Investigation of the Visible Photocatalytic–Fenton Reactive Composite Polishing Process for Single-Crystal SiC Wafers Based on Response Surface Methodology
Abstract
:1. Introduction
2. Experimental Materials and Methods
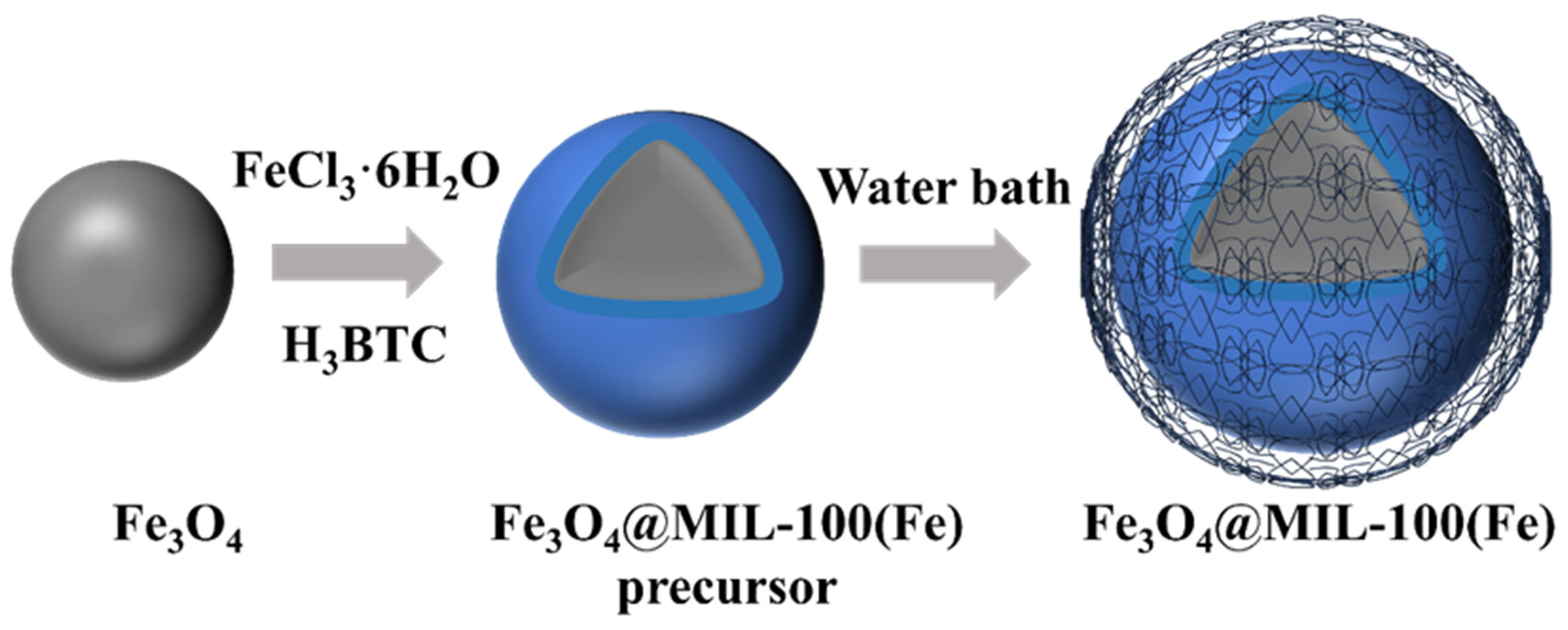
2.1. Synthesis of Fe3O4@MIL-100(Fe)
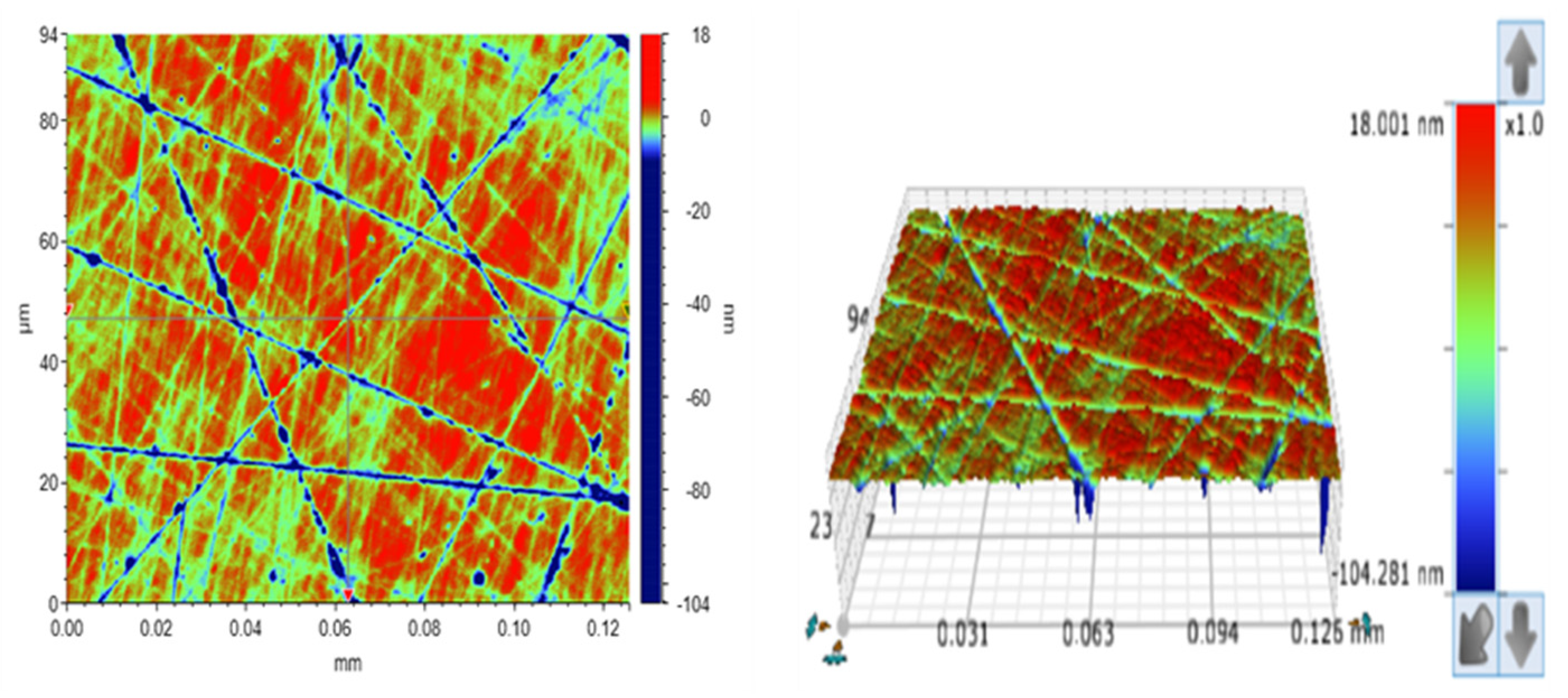
2.2. Characterization of Fe3O4@MIL-100(Fe)
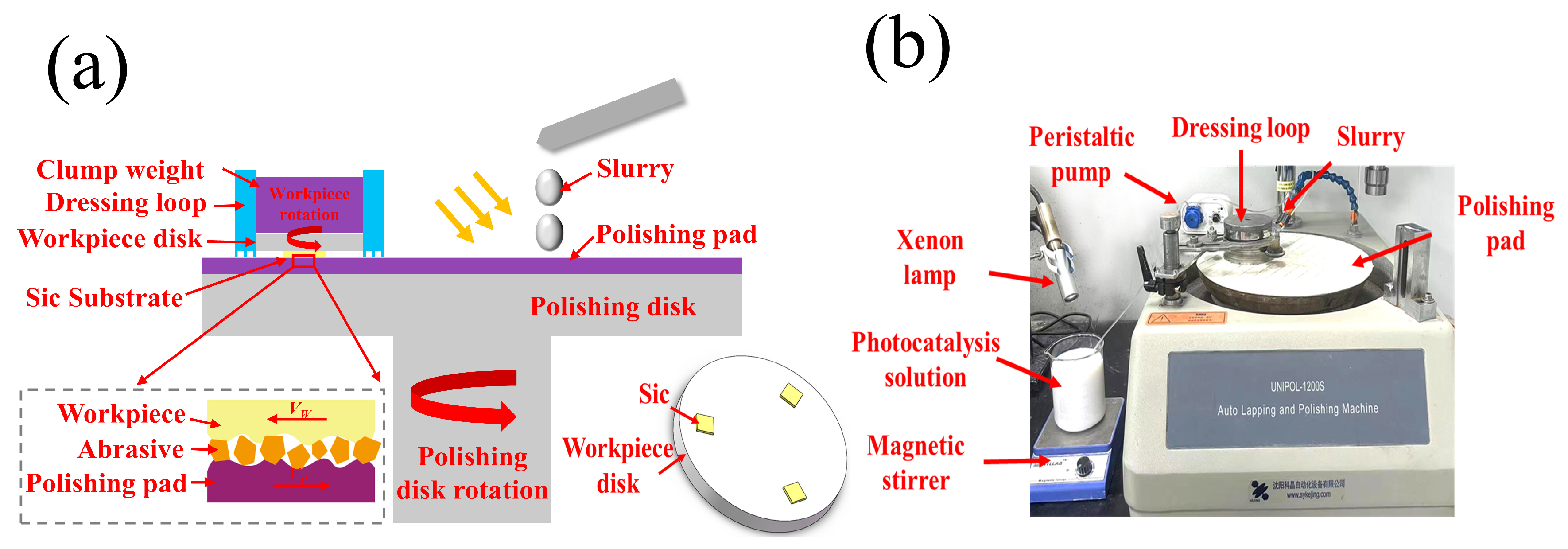
2.3. SiC Chemical Mechanical Polishing Test
