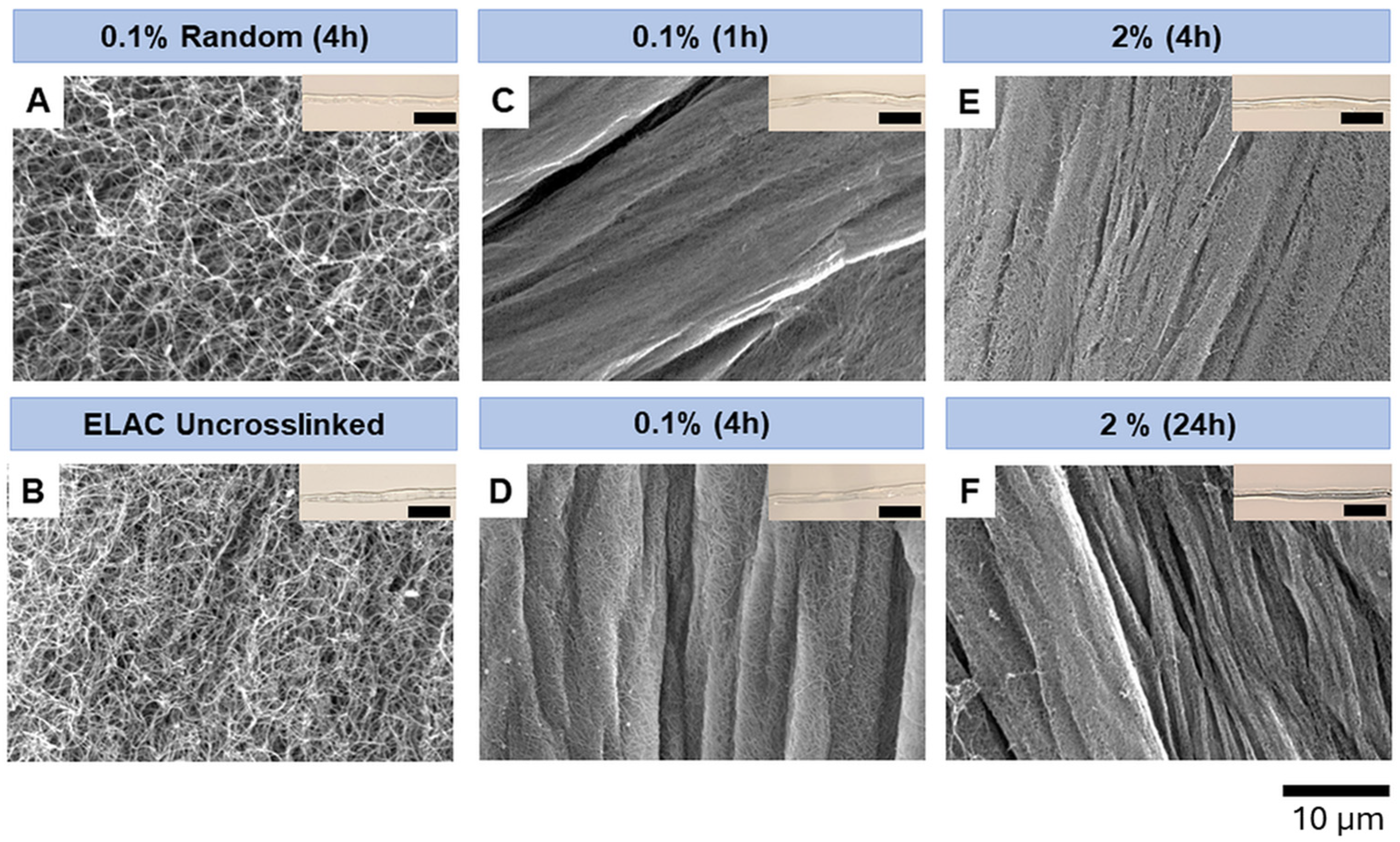
3.2. Effect of Crosslinking Conditions on Surface Microstructure of Electrochemically Aligned Collagen Threads
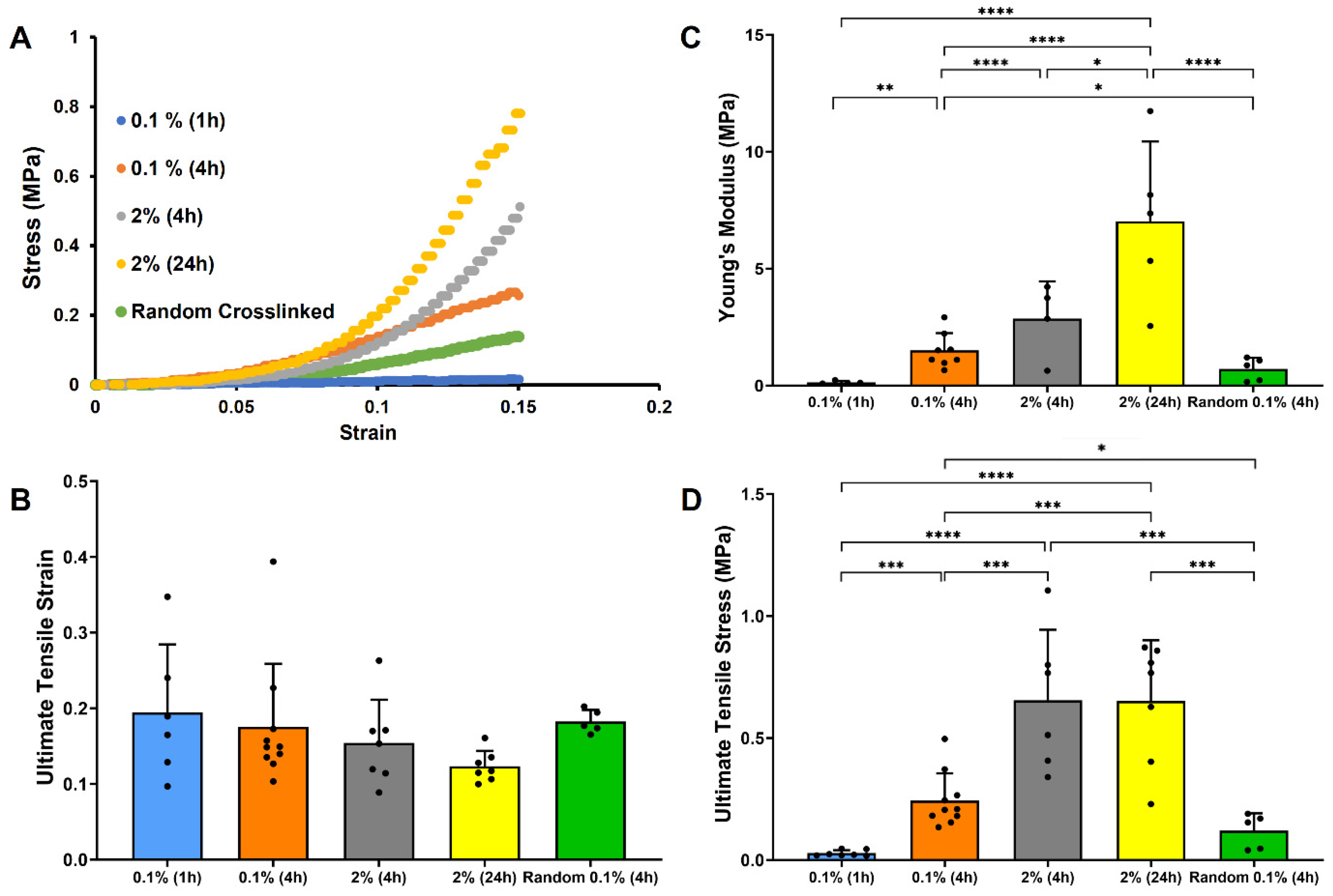
3.3. Effect of Crosslinking Conditions on Tensile Properties of Random and Electrochemical Aligned Collagen Threads
3.4. Simulation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-Based Biomaterials for Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Taufalele, P.V.; VanderBurgh, J.A.; Muñoz, A.; Zanotelli, M.R.; Reinhart-King, C.A. Fiber Alignment Drives Changes in Architectural and Mechanical Features in Collagen Matrices. PLoS ONE 2019, 14, e0216537. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Zhou, C.; Ma, L.; Wu, L.; Xu, X.; Gu, L.; Fan, Y.; Xian, G.; Fan, H.; Zhang, X. Direct 3-D Printing of Ti-6Al-4V/HA Composite Porous Scaffolds for Customized Mechanical Properties and Biological Functions. J. Tissue Eng. Regen. Med. 2020, 14, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.; Kajave, N.; Cai, H.H.; Gu, L.; Albanna, M.; Kishore, V. In Vitro Characterization of Xeno-Free Clinically Relevant Human Collagen and Its Applicability in Cell-Laden 3D Bioprinting. J. Biomater. Appl. 2020, 35, 912–923. [Google Scholar] [CrossRef]
- Cheng, X.; Gurkan, U.A.; Dehen, C.J.; Tate, M.P.; Hillhouse, H.W.; Simpson, G.J.; Akkus, O. An Electrochemical Fabrication Process for the Assembly of Anisotropically Oriented Collagen Bundles. Biomaterials 2008, 29, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Patrawalla, N.Y.; Bock, K.; Liebendorfer, K.; Kishore, V. Decoupling the Effects of Collagen Alignment and Bioceramic Incorporation on Osteoblast Proliferation, Differentiation, and Mineralization. Mater. Today Commun. 2024, 38, 108329. [Google Scholar] [CrossRef] [PubMed]
- Uquillas, J.A.; Kishore, V.; Akkus, O. Effects of Phosphate-Buffered Saline Concentration and Incubation Time on the Mechanical and Structural Properties of Electrochemically Aligned Collagen Threads. Biomed. Mater. 2011, 6, 035008. [Google Scholar] [CrossRef] [PubMed]
- Gurkan, U.A.; Cheng, X.; Kishore, V.; Uquillas, J.A.; Akkus, O. Comparison of Morphology, Orientation, and Migration of Tendon Derived Fibroblasts and Bone Marrow Stromal Cells on Electrochemically Aligned Collagen Constructs. J. Biomed. Mater. Res. 2010, 94, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Cesari, E.; Busilacchi, A.; Manzotti, S.; Kyriakidou, K.; Greco, F.; Primio, R.D.; Mattioli-Belmonte, M. Collagen I Membranes for Tendon Repair: Effect of Collagen Fiber Orientation on Cell Behavior. J. Orthop. Res. 2009, 27, 826–832. