Advances in Microfluidic Systems and Numerical Modeling in Biomedical Applications: A Review
Abstract
1. Introduction
2. Microfluidic Systems for Biomedical Engineering Applications
2.1. Theoretical Principles of Microfluidics
2.2. Techniques to Manufacture Microfluidic Devices
2.2.1. Stereolithography (SLA)
2.2.2. Fused Deposition Modeling (FDM)
2.2.3. Selective Laser Melting (SLM)
2.3. Materials in Microfluidic Devices
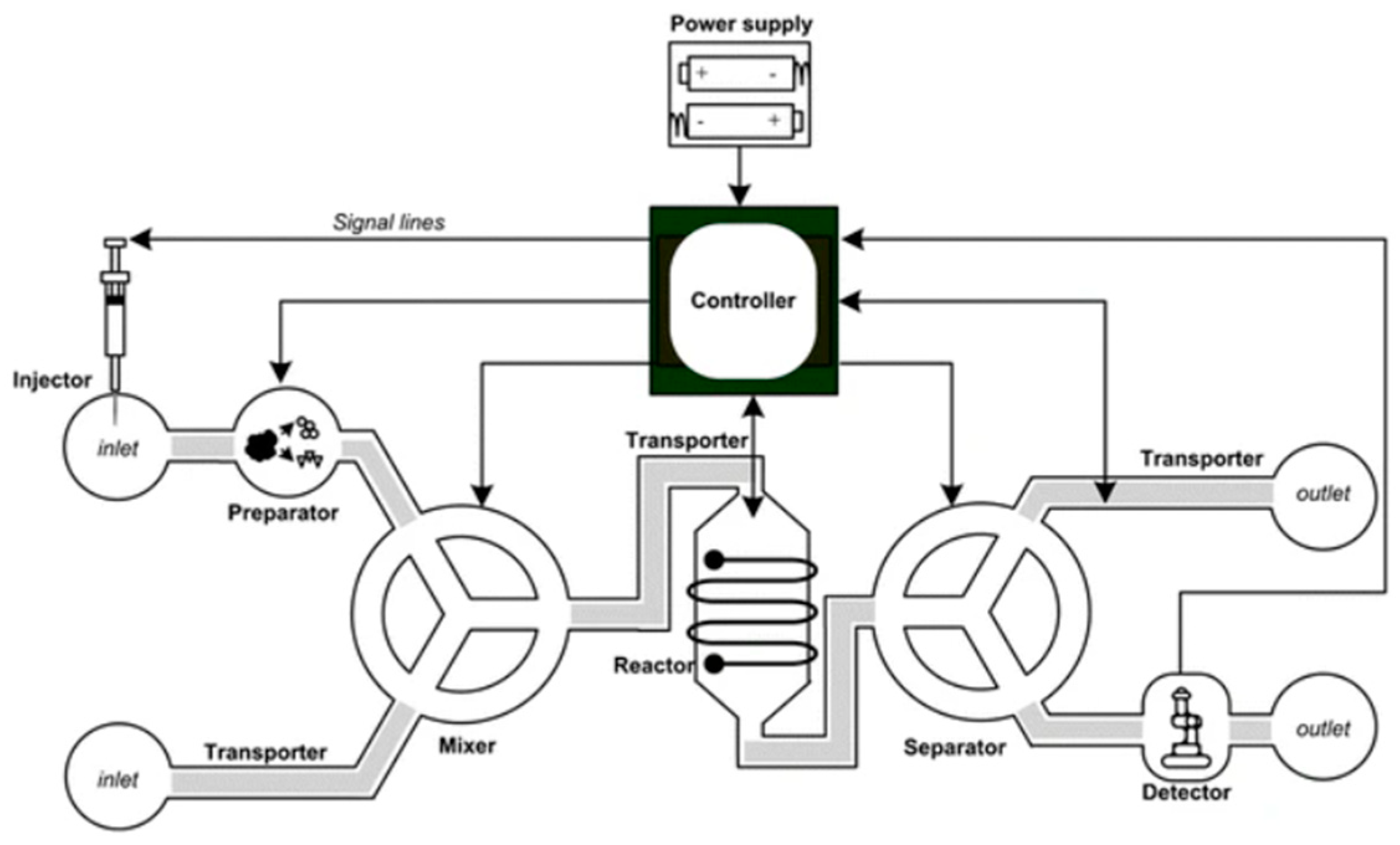
2.4. Microfluidic Systems Applications
2.4.1. Diagnostic Applications
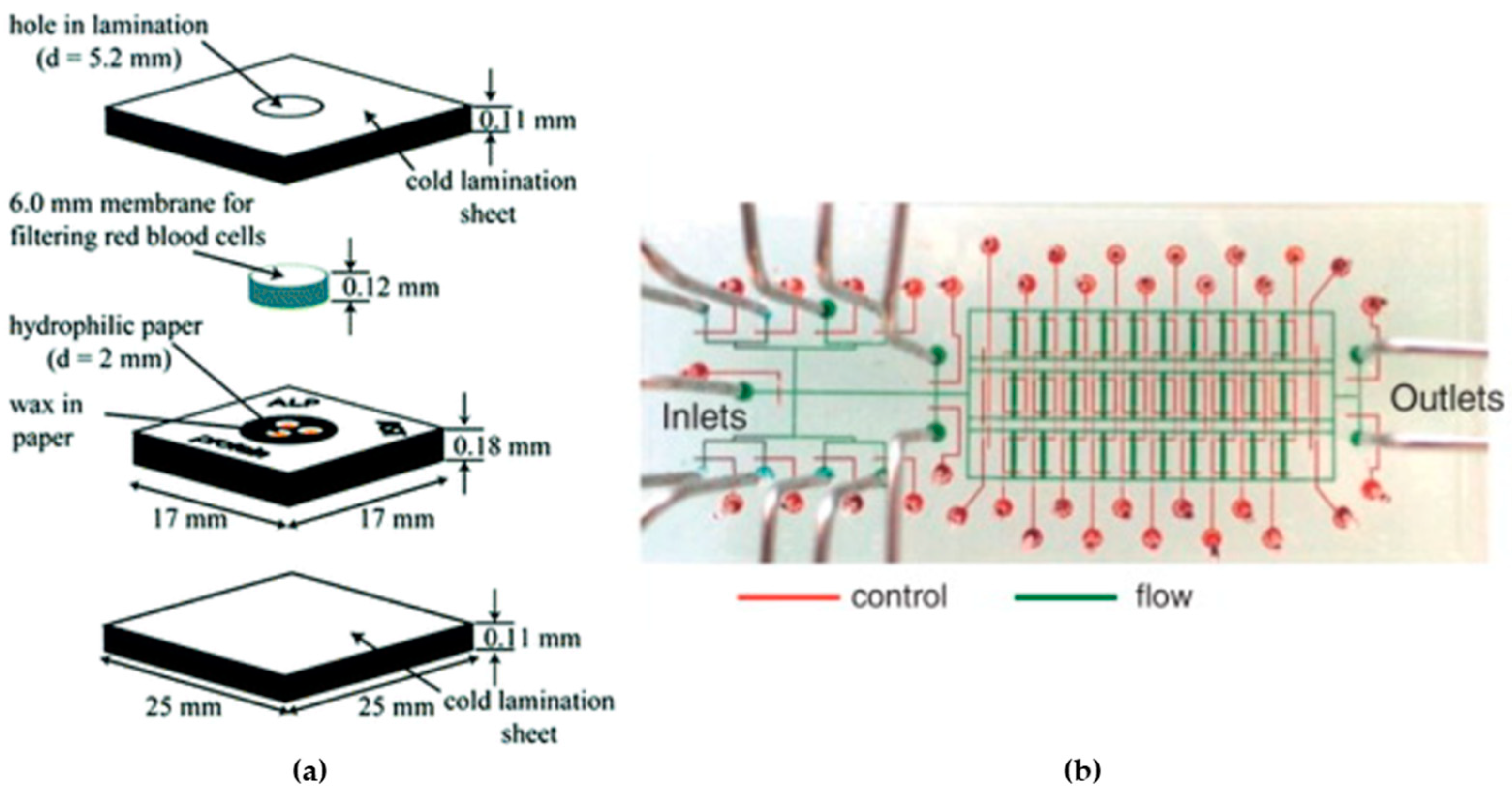
2.4.2. Organ-on-a-Chip
2.4.3. Cell Culture
2.4.4. Drug Testing
2.4.5. Gene Delivery
2.4.6. Microfluidic Cell Sorting
3. Numerical Simulation of Microfluidic Devices
3.1. Fluid Flow Modeling
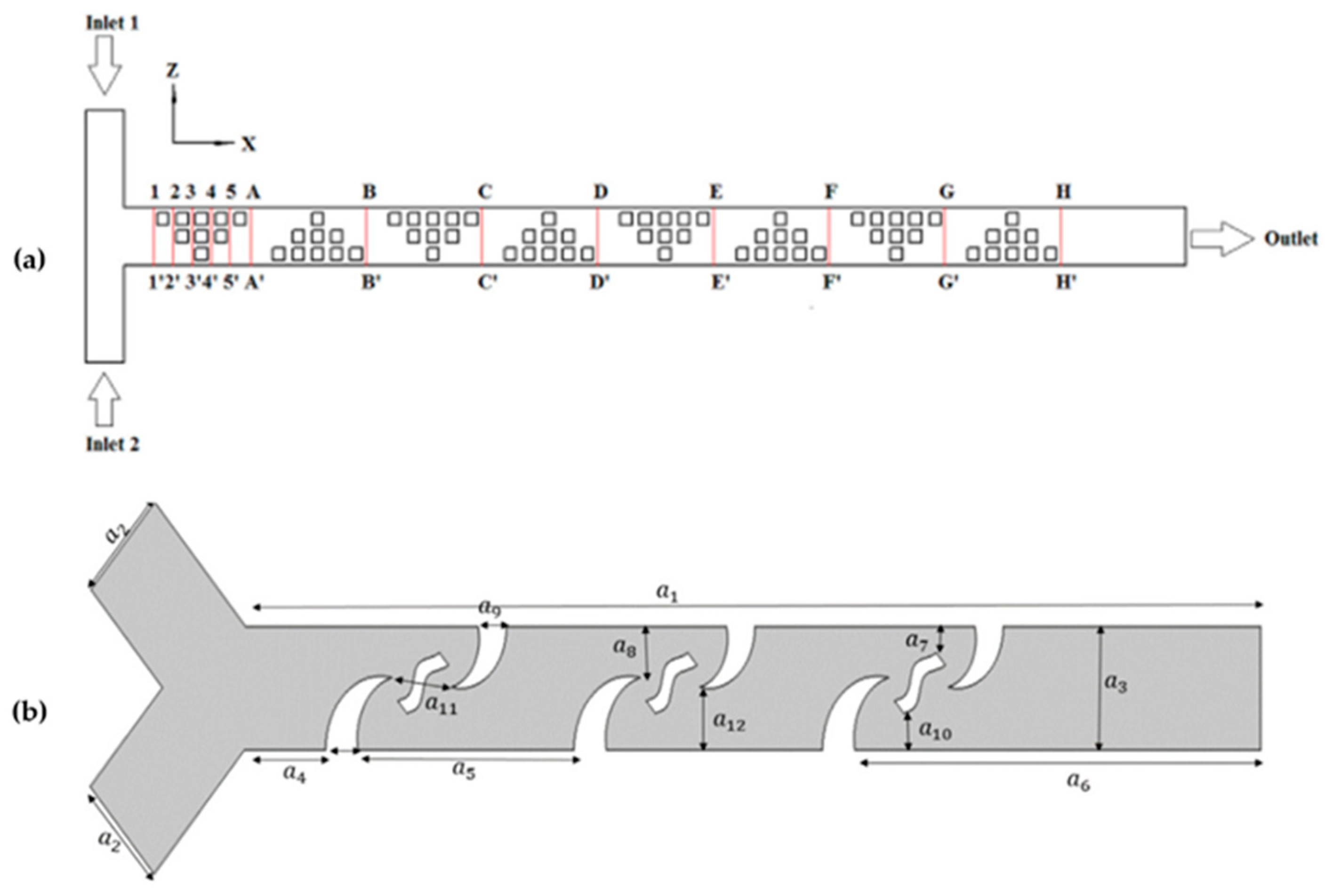
3.2. Microfluidic Devices’ Design Optimizations
3.3. Fluid Flow Analyses
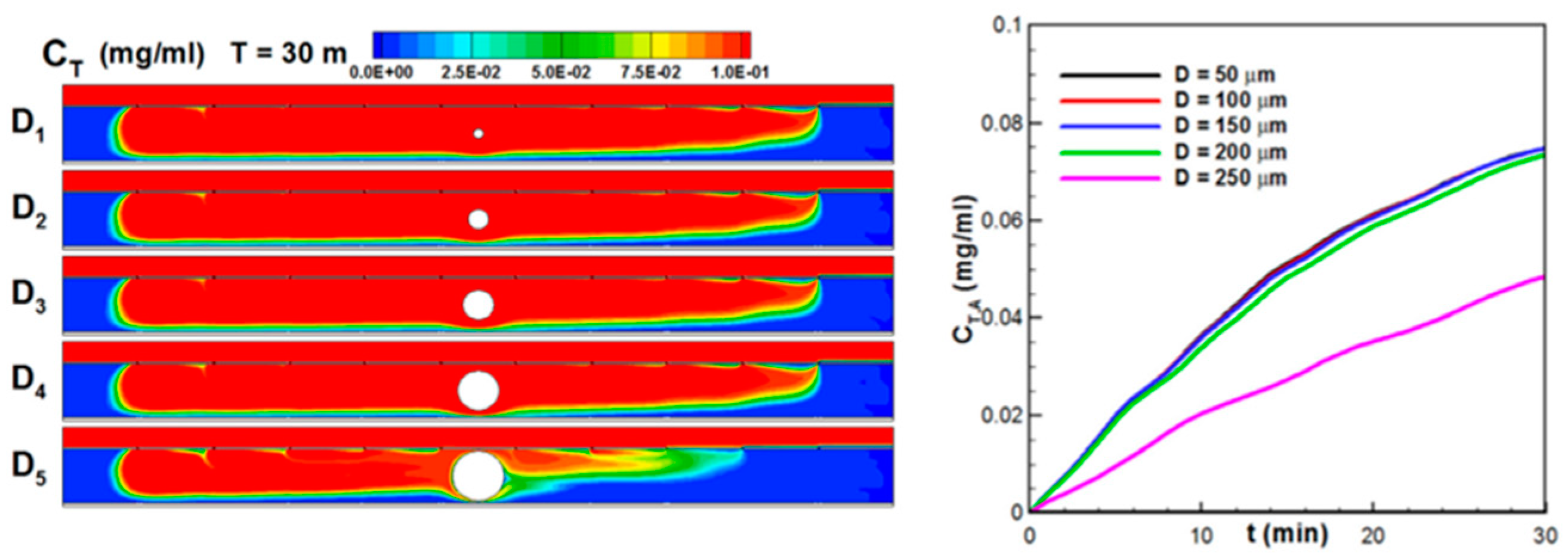
3.4. Microfluidic Mass Transport Phenomena
3.5. Microfluidic Mixing Phenomena
- The flow velocity: Faster flows lead to little interaction between components, and slower flows lead to an uneven distribution of components [118]. The influence of a laminar flow in a microchannel demonstrates that the diffusive mass flow between two miscible streams flowing in a laminar fashion is increased when the velocity at their diffusion interface is increased [119]. The conditions of the flow can be a continuous or pulsatile fluid flow [120].