3. Results and Discussion
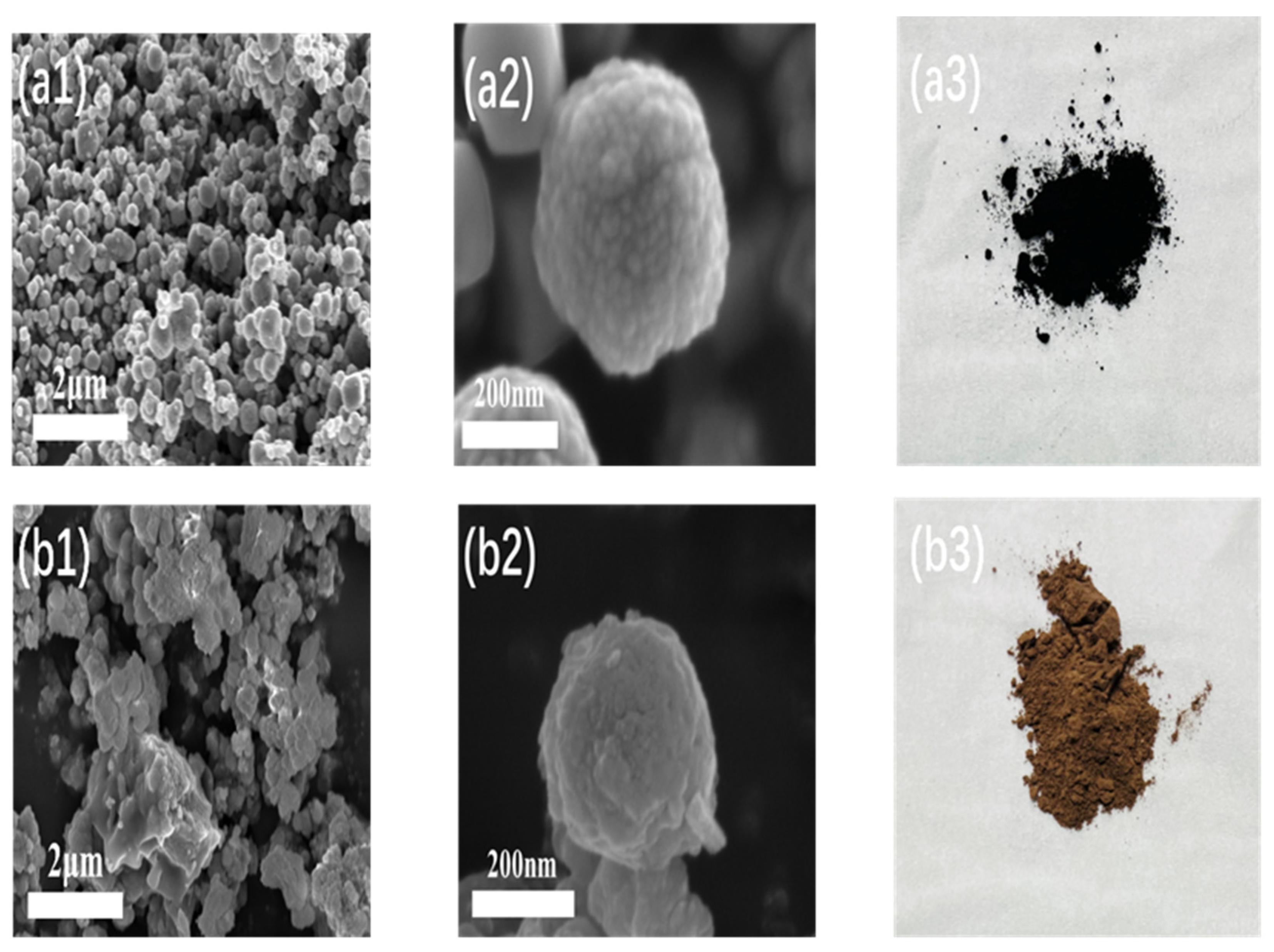
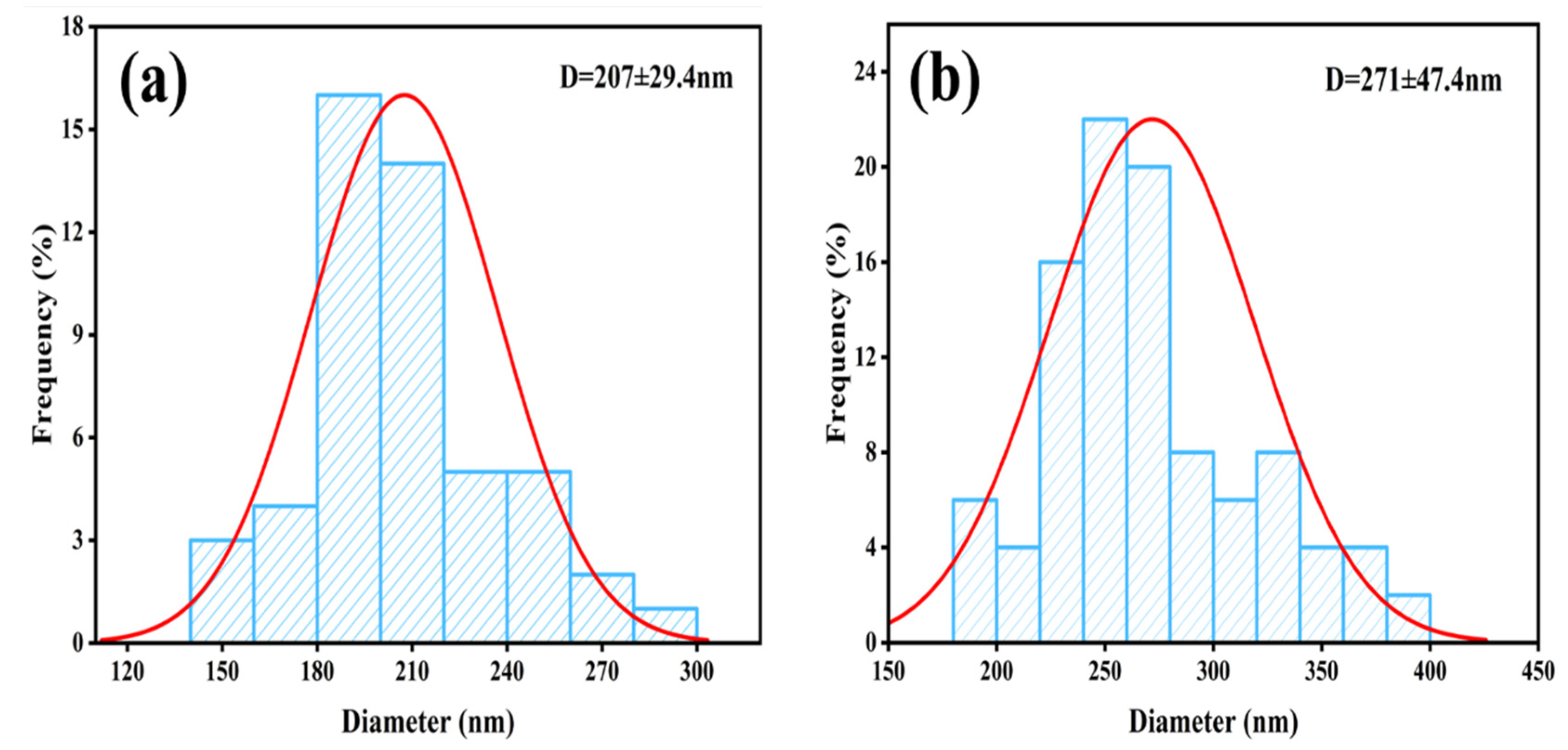
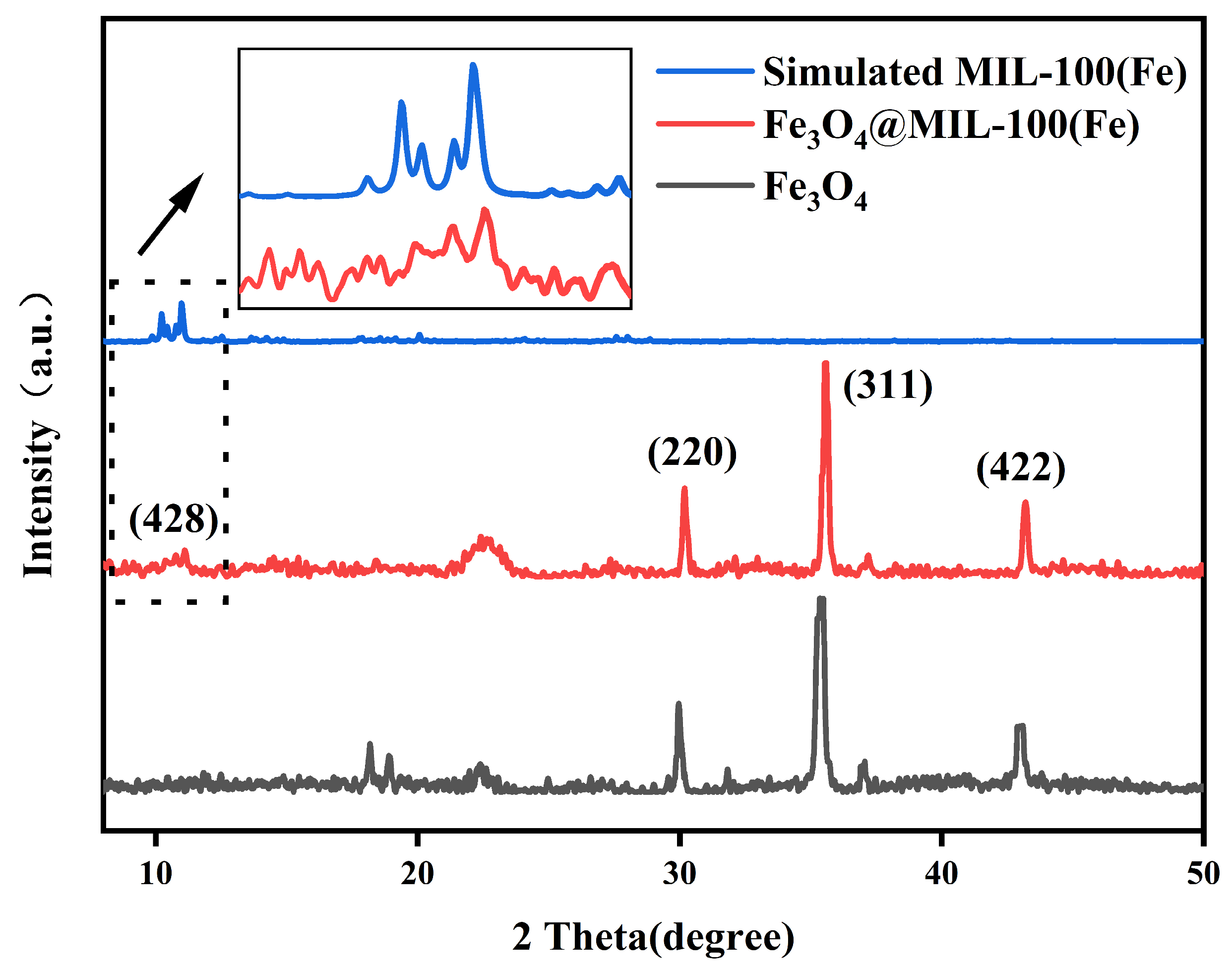
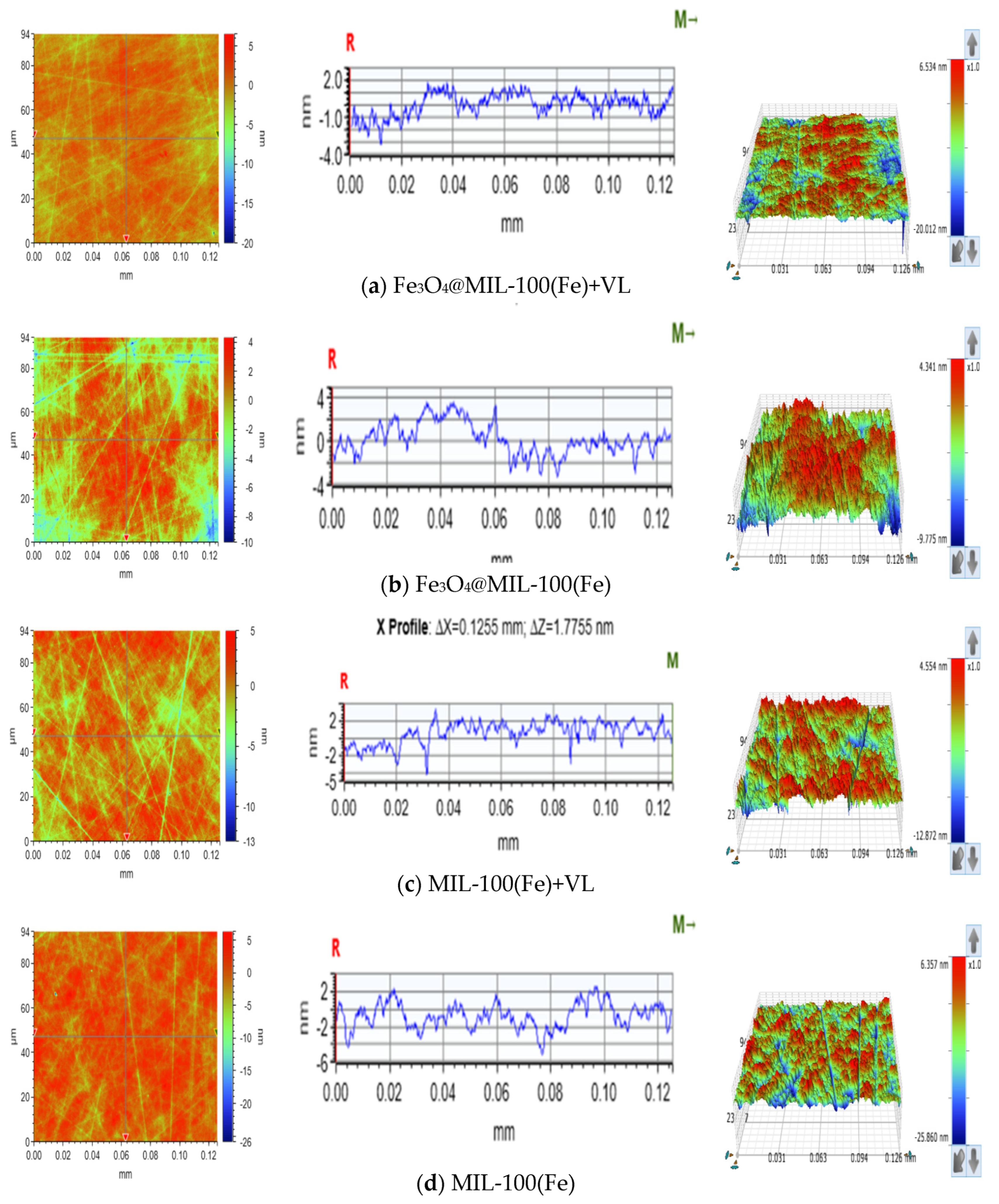
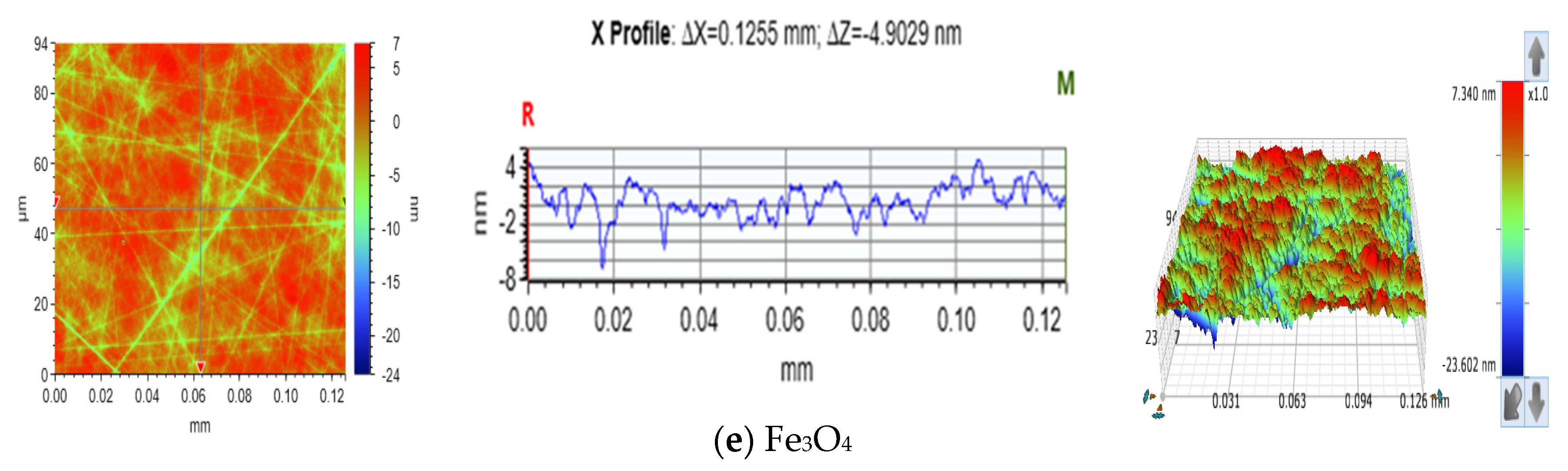
