A Double Emulsion-Based, Plastic-Glass Hybrid Microfluidic Platform for Protein Crystallization
Abstract
:1. Introduction
2. Experimental Section
2.1. Device Fabrication
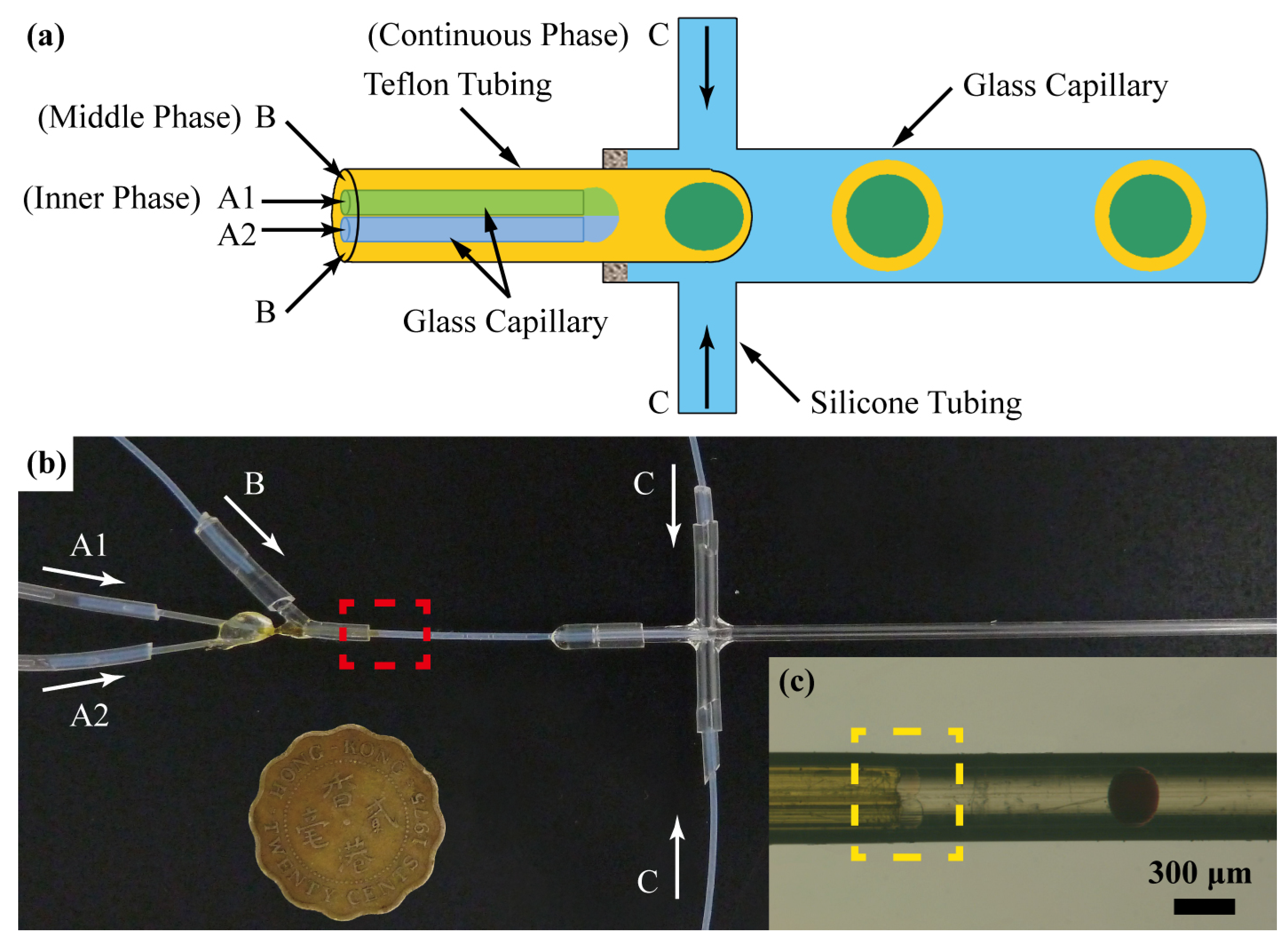
2.2. Microfluidic Production of Emulsions
2.3. Reagents and Data Analysis for Protein Crystallization
3. Results and Discussion
3.1. Performance of the Hybrid Microfluidic Device
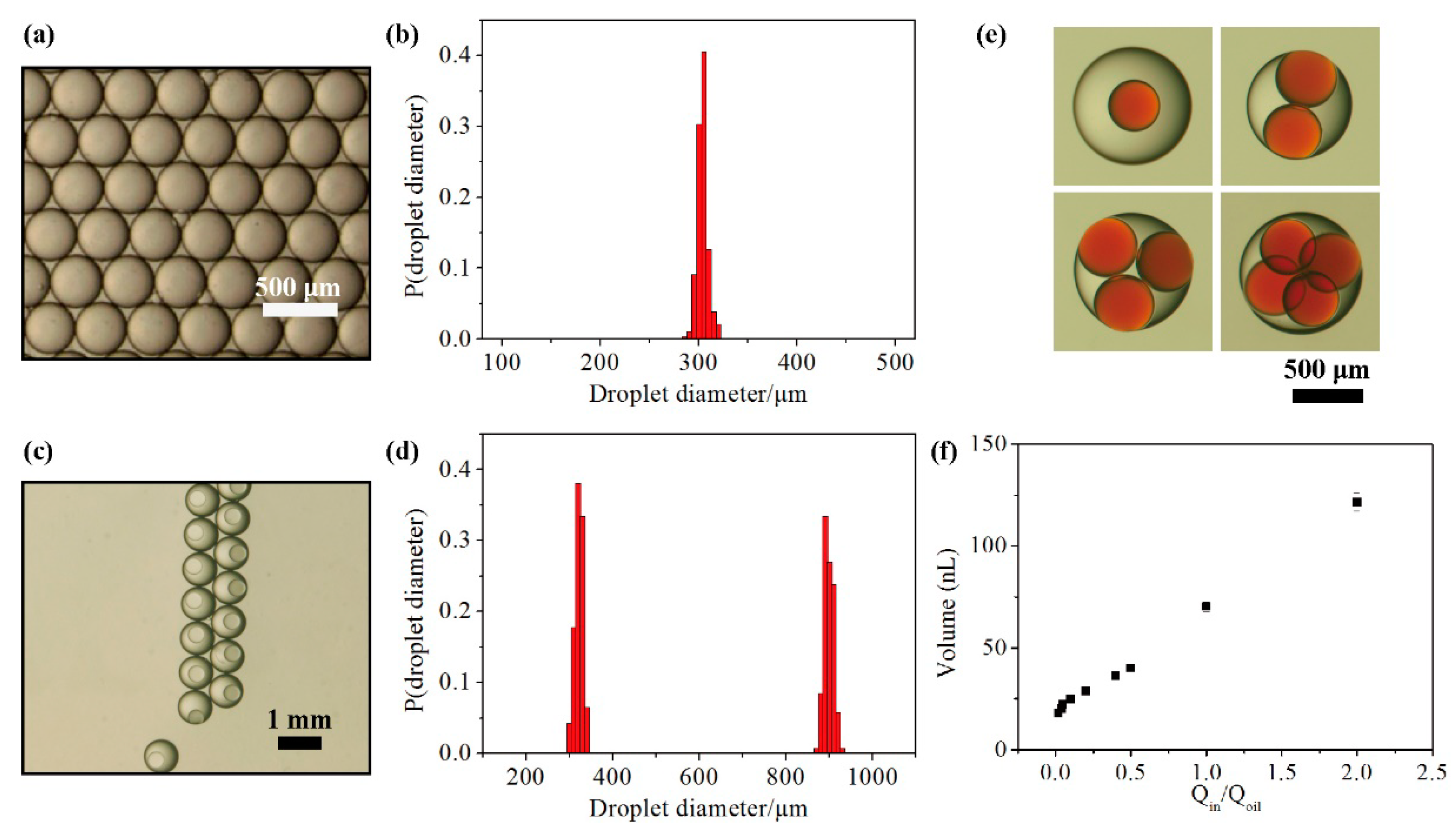
3.2. Stability and Mass Transport of Double Emulsions

3.3. Double Emulsion-Based Approach with the Vapor Diffusion Method


| Protein | Concentration (mg/mL) | Applied Concentration of PEG 8000 (% w/w) |
|---|---|---|
| Lysozyme | 100/80/50 | 40 |
| Thaumatin | 20/10 | 40 |
| Trypsin | 80/60/40 | 30 |
| Horseradish peroxidase | 10/5 | 35 |
3.4. Double Emulsion-Based Approach with the Microbatch Method

3.5. Effect of Protein Adsorption at the Liquid-Liquid Interface on Protein Crystallization


3.6. Volume Effect on Protein Crystallization


3.7. Double Emulsion-Based Approach for Sparse Matrix Screening

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McPherson, A. Introduction to protein crystallization. Methods 2004, 34, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Chayen, N.E. Tackling the bottleneck of protein crystallization in the post-genomic era. Trends Biotechnol. 2002, 20, 98. [Google Scholar] [CrossRef]
- Skolnick, J.; Fetrow, J.S.; Kolinski, A. Structural genomics and its importance for gene function analysis. Nat. Biotechnol. 2000, 18, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Deisenhofer, J.; Epp, O.; Miki, K.; Huber, R.; Michel, H. Structure of the protein subunits in the photosynthetic reaction center of Rhodopseudomonas viridis at 3a resolution. Nature 1985, 318, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.A.; Cabral, J.M.; Pfuetzner, R.A.; Kuo, A.L.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Palczewski, K.; Kumasaka, T.; Hori, T.; Behnke, C.A.; Motoshima, H.; Fox, B.A.; Le Trong, I.; Teller, D.C.; Okada, T.; Stenkamp, R.E.; Yamamoto, M.; Miyano, M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 2000, 289, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Abola, E.; Kuhn, P.; Earnest, T.; Stevens, R.C. Automation of X-ray crystallography. Nat. Struct. Biol. 2000, 7, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E. Chemical-shifts and 3-dimensional protein structures. J. Biomol. NMR 1995, 5, 217–225. [Google Scholar] [PubMed]
