Influence of Pd-Layer Thickness on Bonding Reliability of Pd-Coated Cu Wire
Abstract
:1. Introduction
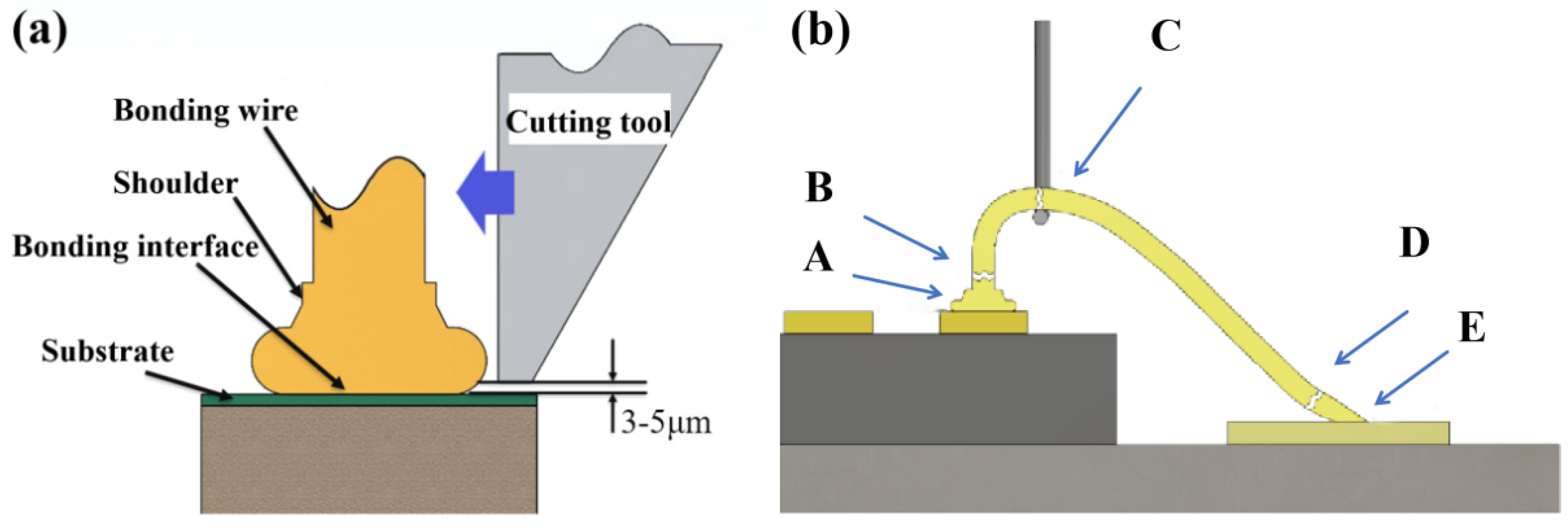
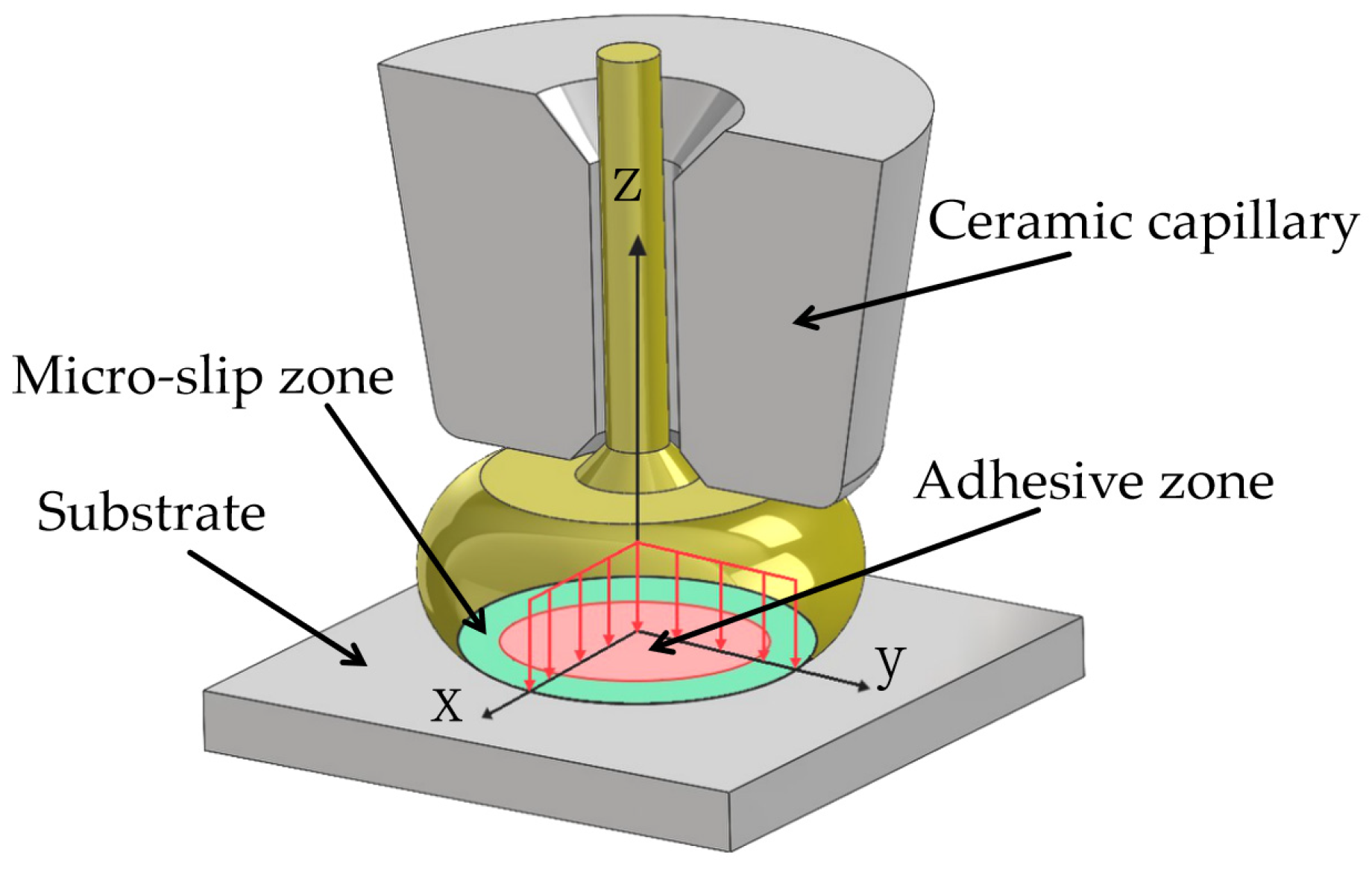
2. Experimental Process
3. Results and Discussion
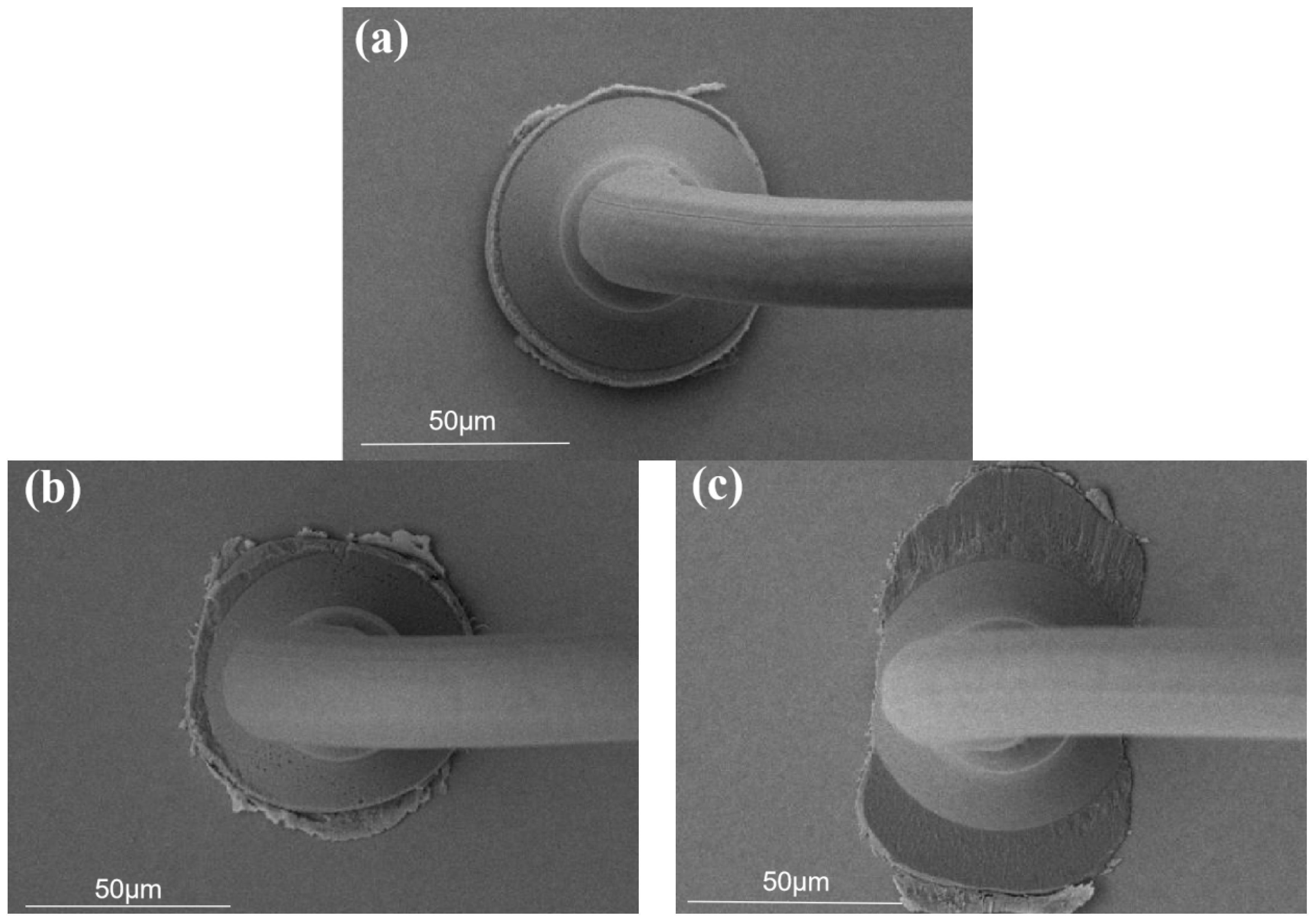
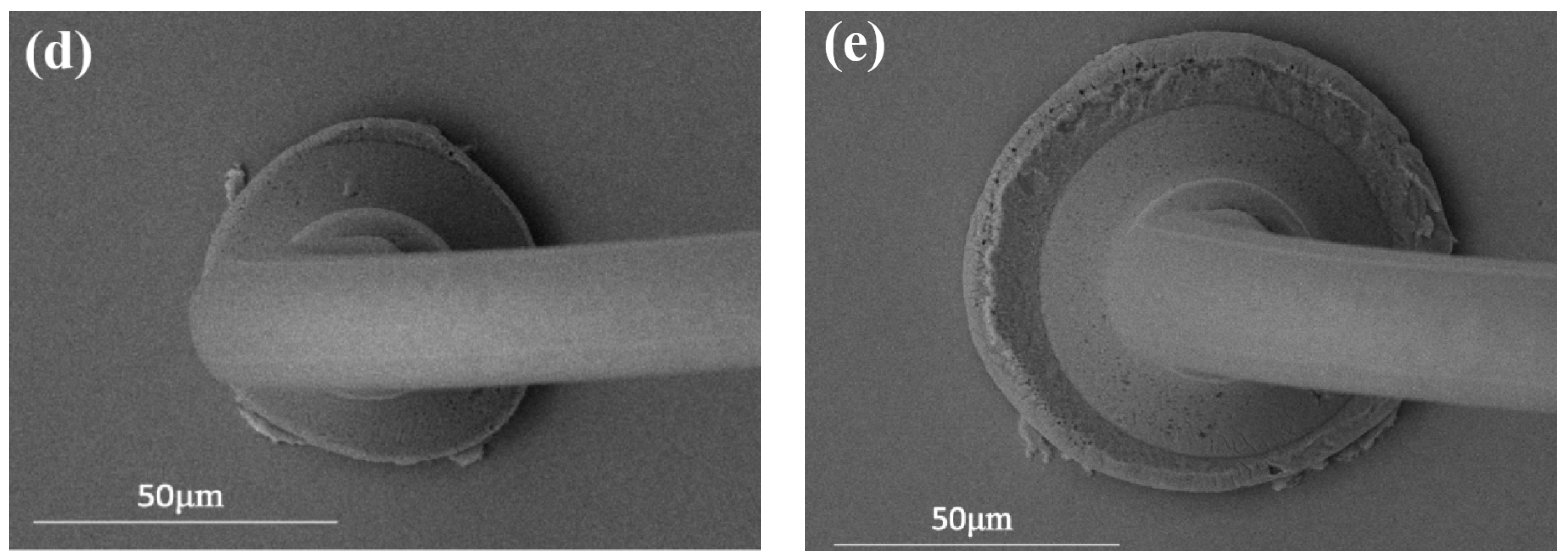
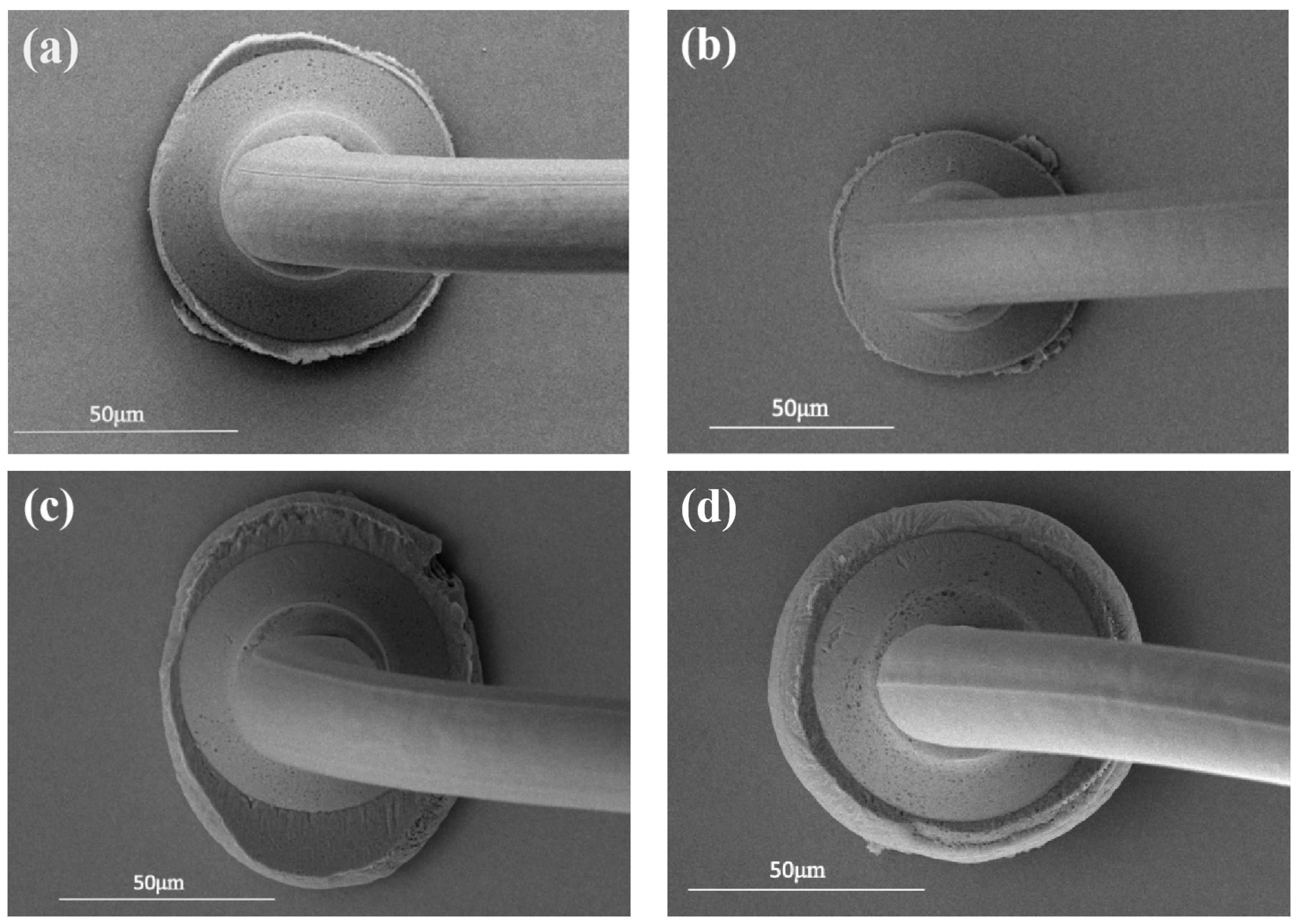
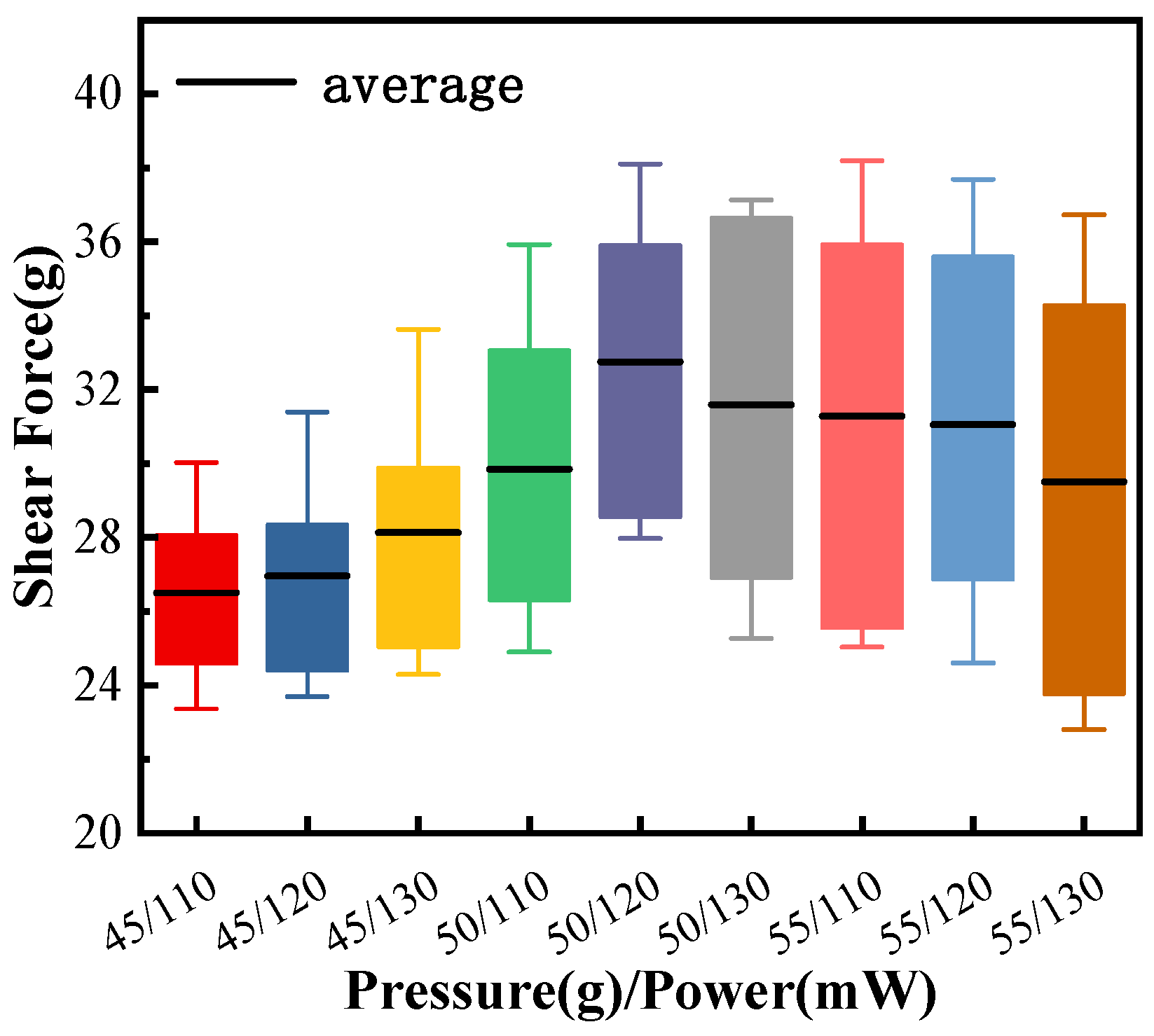
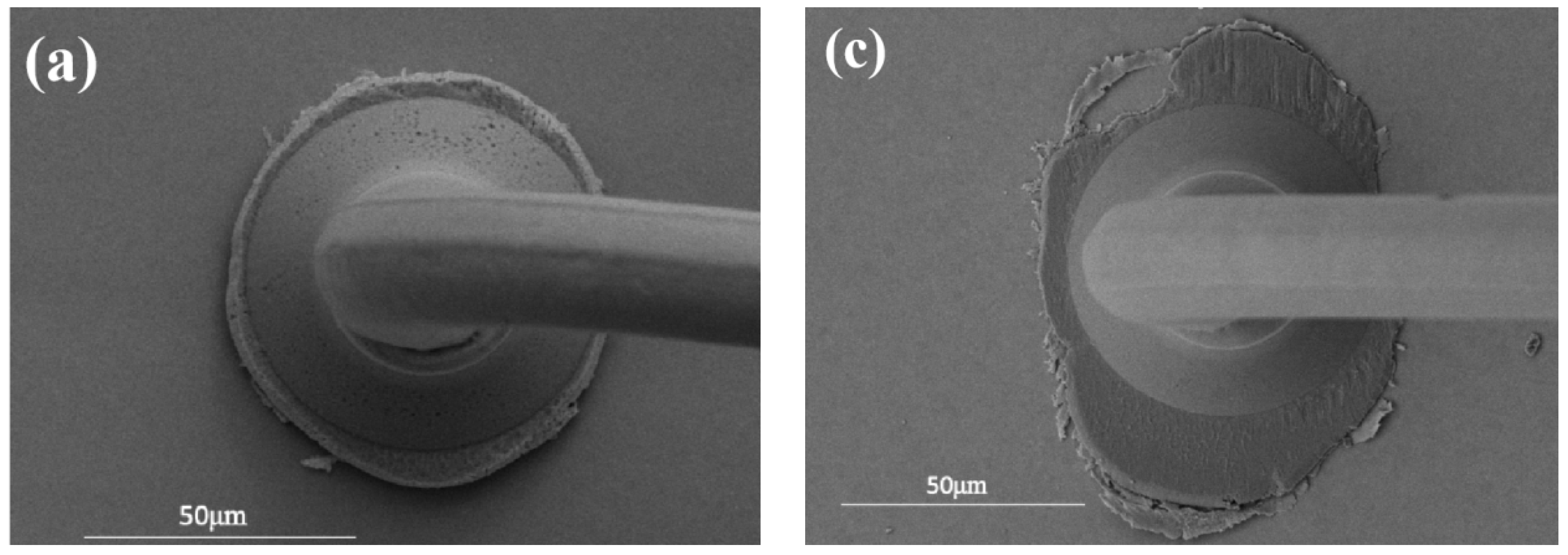
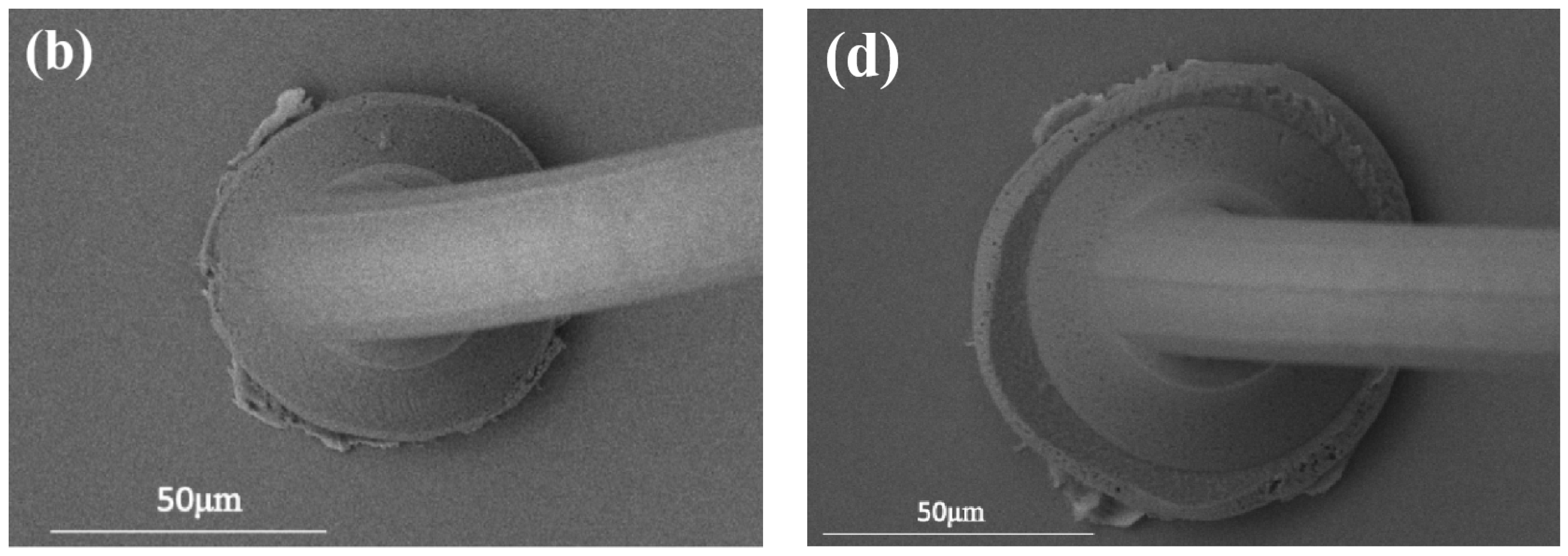
3.1. Study of Pd80 Bonding Parameters
3.2. Study of Pd100 Bonding Parameters
3.3. Study of Pd120 Bonding Parameters
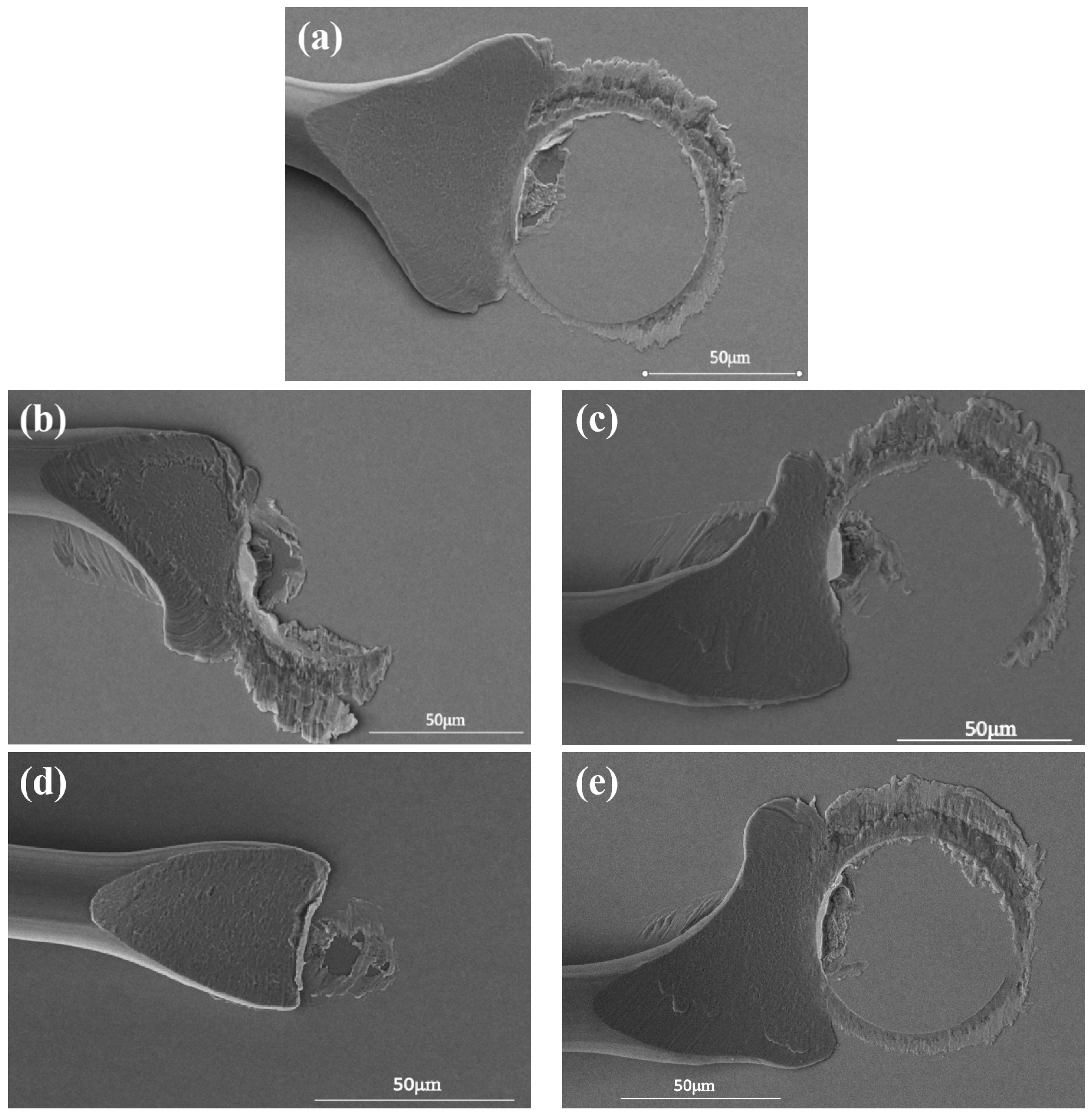
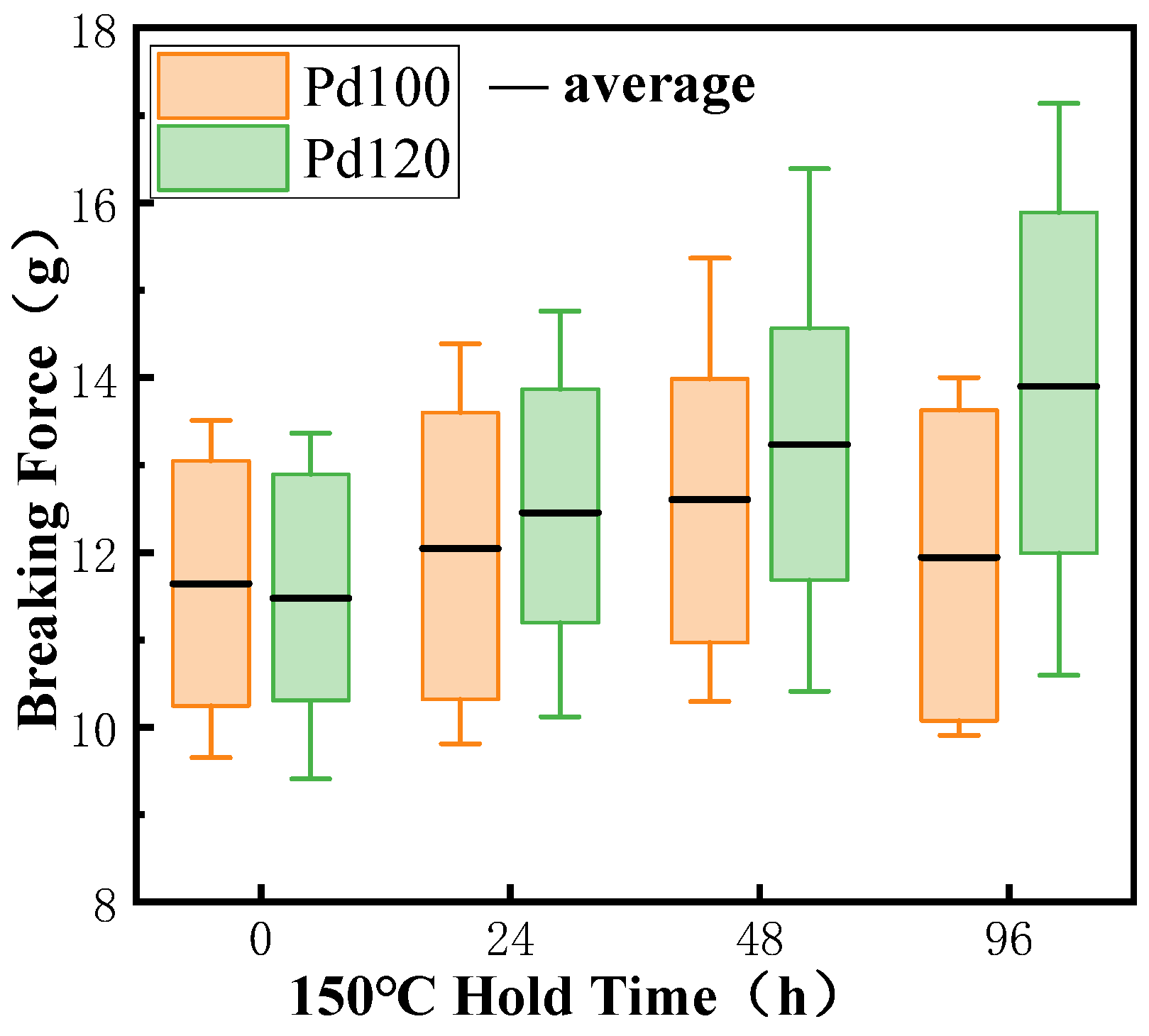
3.4. Effect of Pd-Layer Thickness on Bonding Reliability of Cu-Based Bonding Wire in High-Temperature Environment
3.5. Effect of Pd-Layer Thickness on Bonding Reliability of Cu-Based Bonding Wires in Cold/Hot-Cycle Test
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geissler, U.; Funck, J.; Schneider-Ramelow, M.; Engelmann, H.J.; Rooch, I.; Müller, W.H.; Reichl, H. Interface Formation in the US-Wedge/Wedge-Bond Process of AlSi1/CuNiAu Contacts. J. Electron. Mater. 2011, 40, 239–246. [Google Scholar] [CrossRef]