3.1. Characterization Results of Fe3O4@MIL-100(Fe)
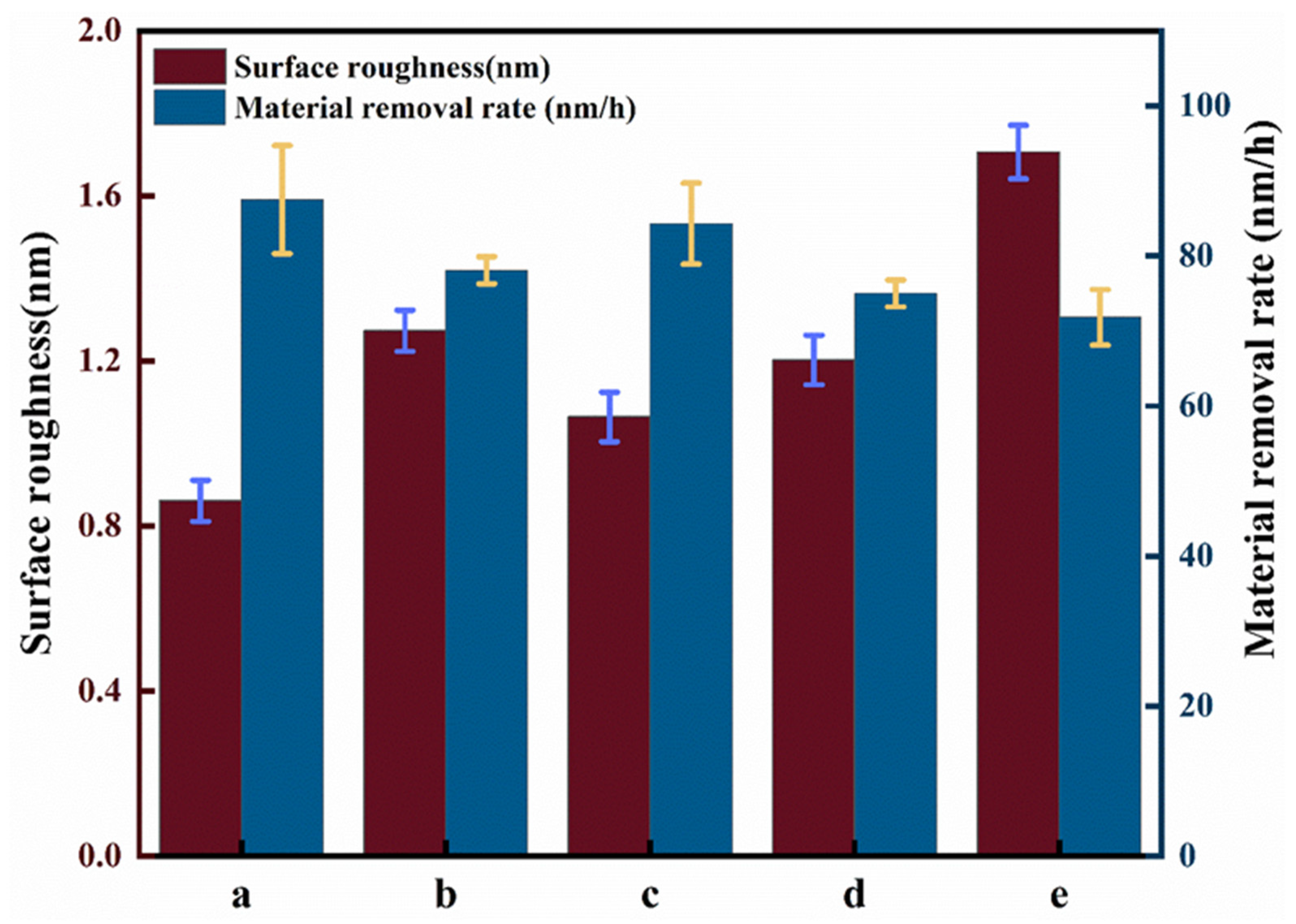
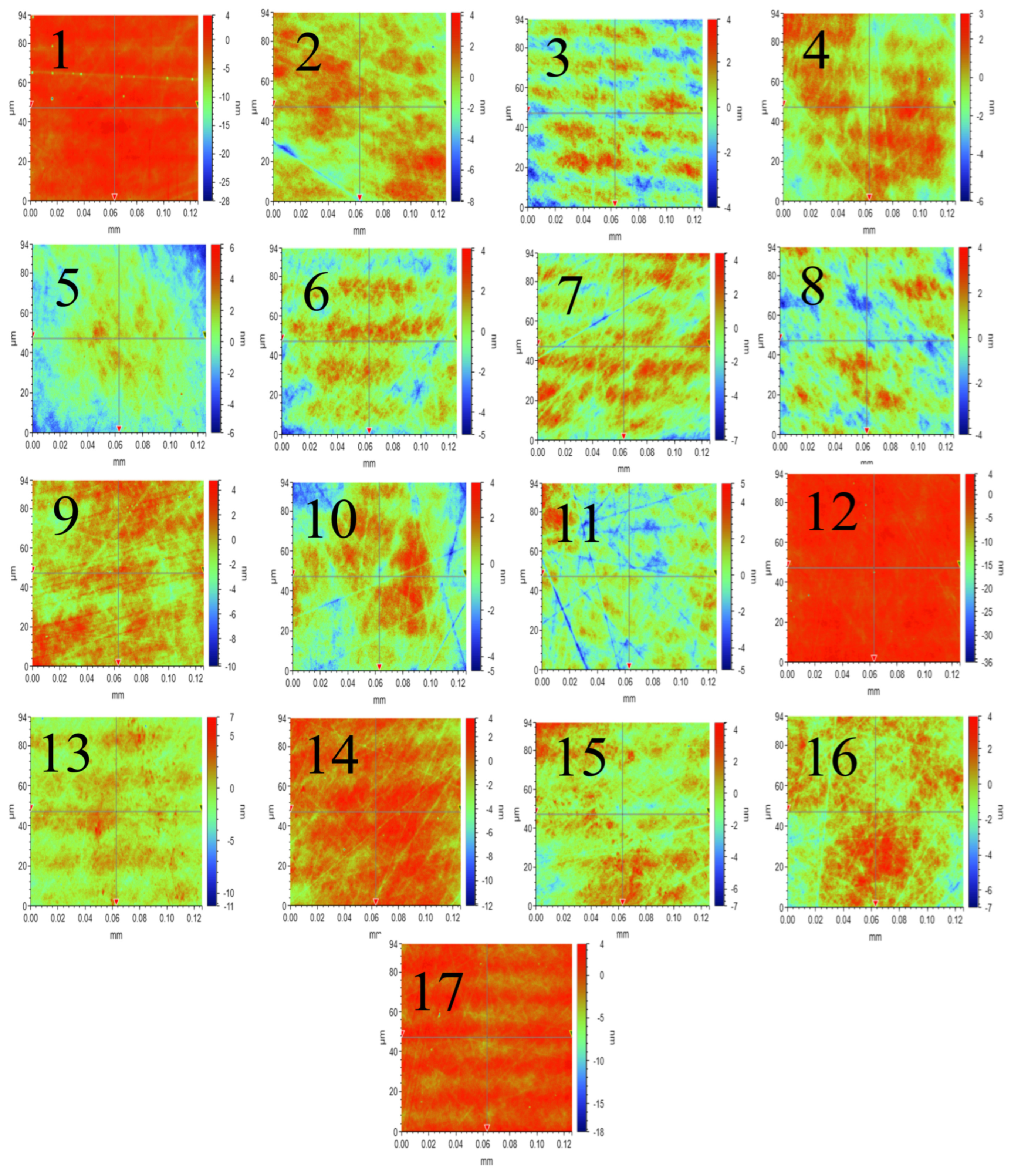
3.2. Effect of Different Catalytic Conditions on Chemical Mechanical Polishing
