Tuning Multi-Wavelength Reflection Properties of Porous Silicon Bragg Reflectors Using Silver-Nanoparticle-Assisted Electrochemical Etching
Abstract
1. Introduction
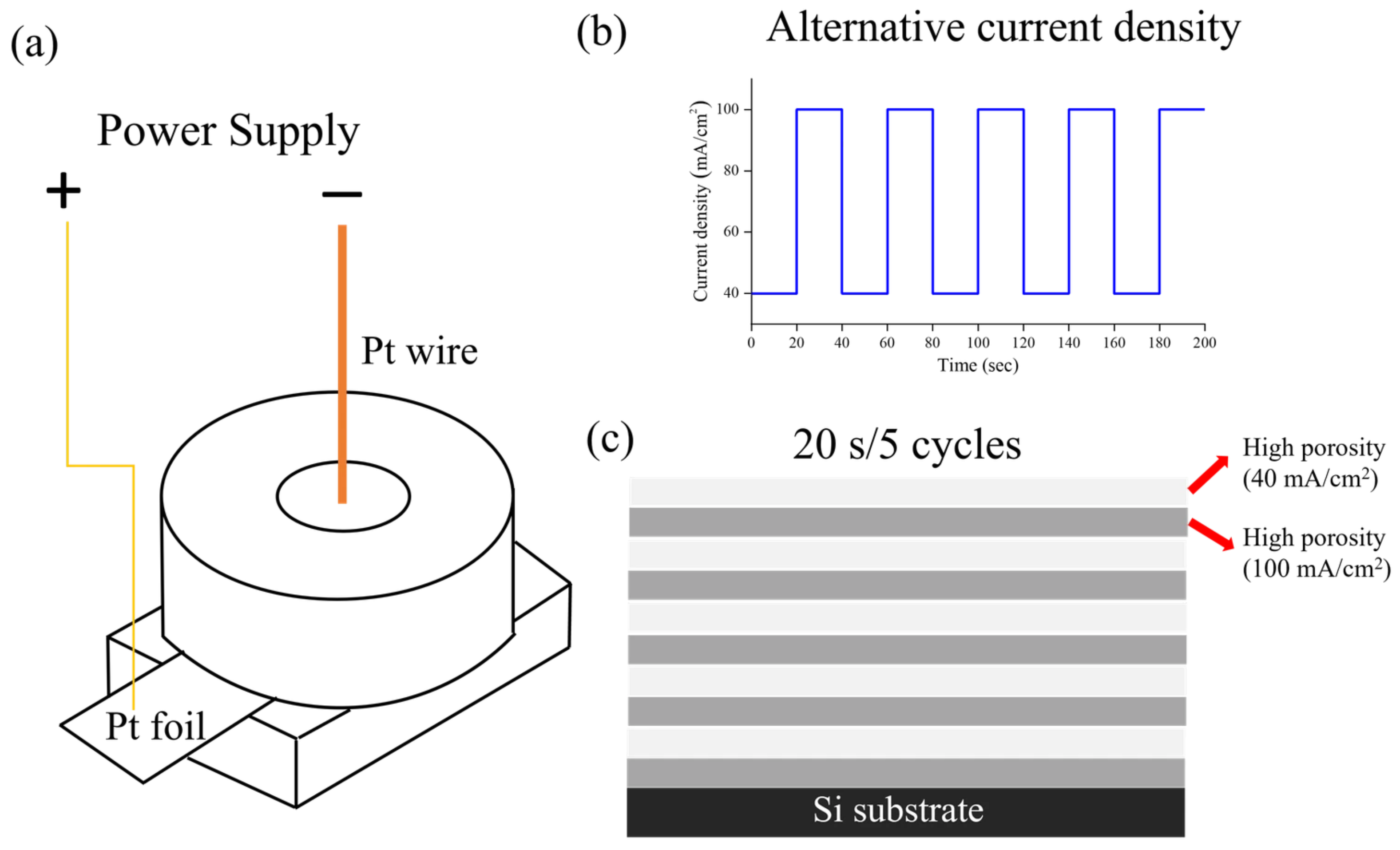
2. Materials and Methods
3. Results and Discussion
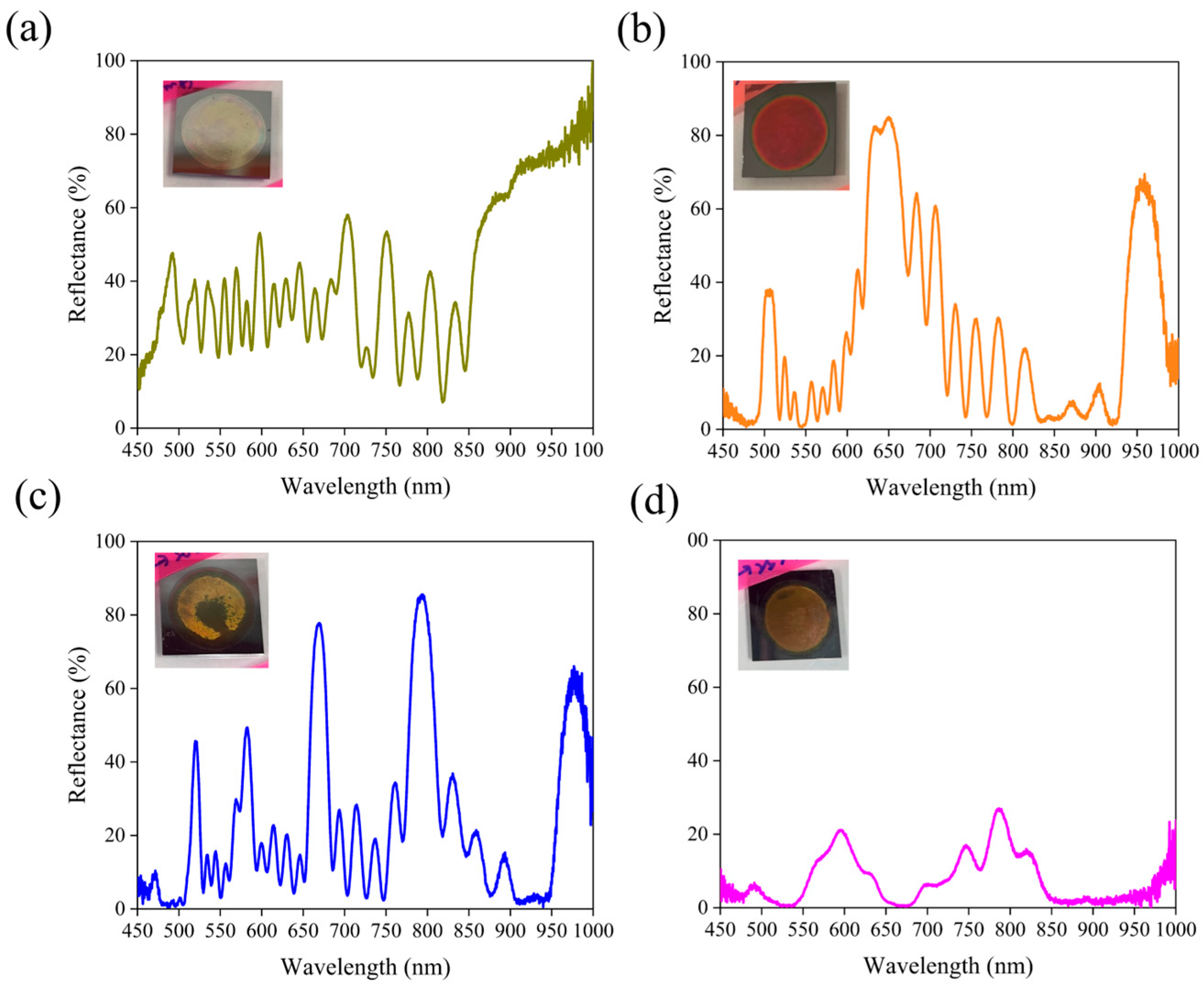
3.1. Effect of Silver Nanoparticles on the Spectral Properties of Porous Silicon Bragg Reflectors
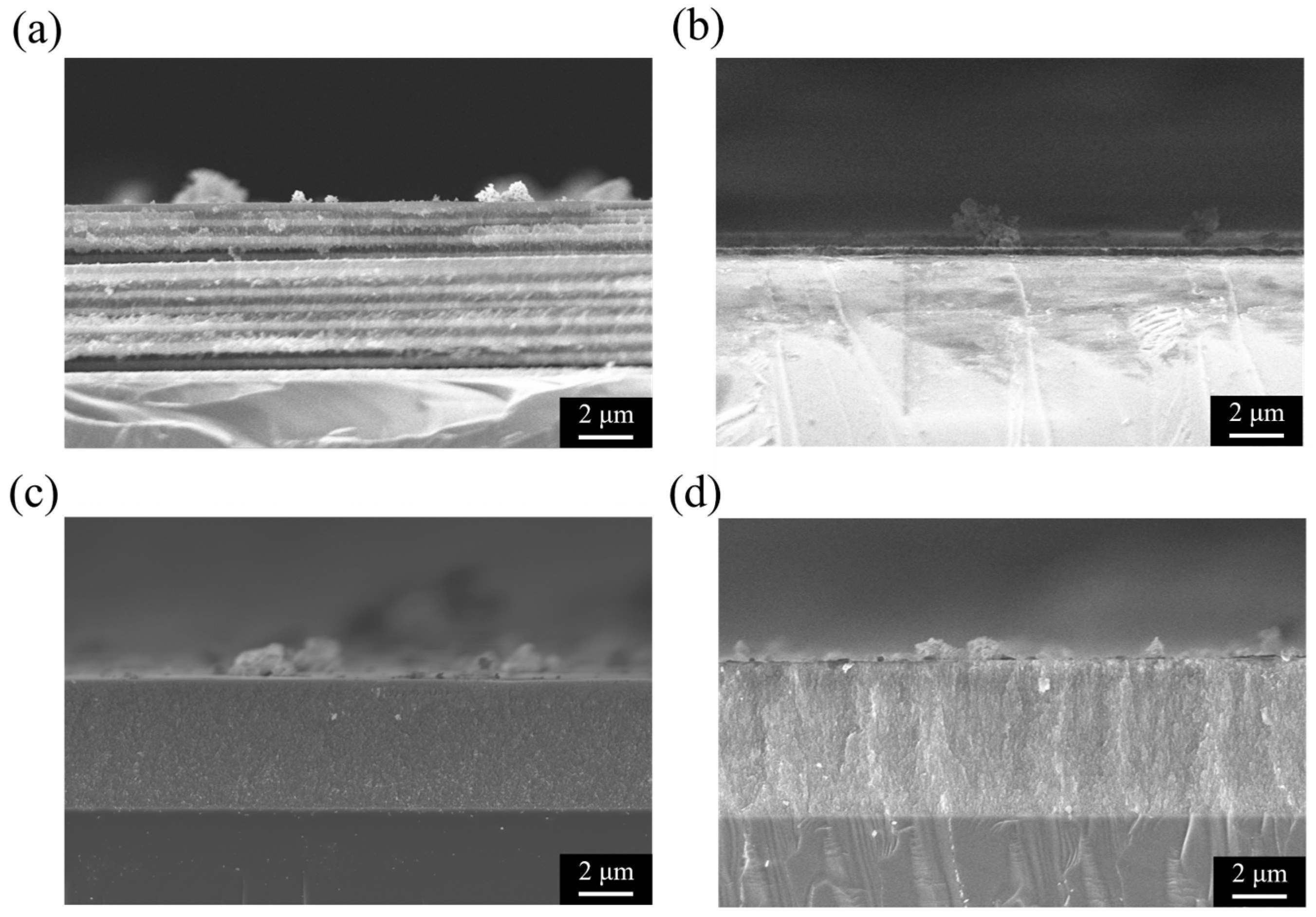
3.2. Cross-Sectional Analysis of Porous Silicon Bragg Reflectors
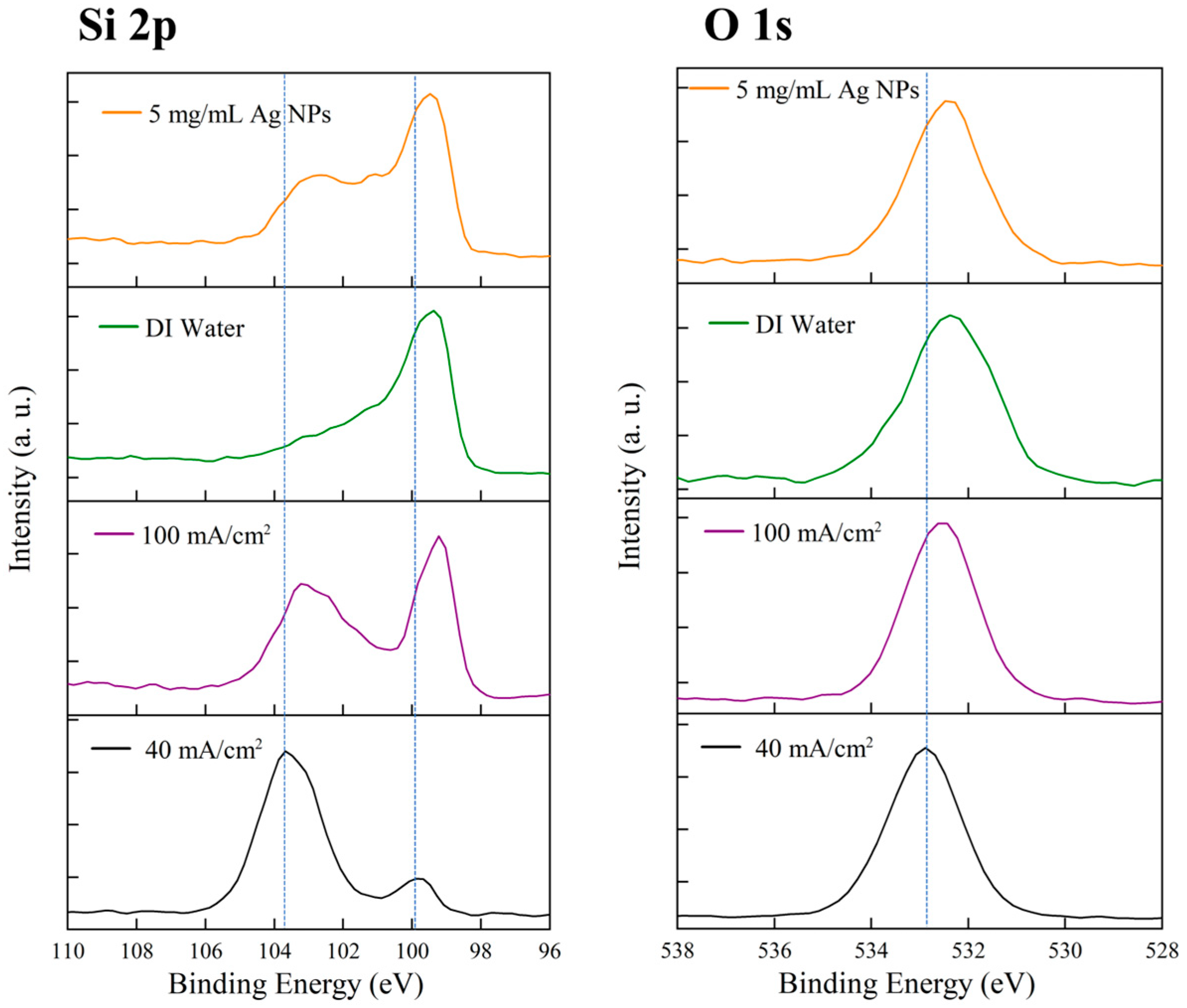
3.3. Influence of Etching Conditions on Surface Oxidation Revealed by XPS
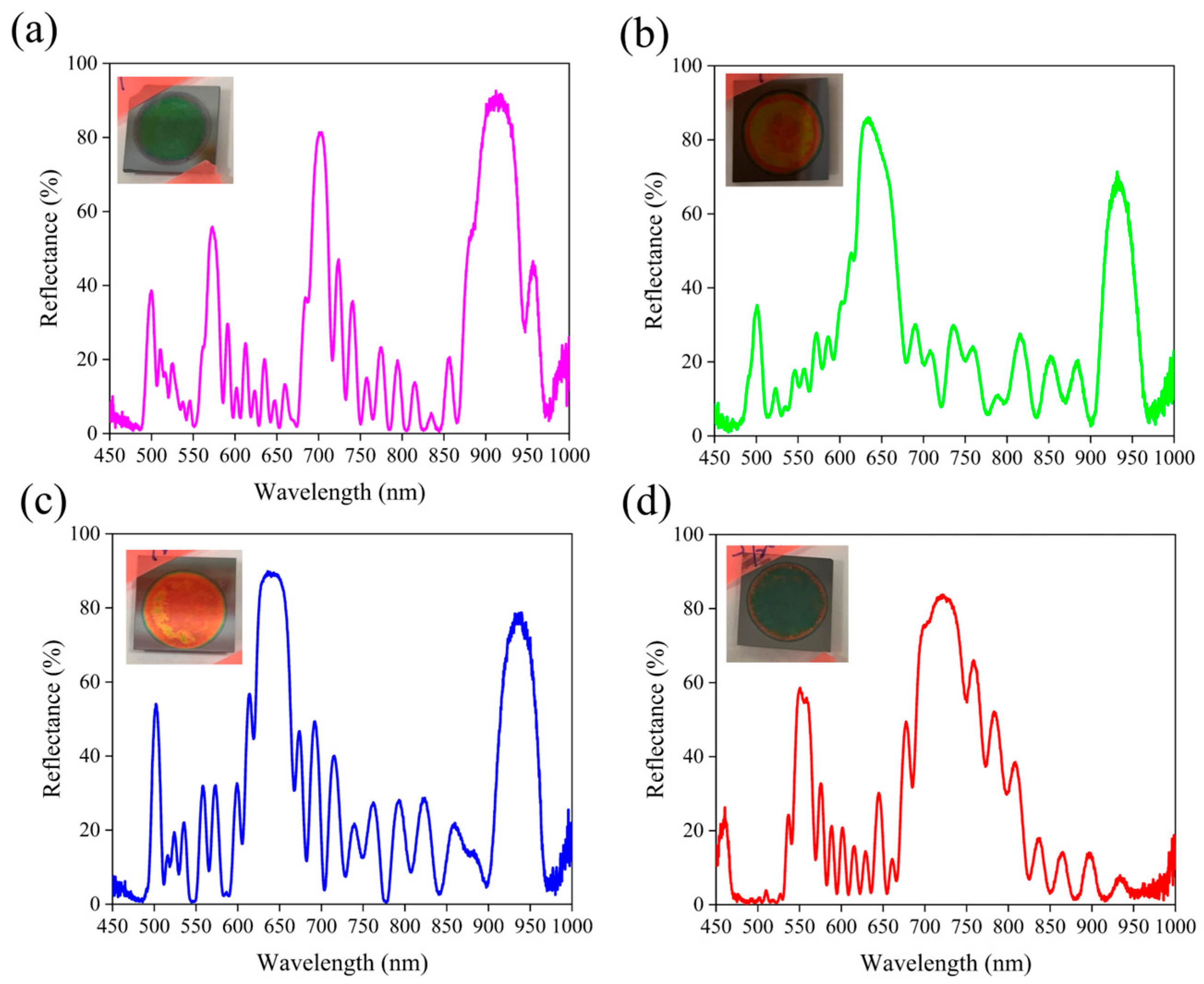
3.4. Influence of Etching Cycles on the Spectral Characteristics of Porous Silicon Bragg Reflectors
3.5. Cross-Sectional Morphology Analysis of Porous Silicon Structures with Different Etching Cycles
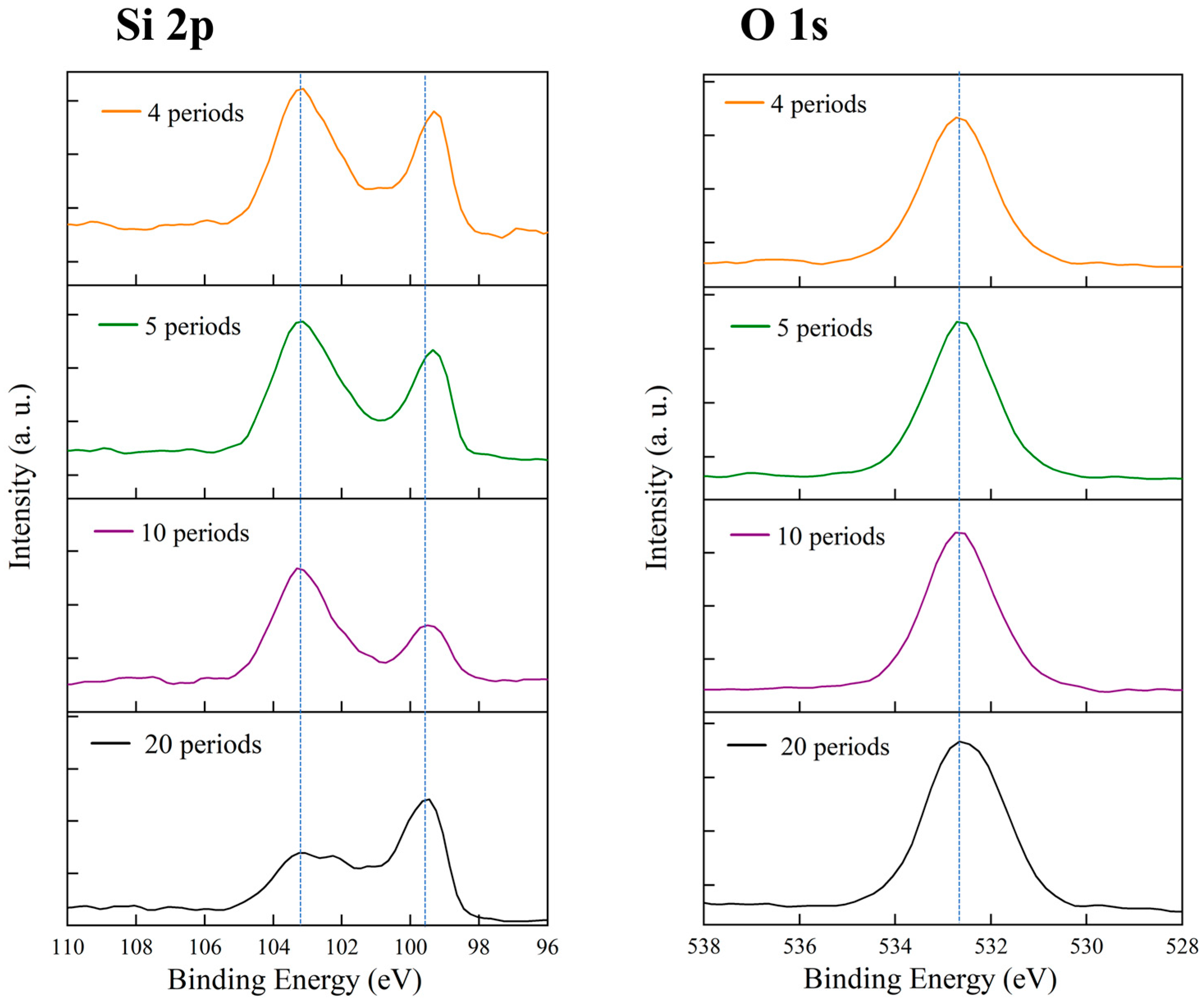
3.6. Comparison of Surface Chemical States at Different Etching Cycles
3.7. Effect of Silver Nanoparticle Concentration on the Optical Properties of Porous Silicon Bragg Reflectors
