Development of a Microbioreactor for Bacillus subtilis Biofilm Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. B. subtilis Growth Media
2.3. B. subtilis Cultivation in Shaken Cultures
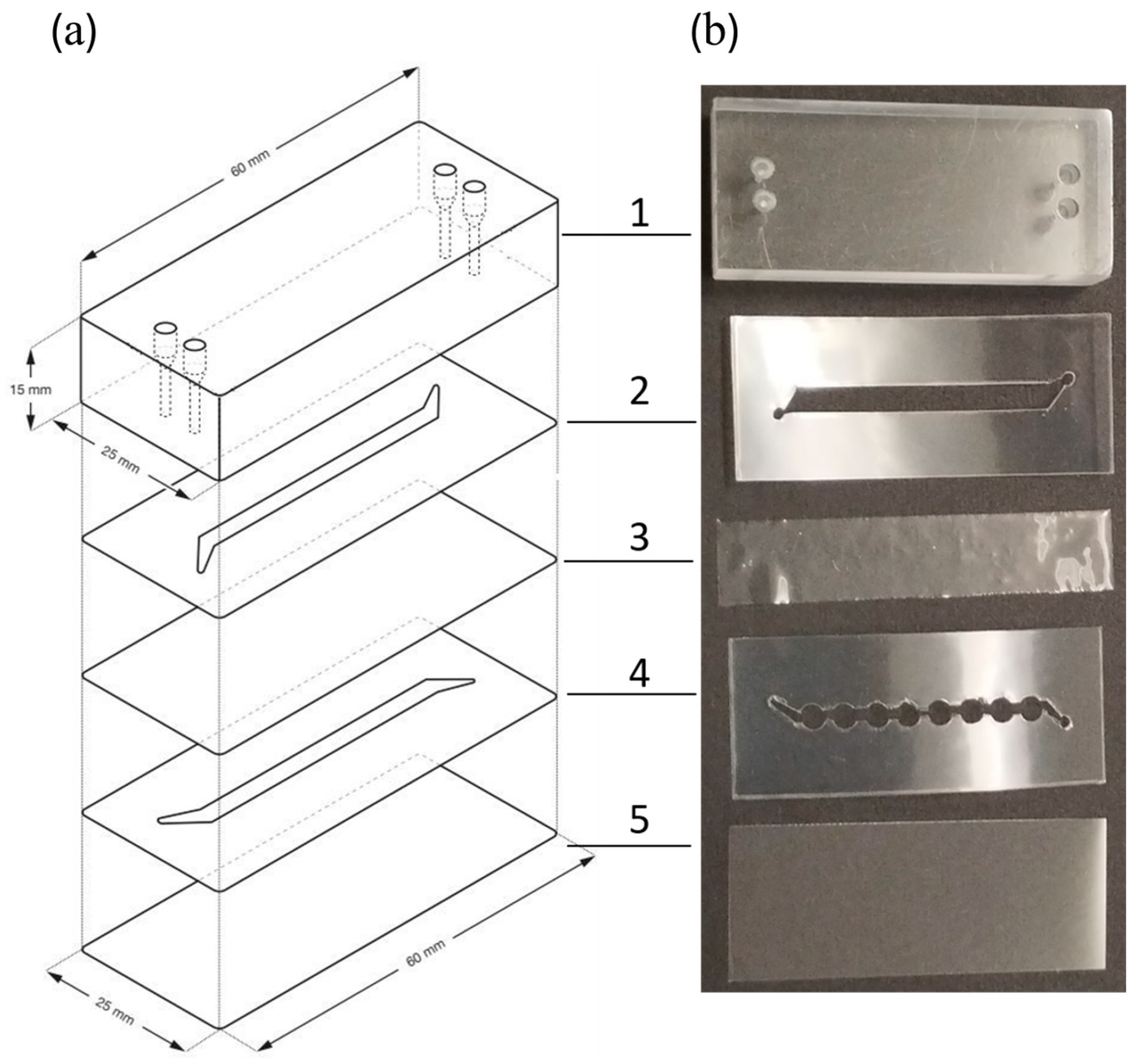
2.4. Microbioreactor Components
2.5. Determination of the Microbioreactor Volume
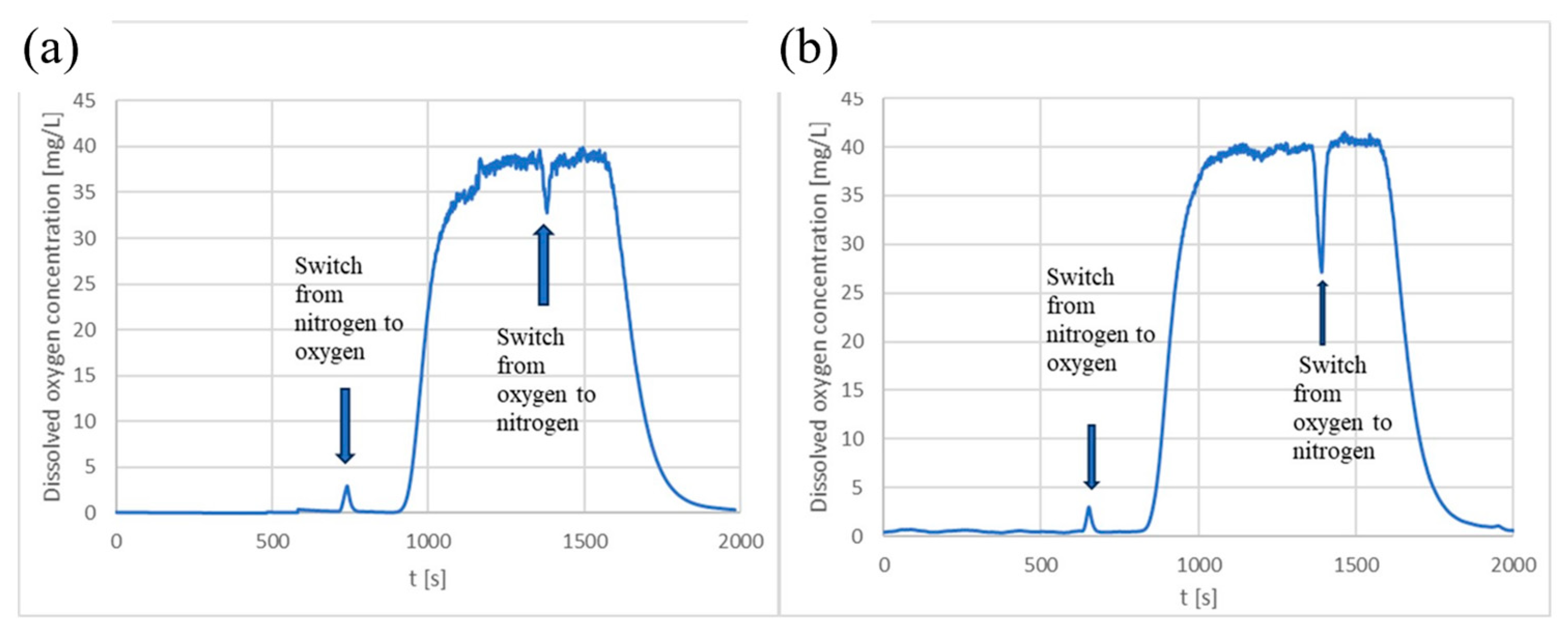
2.6. Evaluation of Oxygen Transfer within the Microbioreactor
2.7. Inoculation and Cultivation of B. subtilis in the Microbioreactor
2.8. Analysis of Biofilm Coverage, Statistics, and Cell Morphology in the Biofilm
3. Results and Discussion
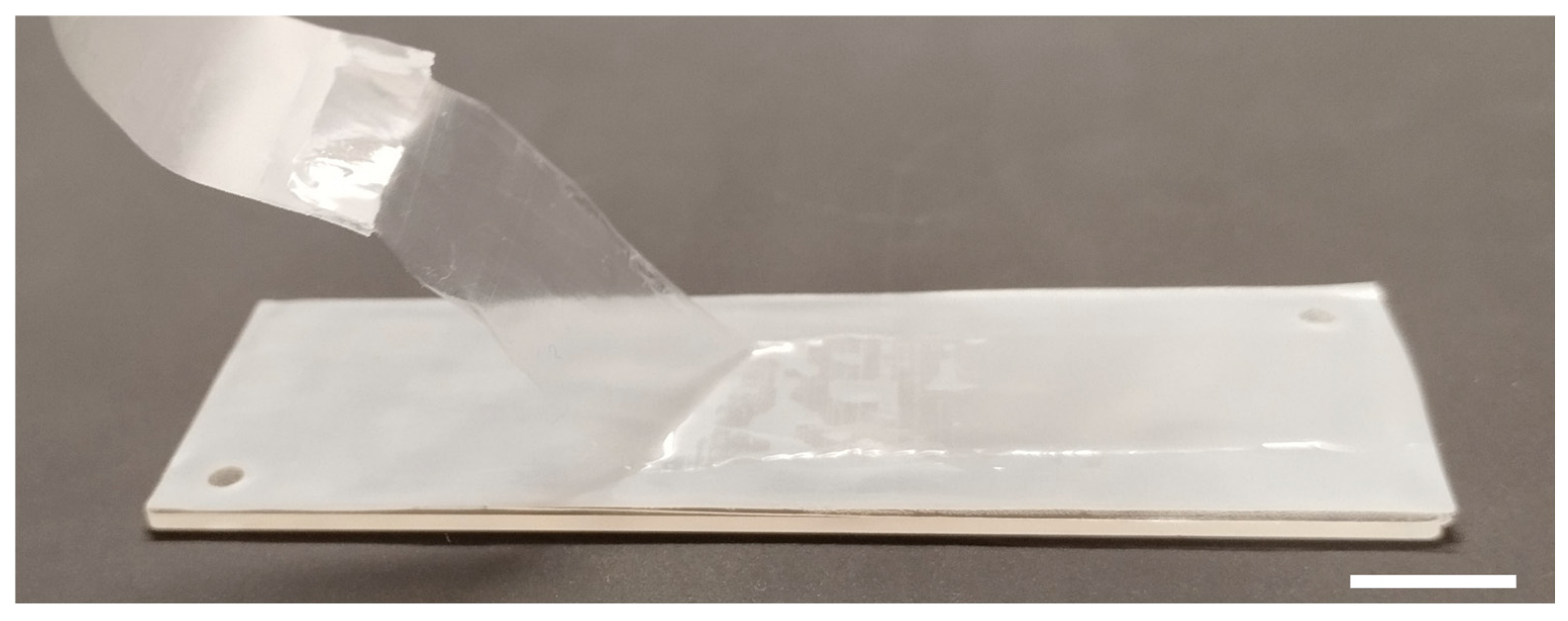
3.1. Development of a Microbioreactor
3.2. Characterization of Oxygen Transfer Rate within the Microbioreactor
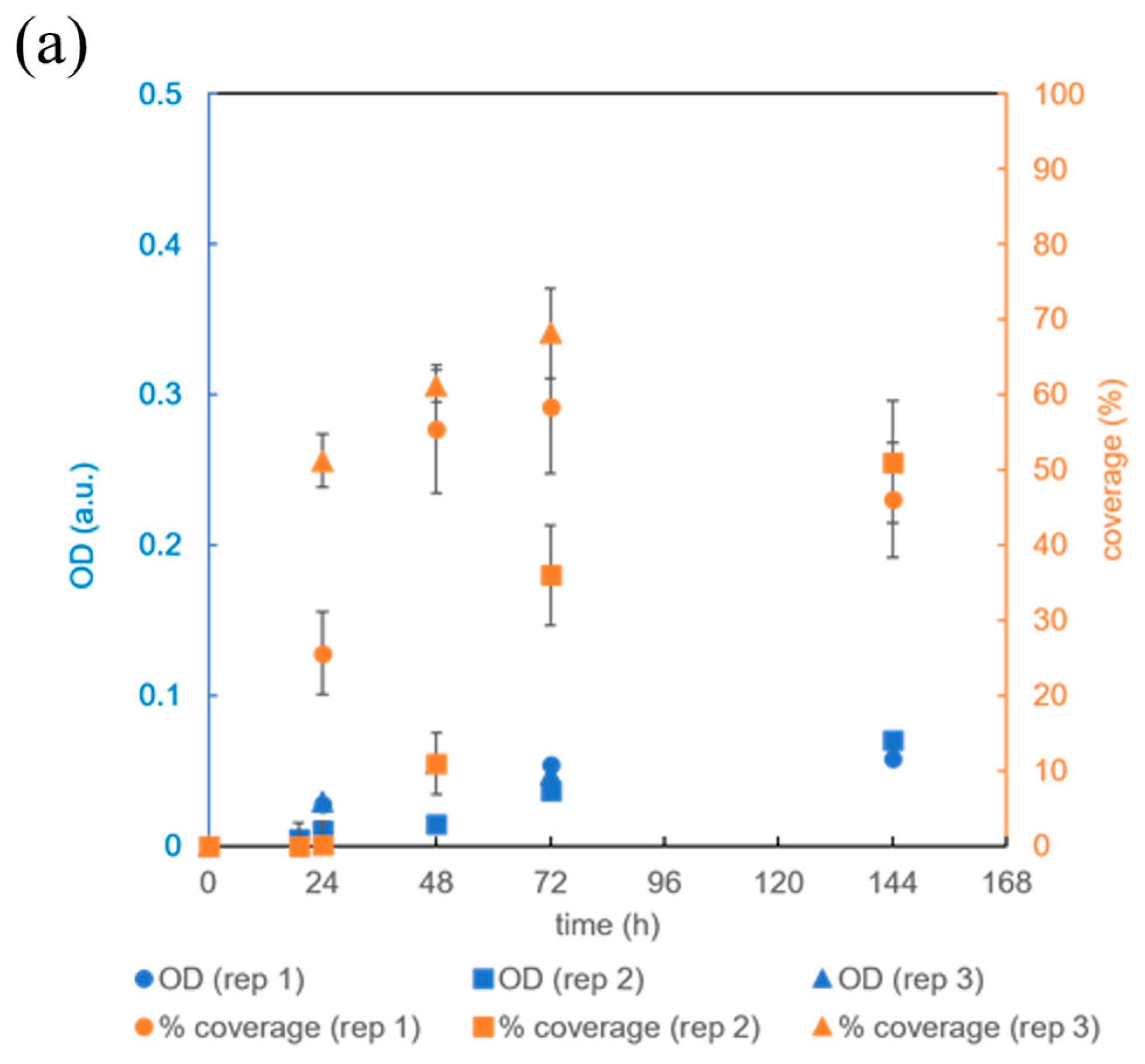
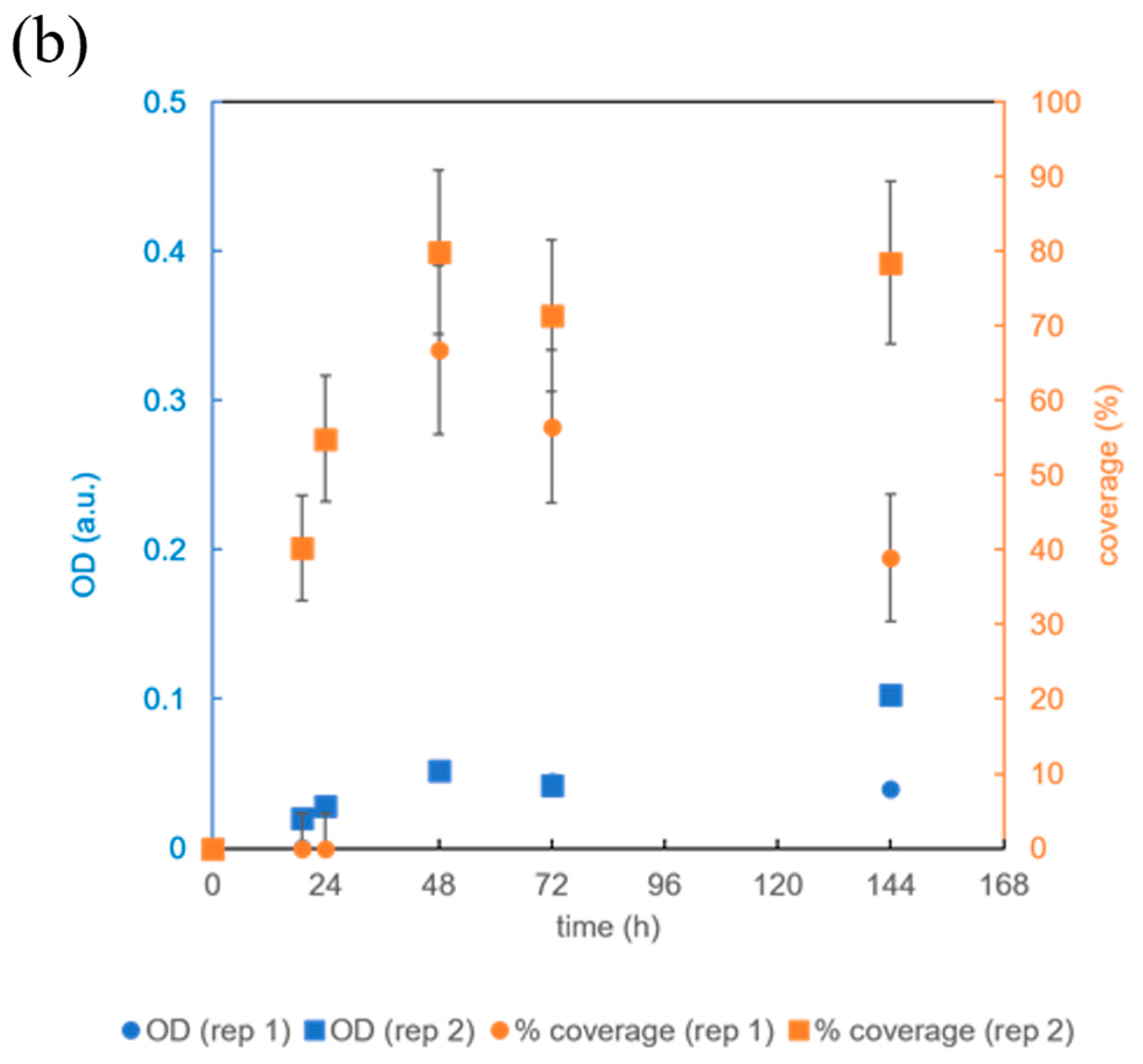
3.3. Testing the Microbioreactor’s Ability to Support Biofilm Growth
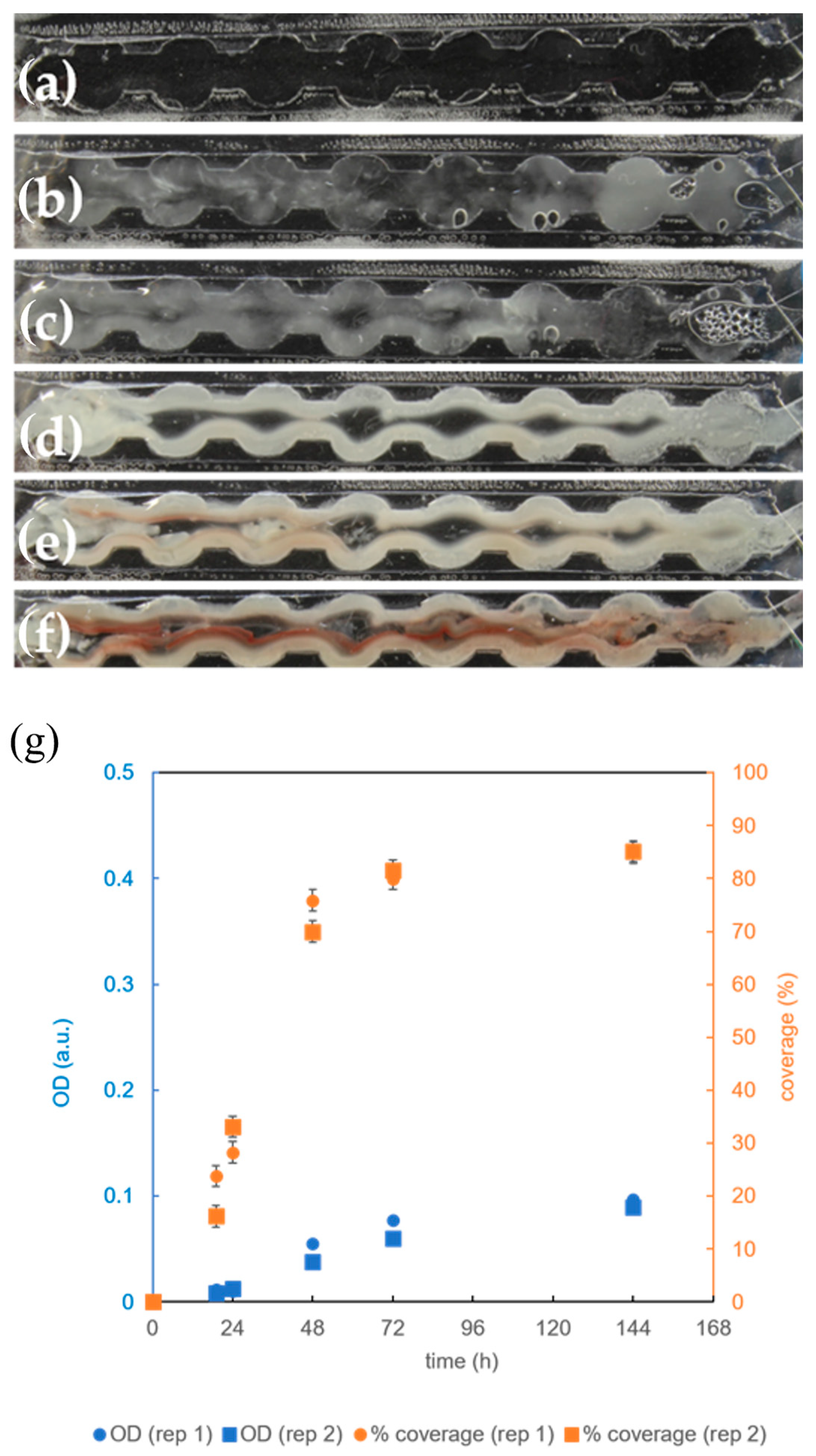
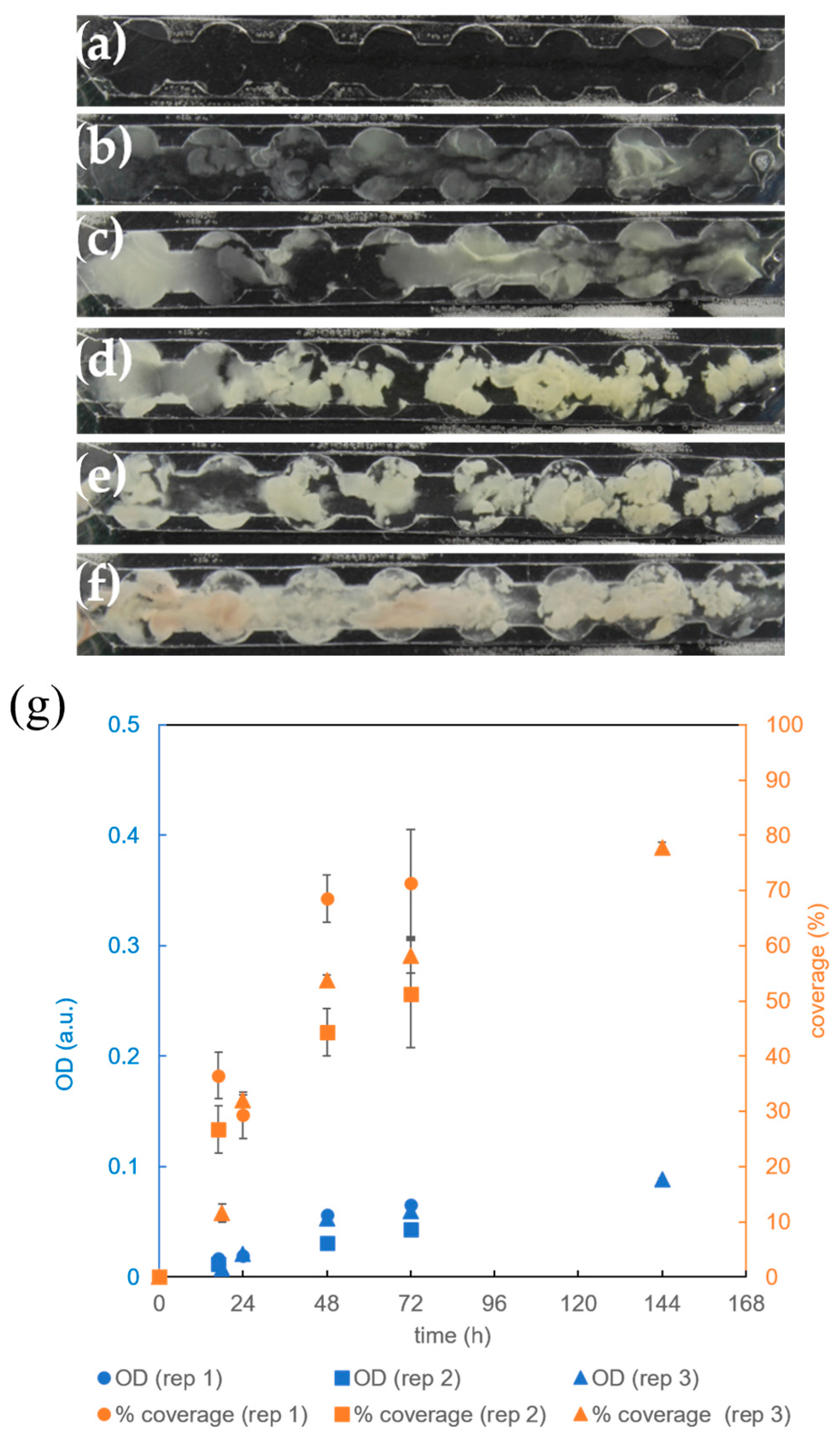
3.4. The Effect of Microbioreactor Geometry on B. subtilis Biofilm Cultivation
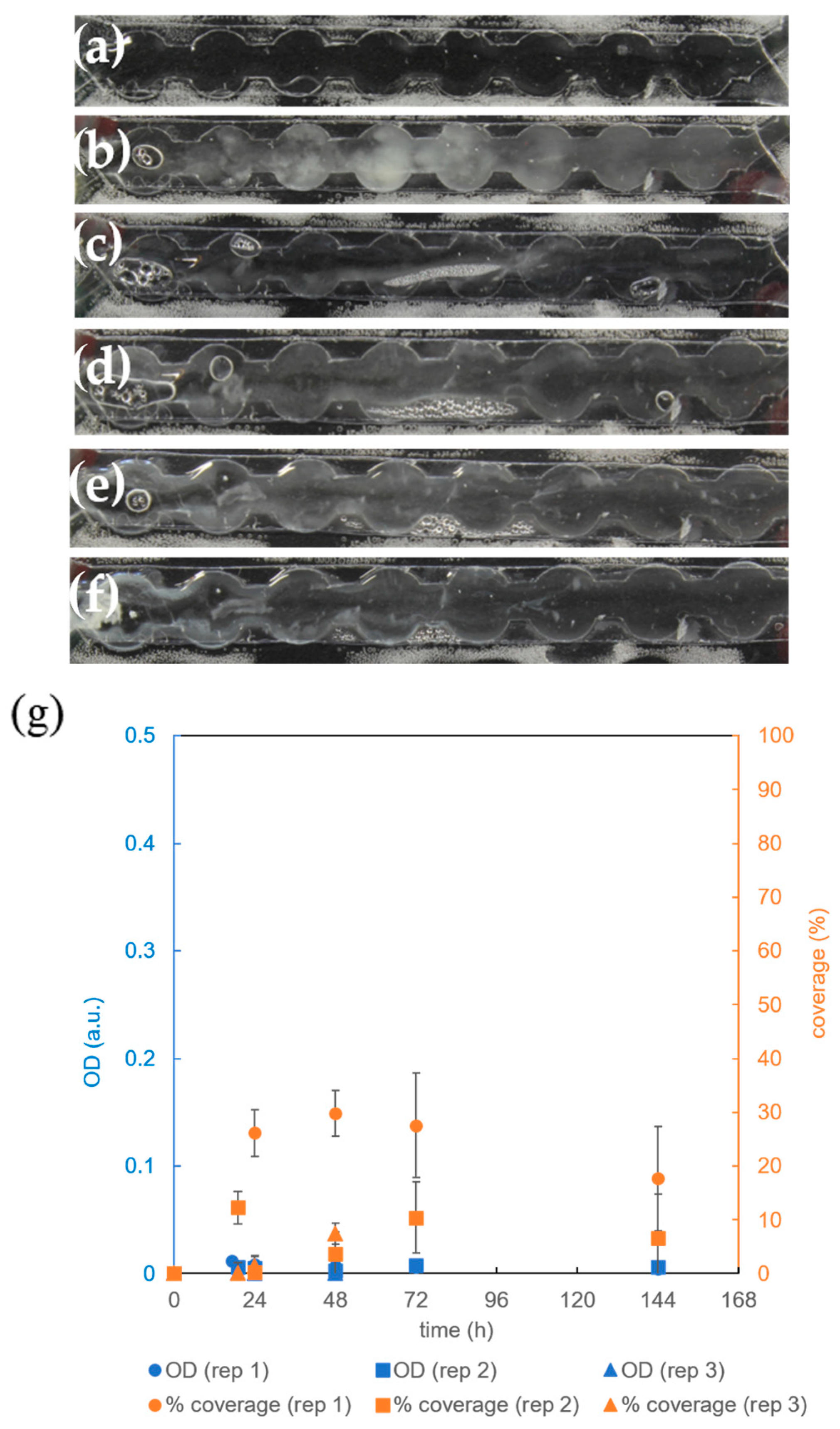
3.5. The Effect of Aeration on B. subtilis Biofilm Cultivation in the Microbioreactor with Circular Extensions
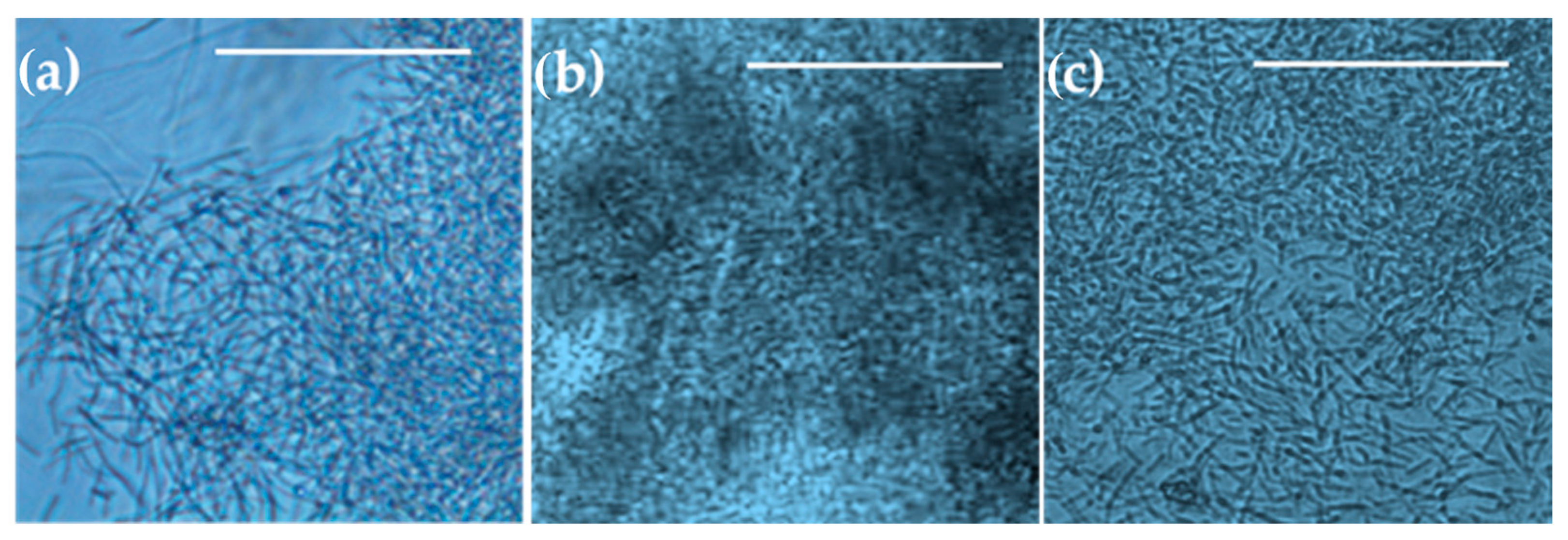
3.6. The Microscopic Properties of B. subtilis Biofilm Cultivated in a Microbioreactor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Žnidaršič-Plazl, P.; Plazl, I. Microbioreactors. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, pp. 414–427. ISBN 978-0-444-64047-5. [Google Scholar] [CrossRef]
