Exploring the Capability of Cu-MoS2 Catalysts for Use in Electrocatalytic Overall Water Splitting
Abstract
:1. Introduction
2. Synthesis Methods
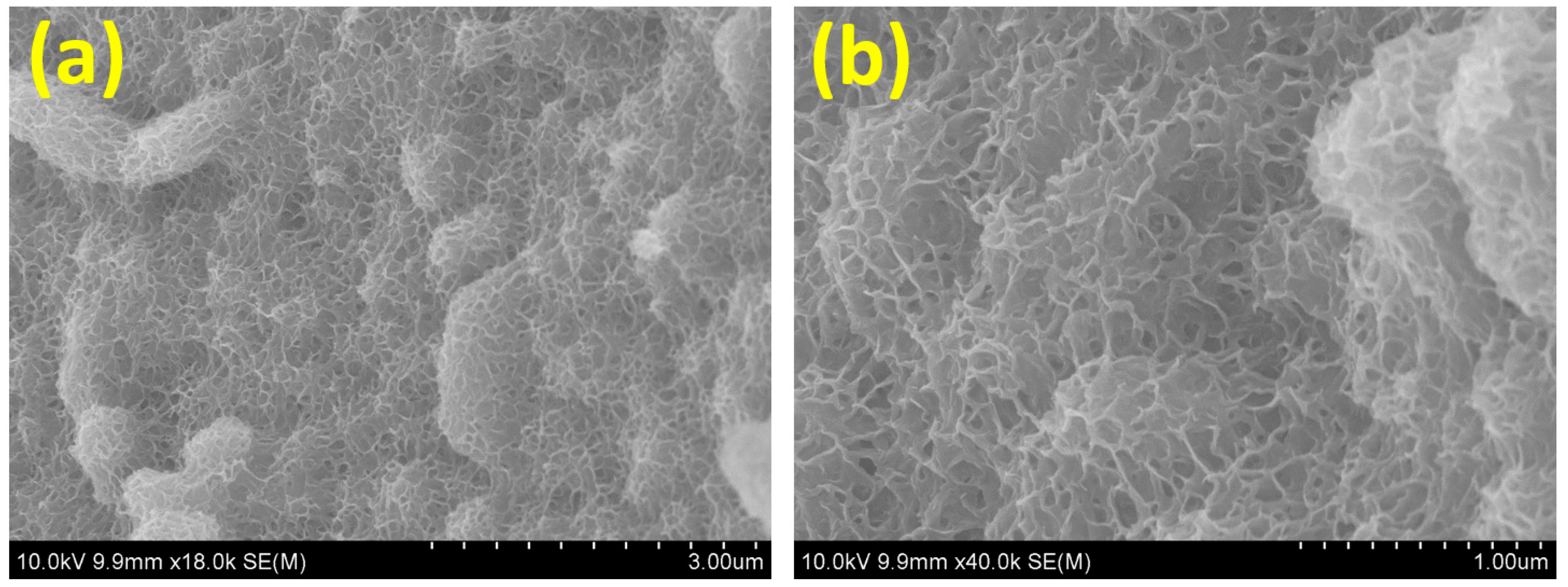
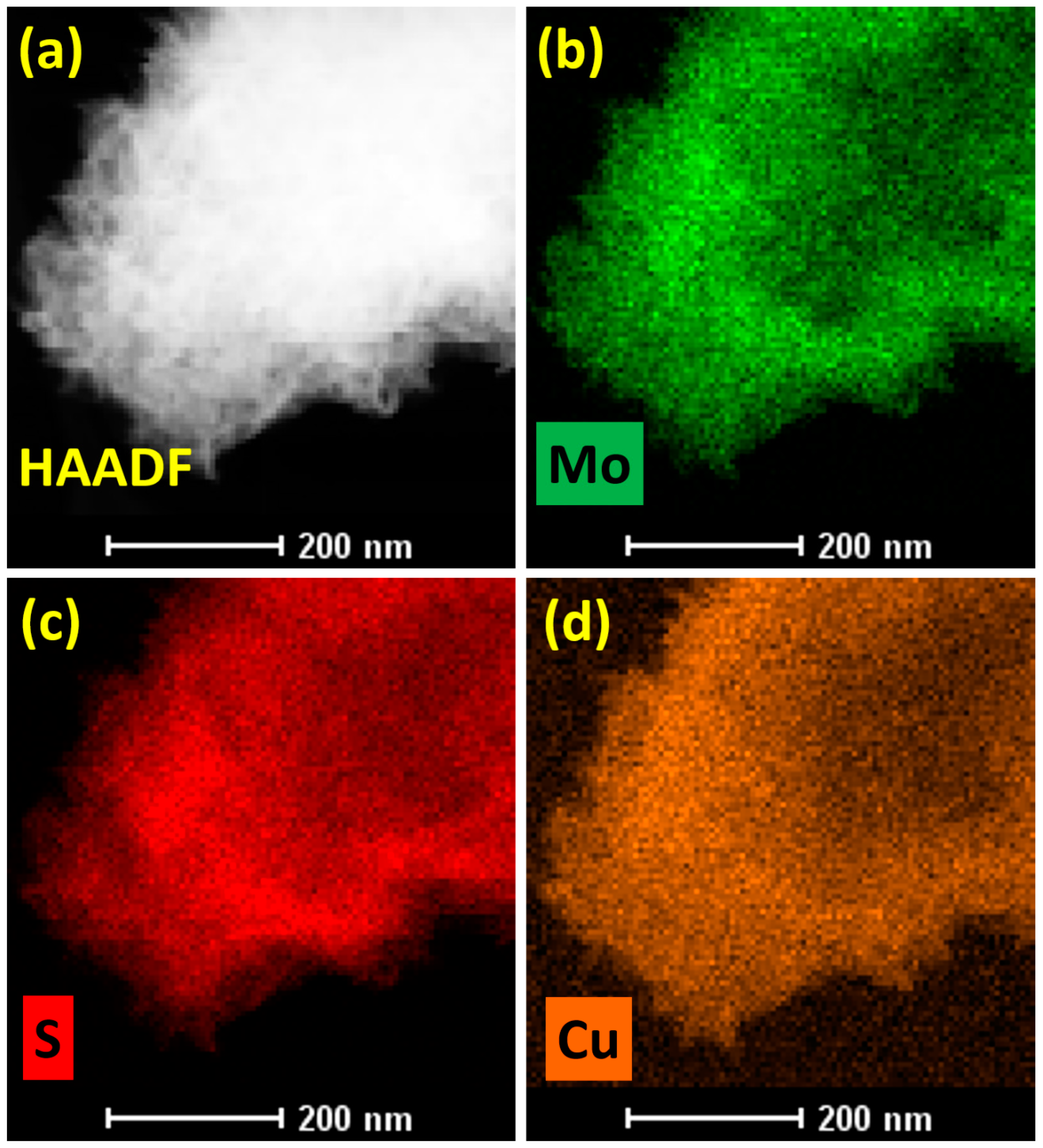
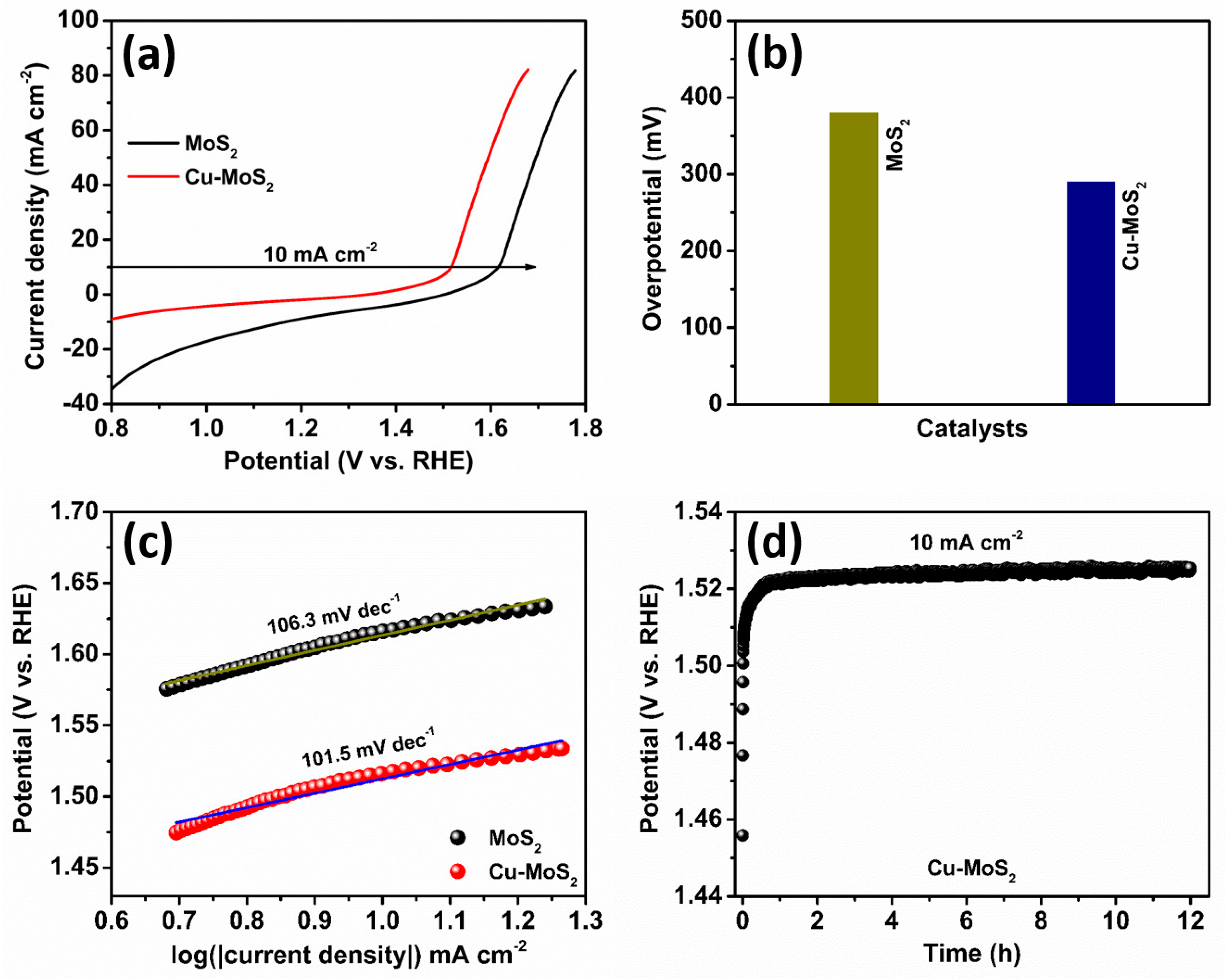
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gautam, A.; Sk, S.; Pal, U. Recent advances in solution assisted synthesis of transition metal chalcogenides for photo-electrocatalytic hydrogen evolution. Phys. Chem. Chem. Phys. 2022, 24, 20638–20673. [Google Scholar] [CrossRef]
- Raveendran, A.; Chandran, M.; Siddiqui, M.R.; Wabaidur, S.M.; Angaiah, S.; Dhanusuraman, R. Binary Ni–Cu nanocomposite-modified MXene-adorned 3D-nickel foam for effective overall water splitting and supercapacitor applications. Sustainable Energy Fuels 2024, 8, 1509–1525. [Google Scholar] [CrossRef]
- Shaikh, N.; Annadata, H.V.; Mishra, A.K.; Urkude, R.R.; Mukhopadhyay, I.; Ray, A. Doping induced mixed polytypic interfaces of MoS2 for superior electrocatalytic hydrogen evolution. Appl. Surf. Sci. 2024, 649, 159195. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, C.; Wang, S.; Lu, K.; Wang, B.; Huang, J.; Peng, H.; Li, N.; Liu, M. Photothermally driven decoupling of gas evolution at the solid-liquid interface for boosted photocatalytic hydrogen production. Nanoscale 2024, 16, 152–162. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Yu, N.; Fan, R.-Y.; Dong, B.; Yan, Z.-F. Design and multilevel regulation of transition metal phosphides for efficient and industrial water electrolysis. Nanoscale 2024, 16, 1080–1101. [Google Scholar] [CrossRef]
- Sharma, S.; Paul, A. One-pot synthesis of CoFe-nanomesh for oxygen evolution reaction. ACS Appl. Nano Mater. 2024, 7, 8567–8857. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water splitting: From electrode to green energy system. Nano-Micro Lett. 2020, 12, 131. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Janák, M.; Abdala, P.M.; Borca, C.N.; Wach, A.; Kierzkowska, A.; Donat, F.; Huthwelker, T.; Kuznetsov, D.A.; Müller, C.R. Probing surface transformations of lanthanum nickelate electrocatalysts during oxygen evolution reaction. J. Am. Chem. Soc. 2024, 146, 11887–11896. [Google Scholar] [CrossRef]
- Paladugu, S.; Abdullahi, I.M.; Singh, H.; Spinuzzi, S.; Nath, M.; Page, K. Mesoporous Re0.5Ce0.5O2–x fluorite electrocatalysts for the oxygen evolution reaction. ACS Appl. Mater. Interfaces 2024, 16, 7014–7025. [Google Scholar] [CrossRef]