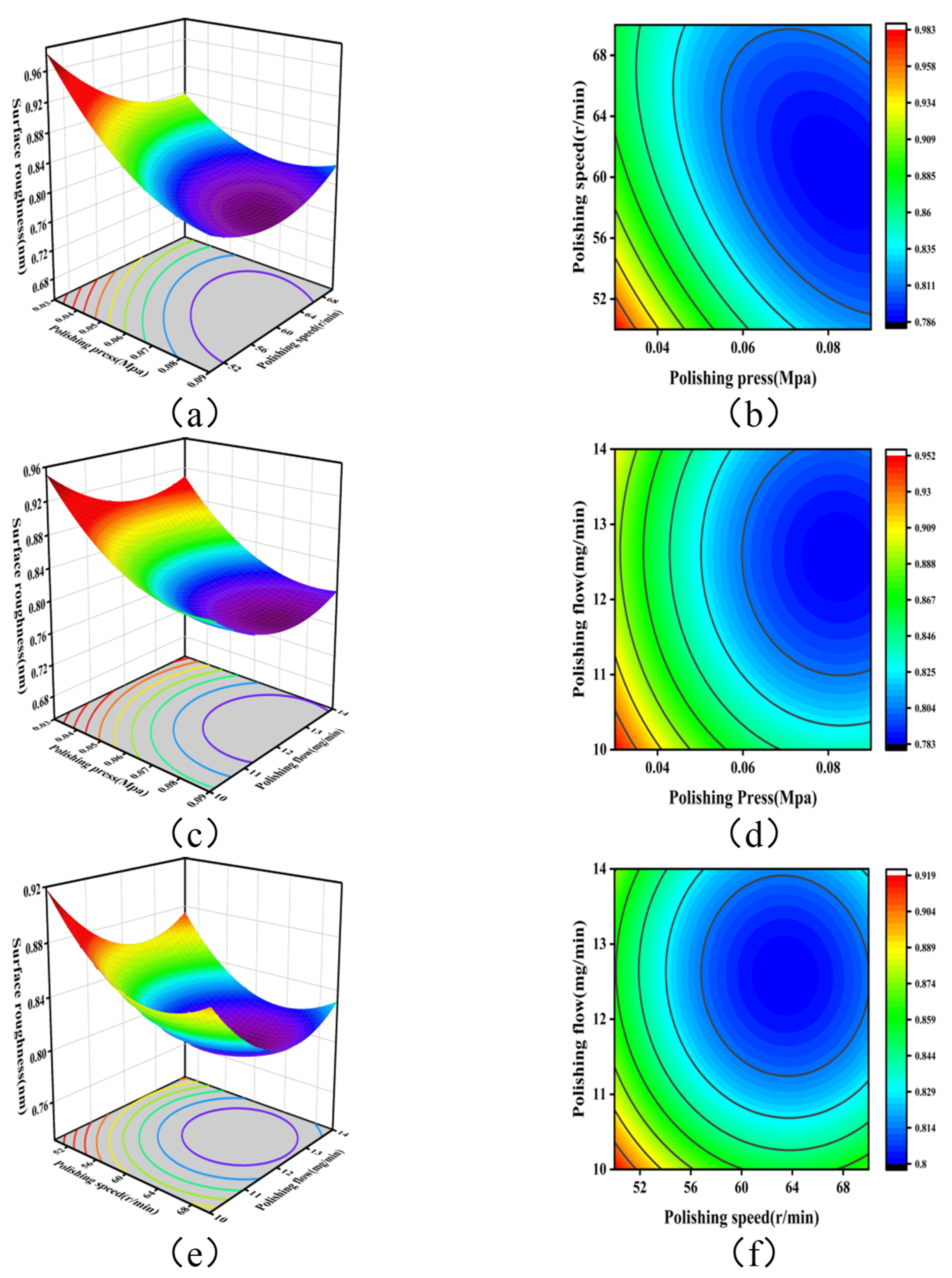
3.3. Analysis of Experimental Results of the Response Surface Method
3.4. Material Removal Mechanisms
4. Conclusions
- Fe3O4@MIL-100(Fe) was synthesized using a hydrothermal method, and the catalyst was fully characterized using an X-ray diffractogram, scanning electron microscopy, X-ray photoelectron spectroscopy, and the results showed the successful synthesis of novel core-shell structure Fe3O4@MIL-100(Fe) magnetic nanoparticles.