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, S.; Luo, B.; Li, H. Engineering Collagen Fiber Templates with Oriented Nanoarchitecture and Concerns on Osteoblast Behaviors. Int. J. Biol. Macromol. 2021, 185, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Kishore, V.; Bullock, W.; Sun, X.; Van Dyke, W.S.; Akkus, O. Tenogenic Differentiation of Human MSCs Induced by the Topography of Electrochemically Aligned Collagen Threads. Biomaterials 2012, 33, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Wiseman, M.; Chuo, C.-B.; Cheema, U.; Nazhat, S.N. Ultrarapid Engineering of Biomimetic Materials and Tissues: Fabrication of Nano- and Microstructures by Plastic Compression. Adv. Funct. Mater. 2005, 15, 1762–1770. [Google Scholar] [CrossRef]
- Younesi, M.; Islam, A.; Kishore, V.; Anderson, J.M.; Akkus, O. Tenogenic Induction of Human MSCs by Anisotropically Aligned Collagen Biotextiles. Adv. Funct. Mater. 2014, 24, 5762–5770. [Google Scholar] [CrossRef] [PubMed]
- Kishore, V.; Paderi, J.E.; Akkus, A.; Smith, K.M.; Balachandran, D.; Beaudoin, S.; Panitch, A.; Akkus, O. Incorporation of a Decorin Biomimetic Enhances the Mechanical Properties of Electrochemically Aligned Collagen Threads. Acta Biomater. 2011, 7, 2428–2436. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-U.; Bashur, C.A.; Kishore, V. Impact of Elastin Incorporation into Electrochemically Aligned Collagen Fibers on Mechanical Properties and Smooth Muscle Cell Phenotype. Biomed. Mater. 2016, 11, 025008. [Google Scholar] [CrossRef] [PubMed]
- Mawad, D.; Stewart, E.; Officer, D.L.; Romeo, T.; Wagner, P.; Wagner, K.; Wallace, G.G. A Single Component Conducting Polymer Hydrogel as a Scaffold for Tissue Engineering. Adv. Funct. Mater. 2012, 22, 2692–2699. [Google Scholar] [CrossRef]
- Weadock, K.; Olson, R.M.; Silver, F.H. Evaluation of Collagen Crosslinking Techniques. Biomater. Med. Devices Artif. Organs 1983, 11, 293–318. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, K.; Sionkowska, A. Current Methods of Collagen Cross-Linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, L. Collagen Cross-Linking: When and How? A Review of the State of the Art of the Technique and New Perspectives. Eye Vis. 2015, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, H.; Luo, W.; Cai, T.; Li, Z.; Liu, Y.; Gao, W.; Wan, Q.; Wang, X.; Wang, J.; et al. Regeneration of Skeletal System with Genipin Crosslinked Biomaterials. J. Tissue