- Li, R.; Chen, L.; Zhang, H.; Humayun, M.; Duan, J.; Xu, X.; Fu, Y.; Bououdina, M.; Wang, C. Exceptional green hydrogen production performance of a ruthenium-modulated nickel selenide. Nanoscale 2023, 15, 19604–19616. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, F.; Guo, W.; He, Z.; Yao, L.; Li, J.; Sun, N.; Wang, Y.; Wang, F. Monolayer MoS2 as a sensitive probe: Exploring the resistive switching mechanism of MoS2/NSTO heterostructures. J. Alloys Compd. 2023, 967, 171712. [Google Scholar] [CrossRef]
- Choi, S.; Oh, G.H.; Kim, T.W.; Hong, S.; Kim, A. Radiation induced changes in chemical and electronic properties of few-layer MoS2 and MoTe2 films. Appl. Surf. Sci. 2024, 652, 159282. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Hedhili, M.N.; Alshareef, H.N. Mechanistic insight into the stability of HfO2-coated MoS2 nanosheet anodes for sodium ion batteries. Small 2015, 11, 4341–4350. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Luo, F.; Chen, S. Porous MoP2/MoS2 hierarchical nanowires for efficient hydrogen evolution reaction in full pH range. J. Alloys Compd. 2024, 985, 174024. [Google Scholar] [CrossRef]
- Dighole, R.P.; Munde, A.V.; Mulik, B.B.; Dhawale, S.C.; Sathe, B.R. Multiwalled carbon nanotubes decorated with molybdenum sulphide (MoS2@MWCNTs) for highly selective electrochemical picric acid (PA) determination. Appl. Surf. Sci. 2024, 659, 159856. [Google Scholar] [CrossRef]
- Gui, T.; Xia, X.; Wei, B.; Zhang, J.; Zhang, K.; Li, Y.; Chen, W.; Yu, W.; Cui, N.; Mu, H.; et al. In-situ fabrication of PtSe2/MoS2 van der Waals heterojunction for self-powered and broadband photodetector. Mater. Des. 2024, 238, 112722. [Google Scholar] [CrossRef]
- Xiong, H.; Nie, X.; Zhao, L.; Deng, S. Engineering symmetry breaking in twisted MoS2-MoSe2 heterostructures for optimal thermoelectric performance. ACS Appl. Mater. Interfaces 2024, 16, 25124–25135. [Google Scholar] [CrossRef]
- Jacobo, J.-R.; Olea-Mejía, O.F.; Martínez-Hernández, A.L.; Carlos, V.-S. Optimization of the optical response of 2D MoS2 materials obtained through liquid-phase exfoliation using a comprehensive multi-objective approach. FlatChem 2024, 45, 100654. [Google Scholar] [CrossRef]
- Liang, S.; Zheng, L.-J.; Song, L.-N.; Wang, X.-X.; Tu, W.-B.; Xu, J.-J. Accelerated confined mass transfer of MoS2 1D nanotube in photo-assisted metal-air batteries. Adv. Mater. 2024, 36, 2307790. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, T.; Li, R.; Chen, Y.; Luo, W.; Wu, Y.; Xie, Y.; Wang, Y.; Zhang, Y. Layered deposited MoS2 nanosheets on acorn leaf like CdS as an efficient anti-photocorrosion photocatalyst for hydrogen production. Fuel 2024, 368, 131621. [Google Scholar] [CrossRef]