- Adrian, M.; Dubochet, J.; Lepault, J.; Mcdowall, A.W. Cryo-electron microscopy of viruses. Nature 1984, 308, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Kuhlbrandt, W. The resolution revolution. Science 2014, 343, 1443–1444. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Narayanan, B.C.; Di Costanzo, L.; Dutta, S.; Ghosh, S.; Hudson, B.P.; Lawson, C.L.; Peisach, E.; Prlic, A.; Rose, P.W.; et al. Trendspotting in the Protein Data Bank. Febs Lett. 2013, 587, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Chayen, N.E. Turning protein crystallisation from an art into a science. Curr. Opin. Struct. Biol. 2004, 14, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Weselak, M.; Patch, M.G.; Selby, T.L.; Knebel, G.; Stevens, R.C. Robotics for automated crystal formation and analysis. Methods Enzymol. 2003, 368, 45–76. [Google Scholar] [PubMed]
- Zhu, Y.; Zhu, L.N.; Guo, R.; Cui, H.J.; Ye, S.; Fang, Q. Nanoliter-scale protein crystallization and screening with a microfluidic droplet robot. Sci. Rep. 2014, 4, 5046. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Gerdts, C.J.; Ismagilov, R.F. Using nanoliter plugs in microfluidics to facilitate and understand protein crystallization. Curr. Opin. Struct. Biol. 2005, 15, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ismagilov, R.F. Protein crystallization using microfluidic technologies based on valves, droplets and SlipChip. Annu. Rev. Biophys. 2010, 39, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Van der Woerd, M.; Ferree, D.; Pusey, M. The promise of macromolecular crystallization in microfluidic chips. J. Struct. Biol. 2003, 142, 180–187. [Google Scholar] [CrossRef]
- Hansen, C.; Quake, S.R. Microfluidics in structural biology: Smaller, faster... better. Curr. Opin. Struct. Biol. 2003, 13, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.L.; Skordalakes, E.; Berger, J.M.; Quake, S.R. A robust and scalable microfluidic metering method that allows protein crystal growth by free interface diffusion. Proc. Natl. Acad. Sci. USA 2002, 99, 16531–16536. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.L.; Roberts, G.W.; Tice, J.D.; Gennis, R.B.; Kenis, P.J.A. Microfluidic generation of lipidic mesophases for membrane protein crystallization. Cryst. Growth Des. 2009, 9, 2566–2569. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Roach, L.S.; Ismagilov, R.F. Screening of protein crystallization conditions on a microfluidic chip using nanoliter-size droplets. J. Am. Chem. Soc. 2003, 125, 11170–11171. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Tice, J.D.; Roach, L.S.; Ismagilov, R.F. A droplet-based, composite PDMS/glass capillary microfluidic system for evaluating protein crystallization conditions by microbatch and vapor-diffusion methods with on-chip X-ray diffraction. Angew. Chem. Int. Ed. 2004, 43, 2508–2511. