Evaluation of Perfusion Cell Culture Conditions in a Double-Layered Microphysiological System Using AI-Assisted Morphological Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microfluidic Chip and Perfusion Setup
2.2. Cell Culture Method Using Microfluidic Chips
2.3. Lucifer Yellow Permeability Test
2.4. Imaging and Analysis
2.4.1. Immunocytochemistry
2.4.2. AI-Assisted Morphological Analysis
3. Results
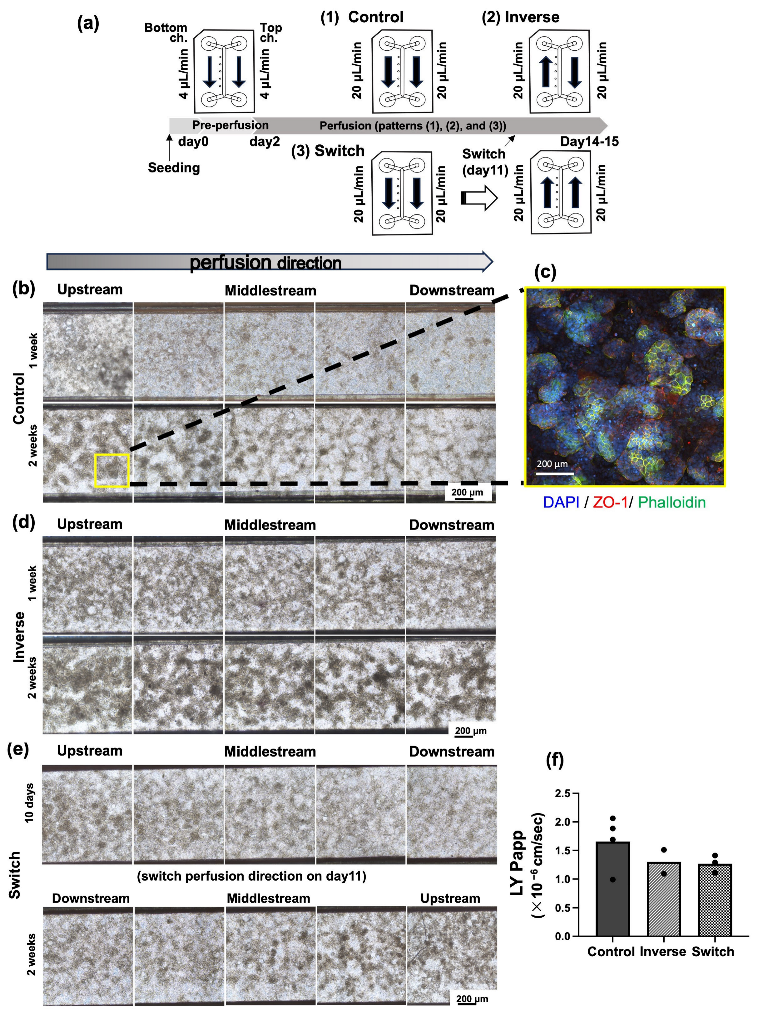
3.1. Alteration of Cell Morphology Depending on the Direction of Medium Perfusion
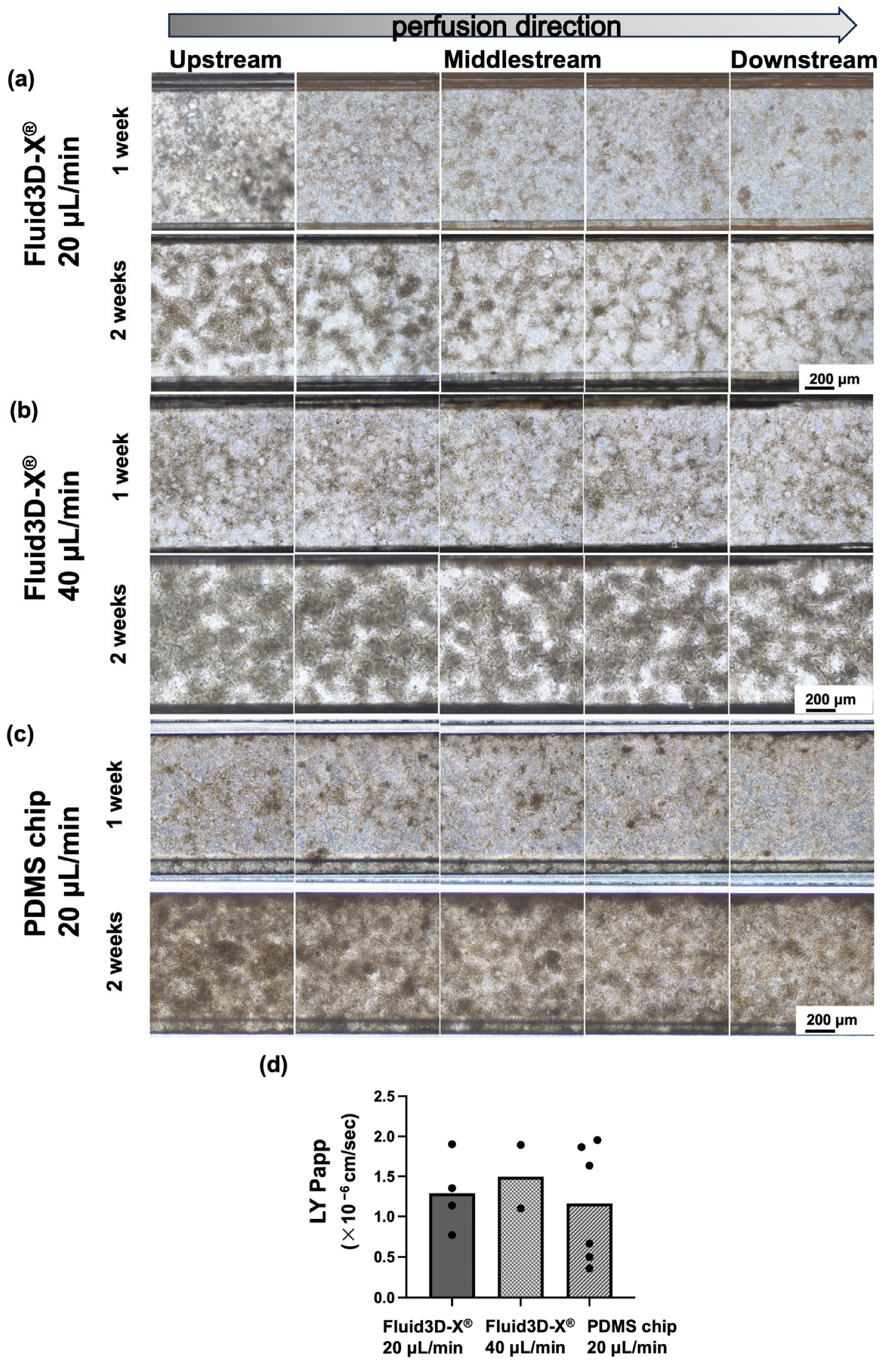
3.2. Alteration of Cell Morphology Depending on the Flow Rate of Medium Perfusion
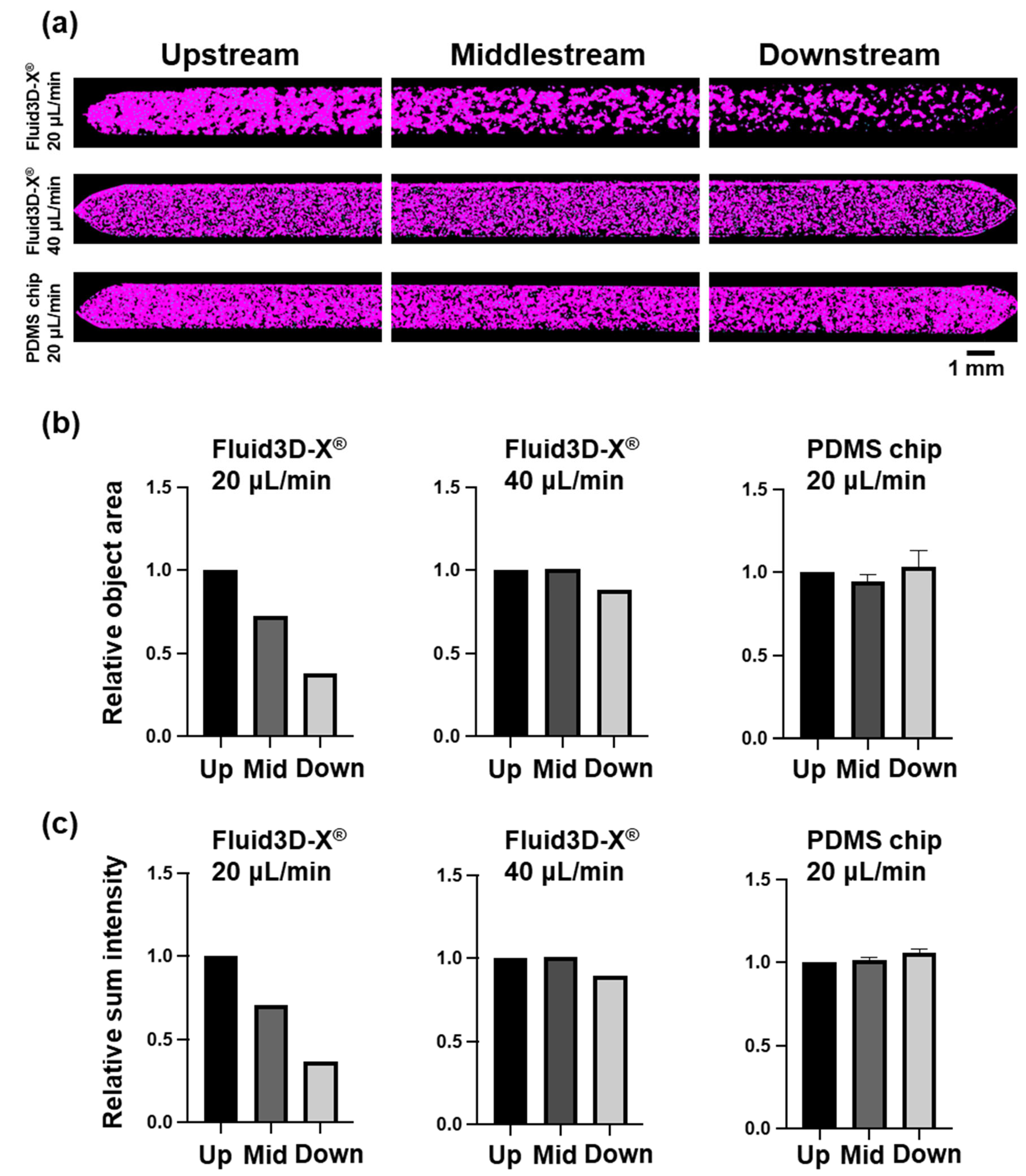
3.3. Alteration of Cell Morphology Depending on the Material of Chips
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marx, U.; Akabane, T.; Andersson, T.B.; Baker, E.; Beilmann, M.; Beken, S.; Brendler-Schwaab, S.; Cirit, M.; David, R.; Dehne, E.M.; et al. Biology-inspired microphysiological systems to advance patient benefit and animal welfare in drug development. Altex 2020, 37, 365–394. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Madia, F.; Corvi, R.; Munn, S.; Grignard, E.; Paini, A.; Worth, A.; Bal-Price, A.; Prieto, P.; Casati, S.; et al. Current EU regulatory requirements for the assessment of chemicals and cosmetic products: Challenges and opportunities for introducing new approach methodologies. Arch. Toxicol. 2021, 95, 1867–1897. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J. FDA Modernization Act 2.0 allows for alternatives to animal testing. Artif. Organs 2023, 47, 449–450. [Google Scholar] [CrossRef]
- Lehman-McKeeman, L.; Davis, M. Toxicology Modernization: Misnomer or Momentum. Med. Chem. Res. 2023, 32, 1235–1238. [Google Scholar] [CrossRef]