- Wohlgemuth, R.; Plazl, I.; Žnidaršič-Plazl, P.; Gernaey, K.V.; Woodley, J.M. Microscale Technology and Biocatalytic Processes: Opportunities and Challenges for Synthesis. Trends Biotechnol. 2015, 33, 302–314. [Google Scholar] [CrossRef]
- Žnidaršič-Plazl, P. Biotransformations in Microflow Systems: Bridging the Gap between Academia and Industry. J. Flow Chem. 2017, 7, 111–117. [Google Scholar] [CrossRef]
- Rivet, C.; Lee, H.; Hirsch, A.; Hamilton, S.; Lu, H. Microfluidics for Medical Diagnostics and Biosensors. Chem. Eng. Sci. 2011, 66, 1490–1507. [Google Scholar] [CrossRef]
- Bolješić, M.; Kraigher, B.; Dogsa, I.; Jerič Kokelj, B.; Mandic-Mulec, I. Kin Discrimination Modifies Strain Distribution, Spatial Segregation, and Incorporation of Extracellular Matrix Polysaccharide Mutants of Bacillus subtilis Strains into Mixed Floating Biofilms. Appl. Environ. Microbiol. 2022, 88, e00871-22. [Google Scholar] [CrossRef] [PubMed]
- Dogsa, I.; Mandic-Mulec, I. Multiscale Spatial Segregation Analysis in Digital Images of Biofilms. Biofilm 2023, 6, 100157. [Google Scholar] [CrossRef]
- Dragoš, A.; Lakshmanan, N.; Martin, M.; Horváth, B.; Maróti, G.; Falcón García, C.; Lieleg, O.; Kovács, Á.T. Evolution of Exploitative Interactions during Diversification in Bacillus subtilis Biofilms. FEMS Microbiol. Ecol. 2018, 94, fix155. [Google Scholar] [CrossRef]