- Noh, B.-I.; Koo, J.-M.; Jo, J.-L.; Jung, S.-B. Application of Underfill for Flip-Chip Package Using Ultrasonic Bonding. Jpn. J. Appl. Phys. 2008, 47, 4257. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, C.; Lum, I.; Mayer, M.; Jung, J.P.; Zhou, Y. Investigation of ultrasonic copper wire wedge bonding on Au/Ni plated Cu substrates at ambient temperature. J. Mater. Process. Tech. 2007, 208, 179–186. [Google Scholar] [CrossRef]
- Murali, S.; Srikanth, N.; Vath, C.J. Effect of wire size on the formation of intermetallics and Kirkendall voids on thermal aging of thermosonic wire bonds. Mater. Lett. 2004, 58, 3096–3101. [Google Scholar] [CrossRef]
- Murali, S.; Srikanth, N.; Vath, C.J. An analysis of intermetallics formation of gold and copper ball bonding on thermal aging. Mater. Res. Bull. 2003, 38, 637–646. [Google Scholar] [CrossRef]
- Xu, H.; Liu, C.; Silberschmidt, V.V.; Chen, Z.; Wei, J. Initial bond formation in thermosonic gold ball bonding on aluminium metallization pads. J. Mater. Process. Technol. 2010, 210, 1035–1042. [Google Scholar] [CrossRef]
- Tian, Y.; Hang, C.; Wang, C.; Ouyang, G.; Yang, D.; Zhao, J. Reliability and failure analysis of fine copper wire bonds encapsulated with commercial epoxy molding compound. Microelectron. Reliab. 2011, 51, 157–165. [Google Scholar] [CrossRef]
- Toyozawa, K.; Fujita, K.; Minamide, S.; Maeda, T. Development of copper wire bonding application technology. IEEE Trans. Compon. Hybrids Manuf. Technol. 1990, 13, 667–672. [Google Scholar] [CrossRef]