- The device materials: The use of hydrophobic materials can lead to the accumulation of fluid in certain areas or bubble formation, which can be improved by using a superficial modification [46].
- The mixing techniques: In general terms, microfluidic mixing methods can be classified into two main categories: “active”, where an external energy force is used to disrupt the sample species, or “passive”, where specially designed microchannel configurations are employed to increase the contact area and contact time of the sample species. Some examples are diffusion, rotation, vibration, pressure gradient, and micromixers [115].
- The size of particles and molecules: Different sizes of particles have different velocities [118].
- Capillarity: Capillarity involves the ability of a liquid to rise/move through narrow spaces due to the attraction between the fluid molecules and the surfaces of the materials that make up these spaces. It occurs due to surface tension and the adhesion and cohesion forces of the liquid molecules [121].
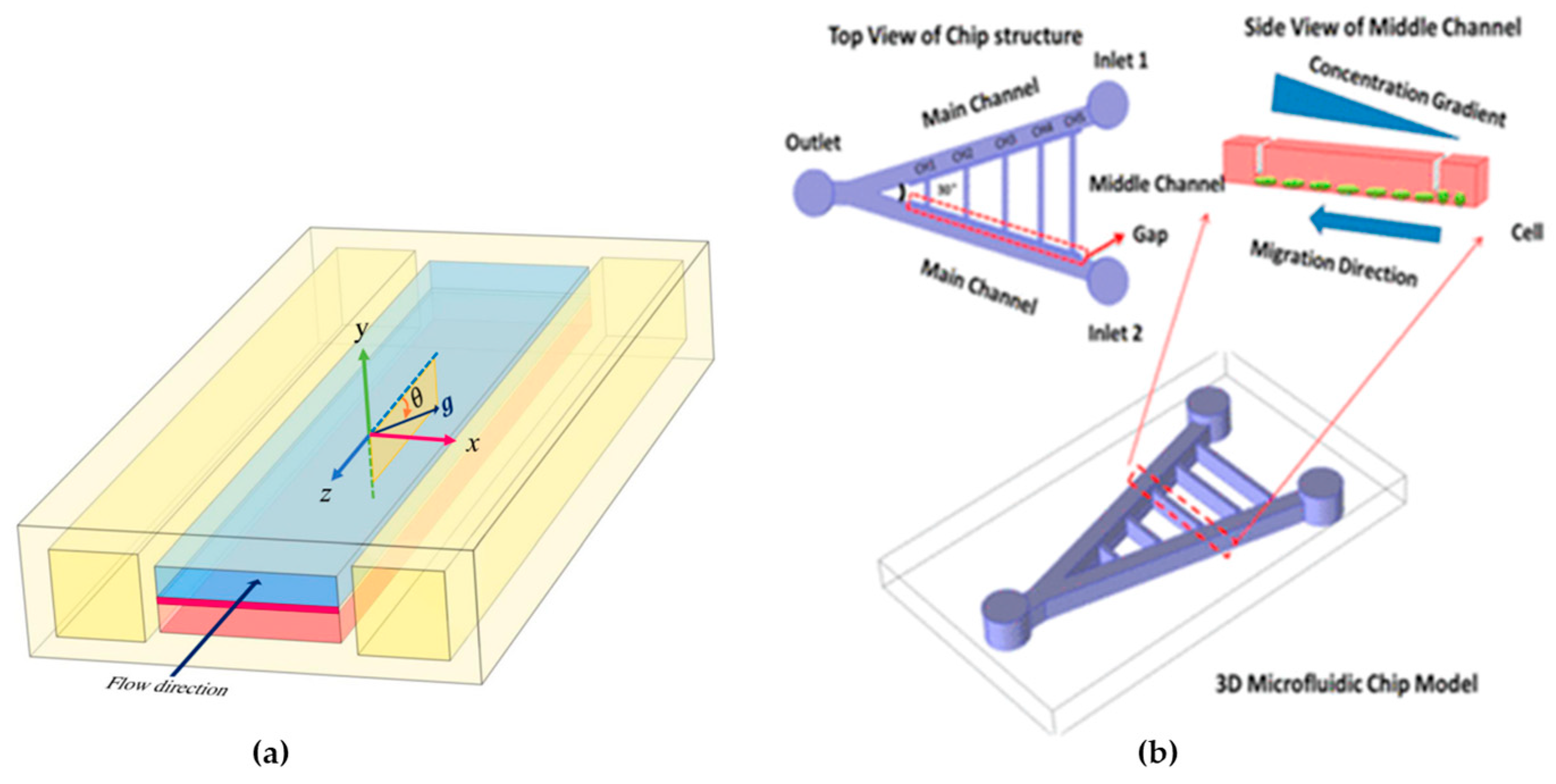
- The geometry: The geometry of a microfluidic device impacts the mixing in several ways. It influences the predominance of convection versus diffusion, determines the residence time of substances, controls the transition between laminar and turbulent flow, creates obstacles and structures that favor mixing, and allows scalability. Furthermore, the introduction of reagents and the specific geometry design can be adjusted for specific applications [92].