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Ismagilov, R.F. A microfluidic approach for screening submicroliter volumes against multiple reagents by using preformed arrays of nanoliter plugs in a three-phase liquid/liquid/gas flow. Angew. Chem. Int. Ed. 2005, 44, 2520–2523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lau, L.; Lam, W.W.L.; Au, S.W.N.; Zheng, B. Nanoliter dispensing method by degassed poly(dimethylsiloxane) microchannels and its application in protein crystallization. Anal. Chem. 2007, 79, 4924–4930. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Guo, D.M.; Zheng, B. Rehydratable gel for rapid loading of nanoliter solution and its application in protein crystallization. RSC Adv. 2012, 2, 4857–4863. [Google Scholar] [CrossRef]
- Guha, S.; Perry, S.L.; Pawate, A.S.; Kenis, P.J.A. Fabrication of X-ray compatible microfluidic platforms for protein crystallization. Sens. Actuators B Chem. 2012, 174, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Selimovic, S.; Gobeaux, F.; Fraden, S. Mapping and manipulating temperature-concentration phase diagrams using microfluidics. Lab Chip 2010, 10, 1696–1699. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.U.; Cristobal, G.; Link, D.R.; Thorsen, T.; Jia, Y.W.; Piattelli, K.; Fraden, S. Control and measurement of the phase behavior of aqueous solutions using microfluidics. J. Am. Chem. Soc. 2007, 129, 8825–8835. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.R.; Weitz, D.A. High-order multiple emulsions formed in poly(dimethylsiloxane) microfluidics. Small 2009, 5, 2030–2032. [Google Scholar] [CrossRef] [PubMed]
- Cournarie, F.; Savelli, M.P.; Rosilio, W.; Bretez, F.; Vauthier, C.; Grossiord, J.L.; Seiller, M. Insulin-loaded W/O/W multiple emulsions: Comparison of the performances of systems prepared with medium-chain-triglycerides and fish oil. Eur. J. Pharm. Biopharm. 2004, 58, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Muschiolik, G. Multiple emulsions for food use. Curr. Opin. Colloid Interface Sci. 2007, 12, 213–220. [Google Scholar] [CrossRef]
- Patravale, V.B.; Mandawgade, S.D. Novel cosmetic delivery systems: An application update. Int. J. Cosmet. Sci. 2008, 30, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bauer, W.A.C.; Fischlechner, M.; Hollfelder, F.; Kaminski, C.F.; Huck, W.T.S. Monodisperse water-in-oil-in-water (W/O/W) double emulsion droplets as uniform compartments for high-throughput analysis via flow cytometry. Micromachines 2013, 4, 402–413. [Google Scholar] [CrossRef]