- Kimura, H.; Nishikawa, M.; Kutsuzawa, N.; Tokito, F.; Kobayashi, T.; Kurniawan, D.A.; Shioda, H.; Cao, W.; Shinha, K.; Nakamura, H.; et al. Advancements in Microphysiological systems: Exploring organoids and organ-on-a-chip technologies in drug development -focus on pharmacokinetics related organs. Drug Metab. Pharmacokinet. 2025, 60, 101046. [Google Scholar] [CrossRef]
- Ebrahimkhani, M.R.; Neiman, J.A.; Raredon, M.S.; Hughes, D.J.; Griffith, L.G. Bioreactor technologies to support liver function in vitro. Adv. Drug Deliv. Rev. 2014, 69–70, 132–157. [Google Scholar] [CrossRef]
- Gallagher, E.M.; Rizzo, G.M.; Dorsey, R.; Dhummakupt, E.S.; Moran, T.S.; Mach, P.M.; Jenkins, C.C. Normalization of organ-on-a-Chip samples for mass spectrometry based proteomics and metabolomics via Dansylation-based assay. Toxicol. In Vitro 2023, 88, 105540. [Google Scholar] [CrossRef]
- Kopp, B.; Khawam, A.; Di Perna, K.; Lenart, D.; Vinette, M.; Silva, R.; Zanoni, T.B.; Rore, C.; Guenigault, G.; Richardson, E.; et al. Liver-on-chip model and application in predictive genotoxicity and mutagenicity of drugs. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2024, 896, 503762. [Google Scholar] [CrossRef]
- Pediaditakis, I.; Kodella, K.R.; Manatakis, D.V.; Le, C.Y.; Barthakur, S.; Sorets, A.; Gravanis, A.; Ewart, L.; Rubin, L.L.; Manolakos, E.S.; et al. A microengineered Brain-Chip to model neuroinflammation in humans. iScience 2022, 25, 104813. [Google Scholar] [CrossRef]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.Y.; Lee, D.; Han, J.; Nguyen, N.T.; Park, S.; Sung, J.H. Microfluidic gut-on-a-chip with three-dimensional villi structure. Biomed. Microdevices 2017, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.C.; Shin, W.; Koh, D.; Wu, A.; Ambrosini, Y.M.; Min, S.; Eckhardt, S.G.; Fleming, R.Y.D.; Kim, S.; Park, S.; et al. Three-Dimensional Regeneration of Patient-Derived Intestinal Organoid Epithelium in a Physiodynamic Mucosal Interface-on-a-Chip. Micromachines 2020, 11, 663. [Google Scholar] [CrossRef]