- Perkins, J.B.; Sloma, A.; Hermann, T.; Theriault, K.; Zachgo, E.; Erdenberger, T.; Hannett, N.; Chatterjee, N.P.; Williams, V., II; Rufo, G., Jr.; et al. Genetic Engineering of Bacillus subtilis for the Commercial Production of Riboflavin. J. Ind. Microbiol. Biotechnol. 1999, 22, 8–18. [Google Scholar] [CrossRef]
- Karande, R.; Schmid, A.; Buehler, K. Applications of Multiphasic Microreactors for Biocatalytic Reactions. Org. Process Res. Dev. 2016, 20, 361–370. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. Relevance of Microbial Extracellular Polymeric Substances (EPSs)—Part I: Structural and Ecological Aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J. Overview of microbial biofilms. J. Ind. Microbiol. 1995, 15, 137–140. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and Archaea on Earth and their Abundance in Biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Vlamakis, H.; Chai, Y.; Beauregard, P.; Losick, R.; Kolter, R. Sticking Together: Building a Biofilm the Bacillus subtilis Way. Nat. Rev. Microbiol. 2013, 11, 157–168. [Google Scholar] [CrossRef]
- Morikawa, M. Beneficial Biofilm Formation by Industrial Bacteria Bacillus subtilis and Related Species. J. Biosci. Bioeng. 2006, 101, 1–8. [Google Scholar] [CrossRef]
- Berlanga, M.; Guerrero, R. Living together in biofilms: The microbial cell factory and its biotechnological implications. Microb. Cell Factories 2016, 15, 165. [Google Scholar] [CrossRef]