- Diffusion: Diffusion plays a key role in mixing in microfluidic devices. It allows the natural homogenization of different components, spreading from areas of higher to lower concentration. This process can be precisely controlled by adjusting the channel width, length, and concentration gradients. Diffusion is particularly effective in laminar flows, reducing the need for turbulent structures and providing controlled, predictable mixing. Its gentle nature minimizes sample loss and dilution, making it ideal for applications where sample integrity is paramount [122].
3.6. Organ-on-a-Chip Modeling Studies
3.6.1. Lung-on-a-Chip
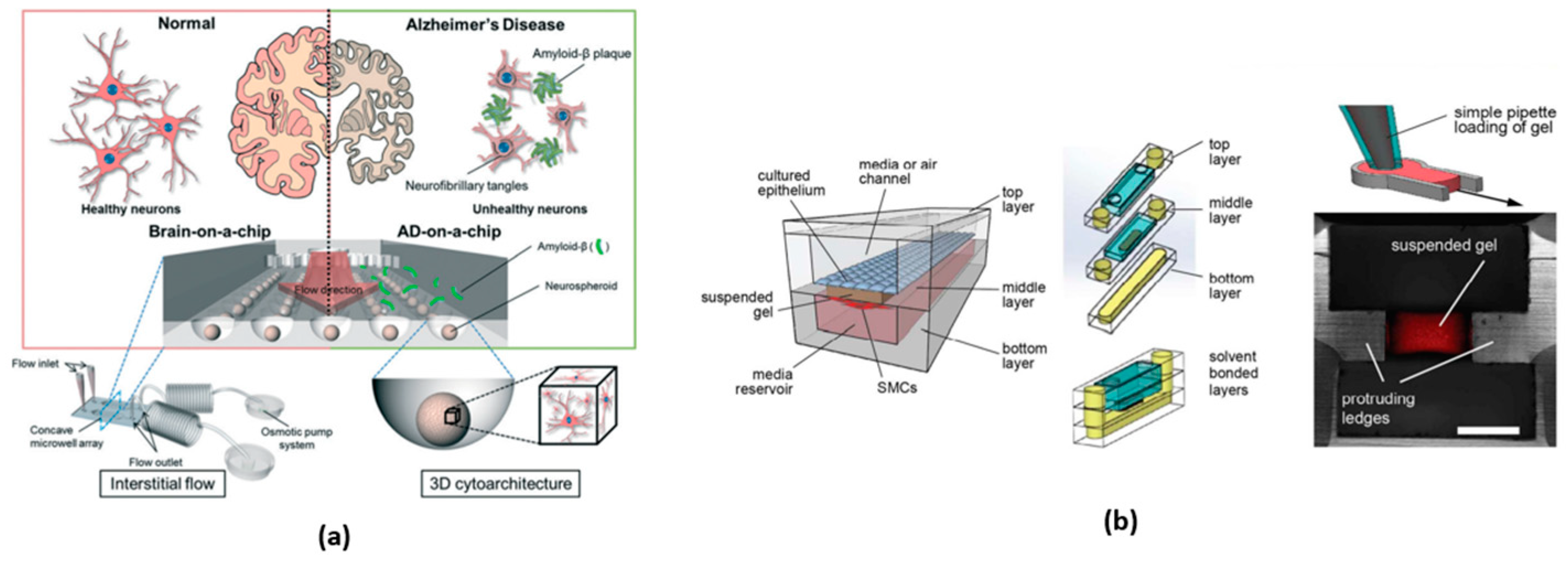
3.6.2. Brain-on-a-Chip
3.6.3. Liver-on-a-Chip
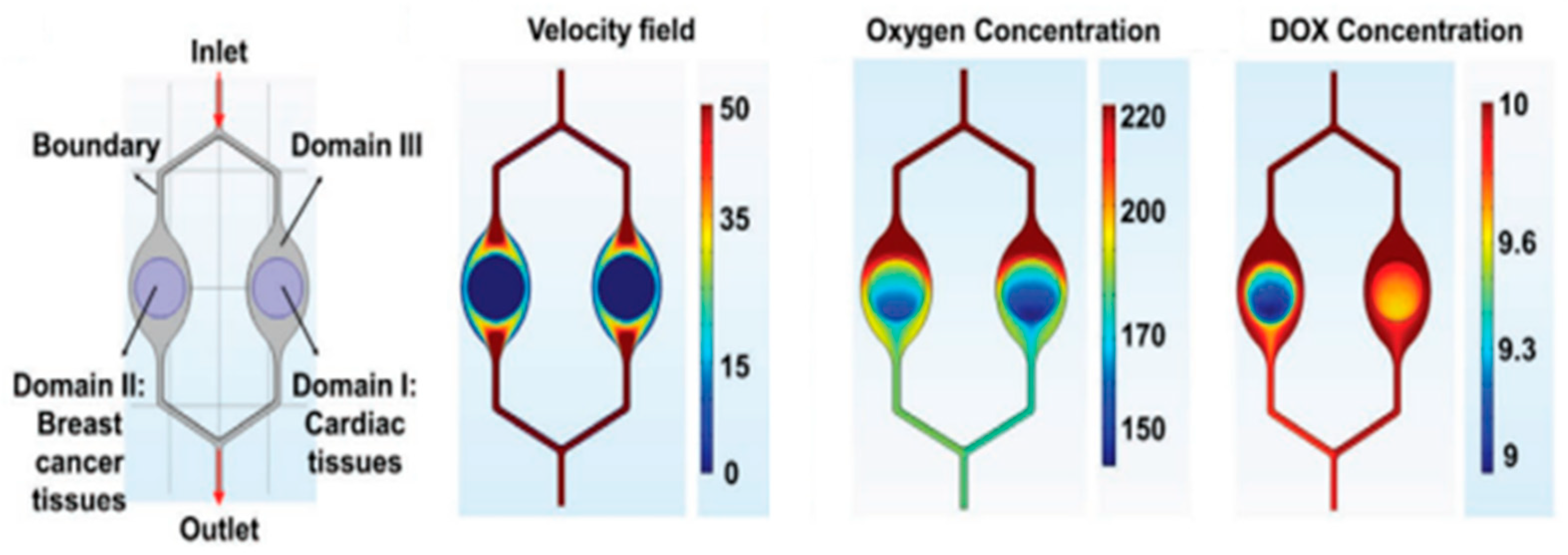
3.6.4. Multi-Organ-on-a-Chip
4. Geometries for Microfluidic Devices
4.1. Geometries to Improve the Mixing
4.1.1. Passive Mixing Methods
- Diffusion and stream splitting/combination: The basis for this method is that two different fluids, when in contact, will mix through molecular diffusion; the mixture between both leads to a uniform mixture at the molecular level. However, in microfluidic devices, this process is typically very slow [133].
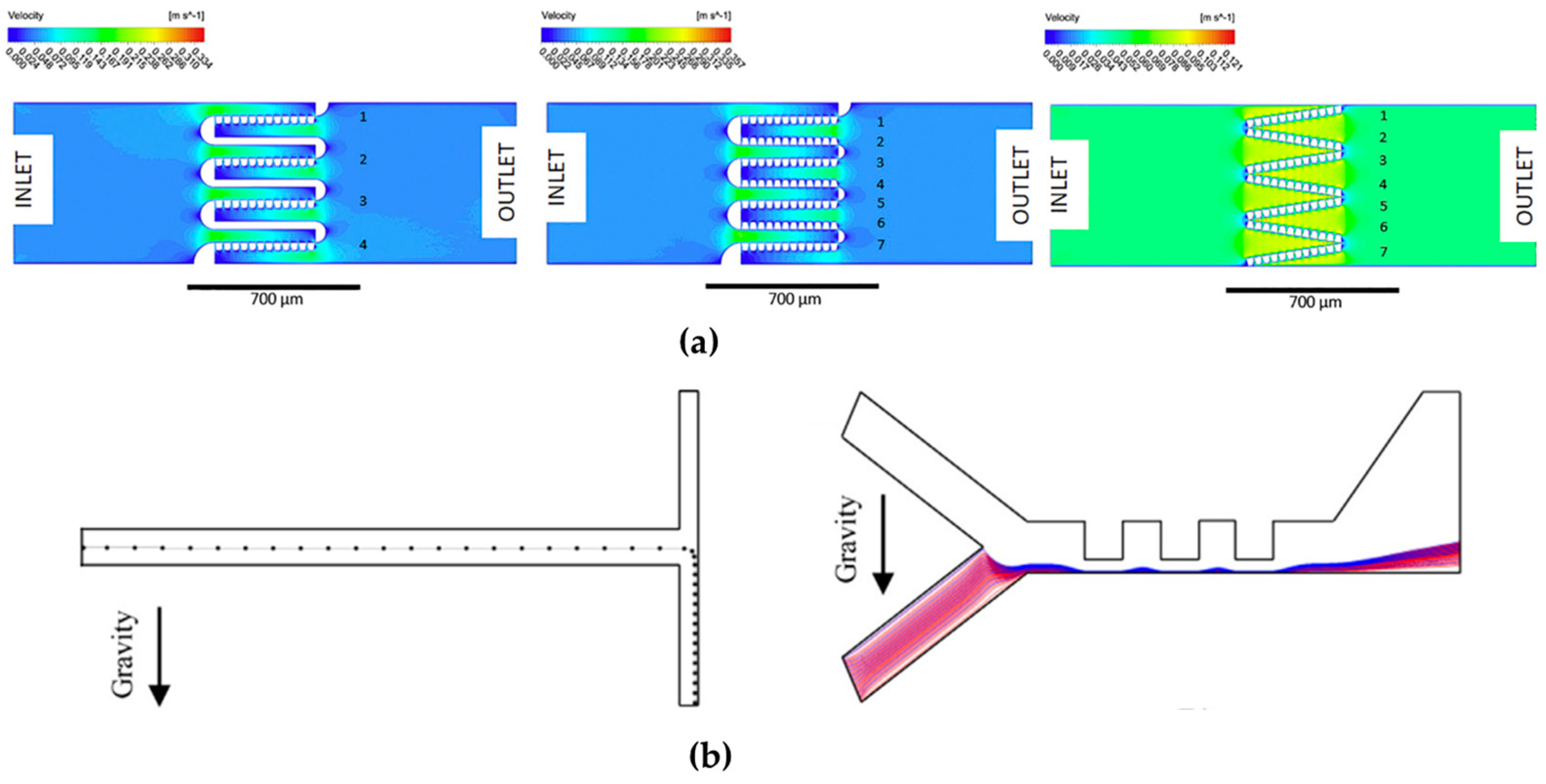
- Slanted wells, ridges, or grooves: This method is normally easy to fabricate within a microfluidic device that can provide additional mixing without the need to increase the channel length. The researchers in this area sought to enhance the rate of mixing beyond what could be achieved through diffusion alone. They aimed to achieve this by amplifying the lateral transport within the channel, with the theoretical expectation of reducing the mixing distance. Johnson et al., 2020 [134], developed a device by using an excimer laser system to create slated wells along a fabricated microchannel [134].