3.8. Cross-Sectional Morphology of Porous Silicon Structures with Different Silver Nanoparticle Concentrations
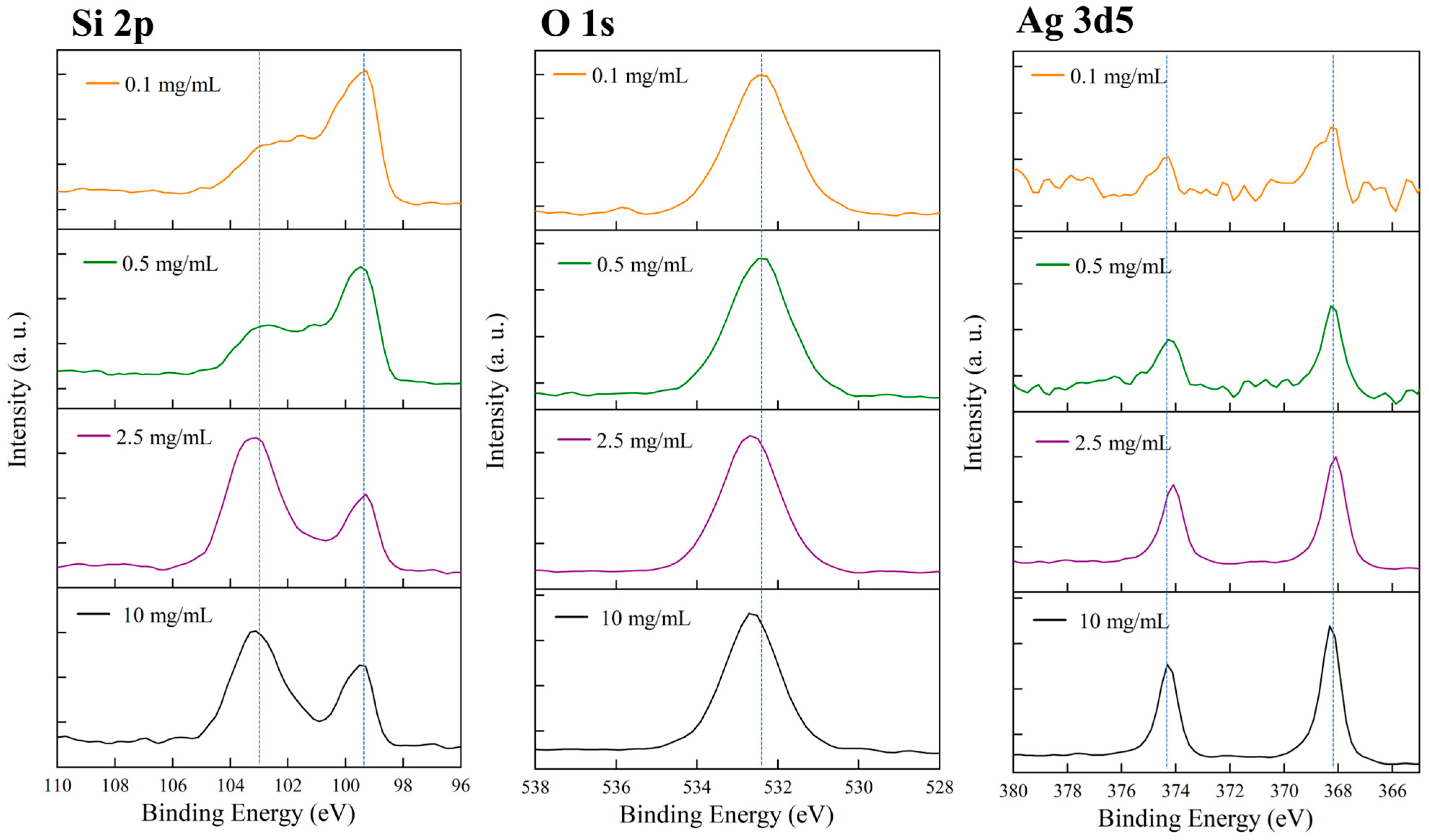
3.9. Surface Chemical Composition Analysis of Samples with Different Silver Nanoparticle Concentrations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Costa, V.; Martín-Palma, R.J. Application of nanostructured porous silicon in the field of optics. A review. J. Mater. Sci. 2010, 45, 2823–2838. [Google Scholar] [CrossRef]
- Torres-Costa, V.; Martín-Palma, R.J. Optical properties of porous silicon materials. In Porous Silicon for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2021; pp. 183–222. [Google Scholar]
- Sanchez-Salcedo, R.; Sharma, P.; Voelcker, N.H. Advancements in porous silicon biosensors for point of care, wearable, and implantable applications. ACS Appl. Mater. Interfaces 2025, 17, 2814–2843. [Google Scholar] [CrossRef] [PubMed]
- Pickering, C.; Beale, M.I.J.; Robbins, D.J.; Pearson, P.J.; Greef, R. Optical studies of the structure of porous silicon films formed in p-type degenerate and non-degenerate silicon. J. Phys. C Solid State Phys. 1984, 17, 6535. [Google Scholar] [CrossRef]