- Arnaouteli, S.; Bamford, N.C.; Stanley-Wall, N.R.; Kovács, Á.T. Bacillus subtilis biofilm formation and social interactions. Nat. Rev. Microbiol. 2021, 19, 600–614. [Google Scholar] [CrossRef]
- Krajnc, M.; Stefanic, P.; Kostanjšek, R.; Mandic-Mulec, I.; Dogsa, I.; Stopar, D. Systems View of Bacillus subtilis Pellicle Development. NPJ Biofilms Microbiomes 2022, 8, 25. [Google Scholar] [CrossRef]
- Sanchez-Vizuete, P.; Dergham, Y.; Bridier, A.; Deschamps, J.; Dervyn, E.; Hamze, K.; Aymerich, S.; Le Coq, D.; Briandet, R. The Coordinated Population Redistribution between Bacillus subtilis Submerged Biofilm and Liquid-Air Pellicle. Biofilm 2022, 4, 100065. [Google Scholar] [CrossRef]
- Podnar, E.; Erega, A.; Danevčič, T.; Kovačec, E.; Lories, B.; Steenackers, H.; Mandic-Mulec, I. Nutrient Availability and Biofilm Polysaccharide Shape the Bacillaene-Dependent Antagonism of Bacillus subtilis against Salmonella Typhimurium. Microbiol. Spectr. 2022, 10, e01836-22. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Corral, R.; Liu, J.; Süel, G.M.; Garcia-Ojalvo, J. Bistable Emergence of Oscillations in Growing Bacillus subtilis Biofilms. Proc. Natl. Acad. Sci. USA 2018, 115, E8333–E8340. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dong, F.; Zhang, D.; Zhang, J.; Wang, X. Effect of Microfluidic Channel Geometry on Bacillus subtilis Biofilm Formation. Biomed. Microdevices 2022, 24, 11. [Google Scholar] [CrossRef]