Eng. 2020, 11, 2041731420974861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Yang, T.; Zhang, N.; Dong, L.; Ma, S.; Liu, X.; Zhou, M.; Li, B. The Effects of Different Crossing-Linking Conditions of Genipin on Type I Collagen Scaffolds: An in Vitro Evaluation. Cell Tissue Bank. 2014, 15, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Alfredo Uquillas, J.; Kishore, V.; Akkus, O. Genipin Crosslinking Elevates the Strength of Electrochemically Aligned Collagen to the Level of Tendons. J. Mech. Behav. Biomed. Mater. 2012, 15, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Kantaros, A.; Ganetsos, T.; Petrescu, F.I.T. Transforming Object Design and Creation: Biomaterials and Contemporary Manufacturing Leading the Way. Biomimetics 2024, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Chung, J.E.; Pui-Yik Chan, P.; Kurisawa, M. Injectable Biodegradable Hydrogels with Tunable Mechanical Properties for the Stimulation of Neurogenesic Differentiation of Human Mesenchymal Stem Cells in 3D Culture. Biomaterials 2010, 31, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Sullivan, P.A.; Martin, C.L.; Shi, H.; Deravi, L.F.; Yeo, J. Probing the Alignment-Dependent Mechanical Behaviors and Time-Evolutional Aligning Process of Collagen Scaffolds. J. Mater. Chem. B 2022, 10, 7052–7061. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chow, M.-J.; Zhang, Y. Experimental and Modeling Study of Collagen Scaffolds with the Effects of Crosslinking and Fiber Alignment. Int. J. Biomater. 2011, 2011, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, B.; Ashton, H.; Muhamed, A.; Yost, M.; Bull, S.; Frankel, D. Nanoscale Viscoelastic Properties of an Aligned Collagen Scaffold. J. Mater. Sci. Mater. Med. 2009, 20, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Paulovich, J.; Webster-Wood, V. Tuning the Mechanical and Geometric Properties of Electrochemically Aligned Collagen Threads Toward Applications in Biohybrid Robotics. J. Biomech. Eng. 2021, 143, 051005. [Google Scholar] [CrossRef] [PubMed]
- Onck, P.R.; Koeman, T.; Van Dillen, T.; Van Der Giessen, E. Alternative Explanation of Stiffening in Cross-Linked Semiflexible Networks. Phys. Rev. Lett. 2005, 95, 178102. [Google Scholar] [CrossRef] [PubMed]
- Head, D.A.; Levine, A.J.; MacKintosh, F.C. Distinct Regimes of Elastic Response and Deformation Modes of Cross-Linked Cytoskeletal and Semiflexible Polymer Networks. Phys. Rev. E 2003, 68, 061907. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Barocas, V.H. Volume-Averaging Theory for the Study of the Mechanics of Collagen Networks. Comput. Methods Appl. Mech. Eng. 2007, 196, 2981–2990. [Google Scholar] [CrossRef]