- Kaushik, R.; Nandi, S.; Mandal, M.; Gupta, A.N. Biocompatible L-cysteine-capped MoS2 nanoflowers for antibacterial applications: Mechanistic insights. ACS Appl. Nano Mater. 2024, 7, 7753–7765. [Google Scholar] [CrossRef]
- Park, D.; Kim, H.; Kim, N. Enhancing valley polarization of a MoS2 zigzag nanoribbon using double magnetic barriers. Phys. E 2024, 159, 115910. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, J.; Ding, L.; Lv, H.; Zhang, K.; Hu, A.; Yang, X.; Sun, W.; Mao, Y. Interfacial engineering for promoting charge transfer in MoS2/CoFeLDH heterostructure electrodes for overall water splitting. Int. J. Hydrogen Energy 2024, 49, 897–906. [Google Scholar] [CrossRef]
- Hai, G.; Xue, X.; Wu, Z.; Zhang, C.; Liu, X.; Huang, X. High-throughput calculation-based rational design of Fe-doped MoS2 nanosheets for electrocatalytic pH-universal overall water splitting. J. Energy Chem. 2024, 91, 194–202. [Google Scholar] [CrossRef]
- Kang, L.; Liu, S.; Zhang, Q.; Zou, J.; Ai, J.; Qiao, D.; Zhong, W.; Liu, Y.; Jun, S.C.; Yamauchi, Y.; et al. Hierarchical spatial confinement unlocking the storage limit of MoS2 for flexible high-energy supercapacitors. ACS Nano 2024, 18, 2149–2161. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, P.; Su, C.; Yan, J.; Zhao, H.; Zhang, Z.; You, Z. Flexible high-temperature MoS2 field-effect transistors and logic gates. ACS Nano 2024, 18, 9627–9635. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Li, Z.; Lampronti, G.I.; Lee, J.-I.; Wang, Y.; Day, J.; Chhowalla, M. Environmental and thermal stability of chemically exfoliated LixMoS2 for lithium-sulfur batteries. Chem. Mater. 2024, 36, 4829–4837. [Google Scholar] [CrossRef]
- Ji, S.; Bae, S.-R.; Hu, L.; Hoang, A.T.; Seol, M.J.; Hong, J.; Katiyar, A.K.; Kim, B.J.; Xu, D.; Kim, S.Y.; et al. Perovskite light-emitting diode display based on MoS2 backplane thin-film transistors. Adv. Mater. 2024, 36, 2309531. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Huang, M. Mechanism of adsorption and gas-sensing of hazardous gases by MoS2 monolayer decorated by Pdn (n = 1–4) clusters. Colloids Surf. A 2024, 695, 134200. [Google Scholar] [CrossRef]
- Chen, P.S.; Hu, Y.; Li, S.-Y.; Mazurkiewicz-Pawlicka, M.; Małolepszy, A. Preparation of a MoS2/carbon nanotube nanocomposite by hydrothermal method for supercapacitor. Int. J. Electrochem. Sci. 2024, 19, 100523. [Google Scholar] [CrossRef]