- Murali, S.; Srikanth, N.; Vath, C.J. Grains, deformation substructures, and slip bands observed in thermosonic copper ball bonding. Mater. Charact. 2003, 50, 39–50. [Google Scholar] [CrossRef]
- Long, Z.; Han, L.; Wu, Y.; Zhong, J. Study of Temperature Parameter in Au–Ag Wire Bonding. IEEE Trans. Electron. Packag. Manuf. 2008, 31, 221–226. [Google Scholar] [CrossRef]
- Hang, C.J.; Wang, C.Q.; Mayer, M.; Tian, Y.H.; Zhou, Y.; Wang, H.H. Growth behavior of Cu/Al intermetallic compounds and cracks in copper ball bonds during isothermal aging. Microelectron. Reliab. 2008, 48, 416–424. [Google Scholar] [CrossRef]
- Yoshitaka, T.; Jian, L.; Russell, S.W.; Mayer, J.W. Thermal and ion beam induced thin film reactions in Cu-Al bilayers. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1992, 64, 130–133. [Google Scholar] [CrossRef]
- Kong, J.; Li, Y.; Gao, G.; Liu, Y.; Zhang, L.; Yan, X.; Zhu, X. Research on the Evolution of Gold Wire Bonding lnterface Used for Microwave Circuit lnter connection in High and Low Space Temperature. Aerosp. Mater. Technol. 2023, 53, 67–73. [Google Scholar]
- Du, Y. Influence of Palladium Additionon the Reliability of Copper Wire Bonding. Ph.D. Thesis, University of Science and Technology of China, Hefei, China, 2020. [Google Scholar]