- Vincent, G. Optical properties of porous silicon superlattices. Appl. Phys. Lett. 1994, 64, 2367–2369. [Google Scholar] [CrossRef]
- Zheng, W.H.; Reece, P.; Sun, B.Q.; Gal, M. Broadband laser mirrors made from porous silicon. Appl. Phys. Lett. 2004, 84, 3519–3521. [Google Scholar] [CrossRef]
- Mazzoleni, C.; Pavesi, L. Application to optical components of dielectric porous silicon multilayers. Appl. Phys. Lett. 1995, 67, 2983–2985. [Google Scholar] [CrossRef]
- Setzu, S.; Ferrand, P.; Romestain, R. Optical properties of multilayered porous silicon. Mater. Sci. Eng. B 2000, 69, 34–42. [Google Scholar] [CrossRef]
- Zettner, J.; Thoenissen, M.; Hierl, T.; Brendel, R.; Schulz, M. Novel porous silicon backside light reflector for thin silicon solar cells. Prog. Photovolt. Res. Appl. 1998, 6, 423–432. [Google Scholar] [CrossRef]
- Duerinckx, F.; Kuzma-Filipek, I.; Van Nieuwenhuysen, K.; Beaucarne, G.; Poortmans, J. Reorganized porous silicon Bragg reflectors for thin-film silicon solar cells. IEEE Electron Device Lett. 2006, 27, 837–839. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Skryshevsky, V.A.; Nychyporuk, T.; Lemiti, M.; Makarov, A.V.; Klyui, N.I.; Tretyak, O.V. Porous silicon Bragg mirrors on single-and multi-crystalline silicon for solar cells. Renew. Energy 2013, 55, 79–84. [Google Scholar] [CrossRef]
- Seba, H.Y.; Hadjersi, T.; Zebbar, N. Bragg mirrors porous silicon back reflector for the light trapping in hydrogenated amorphous silicon. Appl. Surf. Sci. 2015, 350, 57–61. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Skryshevsky, V.A.; Belarouci, A. Porous Bragg reflector based sensors: Ways to increase sensitivity. Sens. Actuators A Phys. 2020, 315, 112234. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.Y.; Lee, K.W.; Park, S.H. A distributed Bragg reflector porous silicon layer for optical interferometric sensing of organic vapor. Sens. Actuators B Chem. 2011, 155, 673–678. [Google Scholar] [CrossRef]