- Stein, A.M.; Vader, D.A.; Weitz, D.A.; Sander, L.M. The Micromechanics of Three-dimensional collagen-I Gels. Complexity 2011, 16, 22–28. [Google Scholar] [CrossRef]
- Kakarla, A.B.; Kong, I.; Nukala, S.G.; Kong, W. Mechanical Behaviour Evaluation of Porous Scaffold for Tissue-Engineering Applications Using Finite Element Analysis. J. Compos. Sci. 2022, 6, 46. [Google Scholar] [CrossRef]
- Lin, S.; Hapach, L.A.; Reinhart-King, C.; Gu, L. Towards Tuning the Mechanical Properties of Three-Dimensional Collagen Scaffolds Using a Coupled Fiber-Matrix Model. Materials 2015, 8, 5376–5384. [Google Scholar] [CrossRef] [PubMed]
- Long, K.; Shi, L.; Zhong, Y.; Luo, G.; Ma, X.; Li, M.; He, X.; Guan, C. Effects of Equivalent Beam Element on the In-Plane Shear Performance of 3D Stochastic Fibrous Networks. Ceram. Int. 2019, 45, 12734–12741. [Google Scholar] [CrossRef]
- Eichinger, J.F.; Grill, M.J.; Kermani, I.D.; Aydin, R.C.; Wall, W.A.; Humphrey, J.D.; Cyron, C.J. A Computational Framework for Modeling Cell–Matrix Interactions in Soft Biological Tissues. Biomech. Model. Mechanobiol. 2021, 20, 1851–1870. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, D.; Ban, E.; Toussaint, K.C.; Janmey, P.A.; Wells, R.G.; Shenoy, V.B. Glycosaminoglycans Modulate Long-Range Mechanical Communication between Cells in Collagen Networks. Proc. Natl. Acad. Sci. USA 2022, 119, e2116718119. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gutierrez, J.; Scanlon, M.G. Rheology and Mechanical Properties of Fats. In Structure-Function Analysis of Edible Fats; Elsevier: Amsterdam, The Netherlands, 2018; pp. 119–168. ISBN 978-0-12-814041-3. [Google Scholar]
- Berer, M.; Major, Z.; Pinter, G.; Constantinescu, D.M.; Marsavina, L. Investigation of the Dynamic Mechanical Behavior of Polyetheretherketone (PEEK) in the High Stress Tensile Regime. Mech. Time-Depend. Mater. 2014, 18, 663–684. [Google Scholar] [CrossRef]
- Lin, S.; Han, X.; Tsui, G.C.P.; Hui, D.; Gu, L. Active Stiffening of F-Actin Network Dominated by Structural Transition of Actin Filaments into Bundles. Compos. Part B Eng. 2017, 116, 377–381. [Google Scholar] [CrossRef]