- Sasaki, Y.; Tatsuoka, H.; Tsuda, M.; Sumi, T.; Eguchi, Y.; So, K.; Higuchi, Y.; Takayama, K.; Torisawa, Y.; Yamashita, F. Intestinal Permeability of Drugs in Caco-2 Cells Cultured in Microfluidic Devices. Biol. Pharm. Bull. 2022, 45, 1246–1253. [Google Scholar] [CrossRef]
- Imaoka, T.; Onuki-Nagasaki, R.; Kimura, H.; Tai, K.; Ishii, M.; Nozue, A.; Kaisaki, I.; Hoshi, M.; Watanabe, K.; Maeda, K.; et al. Development of a novel gut microphysiological system that facilitates assessment of drug absorption kinetics in gut. Sci. Rep. 2024, 14, 29921. [Google Scholar] [CrossRef]
- Kimura, H.; Nakamura, H.; Goto, T.; Uchida, W.; Uozumi, T.; Nishizawa, D.; Shinha, K.; Sakagami, J.; Doi, K. Standalone cell culture microfluidic device-based microphysiological system for automated cell observation and application in nephrotoxicity tests. Lab Chip 2024, 24, 408–421. [Google Scholar] [CrossRef]
- Park, Y.; Depeursinge, C.; Popescu, G. Quantitative phase imaging in biomedicine. Nat. Photonics 2018, 12, 578–589. [Google Scholar] [CrossRef]
- Popescu, G. Quantitative Phase Imaging of Cells and Tissues, 1st ed.; McGraw-Hill Education: New York, NY, USA, 2011. [Google Scholar]
- Lopez-Escalera, S.; Wellejus, A. Evaluation of Caco-2 and human intestinal epithelial cells as in vitro models of colonic and small intestinal integrity. Biochem. Biophys. Rep. 2022, 31, 101314. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, C.; Chen, P.; Hu, J.; Hu, R.; Huang, M.; Bi, H. Development, validation, and application of a novel 7-day Caco-2 cell culture system. J. Pharmacol. Toxicol. Methods 2014, 70, 175–181. [Google Scholar] [CrossRef]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef]
- Chi, M.; Yi, B.; Oh, S.; Park, D.J.; Sung, J.H.; Park, S. A microfluidic cell culture device (μFCCD) to culture epithelial cells with physiological and morphological properties that mimic those of the human intestine. Biomed. Microdevices 2015, 17, 9966. [Google Scholar] [CrossRef] [PubMed]