- Menegatti, T.; Žnidaršič-Plazl, P. Copolymeric Hydrogel-Based Immobilization of Yeast Cells for Continuous Biotransformation of Fumaric Acid in a Microreactor. Micromachines 2019, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Menegatti, T.; Žnidaršič-Plazl, P. Hydrogel-Based Enzyme and Cofactor Co-Immobilization for Efficient Continuous Transamination in a Microbioreactor. Front. Bioeng. Biotechnol. 2021, 9, 752064. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Klimant, I. Optical Nanosensors—Smart Tools in Bioanalytics. Analyst 2008, 133, 1302. [Google Scholar] [CrossRef]
- Ehgartner, D.; Fricke, J.; Schröder, A.; Herwig, C. At-Line Determining Spore Germination of Penicillium chrysogenum Bioprocesses in Complex Media. Appl. Microbiol. Biotechnol. 2016, 100, 8923–8930. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Dogsa, I.; Spacapan, M.; Dragoš, A.; Danevčič, T.; Pandur, Ž.; Mandic-Mulec, I. Peptide Signaling without Feedback in Signal Production Operates as a True Quorum Sensing Communication System in Bacillus subtilis. Commun. Biol. 2021, 4, 58. [Google Scholar] [CrossRef]
- Grant, C.L.; Pramer, D. Minor element composition of yeast extract. J. Bacteriol. 1962, 84, 869–870. [Google Scholar] [CrossRef]
- Uffen, R.L.; Canale-Parola, E. Synthesis of Pulcherriminic Acid by Bacillus subtilis. J. Bacteriol. 1972, 111, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Moeller, R.; Horneck, G.; Facius, R.; Stackebrandt, E. Role of Pigmentation in Protecting Bacillus sp. Endospores against Environmental UV Radiation. FEMS Microbiol. Ecol. 2005, 51, 231–236. [Google Scholar] [CrossRef]