- Kittle, J.D.; Gofus, J.S., III; Abel, A.N.; Evans, B.D. Additive combination of spectra reflected from porous silicon and carbon/porous silicon rugate filters to improve vapor selectivity. ACS Omega 2020, 5, 19820–19826. [Google Scholar] [CrossRef]
- Ivanov, I.; Skryshevsky, V.; Belarouci, A. Engineering porous silicon-based microcavity for chemical sensing. ACS Omega 2023, 8, 21265–21276. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Massad-Ivanir, N.; Segal, E.; Weiss, S. Porous silicon-based photonic biosensors: Current status and emerging applications. Anal. Chem. 2018, 91, 441–467. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Chen, C.; Chen, C.; Wu, L.; Wu, X.; Lu, C.; Zhang, X.; Chao, P.; Lv, X.; Jia, Z.; et al. Coupling annealed silver nanoparticles with a porous silicon Bragg mirror SERS substrate and machine learning for rapid non-invasive disease diagnosis. Anal. Chim. Acta 2023, 1254, 341116. [Google Scholar] [CrossRef]
- Hoang, M.T.; Bui, H.; Hoang, T.H.C.; Pham, V.H.; Loan, N.T.; Le, L.V.; Pham, T.B.; Duc, C.V.; Do, T.C.; Kim, T.J.; et al. Silver Nanoparticles-Decorated Porous Silicon Microcavity as a High-Performance SERS Substrate for Ultrasensitive Detection of Trace-Level Molecules. Nanomaterials 2025, 15, 1007. [Google Scholar] [CrossRef]