- Ratchev, P.; Stoukatch, S.; Swinnen, B. Mechanical reliability of Au and Cu wire bonds to Al, Ni/Au and Ni/Pd/Au capped Cu bond pads. Microelectron. Reliab. 2006, 46, 1315–1325. [Google Scholar] [CrossRef]
- Onuki, J.; Koizumi, M.; Araki, I. Investigation of the Reliability of Copper Ball Bonds to Aluminum Electrodes. IEEE Trans. Compon. Hybrids Manuf. Technol. 1987, 10, 550–555. [Google Scholar] [CrossRef]
- XU, z.; Song, X.; Chen, Z. Advantages and Applications of Silver Plating Process in SMD-LED Packaging. Silicon Val. 2012, 5, 34–35. [Google Scholar]
- Li, J. Research on Cu based nanosolder for low temperature Cu-Cu bonding. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2017. [Google Scholar]
- Tian, Y.; Jiang, Z.; Wang, C.; Ding, S.; Wen, J.; Liu, Z.; Wang, C. Sintering mechanism of the Cu–Ag core–shell nanoparticle paste at low temperature in ambient air. RSC Adv. 2016, 6, 91783–91790. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hwang, W.S. Effect of Annealing on the Interfacial Structure of Aluminum-Copper Joints. Mater. Trans. 2007, 48, 1938–1947. [Google Scholar] [CrossRef]
- Lim, A.B.; Long, X.; Shen, L.; Chen, X.; Ramanujan, R.V.; Gan, C.L.; Chen, Z. Effect of palladium on the mechanical properties of Cu–Al intermetallic compounds. J. Alloy. Compd. 2015, 628, 107–112. [Google Scholar] [CrossRef]