- Angelini, L.L.; Dos Santos, R.A.C.; Fox, G.; Paruthiyil, S.; Gozzi, K.; Shemesh, M.; Chai, Y. Pulcherrimin Protects Bacillus subtilis against Oxidative Stress during Biofilm Development. Npj Biofilms Microbiomes 2023, 9, 50. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.-D.; Chung, S. Microfluidic Approaches to Bacterial Biofilm Formation. Molecules 2012, 17, 9818–9834. [Google Scholar] [CrossRef]
- Alotaibi, G.F. Factors Influencing Bacterial Biofilm Formation and Development. Am. J. Biomed. Sci. Res. 2021, 12, 617–626. [Google Scholar] [CrossRef]
- Yawata, Y.; Nguyen, J.; Stocker, R.; Rusconi, R. Microfluidic Studies of Biofilm Formation in Dynamic Environments. J. Bacteriol. 2016, 198, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Troup, B.; Szabo, A.; Hungerer, C.; Jahn, D. The Anaerobic Life of Bacillus subtilis: Cloning of the Genes Encoding the Respiratory Nitrate Reductase System. FEMS Microbiol. Lett. 1995, 131, 219–225. [Google Scholar] [CrossRef]
- Nakano, M.M.; Zuber, P. Anaerobic growth of a “strict aerobe” (Bacillus subtilis). Annu. Rev. Microbiol. 1998, 52, 165–190. [Google Scholar] [CrossRef]