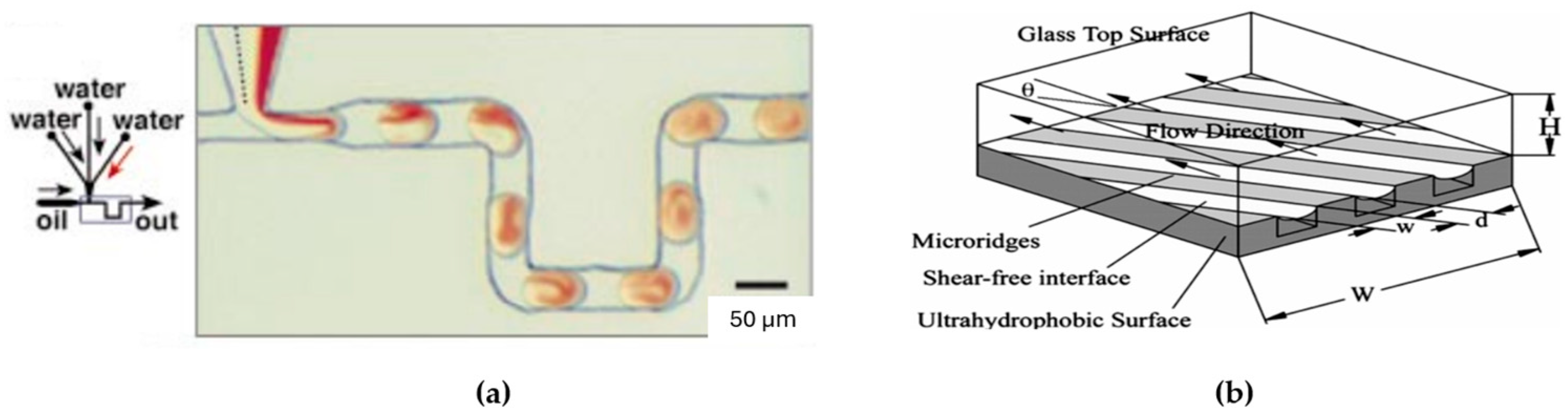
- Multiphase micromixing: Simply, the reagents cannot infiltrate the surrounding fluid because of immiscibility, leading them to retain a concentrated state within their droplets (see Figure 10a). This concentration, coupled with other naturally occurring enhancements like chaotic advection or recirculation, has the potential to significantly improve mixing within the droplets [135].
- Hydrophobic surfaces and other passive mixing enhancements: Ou et al., 2004 and 2007 [136,137], investigated the application of hydrophobic surfaces with micro ridges to enhance mixing in microfluidic systems (see Figure 10b). The hydrophobic surface introduces a shear-free air–liquid interface by increasing the contact angle between the fluid and the channel walls. This, in turn, leads to a reduction in drag forces on the fluid by over 40% [136,137].
4.1.2. Active Mixing Methods
- Microstirrers: These can be used in microfluidic devices to improve mixing, similar to how a magnetic stirring bar is used in larger-scale mixing processes. This method involves a rotating magnetic field that induces the rotation of a microbar within a fluid system, thereby enhancing mixing in the immediate vicinity of the bar. To further improve mixing, multiple bars can be used simultaneously [138].
- Acoustic mixing: Frommelt et al., 2008 [139], researched employing surface acoustic waves to create time-dependent flow patterns aimed at enhancing mixing in microfluidic devices. These surface acoustic waves represent a form of elastic energy propagating along the fluid’s surface, capable of inducing acoustic streaming within the fluid upon excitation. The resulting streamlines have demonstrated the ability to facilitate effective mixing in fluid systems and the manipulation of liquid droplets, even under conditions of low Reynolds numbers [139].
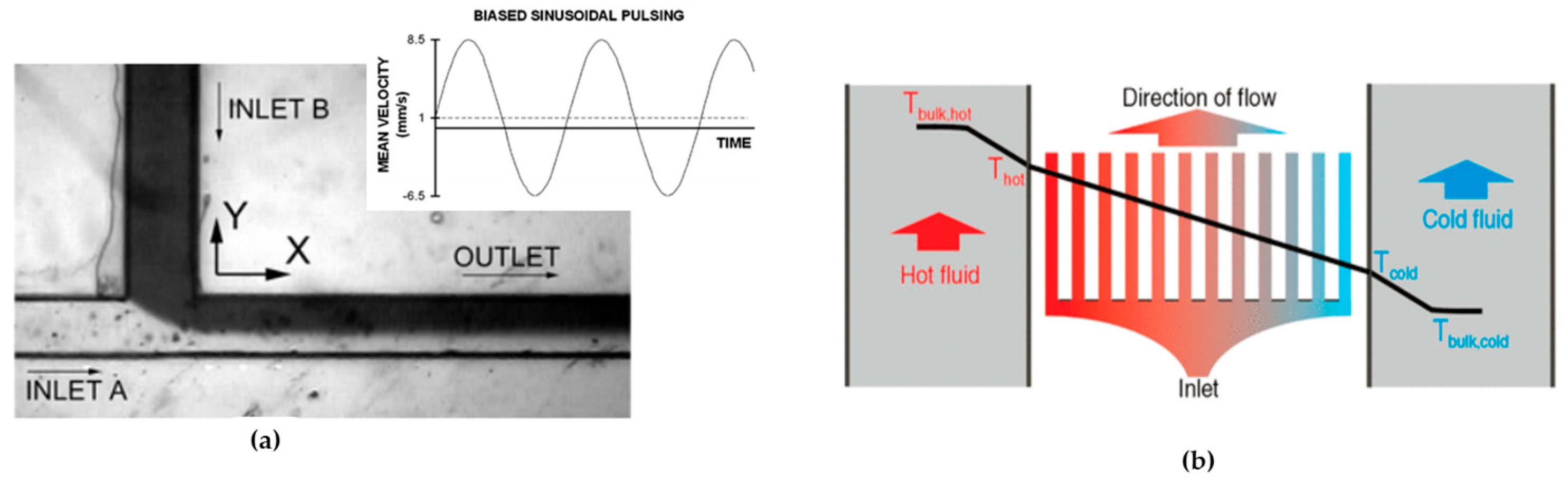
- Periodic fluid pulsation: This approach introduces a ‘pulsed’ flow in one or both channels to enhance mixing at the interface between the two streams. The pulsation is easily achieved by dynamically altering the voltage applied across the channels, particularly when electroosmotic pumping is employed. Because the flow is not constant, the streamlines undergo constant changes, leading to induced alterations in the flow patterns of the fluids within the system (see Figure 11a) [140].
- Thermal mixing enhancement: The usual trend is for diffusion coefficients to rise as the temperature increases. Mao et al., 2002 [141], showcased the creation of linear temperature gradients in a microfluidic system that spans multiple parallel streams simultaneously. While these gradients may not inherently improve mixing in microfluidic devices, the capability to accurately regulate the fluid’s temperature can empower users to manage the diffusion rate within the streams more effectively (see Figure 11b) [141].
4.2. Geometries with Improved Capillarity
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| CAD | Computer-Aided Design |
| CFD | Computer Fluid Dynamics |
| CIC | Cardiotoxicity-on-a-Cip |
| dLCSC | differentiated LCSC |
| DNA | Deoxyribonucleic acid |
| FDM | Fused Depositions Modeling |
| FE | Finite Element |
| Gr | Grashof Number |
| HDPE | high-density polyethylene |
| LCSC | lung cancer stem cells |
| LDPE | low-density polyethylene |
| LOAC | Lung-on-a-Chip |
| MI | Mixing Index |
| OoC | Organ-on-a-Chip |
| PCR | Polymerase Chain Reaction |
| PDMS | Polydimethylsiloxane |
| PMMA | poly (methyl methacrylate) |
| POCT | Point-of-care testing |
| Re | Reynolds number |
| SLA | Stereolithography |
| SLM | Selective Laser Melting |
| ToC | Tumor-on-a-Chip |
References
- Yeo, L.Y.; Chang, H.C.; Chan, P.P.Y.; Friend, J.R. Microfluidic Devices for Bioapplications. Small 2011, 7, 12–48. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Rodrigues, R.O.; Lima, R.A.; Teixeira, S. Computational Simulations in Advanced Microfluidic Devices: A Review. Micromachines 2021, 12, 1149. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering Flows in Small Devices: Microfluidics toward a Lab-on-a-Chip. Annu. Rev. Fluid. Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef]
- Luísa, V.; Faustino, C. Microfluidic System for Cell Separation and Deformation Assessment by Using Passive Methods. Available online: https://hdl.handle.net/1822/80141 (accessed on 22 May 2024).