- Wu, N.; Oakeshott, J.G.; Easton, C.J.; Peat, T.S.; Surjadi, R.; Zhu, Y. A double-emulsion microfluidic platform for in vitro green fluorescent protein expression. J. Micromech. Microeng. 2011, 21, 054032. [Google Scholar] [CrossRef]
- Lamprecht, A.; Ubrich, N.; Perez, M.H.; Lehr, C.M.; Hoffman, M.; Maincent, P. Influences of process parameters on nanoparticle preparation performed by a double emulsion pressure homogenization technique. Int. J. Pharm. 2000, 196, 177–182. [Google Scholar] [CrossRef]
- Chayen, N.E. A novel technique for containerless protein crystallization. Protein Eng. 1996, 9, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Akella, S.V.; Mowitz, A.; Heymann, M.; Fraden, S. Emulsion-based technique to measure protein crystal nucleation rates of lysozyme. Cryst. Growth Design 2014, 14, 4487–4509. [Google Scholar] [CrossRef]
- Hampel, A.; Labanaus, M.; Connors, P.G.; Kirkegar, L.; Rajbhand, U.L.; Sigler, P.B.; Bock, R.M. Single crystals of transfer rna from formylmethionine and phenylalanine transfer rnas. Science 1968, 162, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A. The Growth and Preliminary Investigation of Protein and Nucleic Acid Crystals for X-ray Diffraction Analysis. In Methods of Biochemical Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1976; pp. 249–345. [Google Scholar]
- Chayen, N.E.; Stewart, P.D.S.; Maeder, D.L.; Blow, D.M. An automated-system for microbatch protein crystallization and screening. J. Appl. Crystallogr. 1990, 23, 297–302. [Google Scholar] [CrossRef]
- Chayen, N.E.; Stewart, P.D.S.; Blow, D.M. Microbatch crystallization under oil—A new technique allowing many small-volume crystallization trials. J. Cryst. Growth 1992, 122, 176–180. [Google Scholar] [CrossRef]
- Chapman, H.N.; Fromme, P.; Barty, A.; White, T.A.; Kirian, R.A.; Aquila, A.; Hunter, M.S.; Schulz, J.; DePonte, D.P.; Weierstall, U.; et al. Femtosecond X-ray protein nanocrystallography. Nature 2011, 470, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Gati, C.; Bourenkov, G.; Klinge, M.; Rehders, D.; Stellato, F.; Oberthur, D.; Yefanov, O.; Sommer, B.P.; Mogk, S.; Duszenko, M.; et al. Serial crystallography on in vivo grown microcrystals using synchrotron radiation. IUCrJ 2014, 1, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Olieric, V.; Ma, P.K.; Panepucci, E.; Diederichs, K.; Wang, M.T.; Caffrey, M. In meso in situ serial X-ray crystallography of soluble and membrane proteins. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 1238–1256. [Google Scholar] [CrossRef]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef] [PubMed]
- DArcy, A.; Elmore, C.; Stihle, M.; Johnston, J.E. A novel approach to crystallising proteins under oil. J. Cryst. Growth 1996, 168, 175–180. [Google Scholar] [CrossRef]