- Mondal, K.G.; Rakshit, S.; Kar, B.S.; Saha, S.; Jana, P.C. Study of enhancing photocatalytic activity of solvothermal grown MoS2 nanocrystals under visible light irradiation by the influence of hydrogen peroxide. J. Photochem. Photobiol. A 2024, 447, 115239. [Google Scholar] [CrossRef]
- Al-Namshah, K.S. Synthesis of MoS2-loaded Co3O4 nanocrystals for endorsed photocatalytic reduction of mercury (II) ions under visible light. Opt. Mater. 2023, 142, 114114. [Google Scholar] [CrossRef]
- Khorasanipour, N.; Iranmanesh, P.; Saeednia, S.; Yazdi, S.T. Photocatalytic degradation of Naphthol Green in aqueous solution through the reusable ZnS/MoS2/Fe3O4 magnetic nanocomposite. Surf. Interfaces 2023, 36, 102613. [Google Scholar] [CrossRef]
- Midhun, P.S.; Kumar, K.R.; Jayaraj, M.K. Large area synthesis of mono/few-layer MoS2 thin films on thermal oxide silicon substrate by pulsed laser deposition technique. Thin Solid Films 2023, 782, 140030. [Google Scholar] [CrossRef]
- Tonon, A.; Russo, E.D.; Sgarbossa, F.; Bacci, L.; Argiolas, N.; Scian, C.; Ivanov, Y.P.; Divitini, G.; Sheehan, B.; Salvador, D.D.; et al. Laser induced crystallization of sputtered MoS2 thin films. Mater. Sci. Semicond. Process. 2023, 164, 107616. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.K.; Kang, Y.C. Sodium-ion storage performances of MoS2 nanocrystals coated with N-doped carbon synthesized by flame spray pyrolysis. Appl. Surf. Sci. 2020, 523, 146470. [Google Scholar] [CrossRef]
- Duraisamy, S.; Ganguly, A.; Sharma, P.K.; Benson, J.; Davis, J.; Papakonstantinou, P. One-Step hydrothermal synthesis of phase-engineered MoS2/MoO3 electrocatalysts for hydrogen evolution reaction. ACS Appl. Nano Mater. 2021, 4, 2642–2656. [Google Scholar] [CrossRef]
- Abraham, D.S.; Vinoba, M.; Bhagiyalakshmi, M. NiFe-LDH/MoS2/MXene nanocomposites as an electrode material for battery-type supercapacitors. ACS Appl. Nano Mater. 2024, 7, 5791–5801. [Google Scholar] [CrossRef]
- Park, H.; Liu, N.; Kim, B.H.; Kwon, S.H.; Baek, S.; Kim, S.; Lee, H.-K.; Yoon, Y.J.; Kim, S. Exceptionally uniform and scalable multilayer MoS2 phototransistor array based on large-scale MoS2 grown by RF sputtering, electron beam irradiation, and sulfurization. ACS Appl. Mater. Interfaces 2020, 12, 20645–20652. [Google Scholar] [CrossRef]
- Cho, Y.; Sohn, A.; Kim, S.; Hahm, M.G.; Kim, D.-H.; Cho, B.; Kim, D.-W. Influence of gas adsorption and gold nanoparticles on the electrical properties of CVD-grown MoS2 thin films. ACS Appl. Mater. Interfaces 2016, 8, 21612–21617. [Google Scholar] [CrossRef]