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/Body-on-a-Chip Based on Microfluidic Technology for Drug Discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.F. Microfluidic Systems for Diagnostic Applications: A Review. J. Lab. Autom. 2012, 17, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-Care Microfluidic Devices for Pathogen Detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic Devices for Drug Delivery Systems and Drug Screening. Genes 2018, 9, 103. [Google Scholar] [CrossRef]
- Meyvantsson, I.; Beebe, D.J. Cell Culture Models in Microfluidic Systems. Annu. Rev. Anal. Chem. 2008, 1, 423–449. [Google Scholar] [CrossRef]
- Liu, K.K.; Wu, R.G.; Chuang, Y.J.; Khoo, H.S.; Huang, S.H.; Tseng, F.G. Microfluidic Systems for Biosensing. Sensors 2010, 10, 6623–6661. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, J.; Liu, L.; Xu, L. Application of Microfluidics in Wearable Devices. Small Methods 2019, 3, 1900688. [Google Scholar] [CrossRef]
- Johnson, D.G.; Frisina, R.D.; Borkholder, D.A. In-Plane Biocompatible Microfluidic Interconnects for Implantable Microsystems. IEEE Trans. Biomed. Eng. 2011, 58, 943–948. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urban, G.A. Micro- and Nanobiosensors–State of the Art and Trends. Meas. Sci. Technol. 2009, 20, 012001. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
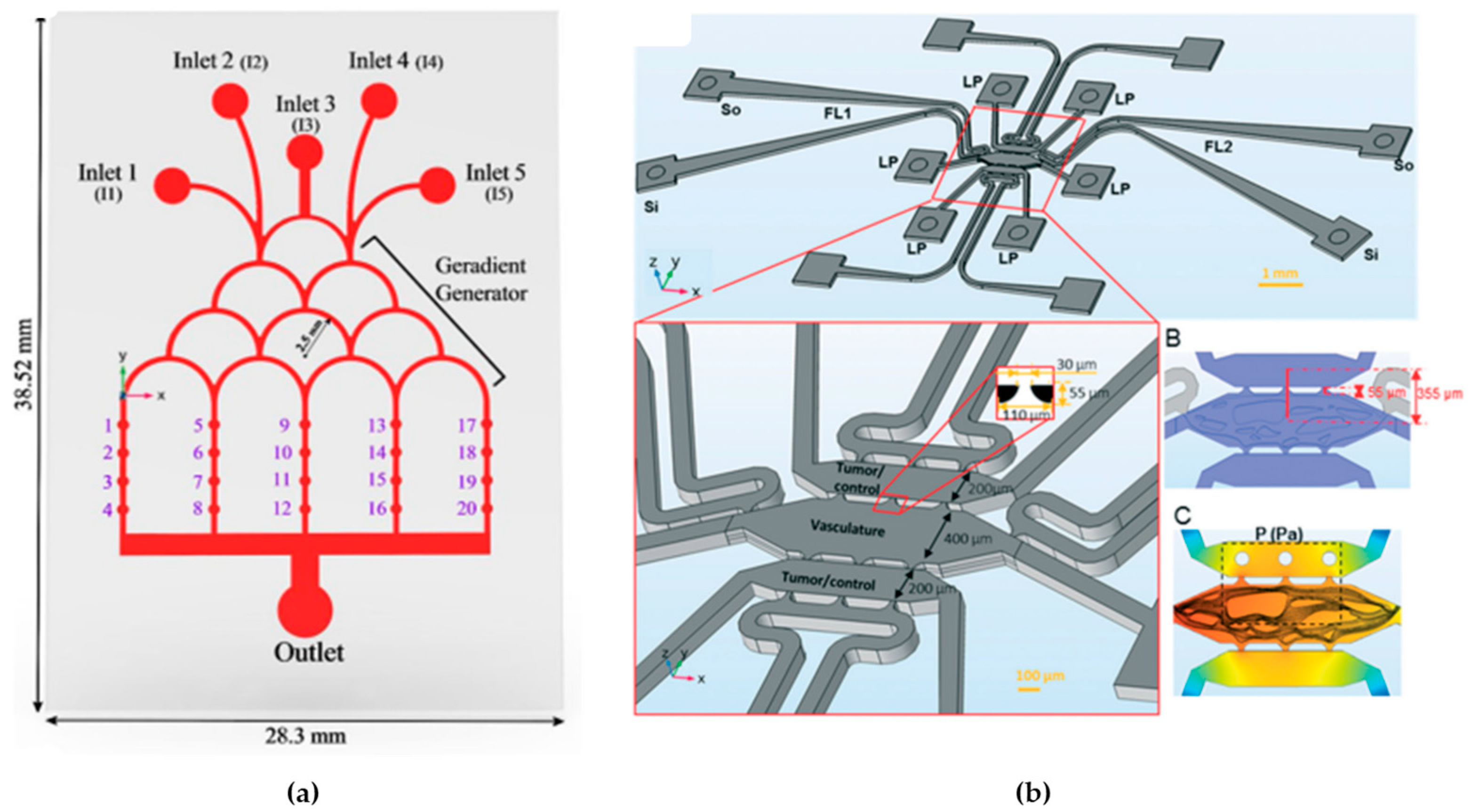
- Carvalho, V.; Gonçalves, I.M.; Rodrigues, N.; Sousa, P.; Pinto, V.; Minas, G.; Kaji, H.; Shin, S.R.; Rodrigues, R.O.; Teixeira, S.F.C.F.; et al. Numerical Evaluation and Experimental Validation of Fluid Flow Behavior within an Organ-on-a-Chip Model. Comput. Methods Programs Biomed. 2024, 243, 107883. [Google Scholar] [CrossRef] [PubMed]
- Sheidaei, Z.; Akbarzadeh, P.; Kashaninejad, N. Advances in Numerical Approaches for Microfluidic Cell Analysis Platforms. J. Sci. Adv. Mater. Devices 2020, 5, 295–307. [Google Scholar] [CrossRef]
- Carvalho, V.; Gonçalves, I.; Lage, T.; Rodrigues, R.O.; Minas, G.; Teixeira, S.F.C.F.; Moita, A.S.; Hori, T.; Kaji, H.; Lima, R.A. 3d Printing Techniques and Their Applications to Organ-on-a-chip Platforms: A Systematic Review. Sensors 2021, 21, 3304. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Fan, Z.H. Mixing in Microfluidic Devices and Enhancement Methods. J. Micromech. Microeng. 2015, 25, 094001. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Chen, W.; Zhang, P. Developing Advanced Polymer Films Based on Microfluidic Laminar Flow. Giant 2022, 9, 100091. [Google Scholar] [CrossRef]
- Brody, J.P.; Yager, P. Low Reynolds Number Micro-Fluidic Devices. Proc. Solid-State Sens. Actuator Workshop 1996, 1996, 105–108. [Google Scholar]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.T.; Ebrahimi Warkiani, M.; Li, W. Fundamentals and Applications of Inertial Microfluidics: A Review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef] [PubMed]
- Van Dinther, A.M.C.; Schroën, C.G.P.H.; Vergeldt, F.J.; Van Der Sman, R.G.M.; Boom, R.M. Suspension Flow in Microfluidic Devices—A Review of Experimental Techniques Focussing on Concentration and Velocity Gradients. Adv. Colloid. Interface Sci. 2012, 173, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Onck, P.; den Toonder, J. A Concise Review of Microfluidic Particle Manipulation Methods. Microfluid. Nanofluidics 2020, 24, 24. [Google Scholar] [CrossRef]
- Stoecklein, D.; Di Carlo, D. Nonlinear Microfluidics. Anal. Chem. 2019, 91, 296–314. [Google Scholar] [CrossRef] [PubMed]
- Ohalete, N.C.; Ayo-Farai, O.; Onwumere, C.; Maduka, C.P.; Olorunsogo, T.O. Navier-Stokes Equations in Biomedical Engineering: A Critical Review of Their Use in medical Device Development in the USA and Africa. World J. Adv. Res. Rev. 2024, 21, 1115–1131. [Google Scholar] [CrossRef]
- Sheng, W. A Revisit of Navier–Stokes Equation. Eur. J. Mech. B/Fluids 2020, 80, 60–71. [Google Scholar] [CrossRef]
- Holmes, A.P.; Ray, C.J.; Kumar, P.; Coney, A.M. A Student Practical to Conceptualize the Importance of Poiseuille’s Law and Flow Control in the Cardiovascular System. Adv. Physiol. Educ. 2020, 44, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Binda, L.; Bolado, M.; D’Onofrio, A.; Freytes, V.M. Analysis of a Microfluidic Device for Diffusion Coefficient Determination of High Molecular Weight Solutes Detectable in the Visible Spectrum. Eur. Phys. J. E 2022, 45, 56. [Google Scholar] [CrossRef]
- Chen, P.; Li, S.; Guo, Y.; Zeng, X.; Liu, B.F. A Review on Microfluidics Manipulation of the Extracellular Chemical Microenvironment and Its Emerging Application to Cell Analysis. Anal. Chim. Acta 2020, 1125, 94–113. [Google Scholar] [CrossRef]
- Kim, P.; Kwon, K.W.; Park, M.C.; Lee, S.H.; Kim, S.M.; Suh, K.Y. Soft Lithography for Microfluidics a Review; The Korean BioChip Society: Seoul, Republic of Korea, 2008. [Google Scholar]
- Amin, R.; Knowlton, S.; Hart, A.; Yenilmez, B.; Ghaderinezhad, F.; Katebifar, S.; Messina, M.; Khademhosseini, A.; Tasoglu, S. 3D-Printed Microfluidic Devices. Biofabrication 2016, 8, 022001. [Google Scholar] [CrossRef]
- Carnero, B.; Bao-Varela, C.; Gómez-Varela, A.I.; Álvarez, E.; Flores-Arias, M.T. Microfluidic Devices Manufacturing with a Stereolithographic Printer for Biological Applications. Mater. Sci. Eng. C 2021, 129, 112388. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, J.; Li, K.; Zhu, L.; Tang, W. Fabrication of PDMS Microfluidic Devices with 3D Wax Jetting. RSC Adv. 2017, 7, 3313–3320. [Google Scholar] [CrossRef]
- Au, A.K.; Lee, W.; Folch, A. Mail-Order Microfluidics: Evaluation of Stereolithography for the Production of Microfluidic Devices. Lab Chip 2014, 14, 1294–1301. [Google Scholar] [CrossRef]
- Gaal, G.; Mendes, M.; de Almeida, T.P.; Piazzetta, M.H.O.; Gobbi, Â.L.; Riul, A.; Rodrigues, V. Simplified Fabrication of Integrated Microfluidic Devices Using Fused Deposition Modeling 3D Printing. Sens. Actuators B Chem. 2017, 242, 35–40. [Google Scholar] [CrossRef]
- Jia, H.; Sun, H.; Wang, H.; Wu, Y.; Wang, H. Scanning Strategy in Selective Laser Melting (SLM): A Review. Int. J. Adv. Manuf. Technol. 2021, 113, 2413–2435. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, J.; Zhang, H.; Kent, N.J.; Diamond, D.; Gilchrist, M.D. 3D Printing of Metallic Microstructured Mould Using Selective Laser Melting for Injection Moulding of Plastic Microfluidic Devices. Micromachines 2019, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; Li, D. Integrated Microfluidic Devices. Anal. Chim. Acta 2004, 507, 11–26. [Google Scholar] [CrossRef]
- Wilding, P.; Shoffner, M.A.; Kricka, L.J. PCR in a Silicon Microstructure. Clin. Chem. 1994, 40, 1815–1818. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(Dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Patel, J.N.; Kaminska, B.; Gray, B.L.; Gates, B.D. PDMS as a Sacrificial Substrate for SU-8-Based Biomedical and Microfluidic Applications. J. Micromech. Microeng. 2008, 18, 095028. [Google Scholar] [CrossRef]
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R.M.T. Advantages and Challenges of Microfluidic Cell Culture in Polydimethylsiloxane Devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.; Locascio, L.E. Polymer Microfluidic Devices. Talanta 2002, 56, 267–287. [Google Scholar] [CrossRef]
- Aralekallu, S.; Boddula, R.; Singh, V. Development of Glass-Based Microfluidic Devices: A Review on Its Fabrication and Biologic Applications. Mater. Des. 2023, 225, 111517. [Google Scholar] [CrossRef]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and Emerging Strategies for the Fabrication and Functionalization of PDMS-Based Microfluidic Devices. Lab Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, R.; Jin, Z.; Fan, Y.; Zhou, X.; Zhang, Y. Injection Molding and Characterization of PMMA-Based Microfluidic Devices. Microsyst. Technol. 2020, 26, 1317–1324. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Y.; Tian, J.; Wu, T.; Li, Z.; Xing, F.; Fu, S. Polymer-Based Microfluidic Devices: A Comprehensive Review on Preparation and Applications. Polym. Eng. Sci. 2022, 62, 3–24. [Google Scholar] [CrossRef]
- Quero, R.F.; Domingos Da Silveira, G.; Fracassi Da Silva, J.A.; Jesus, D.P. De Understanding and Improving FDM 3D Printing to Fabricate High-Resolution and Optically Transparent Microfluidic Devices. Lab Chip 2021, 21, 3715–3729. [Google Scholar] [CrossRef] [PubMed]