- Charrier, J.; Pirasteh, P.; Boucher, Y.G.; Gadonna, M. Bragg reflector formed on oxidised porous silicon. Micro Nano Lett. 2012, 7, 105–108. [Google Scholar] [CrossRef]
- Lorenzo, E.; Oton, C.J.; Capuj, N.E.; Ghulinyan, M.; Navarro-Urrios, D.; Gaburro, Z.; Pavesi, L. Porous silicon-based rugate filters. Appl. Opt. 2005, 44, 5415–5421. [Google Scholar] [CrossRef]
- Maniya, N.H.; Patel, S.R.; Murthy, Z.V.P. Simulation and fabrication study of porous silicon photonic crystal. Optik 2014, 125, 828–831. [Google Scholar] [CrossRef]
- Keshavarzi, S.; Kovacs, A.; Abdo, M.; Badilita, V.; Zhu, R.; Korvink, J.G.; Mescheder, U. Porous silicon based rugate filter wheel for multispectral imaging applications. ECS J. Solid State Sci. Technol. 2019, 8, Q43. [Google Scholar] [CrossRef]
- Vendamani, V.S.; Dang, Z.Y.; Ramana, P.; Pathak, A.P.; Kumar, V.R.K.; Breese, M.B.H.; Rao, S.N. Fabrication of porous silicon based tunable distributed Bragg reflectors by anodic etching of irradiated silicon. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 358, 105–111. [Google Scholar] [CrossRef]
- Verma, C.P.; Asokan, K.; Kanaujia, P.K.; Adnan, M.; Kanjilal, D.; Prakash, G.V. Cavity resonance tunability of porous silicon microcavities by Ar+ ion irradiation. Appl. Surf. Sci. 2021, 535, 147696. [Google Scholar] [CrossRef]
- Verma, C.P.; Asokan, K.; Kanjilal, D.; Prakash, G.V. Tuning the optical properties of porous silicon-based microcavities by energetic oxygen ion beams for optoelectronic applications. Mater. Lett. 2022, 306, 130914. [Google Scholar] [CrossRef]
- Tsai, M.T.; Lee, Y.C.; Lin, Y.M.; Hsiao, V.K.; Chu, C.C. Exploring the influence of solvents on electrochemically etched porous silicon based on photoluminescence and surface morphology analysis. Materials 2024, 17, 989. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-Y.; Hsu, H.-H.; Muhammed Musthafa, A.; Lin, I.-A.; Chou, C.-M.; Hsiao, V.K.S. Tuning Multi-Wavelength Reflection Properties of Porous Silicon Bragg Reflectors Using Silver-Nanoparticle-Assisted Electrochemical Etching. Micromachines 2025, 16, 1198. https://doi.org/10.3390/mi16111198