- Zhang, W.; Phillips, G.N. Structure of the Oxygen Sensor in Bacillus subtilis. Structure 2003, 11, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Baez, A.; Shiloach, J. Effect of Elevated Oxygen Concentration on Bacteria, Yeasts, and Cells Propagated for Production of Biological Compounds. Microb. Cell Factories 2014, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Špacapan, M.; Danevčič, T.; Štefanic, P.; Porter, M.; Stanley-Wall, N.R.; Mandic-Mulec, I. The ComX Quorum Sensing Peptide of Bacillus subtilis Affects Biofilm Formation Negatively and Sporulation Positively. Microorganisms 2020, 8, 1131. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Vlamakis, H.; Losick, R.; Kolter, R. Paracrine Signaling in a Bacterium. Genes Dev. 2009, 23, 1631–1638. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Lopez, D.; Vlamakis, H.; Kolter, R. Generation of Multiple Cell Types in Bacillus subtilis. FEMS Microbiol. Rev. 2009, 33, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Angelini, L.L.; Chai, Y. Bacillus subtilis Cell Differentiation, Biofilm Formation and Environmental Prevalence. Microorganisms 2022, 10, 1108. [Google Scholar] [CrossRef] [PubMed]
- Dogsa, I.; Brloznik, M.; Stopar, D.; Mandic-Mulec, I. Exopolymer Diversity and the Role of Levan in Bacillus subtilis Biofilms. PLoS ONE 2013, 8, e62044. [Google Scholar] [CrossRef]
- Kobayashi, K.; Iwano, M. BslA(YuaB) forms a hydrophobic layer on the surface of Bacillus subtilis biofilms. Mol. Microbiol. 2012, 85, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, F.; Chen, Y.; Jin, C.; Guo, J.H.; Chai, Y. Poly-γ-Glutamic Acids Contribute to Biofilm Formation and Plant Root Colonization in Selected Environmental Isolates of Bacillus subtilis. Front. Microbiol. 2016, 7, 1811. [Google Scholar] [CrossRef]