- Kothuru, A.; Amreen, K.; Goel, S. Electromicrofluidic Device on Multilayered Laser-Induced Polyamide Substrate for Diverse Electrochemical Applications. IEEE Trans. Electron. Devices 2020, 67, 5097–5103. [Google Scholar] [CrossRef]
- Chen, Z.; Lee, J.B. Biocompatibility of Su-8 and Its Biomedical Device Applications. Micromachines 2021, 12, 794. [Google Scholar] [CrossRef]
- Teixeira De Sousa, P.J. Estudo e Otimização de Estruturas Em PDMS Para Dispositivos Microfluídicos. Master’s Thesis, Universidade Do Minho Escola de Engenharia, Guimarães, Portugal, 2011. [Google Scholar]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent Developments in PDMS Surface Modification for Microfluidic Devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef]
- Firpo, G.; Angeli, E.; Repetto, L.; Valbusa, U. Permeability Thickness Dependence of Polydimethylsiloxane (PDMS) Membranes. J. Memb. Sci. 2015, 481, 1–8. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-Based Microfluidic Point-of-Care Diagnostic Devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- Kumar, A.; Parihar, A.; Panda, U.; Parihar, D.S. Microfluidics-Based Point-of-Care Testing (POCT) Devices in Dealing with Waves of COVID-19 Pandemic: The Emerging Solution. ACS Appl. Bio Mater. 2022, 5, 2046–2068. [Google Scholar] [CrossRef] [PubMed]
- Dos-Reis-Delgado, A.A.; Carmona-Dominguez, A.; Sosa-Avalos, G.; Jimenez-Saaib, I.H.; Villegas-Cantu, K.E.; Gallo-Villanueva, R.C.; Perez-Gonzalez, V.H. Recent Advances and Challenges in Temperature Monitoring and Control in Microfluidic Devices. Electrophoresis 2023, 44, 268–297. [Google Scholar] [CrossRef] [PubMed]
- Baluta, S.; Cabaj, J.; Malecha, K. Biosensors Technologies for Point-of-Care Testing—A Review. Period. Polytech. Electr. Eng. Comput. Sci. 2020, 64, 325–333. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Yashas; Vyas, R. A Role of Integrated Microheaters in a Microfluidics Based Point-of-Care-Testing and beyond for Healthcare Applications. Appl. Mater. Today 2024, 38, 102225. [Google Scholar] [CrossRef]
- Ngocho, K.; Yang, X.; Wang, Z.; Hu, C.; Yang, X.; Shi, H.; Wang, K.; Liu, J. Synthetic Cells from Droplet-Based Microfluidics for Biosensing and Biomedical Applications. Small 2024, 2024, 2400086. [Google Scholar] [CrossRef] [PubMed]
- Bruijns, B.; van Asten, A.; Tiggelaar, R.; Gardeniers, H. Microfluidic Devices for Forensic DNA Analysis: A Review. Biosensors 2016, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Mehling, M.; Tay, S. Microfluidic Cell Culture. Curr. Opin. Biotechnol. 2014, 25, 95–102. [Google Scholar] [CrossRef]
- Marle, L.; Greenway, G.M. Microfluidic Devices for Environmental Monitoring. TrAC—Trends Anal. Chem. 2005, 24, 795–802. [Google Scholar] [CrossRef]
- Ha, N.S.; Sadeghi, S.; van Dam, R.M. Recent Progress toward Microfluidic Quality Control Testing of Radiopharmaceuticals. Micromachines 2017, 8, 337. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Hejazian, M.; Ooi, C.H.; Kashaninejad, N. Recent Advances and Future Perspectives on Microfluidic Liquid Handling. Micromachines 2017, 8, 186. [Google Scholar] [CrossRef]
- Lion, N.; Reymond, F.; Girault, H.H.; Rossier, J.S. Why the Move to Microfluidics for Protein Analysis? Curr. Opin. Biotechnol. 2004, 15, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.O.; Sousa, P.C.; Gaspar, J.; Bañobre-López, M.; Lima, R.; Minas, G. Organ-on-a-Chip: A Preclinical Microfluidic Platform for the Progress of Nanomedicine. Small 2020, 16, 2003517. [Google Scholar] [CrossRef]
- Gonçalves, I.M.; Carvalho, V.; Rodrigues, R.O.; Pinho, D.; Teixeira, S.F.C.F.; Moita, A.; Hori, T.; Kaji, H.; Lima, R.; Minas, G. Organ-on-a-Chip Platforms for Drug Screening and Delivery in Tumor Cells: A Systematic Review. Cancers 2022, 14, 935. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A Guide to the Organ-on-a-Chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Driver, R.; Mishra, S. Organ-On-A-Chip Technology: An In-Depth Review of Recent Advancements and Future of Whole Body-on-Chip. BioChip J. 2023, 17, 1–23. [Google Scholar] [CrossRef]
- Park, J.; Lee, B.K.; Jeong, G.S.; Hyun, J.K.; Lee, C.J.; Lee, S.H. Three-Dimensional Brain-on-a-Chip with an Interstitial Level of Flow and Its Application as an in Vitro Model of Alzheimer’s Disease. Lab Chip 2015, 15, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Kunze, A.; Lengacher, S.; Dirren, E.; Aebischer, P.; Magistretti, P.J.; Renaud, P. Astrocyte-Neuron Co-Culture on Microchips Based on the Model of SOD Mutation to Mimic ALS. Integr. Biol. 2013, 5, 964–975. [Google Scholar] [CrossRef]
- Bersini, S.; Jeon, J.S.; Dubini, G.; Arrigoni, C.; Chung, S.; Charest, J.L.; Moretti, M.; Kamm, R.D. A Microfluidic 3D Invitro Model for Specificity of Breast Cancer Metastasis to Bone. Biomaterials 2014, 35, 2454–2461. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Chow, C.W.; Young, E.W.K. Microfluidic Lung Airway-on-a-Chip with Arrayable Suspended Gels for Studying Epithelial and Smooth Muscle Cell Interactions. Lab Chip 2018, 18, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Huang, C.; Lin, Y.D.; Ivanovskaya, A.N.; O’Hara, T.J.; Booth, R.H.; Creek, C.J.; Enright, H.A.; Soscia, D.A.; Belle, A.M.; et al. Simultaneous Electrical Recording of Cardiac Electrophysiology and Contraction on Chip. Lab Chip 2017, 17, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Neumann, C.A.; Leduc, P.R. Tumor-on-a-Chip for Integrating a 3D Tumor Microenvironment: Chemical and Mechanical Factors. Lab Chip 2020, 20, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Sved Skottvoll, F.; Rayner, S.; Pedersen-Bjergaard, S.; Sullivan, G.; Krauss, S.; Ray Wilson, S.; Harrison, S. 3D Cell Culture Models and Organ-on-a-Chip: Meet Separation Science and Mass Spectrometry. Electrophoresis 2020, 41, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.H.; Choi, J.-W. Microfluidic D 18. Microfluidic Devices and Their Applications to Lab-on-a-Chip; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Lim, Y.C.; Kouzani, A.Z.; Duan, W. Lab-on-a-Chip: A Component View. Microsyst. Technol. 2010, 16, 1995–2015. [Google Scholar] [CrossRef]
- Björnmalm, M.; Yan, Y.; Caruso, F. Engineering and Evaluating Drug Delivery Particles in Microfluidic Devices. J. Control. Release 2014, 190, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; Zhang, L.; Ban, L.; Chen, P.; Du, W.; Feng, X.; Liu, B.F. Chemically Edited Exosomes with Dual Ligand Purified by Microfluidic Device for Active Targeted Drug Delivery to Tumor Cells. ACS Appl. Mater. Interfaces 2017, 9, 27441–27452. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, H.; Park, Y.S.; Kaji, N.; Tokeshi, M.; Kogure, K.; Shinohara, Y.; Harashima, H.; Baba, Y. On-Chip Fabrication of Mutifunctional Envelope-Type Nanodevices for Gene Delivery. Anal. Bioanal. Chem. 2008, 391, 2729–2733. [Google Scholar] [CrossRef] [PubMed]
- Wyatt Shields Iv, C.; Reyes, C.D.; López, G.P. Microfluidic Cell Sorting: A Review of the Advances in the Separation of Cells from Debulking to Rare Cell Isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef]
- Kajishima, T.; Taira, K. Numerical Simulation of Fluid Flows. In Computational Fluid Dynamics; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 1–22. [Google Scholar]
- Fishler, R.; Mulligan, M.K.; Sznitman, J. Mapping Low-Reynolds-Number Microcavity Flows Using Microfluidic Screening Devices. Microfluid. Nanofluidics 2013, 15, 491–500. [Google Scholar] [CrossRef]
- Zhang, F.; Erriguible, A.; Marre, S. Investigating Laminar Mixing in High Pressure Microfluidic Systems. Chem. Eng. Sci. 2019, 205, 25–35. [Google Scholar] [CrossRef]
- Li, Z.; Venkataraman, A.; Rosenbaum, M.A.; Angenent, L.T. A Laminar-Flow Microfluidic Device for Quantitative Analysis of Microbial Electrochemical Activity. ChemSusChem 2012, 5, 1119–1123. [Google Scholar] [CrossRef]
- Kamholz, A.E.; Yager, P. Theoretical Analysis of Molecular Diffusion in Pressure-Driven Laminar Flow in Microfluidic Channels. Biophys. J. 2001, 80, 155–160. [Google Scholar] [CrossRef]