- Lin, S.; Gu, L. Influence of Crosslink Density and Stiffness on Mechanical Properties of Type I Collagen Gel. Materials 2015, 8, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Vesentini, S.; Redaelli, A.; Buehler, M.J. Hierarchical Structure and Nanomechanics of Collagen Microfibrils from the Atomistic Scale Up. Nano Lett. 2011, 11, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, K.K.; Lakhani, P.; Kumar, S.; Kumar, N. Effect of Collagen Fibre Orientation on the Poisson’s Ratio and Stress Relaxation of Skin: An Ex Vivo and In Vivo Study. R. Soc. Open Sci. 2022, 9, 211301. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, S.; Matsuda, T. Simultaneous Processing of Fibril Formation and Cross-Linking Improves Mechanical Properties of Collagen. Biomacromolecules 2008, 9, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Gu, L.; Watanabe, H. Micromechanical Analysis of Nanoparticle-Reinforced Dental Composites. Int. J. Eng. Sci. 2013, 69, 69–76. [Google Scholar] [CrossRef][Green Version]
- Suesca, E.; Dias, A.M.A.; Braga, M.E.M.; De Sousa, H.C.; Fontanilla, M.R. Multifactor Analysis on the Effect of Collagen Concentration, Cross-Linking and Fiber/Pore Orientation on Chemical, Microstructural, Mechanical and Biological Properties of Collagen Type I Scaffolds. Mater. Sci. Eng. C 2017, 77, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xie, Y.; Celik, H.; Akkus, O.; Bernacki, S.H.; King, M.W. Engineering Small-Caliber Vascular Grafts from Collagen Filaments and Nanofibers with Comparable Mechanical Properties to Native Vessels. Biofabrication 2019, 11, 035020. [Google Scholar] [CrossRef] [PubMed]
- Chikelu, C.W.; Berns, M.; Conover, D.; Habas, R.; Han, L.; Street, R.M.; Schauer, C.L. Collagen Nanoyarns: Hierarchical Three-Dimensional Biomaterial Constructs. Biomacromolecules 2023, 24, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, G.A.; Ogden, R.W. An Arterial Constitutive Model Accounting for Collagen Content and Cross-Linking. J. Mech. Phys. Solids 2020, 136, 103682. [Google Scholar] [CrossRef]
- Kantaros, A.; Chatzidai, N.; Karalekas, D. 3D Printing-Assisted Design of Scaffold Structures. Int. J. Adv. Manuf. Technol. 2016, 82, 559–571. [Google Scholar] [CrossRef]
- Nachtrab, S.; Kapfer, S.C.; Arns, C.H.; Madadi, M.; Mecke, K.; Schröder-Turk, G.E. Morphology and Linear-Elastic Moduli of Random Network Solids. Adv. Mater. 2011, 23, 2633–2637. [Google Scholar] [CrossRef] [PubMed]