- Ficheux, M.F.; Bonakdar, L.; Leal-Calderon, F.; Bibette, J. Some stability criteria for double emulsions. Langmuir 1998, 14, 2702–2706. [Google Scholar] [CrossRef]
- Matsumoto, S.; Inoue, T.; Kohda, M.; Ikura, K. Water permeability of oil layers in W/O/W emulsions under osmotic pressure gradients. J. Colloid Interface Sci. 1980, 77, 555–563. [Google Scholar] [CrossRef]
- Colinart, P.; Delepine, S.; Trouve, G.; Renon, H. Water transfer in emulsified liquid membrane processes. J. Membr. Sci. 1984, 20, 167–187. [Google Scholar] [CrossRef]
- Wen, L.X.; Papadopoulos, K.D. Visualization of water transport in W1/O/W2 emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2000, 174, 159–167. [Google Scholar] [CrossRef]
- Wen, L.X.; Papadopoulos, K.D. Effects of surfactants on water transport in W1/O/W2 emulsions. Langmuir 2000, 16, 7612–7617. [Google Scholar] [CrossRef]
- Bahtz, J.; Gunes, D.Z.; Hughes, E.; Pokorny, L.; Riesch, F.; Syrbe, A.; Fischer, P.; Windhab, E.J. Decoupling of mass transport mechanisms in the stagewise swelling of multiple emulsions. Langmuir 2015, 31, 5265–5273. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.J.; Song, J.J.; Chaumont, F.; Ye, Q. Differential responses of plasma membrane aquaporins in mediating water transport of cucumber seedlings under osmotic and salt stresses. Plant Cell Environ. 2015, 38, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Chayen, N.E.; Saridakis, E. Protein crystallization: From purified protein to diffraction-quality crystal. Nat. Methods 2008, 5, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Roach, L.S.; Song, H.; Ismagilov, R.F. Controlling nonspecific protein adsorption in a plug-based microfluidic system by controlling interfacial chemistry using fluorous-phase surfactants. Anal. Chem. 2005, 77, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Kalyanikutty, K.P. The liquid-liquid interface as a medium to generate nanocrystalline films of inorganic materials. Acc. Chem. Res. 2008, 41, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Tong, L.M.; Xia, Y.A. Synthesis of colloidal metal nanocrystals in droplet reactors: The pros and cons of interfacial adsorption. Nano Lett. 2014, 14, 4189–4194. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, M.K.; Agrawal, V.V.; Bera, M.K.; Kalyanikutty, K.P.; Daillant, J.; Blot, C.; Kubowicz, S.; Konovalov, O.; Rao, C.N.R. Formation and ordering of gold nanoparticles at the toluene-water interface. J. Phys. Chem. C 2008, 112, 1739–1743. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, A.P.; Peng, S.; Duan, H.W. Responsive plasmonic assemblies of amphiphilic nanocrystals at oil-water interfaces. Acs Nano 2010, 4, 6098–6104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.T.; Wu, F.X.; Peng, M.H.; Wang, X.Y.; Xia, D.G.; Guo, G.S. One-step, facile and ultrafast synthesis of phase- and size-controlled Pt-Bi intermetallic nanocatalysts through continuous-flow microfluidics. J. Am. Chem. Soc. 2015, 137, 6263–6269. [Google Scholar] [CrossRef] [PubMed]