- CMP experiments were carried out under different catalytic conditions, and the results showed that under the synergistic conditions of visible light catalyzed-Fenton reaction, the surface roughness of SiC reached an optimal value of 0.861 nm, which improved the surface quality by 50% compared to the Fenton group.
- A mathematical prediction model for surface roughness was established. When the actual process parameters were as follows: polishing pressure of 0.06 MPa, polishing speed of 60 rpm, and polishing flow rate of 12 mL/min, the surface roughness achieved was as low as 0.78 nm.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Zhang, B.; Shi, Y.; Zhou, J.; Wang, R.; Zeng, N. Improved chemical mechanical polishing performance in 4H-SiC substrate by combining novel mixed abrasive slurry and photocatalytic effect. Appl. Surf. Sci. 2022, 575, 151676. [Google Scholar]
- Xue, M.; Xiao, W.; Zhang, T.; Wang, Z.; Su, J. Catalytic mechanism of tribochemical mechanical polishing on (0001) C-face of single crystal 6H-SiC substrate. Int. J. Adv. Manuf. Technol. 2024, 131, 2311–2323. [Google Scholar]
- Luo, Y.; Xiong, Q.; Lu, J.; Yan, Q.; Hu, D. Chemical mechanical polishing exploiting metal electrochemical corrosion of single-crystal SiC. Mater. Sci. Semicond. Process. 2022, 152, 107067. [Google Scholar]
- Datta, D.; Rai, H.; Singh, S.; Srivastava, M.; Sharma, R.K.; Gosvami, N.N. Nanoscale tribological aspects of chemical mechanical polishing: A review. Appl. Surf. Sci. Adv. 2022, 11, 100286. [Google Scholar]
- Kamiński, P.; Budzich, R.; Gaca, J.; Michałowski, P.P.; Kozłowski, R.; Harmasz, A.; Ciuk, T.; Płocharski, J. Effect of oxidation temperature on the inhomogeneity of chemical composition and density in nanometric SiO2 films grown on 4H-SiC. J. Mater. Chem. C 2021, 9, 4393–4404. [Google Scholar]
- Luo, Q.F.; Lu, J.; Xu, X.P. A comparative study on the material removal mechanisms of 6H-SiC polished by semi-fixed and fixed diamond abrasive tools. Wear 2016, 350–351, 99–106. [Google Scholar]
- Lu, J.; Huang, Y.; Fu, Y.; Yan, Q.; Zeng, S. Synergistic Effect of Photocatalysis and Fenton on Improving the Removal Rate of 4H-SiC during CMP. ECS J. Solid State Sci. Technol. 2021, 10, 044001. [Google Scholar]
- Zhou, Y.; Luo, H.; Chen, G.; Luo, G.; Pan, L.; Pan, G. Photocatalysis-Assisted Chemical Mechanical Polishing of SiC Wafer using a Novel SiO2@TiO2 Core-Shell Composite Nanoparticles Slurry. ECS J. Solid State Sci. Technol. 2023, 12, 104001. [Google Scholar]
- Yang, H.; Jin, Z.; Niu, L.; Wang, H.; Niu, H. Visible-light catalyzed assisted chemical mechanical polishing of single crystal diamond. Diam. Relat. Mater. 2022, 125, 108982. [Google Scholar]
- Qiu, J.; Zhang, X.; Feng, Y.; Zhang, X.; Wang, H.; Yao, J. Modified metal-organic frameworks as photocatalysts. Appl. Catal. B Environ. 2018, 231, 317–342. [Google Scholar]
- Li, S.; Cui, J.; Wu, X.; Zhang, X.; Hu, Q.; Hou, X. Rapid in situ microwave synthesis of Fe3O4@MIL-100(Fe) for aqueous diclofenac sodium removal through integrated adsorption and photodegradation. J. Hazard. Mater. 2019, 373, 408–416. [Google Scholar] [CrossRef]
- Aslam, S.; Zeng, J.; Subhan, F.; Li, M.; Lyu, F.; Li, Y.; Yan, Z. In situ one-step synthesis of Fe3O4@MIL-100(Fe) core-shells for adsorption of methylene blue from water. J. Colloid Interface Sci. 2017, 505, 186–195. [Google Scholar] [CrossRef]
- Abbas, M.; Rao, B.P.; Reddy, V.; Kim, C. Fe3O4/TiO2 core/shell nanocubes: Single-batch surfactantless synthesis, characterization and efficient catalysts for methylene blue degradation. Ceram. Int. 2014, 40, 11177–11186. [Google Scholar] [CrossRef]
- Gong, Q.J.; Liu, Y.; Dang, Z. Core-shell structured Fe3O4@GO@MIL-100(Fe) magnetic nanoparticles as heterogeneous photo-Fenton catalyst for 2,4-dichlorophenol degradation under visible light. J. Hazard. Mater. 2019, 371, 677–686. [Google Scholar] [CrossRef]
- Zhang, C.F.; Qiu, L.G.; Ke, F.; Zhu, Y.J.; Yuan, Y.P.; Xu, G.S.; Jiang, X. A novel magnetic recyclable photocatalyst based on a core–shell metal–organic framework Fe3O4@MIL-100(Fe) for the decolorization of methylene blue dye. J. Mater. Chem. A 2013, 1, 14329–14334. [Google Scholar] [CrossRef]
- Hu, S.Z.; Chen, X.; Li, Q.; Li, F.Y.; Fan, Z.P.; Wang, H.; Wang, Y.J.; Zheng, B.H.; Wu, G. Fe3+ doping promoted N2 photofixation ability of honeycombed graphitic carbon nitride: The experimental and density functional theory simulation analysis. Appl. Catal. B-Environ. 2017, 201, 58–69. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically Derived Graphene Oxide: Towards Large-Area Thin-Film Electronics and Optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Guo, W.; Sun, W.; Lv, L.P.; Kong, S.; Wang, Y. Microwave-Assisted Morphology Evolution of Fe-Based Metal–Organic Frameworks and Their Derived Fe2O3 Nanostructures for Li-Ion Storage. ACS Nano 2017, 11, 4198–4205. [Google Scholar] [CrossRef]