- Yu, N.; Ke, H.; Yu, H.; Wu, X.; Li, S.; Chen, G.; Wang, J.; Cai, N.; Xue, Y.; Yu, F. Polysulfide-induced synthesis of coral-like MoS2/NiS2 nanostructures for overall water splitting. ACS Appl. Nano Mater. 2023, 6, 5136–5144. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, X.; Song, R.; Liang, W. Cu doped MoS2 nanosheets/NiS2 nanowires heterogeneous structure for enhanced hydrogen evolution reaction. J. Phys. Chem. Solids 2023, 181, 111540. [Google Scholar] [CrossRef]
- Wang, Z.; Kannan, H.; Su, T.; Swaminathan, J.; Shirodkar, S.N.; Hernandez, F.C.R.; Benavides, H.C.; Vajtai, R.; Yakobson, B.I.; Meiyazhagan, A.; et al. Substitution of copper atoms into defect-rich molybdenum sulfides and their electrocatalytic activity. Nanoscale Adv. 2021, 3, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, Y.; Lim, H.Y.; Son, S.; Cho, Y.; Park, J.B.; Cho, H.-S.; Jang, A.-R.; Lee, Y.-W. Bimetallic metal organic framework-derived Co9S8-MoS2 nanohybrids as an efficient dual functional electrocatalyst towards the hydrogen and oxygen evolution reactions. J. Ind. Eng. Chem. 2024, 130, 317–323. [Google Scholar] [CrossRef]
- Ghanashyam, G.; Kim, H. Co-doped 1T-MoS2 microspheres embedded in N-doped reduced graphene oxide for efficient electrocatalysis toward hydrogen and oxygen evolution reactions. J. Power Sources 2024, 596, 234088. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Song, M.; Qiu, Z.; Wang, S.; Sun, L. Hierarchical interfaces engineering-driven of the CoS2/MoS2/Ni3S2/NF electrode for high-efficient and stable oxygen evolution and urea oxidation reactions. Appl. Surf. Sci. 2023, 617, 156621. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.G.; Chun, D.-M. Nanosized Co3O4-MoS2 heterostructure electrodes for improving the oxygen evolution reaction in an alkaline medium. J. Alloys Compd. 2021, 853, 156946. [Google Scholar] [CrossRef]
- Rasool, F.; Pirzada, B.M.; Uddin, M.M.; Mohideen, M.I.H.; Yildiz, I.; Elkadi, M.; Qurashi, A. Interfacial engineering of ZnS-ZnO decorated MoS2 supported on 2D Ti3C2Tx MXene sheets for enhanced hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 59, 63–73. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Z.; Xiang, C.; Zou, Y.; Xu, F.; Sun, L. MoS2-CuCo2S4 nanosheets with a honeycomb structure formed on Ni foam: An efficient electrocatalyst for hydrogen evolution reaction. J. Alloys Compd. 2024, 988, 174300. [Google Scholar] [CrossRef]