- Silver, B.R.; Fulop, V.; Unwin, P.R. Protein crystallization at oil/water interfaces. New J. Chem. 2011, 35, 602–606. [Google Scholar] [CrossRef]
- Zhang, Y.; Ho, Y.P.; Chiu, Y.L.; Chan, H.F.; Chlebina, B.; Schuhmann, T.; You, L.C.; Leong, K.W. A programmable microenvironment for cellular studies via microfluidics-generated double emulsions. Biomaterials 2013, 34, 4564–4572. [Google Scholar] [CrossRef] [PubMed]
- Heymann, M.; Opthalage, A.; Wierman, J.L.; Akella, S.; Szebenyi, D.M.E.; Gruner, S.M.; Fraden, S. Room-temperature serial crystallography using a kinetically optimized microfluidic device for protein crystallization and on-chip X-ray diffraction. IUCrJ 2014, 1, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Kashchiev, D.; Verdoes, D.; van Rosmalen, G.M. Induction time and metastability limit in new phase formation. J. Cryst. Growth 1991, 110, 373–380. [Google Scholar] [CrossRef]
- Laval, P.; Salmon, J.B.; Joanicot, M. A microfluidic device for investigating crystal nucleation kinetics. J. Cryst. Growth 2007, 303, 622–628. [Google Scholar] [CrossRef]
- Salmon, J.B.; Leng, J. Microfluidics for kinetic inspection of phase diagrams. Comptes Rendus Chim. 2009, 12, 258–269. [Google Scholar] [CrossRef]
- Jiang, S.F.; ter Horst, J.H. Crystal nucleation rates from probability distributions of induction times. Cryst. Growth Des. 2011, 11, 256–261. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Zhou, X.; Zheng, B. A Double Emulsion-Based, Plastic-Glass Hybrid Microfluidic Platform for Protein Crystallization. Micromachines 2015, 6, 1629-1644. https://doi.org/10.3390/mi6111446
Zhu D, Zhou X, Zheng B. A Double Emulsion-Based, Plastic-Glass Hybrid Microfluidic Platform for Protein Crystallization. Micromachines. 2015; 6(11):1629-1644. https://doi.org/10.3390/mi6111446
Chicago/Turabian StyleZhu, Deyong, Xiaohu Zhou, and Bo Zheng. 2015. "A Double Emulsion-Based, Plastic-Glass Hybrid Microfluidic Platform for Protein Crystallization" Micromachines 6, no. 11: 1629-1644. https://doi.org/10.3390/mi6111446
APA StyleZhu, D., Zhou, X., & Zheng, B. (2015). A Double Emulsion-Based, Plastic-Glass Hybrid Microfluidic Platform for Protein Crystallization. Micromachines, 6(11), 1629-1644. https://doi.org/10.3390/mi6111446