- Kamholz, A.E.; Yager, P. Molecular Diffusive Scaling Laws in Pressure-Driven Microuidic Channels: Deviation from One-Dimensional Einstein Approximations. Sens. Actuators B Chem. 2002, 82, 117–121. [Google Scholar] [CrossRef]
- Glatzel, T.; Litterst, C.; Cupelli, C.; Lindemann, T.; Moosmann, C.; Niekrawietz, R.; Streule, W.; Zengerle, R.; Koltay, P. Computational Fluid Dynamics (CFD) Software Tools for Microfluidic Applications—A Case Study. Comput. Fluids 2008, 37, 218–235. [Google Scholar] [CrossRef]
- Venkateshwarlu, A.; Bharti, R.P. Effects of Capillary Number and Flow Rates on the Hydrodynamics of Droplet Generation in Two-Phase Cross-Flow Microfluidic Systems: Hydrodynamics of Droplet Generation in Two-Phase Cross-Flow Microfluidic Systems. J. Taiwan Inst. Chem. Eng. 2021, 129, 64–79. [Google Scholar] [CrossRef]
- Jeon, W.; Shin, C.B. Design and Simulation of Passive Mixing in Microfluidic Systems with Geometric Variations. Chem. Eng. J. 2009, 152, 575–582. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Nguyen, N.; Baratchi, S.; Thurgood, P.; Ghorbani, K.; Khoshmanesh, K. Temperature-Controlled Microfluidic System Incorporating Polymer Tubes. Anal. Chem. 2019, 91, 2498–2505. [Google Scholar] [CrossRef]
- Bayraktar, T.; Pidugu, S.B. Characterization of Liquid Flows in Microfluidic Systems. Int. J. Heat Mass Transf. 2006, 49, 815–824. [Google Scholar] [CrossRef]
- Aswin, V.S.; Awasthi, A.; Anu, C. A Comparative Study of Numerical Schemes for Convection-Diffusion Equation. In Proceedings of the Procedia Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 127, pp. 621–627. [Google Scholar]
- Smith, R.; Peters, C.; Inomata, H. Heat Transfer and Finite-Difference Methods. In Supercritical Fluid Science and Technology; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 4, pp. 557–615. [Google Scholar]
- Galindo-Rosales, F.J.; Campo-Deaño, L.; Pinho, F.T.; Van Bokhorst, E.; Hamersma, P.J.; Oliveira, M.S.N.; Alves, M.A. Microfluidic Systems for the Analysis of Viscoelastic Fluid Flow Phenomena in Porous Media. Microfluid. Nanofluidics 2012, 12, 485–498. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Z. Numerical Investigation on Layout Optimization of Obstacles in a Three-Dimensional Passive Micromixer. Anal. Chim. Acta 2017, 964, 142–149. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Li, X. Numerical Research on Shape Optimization of Microchannels of Passive Micromixers. IEEE Sens. J. 2016, 16, 6527–6532. [Google Scholar] [CrossRef]
- Dehghani, T.; Sadegh Moghanlou, F.; Vajdi, M.; Shahedi Asl, M.; Shokouhimehr, M.; Mohammadi, M. Mixing Enhancement through a Micromixer Using Topology Optimization. Chem. Eng. Res. Des. 2020, 161, 187–196. [Google Scholar] [CrossRef]
- IEEE Malaysia Section; Electron Devices Chapter; Universiti Kebangsaan Malaysia; Institute of Microengineering and Nanoelectronics; Institute of Electrical and Electronics Engineers. Annual Report 2012. In Proceedings of the ICSE 2012: 2012 10th IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 19–21 September 2012; ISBN 9781467323963. [Google Scholar]
- Ahmed, F.; Yoshida, Y.; Wang, J.; Sakai, K.; Kiwa, T. Design and Validation of Microfluidic Parameters of a Microfluidic Chip Using Fluid Dynamics. AIP Adv. 2021, 11, 075224. [Google Scholar] [CrossRef]
- Spigarelli, L.; Bertana, V.; Marchisio, D.; Scaltrito, L.; Ferrero, S.; Cocuzza, M.; Marasso, S.L.; Canavese, G.; Pirri, C.F. A Passive Two-Way Microfluidic Device for Low Volume Blood-Plasma Separation. Microelectron. Eng. 2019, 209, 28–34. [Google Scholar] [CrossRef]
- Sanjuan, A.; Errarte, A.; Bou-Ali, M.M. Analysis of Thermophoresis for Separation of Polystyrene Microparticles in Microfluidic Devices. Int. J. Heat Mass Transf. 2022, 189, 122690. [Google Scholar] [CrossRef]
- Jeong, S.; Seo, J.H.; Garud, K.S.; Park, S.W.; Lee, M.Y. Numerical Approach-Based Simulation to Predict Cerebrovascular Shear Stress in a Blood-Brain Barrier Organ-on-a-Chip. Biosens. Bioelectron. 2021, 183, 113197. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.F.; Li, Z.Y.; Wang, T.; Zhao, C.X. Numerical Investigation of Drug Transport from Blood Vessels to Tumour Tissue Using a Tumour-Vasculature-on-a-Chip. Chem. Eng. Sci. 2019, 208, 115155. [Google Scholar] [CrossRef]
- Müller, T.; Arosio, P.; Rajah, L.; Cohen, S.I.A.; Yates, E.V.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J. Particle-Based Monte-Carlo Simulations of Steady-State Mass Transport at Intermediate Péclet Numbers. Int. J. Nonlinear Sci. Numer. Simul. 2016, 17, 175–183. [Google Scholar] [CrossRef]
- Jen, C.P.; Wu, C.Y.; Lin, Y.C.; Wu, C.Y. Design and Simulation of the Micromixer with Chaotic Advection in Twisted Microchannels. Lab Chip 2003, 3, 77–81. [Google Scholar] [CrossRef]
- Alexandre, A.; Guérin, T.; Dean, D.S. Generalized Taylor Dispersion for Translationally Invariant Microfluidic Systems. Phys. Fluids 2021, 33, 082004. [Google Scholar] [CrossRef]
- Gervais, T.; Jensen, K.F. Mass Transport and Surface Reactions in Microfluidic Systems. Chem. Eng. Sci. 2006, 61, 1102–1121. [Google Scholar] [CrossRef]
- Razavi Bazaz, S.; Mashhadian, A.; Ehsani, A.; Saha, S.C.; Krüger, T.; Ebrahimi Warkiani, M. Computational Inertial Microfluidics: A Review. Lab Chip 2020, 20, 1023–1048. [Google Scholar] [CrossRef]
- Ostrowski, Z.; Melka, B.; Adamczyk, W.; Rojczyk, M.; Golda, A.; Nowak, A.J. CFD Analysis of Multiphase Blood Flow within Aorta and Its Thoracic Branches of Patient with Coarctation of Aorta Using Multiphase Euler—Euler Approach. In Proceedings of the Journal of Physics: Conference Series; Institute of Physics Publishing: Bristol, UK, 2016; Volume 745. [Google Scholar]
- Hajji, H.; Kolsi, L.; Hassen, W.; Al-Rashed, A.A.A.A.; Borjini, M.N.; Aichouni, M.A. Finite Element Simulation of Antigen-Antibody Transport and Adsorption in a Microfluidic Chip. Phys. E Low Dimens. Syst. Nanostruct. 2018, 104, 177–186. [Google Scholar] [CrossRef]
- Shackelford, C.D.; Moore, S.M. Fickian Diffusion of Radionuclides for Engineered Containment Barriers: Diffusion Coefficients, Porosities, And Complicating Issues. Eng. Geol. 2013, 152, 133–147. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chang, C.L.; Wang, Y.N.; Fu, L.M. Microfluidic Mixing: A Review. Int. J. Mol. Sci. 2011, 12, 3263–3287. [Google Scholar] [CrossRef]
- Suh, Y.K.; Kang, S. A Review on Mixing in Microfluidics. Micromachines 2010, 1, 82–111. [Google Scholar] [CrossRef]
- Antognoli, M.; Stoecklein, D.; Galletti, C.; Brunazzi, E.; Di Carlo, D. Optimized Design of Obstacle Sequences for Microfluidic Mixing in an Inertial Regime. Lab Chip 2021, 21, 3910–3923. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Bagdi, P.; Sen, A.K. Microfluidic Device Based on a Micro-Hydrocyclone for Particle-Liquid Separation. Lab Chip 2011, 11, 4012–4021. [Google Scholar] [CrossRef]
- Tafti, E.Y.; Kumar, R.; Cho, H.J. Effect of Laminar Velocity Profile Variation on Mixing in Microfluidic Devices: The Sigma Micromixer. Appl. Phys. Lett. 2008, 93, 143504. [Google Scholar] [CrossRef]
- Dincau, B.; Dressaire, E.; Sauret, A. Pulsatile Flow in Microfluidic Systems. Small 2020, 16, e1904032. [Google Scholar] [CrossRef]
- Geng, X.; Heiss, J.W.; Michael, H.A.; Boufadel, M.C.; Lee, K. Groundwater Flow and Moisture Dynamics in the Swash Zone: Effects of Heterogeneous Hydraulic Conductivity and Capillarity. Water Resour. Res. 2020, 56, e2020WR028401. [Google Scholar] [CrossRef]
- Cao, X.; Ashfaq, R.; Cheng, F.; Maharjan, S.; Li, J.; Ying, G.; Hassan, S.; Xiao, H.; Yue, K.; Zhang, Y.S. A Tumor-on-a-Chip System with Bioprinted Blood and Lymphatic Vessel Pair. Adv. Funct. Mater. 2019, 29, 1807173. [Google Scholar] [CrossRef]
- Seidi, S.; Eftekhari, A.; Khusro, A.; Heris, R.S.; Sahibzada, M.U.K.; Gajdács, M. Simulation and Modeling of Physiological Processes of Vital Organs in Organ-on-a-Chip Biosystem. J. King Saud Univ. Sci. 2022, 34, 101710. [Google Scholar] [CrossRef]
- Amin Arefi, S.M.; Tony Yang, C.W.; Sin, D.D.; Feng, J.J. Simulation of Nanoparticle Transport and Adsorption in a Microfluidic Lung-on-a-Chip Device. Biomicrofluidics 2020, 14, 044117. [Google Scholar] [CrossRef]
- Zou, H.; Yue, W.; Yu, W.K.; Liu, D.; Fong, C.C.; Zhao, J.; Yang, M. Microfluidic Platform for Studying Chemotaxis of Adhesive Cells Revealed a Gradient-Dependent Migration and Acceleration of Cancer Stem Cells. Anal. Chem. 2015, 87, 7098–7108. [Google Scholar] [CrossRef]