- Lake, S.P.; Barocas, V.H. Mechanical and Structural Contribution of Non-Fibrillar Matrix in Uniaxial Tension: A Collagen-Agarose Co-Gel Model. Ann. Biomed. Eng. 2011, 39, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- Lake, S.P.; Hadi, M.F.; Lai, V.K.; Barocas, V.H. Mechanics of a Fiber Network Within a Non-Fibrillar Matrix: Model and Comparison with Collagen-Agarose Co-Gels. Ann. Biomed. Eng. 2012, 40, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J. Biomechanics of Cellular Solids. J. Biomech. 2005, 38, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, Y.; Dong, P.; Gamage, P.T.; Patrawalla, N.; Kishore, V.; Mozafari, H.; Gu, L. Finite Element-Based Machine Learning Model for Predicting the Mechanical Properties of Composite Hydrogels. Appl. Sci. 2022, 12, 10835. [Google Scholar] [CrossRef]
- Bersie-Larson, L.M.; Lai, V.K.; Dhume, R.Y.; Provenzano, P.P.; Barocas, V.H.; Tranquillo, R.T. Elucidating the Signal for Contact Guidance Contained in Aligned Fibrils with a Microstructural–Mechanical Model. J. R. Soc. Interface 2022, 19, 20210951. [Google Scholar] [CrossRef] [PubMed]
- Kantaros, A.; Piromalis, D. Fabricating Lattice Structures via 3D Printing: The Case of Porous Bio-Engineered Scaffolds. Appl. Mech. 2021, 2, 289–302. [Google Scholar] [CrossRef]
- Chen, X.; Fan, H.; Deng, X.; Wu, L.; Yi, T.; Gu, L.; Zhou, C.; Fan, Y.; Zhang, X. Scaffold Structural Microenvironmental Cues to Guide Tissue Regeneration in Bone Tissue Applications. Nanomaterials 2018, 8, 960. [Google Scholar] [CrossRef] [PubMed]


| Experimental Result | Simulation Result | |
|---|---|---|
| 0.1% (4 h) ELAC | 1.51 ± 0.74 MPa | 1.53 MPa |
| 2% (4 h) ELAC | 2.87 ± 1.59 MPa | 2.68 MPa |
| 0.1% (4 h) random | 0.71 ± 0.48 MPa | 0.78 MPa |
| 2% (4 h) random | - | 1.36 MPa (prediction) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Patrawalla, N.Y.; Zhai, Y.; Dong, P.; Kishore, V.; Gu, L. Computational and Experimental Characterization of Aligned Collagen across Varied Crosslinking Degrees. Micromachines 2024, 15, 851. https://doi.org/10.3390/mi15070851
Lin S, Patrawalla NY, Zhai Y, Dong P, Kishore V, Gu L. Computational and Experimental Characterization of Aligned Collagen across Varied Crosslinking Degrees. Micromachines. 2024; 15(7):851. https://doi.org/10.3390/mi15070851
Chicago/Turabian StyleLin, Shengmao, Nashaita Y. Patrawalla, Yingnan Zhai, Pengfei Dong, Vipuil Kishore, and Linxia Gu. 2024. "Computational and Experimental Characterization of Aligned Collagen across Varied Crosslinking Degrees" Micromachines 15, no. 7: 851. https://doi.org/10.3390/mi15070851
APA StyleLin, S., Patrawalla, N. Y., Zhai, Y., Dong, P., Kishore, V., & Gu, L. (2024). Computational and Experimental Characterization of Aligned Collagen across Varied Crosslinking Degrees. Micromachines, 15(7), 851. https://doi.org/10.3390/mi15070851