- Valiei, A.; Aminian-Dehkordi, J.; Mofrad, M.R.K. Gut-on-a-chip models for dissecting the gut microbiology and physiology. APL Bioeng. 2023, 7, 011502. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Kim, H.J. 3D in vitro morphogenesis of human intestinal epithelium in a gut-on-a-chip or a hybrid chip with a cell culture insert. Nat. Protoc. 2022, 17, 910–939. [Google Scholar] [CrossRef] [PubMed]
- Jalili-Firoozinezhad, S.; Prantil-Baun, R.; Jiang, A.; Potla, R.; Mammoto, T.; Weaver, J.C.; Ferrante, T.C.; Kim, H.J.; Cabral, J.M.S.; Levy, O.; et al. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Cell Death Dis. 2018, 9, 223. [Google Scholar] [CrossRef]
- Kim, H.J.; Li, H.; Collins, J.J.; Ingber, D.E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl. Acad. Sci. USA 2016, 113, E7–E15. [Google Scholar] [CrossRef]
- Pocock, K.; Delon, L.; Bala, V.; Rao, S.; Priest, C.; Prestidge, C.; Thierry, B. Intestine-on-a-Chip Microfluidic Model for Efficient in Vitro Screening of Oral Chemotherapeutic Uptake. ACS Biomater. Sci. Eng. 2017, 3, 951–959. [Google Scholar] [CrossRef]
- Guo, K.T.; Fu, P.; Juerchott, K.; Motaln, H.; Selbig, J.; Lah, T.; Tonn, J.C.; Schichor, C. The expression of Wnt-inhibitor DKK1 (Dickkopf 1) is determined by intercellular crosstalk and hypoxia in human malignant gliomas. J. Cancer Res. Clin. Oncol. 2014, 140, 1261–1270. [Google Scholar] [CrossRef]
- Leijten, J.C.; Moreira Teixeira, L.S.; Landman, E.B.; van Blitterswijk, C.A.; Karperien, M. Hypoxia inhibits hypertrophic differentiation and endochondral ossification in explanted tibiae. PLoS ONE 2012, 7, e49896. [Google Scholar] [CrossRef]
- Matsumoto, S.; Safitri, A.R.; Danoy, M.; Maekawa, T.; Kinoshita, H.; Shinohara, M.; Sakai, Y.; Fujii, T.; Leclerc, E. Investigation of the hepatic respiration and liver zonation on rat hepatocytes using an integrated oxygen biosensor in a microscale device. Biotechnol. Prog. 2019, 35, e2854. [Google Scholar] [CrossRef]