- Hanslin, S.Ø.; Jónsson, H.; Akola, J. Is the doped MoS2 basal plane an efficient hydrogen evolution catalyst? Calculations of voltage-dependent activation energy. Phys. Chem. Chem. Phys. 2023, 25, 15162–15172. [Google Scholar] [CrossRef]
- Hu, W.; Liu, H.; Dong, W.; Munir, H.A.; Fan, X.; Tian, X.; Pang, L. Ammonium ions intercalated 1T/2H-MoS2 with increased interlayer spacing for high-efficient electrocatalytic hydrogen evolution reaction. J. Electroanal. Chem. 2023, 949, 117882. [Google Scholar] [CrossRef]
- Liang, J.; Yang, Y.; Zhang, J.; Dong, P.; Lou, J. Ultrasmall CoSe2 Nanoparticles Grown on MoS2 Nanofilms: A New Catalyst for Hydrogen Evolution Reaction. Phys. Status Solidi RRL 2023, 2300169. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Wu, Z.; Chen, Q.; Zheng, R.; Huan, J.; Huang, Y.; Zhu, X.; Sun, Y. Phase engineering of W-doped MoS2 by magneto-hydrothermal synthesis for hydrogen evolution reaction. Small 2023, 19, 2303646. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Tao, T.; Zhang, Y.; Lu, S.; Xie, J.; Ding, Z.; Wu, Z. Amidoximated polyacrylonitrile extended the interlayer spacing of MoS2/graphite for hydrogen evolution reaction. ChemistrySelect 2023, 8, e202204699. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, K.; Lin, H.; Li, X.; Chan, H.C.; Yang, L.; Gao, Q. MoS2-Ni3S2 heteronanorods as efficient and stable bifunctional electrocatalysts for overall water splitting. ACS Catal. 2017, 7, 2357–2366. [Google Scholar] [CrossRef]
- Hanan, A.; Lakhan, M.N.; Walvekar, R.; Khalid, M.; Prakash, C. Heteroatom-doped MXenes as electrocatalysts for hydrogen evolution reaction: A review on the recent advances, mechanisms and prospects. Chem. Eng. J. 2024, 483, 149107. [Google Scholar] [CrossRef]
- Xiao, C.; Hong, T.; Jia, J.; Jia, H.; Li, J.; Zhu, Y.; Ge, S.; Liu, C.; Zhu, G. Unlocking the potential of hydrogen evolution: Advancements in 3D nanostructured electrocatalysts supported on nickel foam. Appl. Catal. B 2024, 355, 124197. [Google Scholar] [CrossRef]
- Cao, X.; Gao, Y.; Li, Y.; Weragoda, D.M.; Tian, G.; Zhang, W.; Zhang, Z.; Zhao, X.; Chen, B. Research progress on MOFs and their derivatives as promising and efficient electrode materials for electrocatalytic hydrogen production from water. RSC Adv. 2023, 13, 24393–24411. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fan, K.; Zou, Y.; Fu, H.; Dong, M.; Dou, Y.; Wang, Y.; Chen, S.; Yin, H.; Al-Mamun, M.; et al. Rational design of metal oxide catalysts for electrocatalytic water splitting. Nanoscale 2021, 13, 20324–20353. [Google Scholar] [CrossRef]