Huang S-Y, Hsu H-H, Muhammed Musthafa A, Lin I-A, Chou C-M, Hsiao VKS. Tuning Multi-Wavelength Reflection Properties of Porous Silicon Bragg Reflectors Using Silver-Nanoparticle-Assisted Electrochemical Etching. Micromachines. 2025; 16(11):1198. https://doi.org/10.3390/mi16111198
Chicago/Turabian StyleHuang, Sheng-Yang, Hsiao-Han Hsu, Amal Muhammed Musthafa, I-An Lin, Chia-Man Chou, and Vincent K. S. Hsiao. 2025. "Tuning Multi-Wavelength Reflection Properties of Porous Silicon Bragg Reflectors Using Silver-Nanoparticle-Assisted Electrochemical Etching" Micromachines 16, no. 11: 1198. https://doi.org/10.3390/mi16111198
APA StyleHuang, S.-Y., Hsu, H.-H., Muhammed Musthafa, A., Lin, I.-A., Chou, C.-M., & Hsiao, V. K. S. (2025). Tuning Multi-Wavelength Reflection Properties of Porous Silicon Bragg Reflectors Using Silver-Nanoparticle-Assisted Electrochemical Etching. Micromachines, 16(11), 1198. https://doi.org/10.3390/mi16111198