| Liquid flow rate [µL/min] | 5 | 10 |
| [mg/(L s)] | 0.279 ± 0.012 | 0.285 ± 0.001 |
| Microreactor Growth Channel | Volume [µL] | Single Surface Area [mm2] |
|---|---|---|
| without circular extensions | 17 ± 4 | 73 ± 1 |
| with circular extensions | 28 ± 4 | 112 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seručnik, M.; Dogsa, I.; Zadravec, L.J.; Mandic-Mulec, I.; Žnidaršič-Plazl, P. Development of a Microbioreactor for Bacillus subtilis Biofilm Cultivation. Micromachines 2024, 15, 1037. https://doi.org/10.3390/mi15081037
Seručnik M, Dogsa I, Zadravec LJ, Mandic-Mulec I, Žnidaršič-Plazl P. Development of a Microbioreactor for Bacillus subtilis Biofilm Cultivation. Micromachines. 2024; 15(8):1037. https://doi.org/10.3390/mi15081037
Chicago/Turabian StyleSeručnik, Mojca, Iztok Dogsa, Lan Julij Zadravec, Ines Mandic-Mulec, and Polona Žnidaršič-Plazl. 2024. "Development of a Microbioreactor for Bacillus subtilis Biofilm Cultivation" Micromachines 15, no. 8: 1037. https://doi.org/10.3390/mi15081037
APA StyleSeručnik, M., Dogsa, I., Zadravec, L. J., Mandic-Mulec, I., & Žnidaršič-Plazl, P. (2024). Development of a Microbioreactor for Bacillus subtilis Biofilm Cultivation. Micromachines, 15(8), 1037. https://doi.org/10.3390/mi15081037