- Shah, P.; Fritz, J.V.; Glaab, E.; Desai, M.S.; Greenhalgh, K.; Frachet, A.; Niegowska, M.; Estes, M.; Jäger, C.; Seguin-Devaux, C.; et al. A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat. Commun. 2016, 7, 11535. [Google Scholar] [CrossRef]
- Chen, S.L.; Xie, R.H.; Chen, C.Y.; Yang, J.W.; Hsieh, K.Y.; Liu, X.Y.; Xin, J.Y.; Kung, C.K.; Chung, J.H.Y.; Chen, G.Y. Revolutionizing Epithelial Differentiability Analysis in Small Airway-on-a-Chip Models Using Label-Free Imaging and Computational Techniques. Biosensors 2024, 14, 581. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Matsuda, N.; Yamanaka, M.; Suzuki, I. Development of a Novel Microphysiological System for Peripheral Neurotoxicity Prediction Using Human iPSC-Derived Neurons with Morphological Deep Learning. Toxics 2024, 12, 809. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutsuzawa, N.; Goto, T.; Nakamura, H.; Maeda, M.; Kinehara, M.; Sakagami, J.; Kimura, H. Evaluation of Perfusion Cell Culture Conditions in a Double-Layered Microphysiological System Using AI-Assisted Morphological Analysis. Micromachines 2025, 16, 327. https://doi.org/10.3390/mi16030327
Kutsuzawa N, Goto T, Nakamura H, Maeda M, Kinehara M, Sakagami J, Kimura H. Evaluation of Perfusion Cell Culture Conditions in a Double-Layered Microphysiological System Using AI-Assisted Morphological Analysis. Micromachines. 2025; 16(3):327. https://doi.org/10.3390/mi16030327
Chicago/Turabian StyleKutsuzawa, Naokata, Tomomi Goto, Hiroko Nakamura, Miwa Maeda, Masaki Kinehara, Junko Sakagami, and Hiroshi Kimura. 2025. "Evaluation of Perfusion Cell Culture Conditions in a Double-Layered Microphysiological System Using AI-Assisted Morphological Analysis" Micromachines 16, no. 3: 327. https://doi.org/10.3390/mi16030327
APA StyleKutsuzawa, N., Goto, T., Nakamura, H., Maeda, M., Kinehara, M., Sakagami, J., & Kimura, H. (2025). Evaluation of Perfusion Cell Culture Conditions in a Double-Layered Microphysiological System Using AI-Assisted Morphological Analysis. Micromachines, 16(3), 327. https://doi.org/10.3390/mi16030327