- Drapaca, C.S. Brain-on-a-Chip: Design and Modeling. DCDIS Ser. B Appl. Algorithms 2018, 25, 3–4. [Google Scholar]
- Docci, L.; Milani, N.; Ramp, T.; Romeo, A.A.; Godoy, P.; Franyuti, D.O.; Krähenbühl, S.; Gertz, M.; Galetin, A.; Parrott, N.; et al. Exploration and Application of a Liver-on-a-Chip Device in Combination with Modelling and Simulation for Quantitative Drug Metabolism Studies. Lab Chip 2022, 22, 1187–1205. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mehrotra, S.; Zare-Eelanjegh, E.; Rodrigues, R.O.; Akbarinejad, A.; Ge, D.; Amato, L.; Kiaee, K.; Fang, Y.C.; Rosenkranz, A.; et al. A Heart-Breast Cancer-on-a-Chip Platform for Disease Modeling and Monitoring of Cardiotoxicity Induced by Cancer Chemotherapy. Small 2021, 17, 2004258. [Google Scholar] [CrossRef]
- Hajari, M.A.; Baheri Islami, S.; Chen, X. A Numerical Study on Tumor-on-Chip Performance and Its Optimization for Nanodrug-Based Combination Therapy. Biomech. Model. Mechanobiol. 2021, 20, 983–1002. [Google Scholar] [CrossRef]
- Shirure, V.S.; Bi, Y.; Curtis, M.B.; Lezia, A.; Goedegebuure, M.M.; Goedegebuure, S.P.; Aft, R.; Fields, R.C.; George, S.C. Tumor-on-a-Chip Platform to Investigate Progression and Drug Sensitivity in Cell Lines and Patient-Derived Organoids. Lab Chip 2018, 18, 3687–3702. [Google Scholar] [CrossRef]
- Pisapia, F.; Balachandran, W.; Rasekh, M. Organ-on-a-Chip: Design and Simulation of Various Microfluidic Channel Geometries for the Influence of Fluid Dynamic Parameters. Appl. Sci. 2022, 12, 3829. [Google Scholar] [CrossRef]
- Kotsifaki, D.G.; Mackenzie, M.D.; Polydefki, G.; Kar, A.K.; Makropoulou, M.; Serafetinides, A.A. Geometrical Effect Characterization of Femtosecond-Laser Manufactured Glass Microfluidic Chips Based on Optical Manipulation of Submicroparticles. Opt. Eng. 2017, 56, 124111. [Google Scholar] [CrossRef]
- Bessoth, F.G.; Demello, A.J.; Manz, A. Microstructure for Efficient Continuous Flow Mixing. Anal. Commun. 1999, 36, 213–215. [Google Scholar] [CrossRef]
- Johnson, T.J.; Ross, D.; Locascio, L.E. Rapid Microfluidic Mixing. Anal. Chem. 2002, 74, 45–51. [Google Scholar] [CrossRef]
- Song, H.; Bringer, M.R.; Tice, J.D.; Gerdts, C.J.; Ismagilov, R.F. Experimental Test of Scaling of Mixing by Chaotic Advection in Droplets Moving through Microfluidic Channels. Appl. Phys. Lett. 2003, 83, 4664–4666. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Moss, G.R.; Rothstein, J.P. Enhanced Mixing in Laminar Flows Using Ultrahydrophobic Surfaces. Phys. Rev. E 2007, 76, 016304. [Google Scholar] [CrossRef]
- Ou, J.; Perot, B.; Rothstein, J.P. Laminar Drag Reduction in Microchannels Using Ultrahydrophobic Surfaces. Phys. Fluids 2004, 16, 4635–4643. [Google Scholar] [CrossRef]
- Lu, L.H.; Ryu, K.S.; Liu, C. A Magnetic Microstirrer and Array for Microfluidic Mixing. J. Microelectromech. Syst. 2002, 11, 462–469. [Google Scholar] [CrossRef]
- Frommelt, T.; Kostur, M.; Wenzel-Schäfer, M.; Talkner, P.; Hänggi, P.; Wixforth, A. Microfluidic Mixing via Acoustically Driven Chaotic Advection. Phys. Rev. Lett. 2008, 100, 034502. [Google Scholar] [CrossRef]
- Glasgow, I.; Aubry, N. Enhancement of Microfluidic Mixing Using Time Pulsing. Lab Chip 2003, 3, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Yang, T.; Cremer, P.S. A Microfluidic Device with a Linear Temperature Gradient for Parallel and Combinatorial Measurements. J. Am. Chem. Soc. 2002, 124, 4432–4435. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chung, C.K. PDMS Microfabrication and Design for Microfluidics and Sustainable Energy Application: Review. Micromachines 2021, 12, 1350. [Google Scholar] [CrossRef] [PubMed]
- Pungjunun, K.; Yakoh, A.; Chaiyo, S.; Praphairaksit, N.; Siangproh, W.; Kalcher, K.; Chailapakul, O. Laser engraved microapillary pump paper-based microfluidic device for colorimetric and electrochemical detection of salivary thiocyanate. Microchim. Acta 2021, 188, 140. [Google Scholar] [CrossRef]
- Huang, E.; Huang, D.; Wang, Y.; Cai, D.; Luo, Y.; Zhong, Z.; Liu, D. Active droplet-array microfluidics-based chemiluminescence immunoassay for point-of-care detection of procalcitonin. Biosens. Bioelectron. 2022, 195, 113684. [Google Scholar] [CrossRef]
- Boonkaew, S.; Szot-Karpińska, K.; Niedziółka-Jönsson, J.; Pałys, B.; Jönsson-Niedziółk, M. Point-of-care testing for C-reactive protein in a sequential microfluidic device. Sens. Actuators B Chem. 2023, 397, 134659. [Google Scholar] [CrossRef]
| Techniques | Advantages | Disadvantages |
|---|---|---|
| SLA | High precision and detail Simplified process Variety of resins Automation and medium-scale production Ease of design | Cost of resins Production scale limitations Mechanical properties of resins Material toxicity |
| FDM | Integration of various materials Lower cost Simplified assembly Versatility and accessibility | Lower resolution Surface roughness Adhesion and sealing Low material resistance (temperature and mechanical stress) |
| SLM | High precision and detail Rapid prototyping Material versatility Flexibility and cost-effectiveness | Accuracy and surface roughness Equipment cost Material limitation |
| Materials | Advantages | Disadvantages |
|---|---|---|
| Glass [45] | Transparency; Chemical compatibility; Dimensional stability; Withstands high temperatures; Biocompatibility. | High cost; Fragile; Difficult to fabricate; Does not support the integration of electronic components. |
| Silicon [15] | Flexibility and elasticity; Biocompatibility; Easy to fabricate; Chemical resistance; Optical transparency. | Gas permeability; Hydrophobicity; More significant molecule diffusion; Limited thermal compatibility; Limited mechanical resistance. |
| PDMS [46] | Easy to fabricate; Elasticity and deformity; Optical transparency; Biocompatibility; Selective permeability. | Sorption of molecules; Gas permeability; Limited thermal compatibility; Limited durability; Difficult to integrate electronics. |
| PMMA [47] | Optical transparency; Easy to fabricate; Chemical compatibility; Biocompatibility; Durability. | Gas permeability; Sorption of molecules; Limited thermal compatibility; Difficult to integrate electronics; Relative stiffness. |
| HDPE [48] | Low cost; Easy to fabricate; Durability; Chemical compatibility; Electrical isolation. | Permeability; Sorption of molecules; Limited thermal compatibility; Difficult to weld; Relative stiffness. |
| LDPE [49] | Low cost; Easy to fabricate; Flexibility; Chemical compatibility; Electrical isolation. | Permeability; Sorption of molecules; Limited thermal compatibility; Difficult to weld; Relative stiffness. |
| Polyamide 6 [50] | Mechanical resistance; Dimensional stability; Chemical compatibility; Easy to fabricate; Biocompatibility. | Gas permeability; Limited thermal compatibility; Difficult to weld; Sorption of molecules; Difficult to weld. |
| SU-8 [51] | High resolution; Photolithography compatibility; Design flexibility; Chemical compatibility; Mechanically stable. | Difficult to fabricate; Long fabrication time; Gas permeability; High cost; Limited thermal compatibility. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.; Carvalho, V.; Ribeiro, J.; Lima, R.A.; Teixeira, S.; Pinho, D. Advances in Microfluidic Systems and Numerical Modeling in Biomedical Applications: A Review. Micromachines 2024, 15, 873. https://doi.org/10.3390/mi15070873
Ferreira M, Carvalho V, Ribeiro J, Lima RA, Teixeira S, Pinho D. Advances in Microfluidic Systems and Numerical Modeling in Biomedical Applications: A Review. Micromachines. 2024; 15(7):873. https://doi.org/10.3390/mi15070873
Chicago/Turabian StyleFerreira, Mariana, Violeta Carvalho, João Ribeiro, Rui A. Lima, Senhorinha Teixeira, and Diana Pinho. 2024. "Advances in Microfluidic Systems and Numerical Modeling in Biomedical Applications: A Review" Micromachines 15, no. 7: 873. https://doi.org/10.3390/mi15070873
APA StyleFerreira, M., Carvalho, V., Ribeiro, J., Lima, R. A., Teixeira, S., & Pinho, D. (2024). Advances in Microfluidic Systems and Numerical Modeling in Biomedical Applications: A Review. Micromachines, 15(7), 873. https://doi.org/10.3390/mi15070873