- Tavassoli, S.; Abbasi, M.; Tahavvori, R. Controlling of IMCs layers formation sequence, bond strength and electrical resistance in AlCu bimetal compound casting process. Mater. Des. 2016, 108, 343–353. [Google Scholar] [CrossRef]
- Xu, H.; Liu, C.; Silberschmidt, V.V.; Chen, Z.; Wei, J.; Sivakumar, M. Effect of bonding duration and substrate temperature in copper ball bonded on aluminium pads: A TEM study of interfacial evolution. Microelectron. Reliab. 2011, 51, 113–118. [Google Scholar] [CrossRef]
- Lim, A.B.; Neo, W.J.; Yauw, O.; Chylak, B.; Gan, C.L.; Chen, Z. Evaluation of the corrosion performance of Cu–Al intermetallic compounds and the effect of Pd addition. Microelectron. Reliab. 2016, 56, 155–161. [Google Scholar] [CrossRef]
- Park, H.-W.; Cho, D.-C.; Lee, S.-H.; Kim, J.-K.; Lee, J.-H.; Jung, S.-K.; Nam, H.-S.; Hsu, P.; Low, S.; Lim, S.-H. Behavior of Au and Pd and the effects of these metals on IMCs in Pd-Au-coated copper wire. Microelectron. Reliab. 2018, 91, 283–290. [Google Scholar] [CrossRef]
- Na, S.; Hwang, T.; Park, J.; Kim, J.; Yoo, H.; Lee, C. Characterization of intermetallic compound (IMC) growth in Cu wire ball bonded on Al pad metallization. In Proceedings of the 2011 IEEE 61st Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 31 May–3 June 2011. [Google Scholar]