- He, W.; Li, Z.; Lv, S.; Niu, M.; Zhou, W.; Li, J.; Lu, R.; Gao, H.; Pan, C.; Zhang, S. Facile synthesis of Fe3O4@ MIL-100 (Fe) towards enhancing photo-Fenton like degradation of levofloxacin via a synergistic effect between Fe3O4 and MIL-100 (Fe). Chem. Eng. J. 2021, 409, 128274. [Google Scholar] [CrossRef]
- Zhang, N.; Mu, M.; Zhu, S.; Gao, Y.; Lu, M. Well-defined Fe3O4@ MIL-100 (Fe) hollow nanoflower heterostructures for selective dection and monitoring of benzoylurea insecticides from food and water. Food Chem. 2024, 435, 137579. [Google Scholar] [CrossRef]
- Jain, R.; Luthra, V.; Gokhale, S. Dysprosium doping induced shape and magnetic anisotropy of Fe3−xDyx O4 (x=0.01–0.1) nanoparticles. J. Magn. Magn. Mater. 2016, 414, 111–115. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Wang, Y.; Li, Y. Sr2Fe2−xMox O6−ᵹ perovskite as an anode in a solid oxide fuel cell: Effect of the substitution ratio. Catalysis Today 2016, 259, 417–422. [Google Scholar] [CrossRef]
- Gong, J.; Wang, W.; Liu, W.; Song, Z. Polishing Mechanism of CMP 4H-SiC Crystal Substrate (0001) Si Surface Based on an Alumina (Al2O3) Abrasive. Mater. Des. 2024, 17, 679. [Google Scholar]
- Wang, W.; Lu, X.; Wu, X.; Zhang, Y.; Wang, R.; Yang, D.; Pi, X. Chemical–mechanical polishing of 4H silicon carbide wafers. Adv. Mater. Interfaces 2023, 10, 2202369. [Google Scholar] [CrossRef]
- Ahmad, M.; Chen, S.; Ye, F.; Quan, X.; Afzal, S.; Yu, H.; Zhao, X. Efficient photo-Fenton activity in mesoporous MIL-100(Fe) decorated with ZnO nanosphere for pollutants degradation. Appl. Catal. B Environ. 2019, 245, 428–438. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, B.; Baruah, A.; Shanker, V. Synthesis of Magnetically Separable and Recyclable g-C3N4–Fe3O4 Hybrid Nanocomposites with Enhanced Photocatalytic Performance under Visible-Light Irradiation. J. Phys. Chem. C 2013, 117, 26135–26143. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Peng, Y.; Guo, J.; Ding, C. Silica-assisted fixed agglomerated diamond abrasive polishing. J. Manuf. Process. 2020, 59, 595–603. [Google Scholar]
- Lipkin, L.A.; Palmour, J.W. Improved oxidation procedures for reduced SiO2/SiC defects. J. Electron. Mater. 1996, 25, 909–915. [Google Scholar]
- Liu, G.; Huang, Z.; Liu, X.; Jiang, D. Removal behaviors of different SiC ceramics during polishing. J. Mater. Sci. Technol. 2010, 26, 125–130. [Google Scholar] [CrossRef]


| Level | Polishing Pressure (MPa) | Polishing Speed (r/min) | Polishing Flow Rate (mL/min) |
|---|---|---|---|
| −1 | 0.03 | 50 | 10 |
| 0 | 0.06 | 60 | 12 |
| 1 | 0.09 | 70 | 14 |
| Num | A (MPa) | B (r/min) | C (mL/min) | X (nm) |
|---|---|---|---|---|
| 1 | 0.03 | 50 | 12 | 0.983 |
| 2 | 0.09 | 50 | 12 | 0.842 |
| 3 | 0.03 | 70 | 12 | 0.856 |
| 4 | 0.09 | 70 | 12 | 0.831 |
| 5 | 0.03 | 60 | 10 | 0.968 |
| 6 | 0.09 | 60 | 10 | 0.831 |
| 7 | 0.03 | 60 | 14 | 0.921 |
| 8 | 0.09 | 60 | 14 | 0.790 |
| 9 | 0.06 | 50 | 10 | 0.906 |
| 10 | 0.06 | 70 | 10 | 0.879 |
| 11 | 0.06 | 50 | 14 | 0.867 |
| 12 | 0.06 | 70 | 14 | 0.848 |
| 13 | 0.06 | 60 | 12 | 0.809 |
| 14 | 0.06 | 60 | 12 | 0.811 |
| 15 | 0.06 | 60 | 12 | 0.783 |
| 16 | 0.06 | 60 | 12 | 0.816 |
| 17 | 0.06 | 60 | 12 | 0.823 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.0508 | 9 | 0.0056 | 11.90 | 0.0018 | significant |
| A | 0.0235 | 1 | 0.0235 | 49.61 | 0.0002 | |
| B | 0.0042 | 1 | 0.0042 | 8.92 | 0.0203 | |
| C | 0.0031 | 1 | 0.0031 | 6.58 | 0.0373 | |
| AB | 0.0034 | 1 | 0.0034 | 7.09 | 0.0324 | |
| AC | 9.000 × 10−6 | 1 | 9.000 × 10−6 | 0.0190 | 0.8943 | |
| BC | 0.0000 | 1 | 0.0000 | 0.0337 | 0.8595 | |
| A2 | 0.0055 | 1 | 0.0055 | 11.53 | 0.0115 | |
| B2 | 0.0047 | 1 | 0.0047 | 9.99 | 0.0159 | |
| C2 | 0.0046 | 1 | 0.0046 | 9.69 | 0.0170 | |
| Residual | 0.0033 | 7 | 0.0005 | |||
| Lack of fit | 0.0024 | 3 | 0.0008 | 3.46 | 0.1306 | not significant |
| Pure error | 0.0009 | 4 | 0.0002 | |||
| Cor total | 0.0542 | 16 |
| Target Parameters | Projected Value | Actual Value | Relative Error |
|---|---|---|---|
| Surface roughness (nm) | 0.803 | 0.78 | 2.86% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Ran, B.; Pan, J.; Zhuang, R. Investigation of the Visible Photocatalytic–Fenton Reactive Composite Polishing Process for Single-Crystal SiC Wafers Based on Response Surface Methodology. Micromachines 2025, 16, 380. https://doi.org/10.3390/mi16040380
Han Z, Ran B, Pan J, Zhuang R. Investigation of the Visible Photocatalytic–Fenton Reactive Composite Polishing Process for Single-Crystal SiC Wafers Based on Response Surface Methodology. Micromachines. 2025; 16(4):380. https://doi.org/10.3390/mi16040380
Chicago/Turabian StyleHan, Zijuan, Bo Ran, Jisheng Pan, and Rongji Zhuang. 2025. "Investigation of the Visible Photocatalytic–Fenton Reactive Composite Polishing Process for Single-Crystal SiC Wafers Based on Response Surface Methodology" Micromachines 16, no. 4: 380. https://doi.org/10.3390/mi16040380
APA StyleHan, Z., Ran, B., Pan, J., & Zhuang, R. (2025). Investigation of the Visible Photocatalytic–Fenton Reactive Composite Polishing Process for Single-Crystal SiC Wafers Based on Response Surface Methodology. Micromachines, 16(4), 380. https://doi.org/10.3390/mi16040380