- Mohanty, B.; Ghorbani-Asl, M.; Kretschmer, S.; Ghosh, A.; Guha, P.; Panda, S.K.; Jena, B.; Krasheninnikov, A.V.; Jena, B.K. MoS2 quantum dots as efficient catalyst materials for the oxygen evolution reaction. ACS Catal. 2018, 8, 1683–1689. [Google Scholar] [CrossRef]
- Muthurasu, A.; Maruthapandian, V.; Kim, H.Y. Metal-organic framework derived Co3O4/MoS2 heterostructure for efficient bifunctional electrocatalysts for oxygen evolution reaction and hydrogen evolution reaction. Appl. Catal. B 2019, 248, 202–210. [Google Scholar] [CrossRef]
- Ma, W.; Li, W.; Zhang, H.; Wang, Y. N-doped carbon wrapped CoFe alloy nanoparticles with MoS2 nanosheets as electrocatalyst for hydrogen and oxygen evolution reactions. Int. J. Hydrogen Energy 2023, 48, 22032–22043. [Google Scholar] [CrossRef]
- Rong, J.; Ye, Y.; Cao, J.; Liu, X.; Fan, H.; Yang, S.; Wei, M.; Yang, L.; Yang, J.; Chen, Y. Restructuring electronic structure via W doped 1T MoS2 for enhancing hydrogen evolution reaction. Appl. Surf. Sci. 2022, 579, 152216. [Google Scholar] [CrossRef]
- Younis, A.; Sehar, S.; Guan, X.; Aftab, S.; Manaa, H.; Mahmood, T.; Iqbal, J.; Akram, F.; Ali, N.; Wu, T. Four-in-one strategy to boost the performance of 3-dimensional MoS2 nanostructures for industrial effluent treatment and hydrogen evolution reactions. J. Alloys Compd. 2024, 976, 173104. [Google Scholar] [CrossRef]
- Homayounfard, A.M.; Maleki, M.; Ghanbari, H.; Kahnamouei, M.H.; Safaei, B. Growth of few-layer flower-like MoS2 on heteroatom-doped activated carbon as a hydrogen evolution reaction electrode. Int. J. Hydrogen Energy 2024, 55, 1360–1370. [Google Scholar] [CrossRef]
- Huang, W.-H.; Li, X.-M.; Yang, X.-F.; Zhang, H.-B.; Wang, F.; Zhang, J. Highly efficient electrocatalysts for overall water splitting: Mesoporous CoS/MoS2 with hetero-interfaces. Chem. Commun. 2021, 57, 4847–4850. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Rahman, G.; Chae, S.Y.; Shah, A.U.H.A.; Joo, O.S.; Mian, S.A.; Hussain, A. Boosting electrocatalytic hydrogen generation from water splitting with heterostructured MoS2/NiFe2O4 composite in alkaline media. Int. J. Hydrogen Energy 2024, 69, 261–271. [Google Scholar] [CrossRef]
- Liu, W.; Dong, J.; An, B.; Su, H.; Teng, Z.; Li, N.; Gao, Y.; Ge, L. Synergistic dual built-in electric fields in 1T-MoS2/Ni3S2/LDH for efficient electrocatalytic overall water splitting reactions. J. Colloid Interface Sci. 2024, 673, 228–238. [Google Scholar] [CrossRef]




| S. No. | Catalysts | Electrolyte | Overpotential | Stability | Ref. |
|---|---|---|---|---|---|
| Oxygen Evolution Reaction | |||||