- Qin, I.; Xu, H.; Clauberg, H.; Cathcart, R.; Acoff, V.L.; Chylak, B.; Huynh, C. Wire bonding of Cu and Pd coated Cu wire: Bondability, reliability, and IMC formation. In Proceedings of the 2011 IEEE 61st Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 31 May–3 June 2011. [Google Scholar]
- Yamaji, Y.; Hori, M.; Ikenosako, H.; Oshima, Y.; Suda, T.; Umeki, A.; Kandori, M.; Oida, M.; Goto, H.; Katsumata, A.; et al. IMC study on Cu wirebond failures under high humidity conditions. In Proceedings of the 2011 IEEE 13th Electronics Packaging Technology Conference, Singapore, 7–9 December 2011. [Google Scholar]
- Lim, A.B.; Boothroyd, C.B.; Yauw, O.; Chylak, B.; Gan, C.L.; Chen, Z. Interfacial evolution and bond reliability in thermosonic Pd coated Cu wire bonding on aluminum metallization: Effect of palladium distribution. Microelectron. Reliab. 2016, 63, 214–223. [Google Scholar] [CrossRef]
- Abe, H.; Kang, D.C.; Yamamoto, T.; Yagihashi, T.; Endo, Y.; Saito, H.; Horie, T.; Tamate, H.; Ejiri, Y.; Watanabe, N.; et al. Cu wire and Pd-Cu wire package reliability and molding compounds. In Proceedings of the 2012 IEEE 62nd Electronic Components and Technology Conference, San Diego, CA, USA, 29 May–1 June 2012. [Google Scholar]
- Lee CC, S.; Tran, T.; Boyne, D.; Higgins, L.; Mawer, A. Copper versus palladium coated copper wire process and reliability differences. In Proceedings of the 2014 IEEE 64th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 27–30 May 2014. [Google Scholar]
- Xu, H.; Qin, I.; Clauberg, H.; Chylak, B.; Acoff, V.L. Behavior of palladium and its impact on intermetallic growth in palladium-coated Cu wire bonding. Acta Mater. 2013, 61, 79–88. [Google Scholar] [CrossRef]
- Schneider-Ramelow, M.; Geißler, U.; Schmitz, S.; Grübl, W.; Schuch, B. Development and Status of Cu Ball/wedge bonded in 2012. J. Electron. Mater. 2013, 42, 558–595. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wang, Y.W.; Appelt, B.K.; Lai, Y.S.; Kao, C.R. Growth of CuAl intermetallic compounds in Cu and Cu(Pd) wire bonding. In Proceedings of the 2011 IEEE 61st Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 31 May–3 June 2011. [Google Scholar]
- Lin, Y.; Ke, W.; Wang, R.; Wang, I.; Chiu, Y.; Lu, K.; Lin, K.; Lai, Y. The influence of Pd on the interfacial reactions between the PCC ball bond and Al pad. Surf. Coat. Technol. 2013, 231, 599–603. [Google Scholar] [CrossRef]
- Xu, H.; Qin, I.; Shah, A.; Clauberg, H.; Chylak, B.; Acoff, V.L. TEM study on interface of palladium coated copper wire bonding on aluminum metallization. In Proceedings of the 2012 13th International Conference on Electronic Packaging Technology & High Density Packaging, Guilin, China, 13–16 August 2012. [Google Scholar]




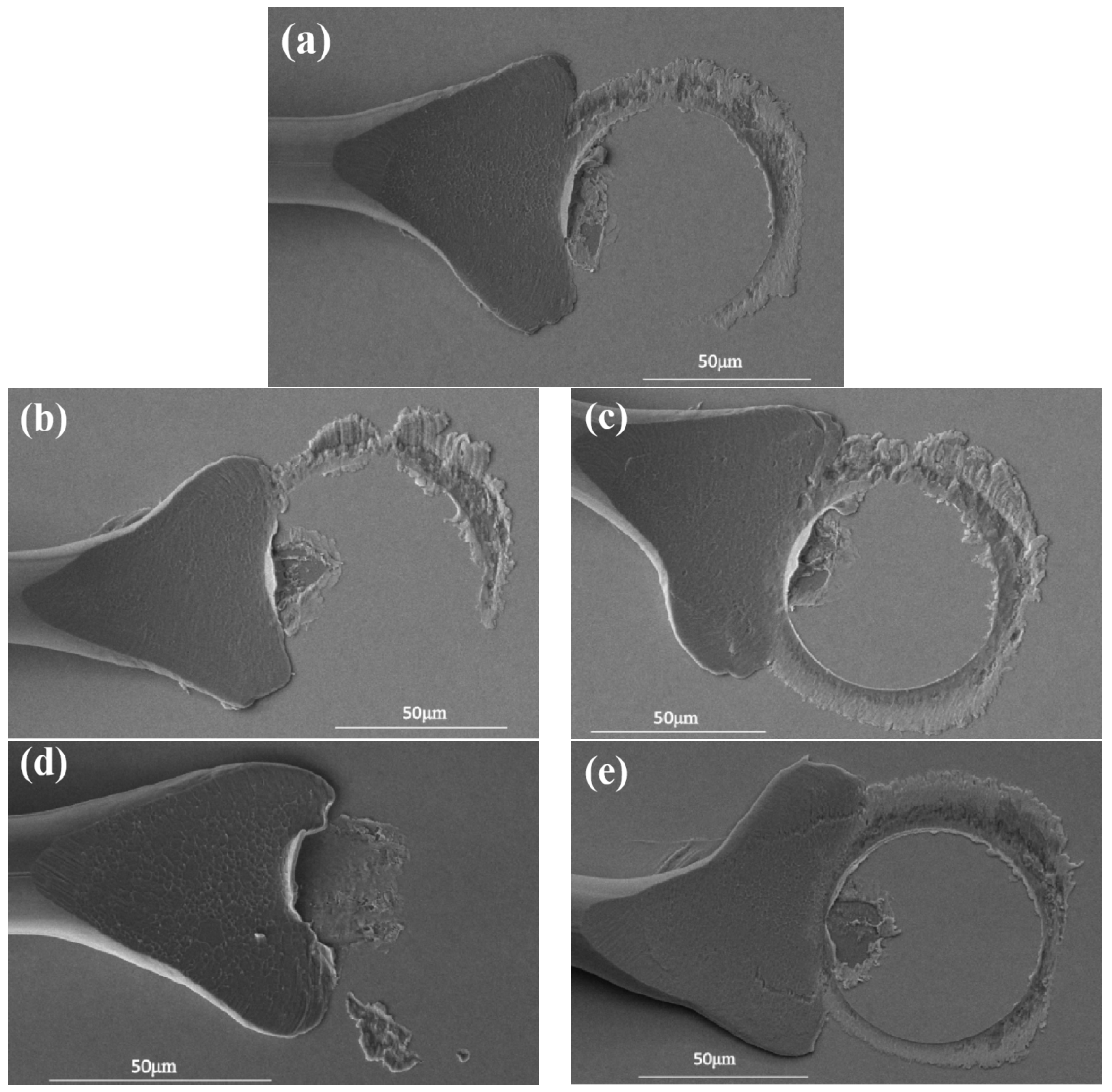


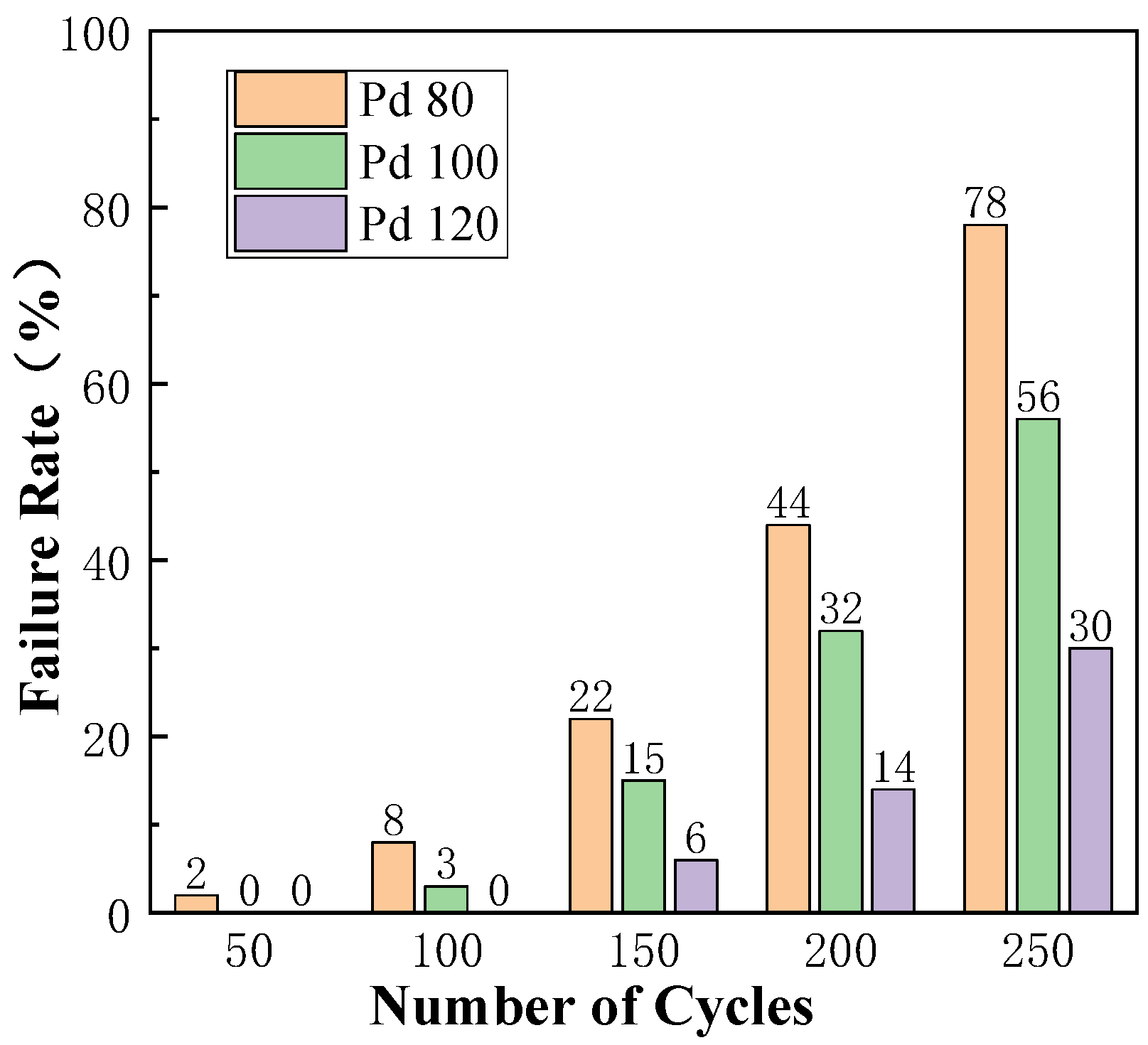
| Number | Bonding Pressure/g | Ultrasonic Power/mW |