| 1 | MoS2 quantum dots | 1.0 M KOH | 370 mV (10 mA cm−2) | 2 h (10 mA cm−2) | [60] |
| 2 | MoS2 nanosheets wrapped MOF-based Co3O4 | 1.0 M KOH | 230 mV (10 mA cm−2) | 13 h (10 mA cm−2) | [61] |
| 3 | Metal–organic-framework-derived Co9S8-MoS2 | 1.0 M KOH | 270 mV (10 mA cm−2) | 24 h (10 mA cm−2) | [44] |
| 4 | MoS2-based hybrid with N-doped carbon-wrapped CoFe alloy | 1.0 M KOH | 337 mV (10 mA cm−2) | 24 h (10 mA cm−2) | [62] |
| 5 | Cu-MoS2 | 1.0 M KOH | 290 mV (10 mA cm−2) | 12 h (10 mA cm−2) | This work |
| Hydrogen Evolution Reaction | |||||
| 6 | Cu-MoS2/NiS2 | 1.0 M KOH | 105 mV (−10 mA cm−2) | -- | [42] |
| 7 | W-1T MoS2-15 | 0.5 M H2SO4 | 292 mV (−10 mA cm−2) | 14 h (−10 mA cm−2) | [63] |
| 8 | Mix-phased 1 T/2 H MoS2 | 1.0 M KOH | 145 mV (−10 mA cm−2) | 24 h (−10 mA cm−2) | [64] |
| 9 | AC/MoS2–F | 0.5 M H2SO4 | 136 mV (−10 mA cm−2) | 24 h (−10 mA cm−2) | [65] |
| 10 | Cu-MoS2 | 1.0 M KOH | 167.7 mV (−10 mA cm−2) | 12 h (−10 mA cm−2) | This work |
| Overall water splitting | |||||
| 11 | CoS/MoS2||CoS/MoS2 | 1.0 M KOH | 1.61 V (cell potential) (10 mA cm−2) | 12 h (10 mA cm−2) | [66] |
| 12 | MoS2-CoFeLDH/NF|| MoS2-CoFeLDH/NF | 1.0 M KOH | 1.55 V (cell potential) (10 mA cm−2) | 48 h (10 mA cm−2) | [23] |
| 13 | MoS2/NiFe2O4|| MoS2/NiFe2O4 | 1.0 M KOH | 1.69 V (cell potential) (10 mA cm−2) | -- | [67] |
| 14 | 1T-MoS2/Ni3S2/LDH|| 1T-MoS2/Ni3S2/LDH | 1.0 M KOH | 1.55 V (cell potential) (10 mA cm−2) | 20 h (10 mA cm−2) | [68] |
| 15 | Cu-MoS2||Cu-MoS2 | 1.0 M KOH | 1.69 V (cell potential) (10 mA cm−2) | 18 h (10 mA cm−2) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teli, A.M.; Mishra, R.K.; Shin, J.C.; Jeon, W. Exploring the Capability of Cu-MoS2 Catalysts for Use in Electrocatalytic Overall Water Splitting. Micromachines 2024, 15, 876. https://doi.org/10.3390/mi15070876
Teli AM, Mishra RK, Shin JC, Jeon W. Exploring the Capability of Cu-MoS2 Catalysts for Use in Electrocatalytic Overall Water Splitting. Micromachines. 2024; 15(7):876. https://doi.org/10.3390/mi15070876
Chicago/Turabian StyleTeli, Aviraj M., Rajneesh Kumar Mishra, Jae Cheol Shin, and Wookhee Jeon. 2024. "Exploring the Capability of Cu-MoS2 Catalysts for Use in Electrocatalytic Overall Water Splitting" Micromachines 15, no. 7: 876. https://doi.org/10.3390/mi15070876
APA StyleTeli, A. M., Mishra, R. K., Shin, J. C., & Jeon, W. (2024). Exploring the Capability of Cu-MoS2 Catalysts for Use in Electrocatalytic Overall Water Splitting. Micromachines, 15(7), 876. https://doi.org/10.3390/mi15070876