|---|---|---|
| 1 | 40 | 95 |
| 2 | 40 | 105 |
| 3 | 40 | 115 |
| 4 | 45 | 95 |
| 5 | 45 | 105 |
| 6 | 45 | 115 |
| 7 | 50 | 95 |
| 8 | 50 | 105 |
| 9 | 50 | 115 |
| Number | Bonding Pressure/g | Ultrasonic Power/mW |
|---|---|---|
| 1 | 45 | 100 |
| 2 | 45 | 110 |
| 3 | 45 | 120 |
| 4 | 50 | 100 |
| 5 | 50 | 110 |
| 6 | 50 | 120 |
| 7 | 55 | 100 |
| 8 | 55 | 110 |
| 9 | 55 | 120 |
| Number | Bonding Pressure/g | Ultrasonic Power/mW |
|---|---|---|
| 1 | 50 | 105 |
| 2 | 50 | 115 |
| 3 | 50 | 125 |
| 4 | 55 | 105 |
| 5 | 55 | 115 |
| 6 | 55 | 125 |
| 7 | 60 | 105 |
| 8 | 60 | 115 |
| 9 | 60 | 125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Yuan, D.; Du, J.; Hou, T.; Wang, F.; Cao, J.; Yang, X.; Zhang, Y. Influence of Pd-Layer Thickness on Bonding Reliability of Pd-Coated Cu Wire. Micromachines 2024, 15, 931. https://doi.org/10.3390/mi15070931
Fan J, Yuan D, Du J, Hou T, Wang F, Cao J, Yang X, Zhang Y. Influence of Pd-Layer Thickness on Bonding Reliability of Pd-Coated Cu Wire. Micromachines. 2024; 15(7):931. https://doi.org/10.3390/mi15070931
Chicago/Turabian StyleFan, Junling, Donglin Yuan, Juan Du, Tao Hou, Furong Wang, Jun Cao, Xuemei Yang, and Yuemin Zhang. 2024. "Influence of Pd-Layer Thickness on Bonding Reliability of Pd-Coated Cu Wire" Micromachines 15, no. 7: 931. https://doi.org/10.3390/mi15070931





