Nafamostat Mesilate Enhances the Radiosensitivity and Reduces the Radiation-Induced Invasive Ability of Colorectal Cancer Cells
Abstract
:1. Introduction
2. Results
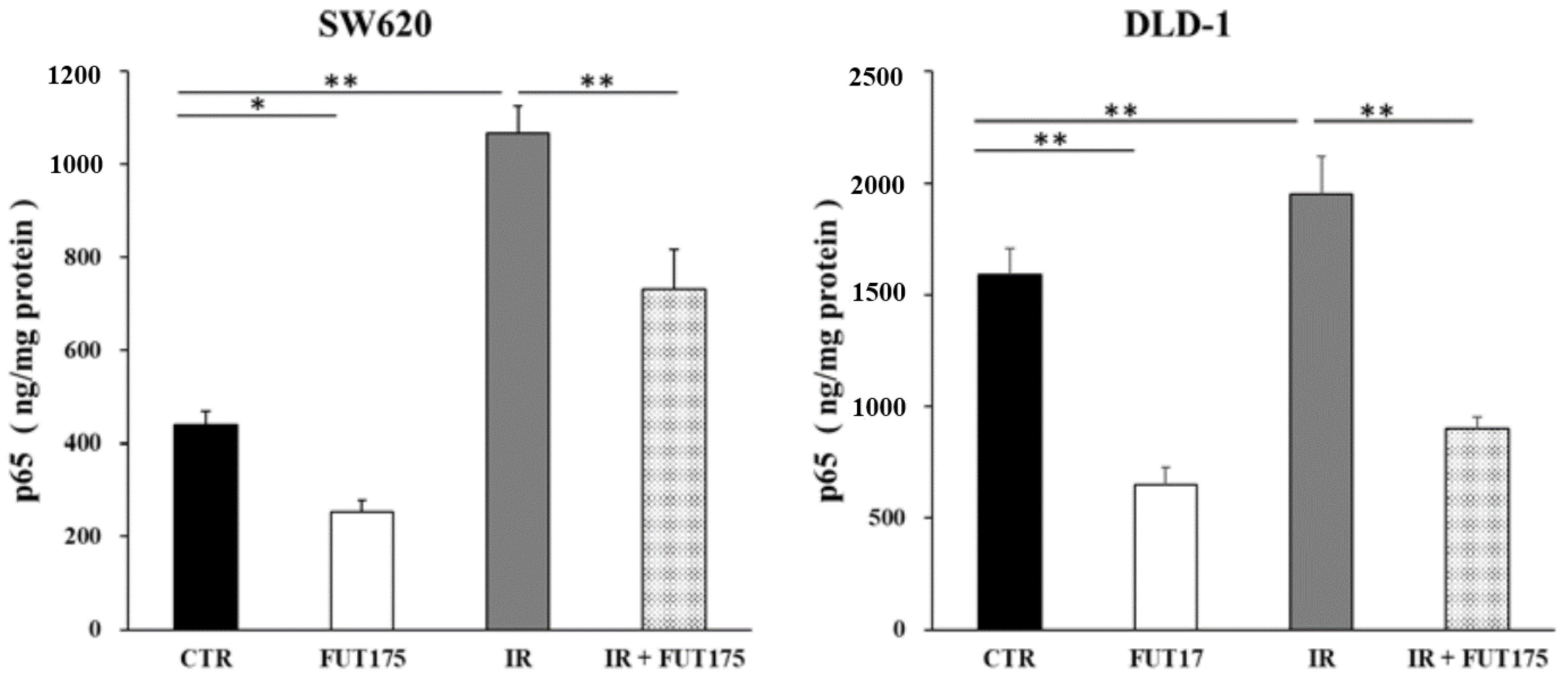
2.1. Nafamostat Mesilate (FUT175) Prevents NF-κB Activation and Induces Apoptosis in Irradiated Colorectal Cancer (CRC) Cells
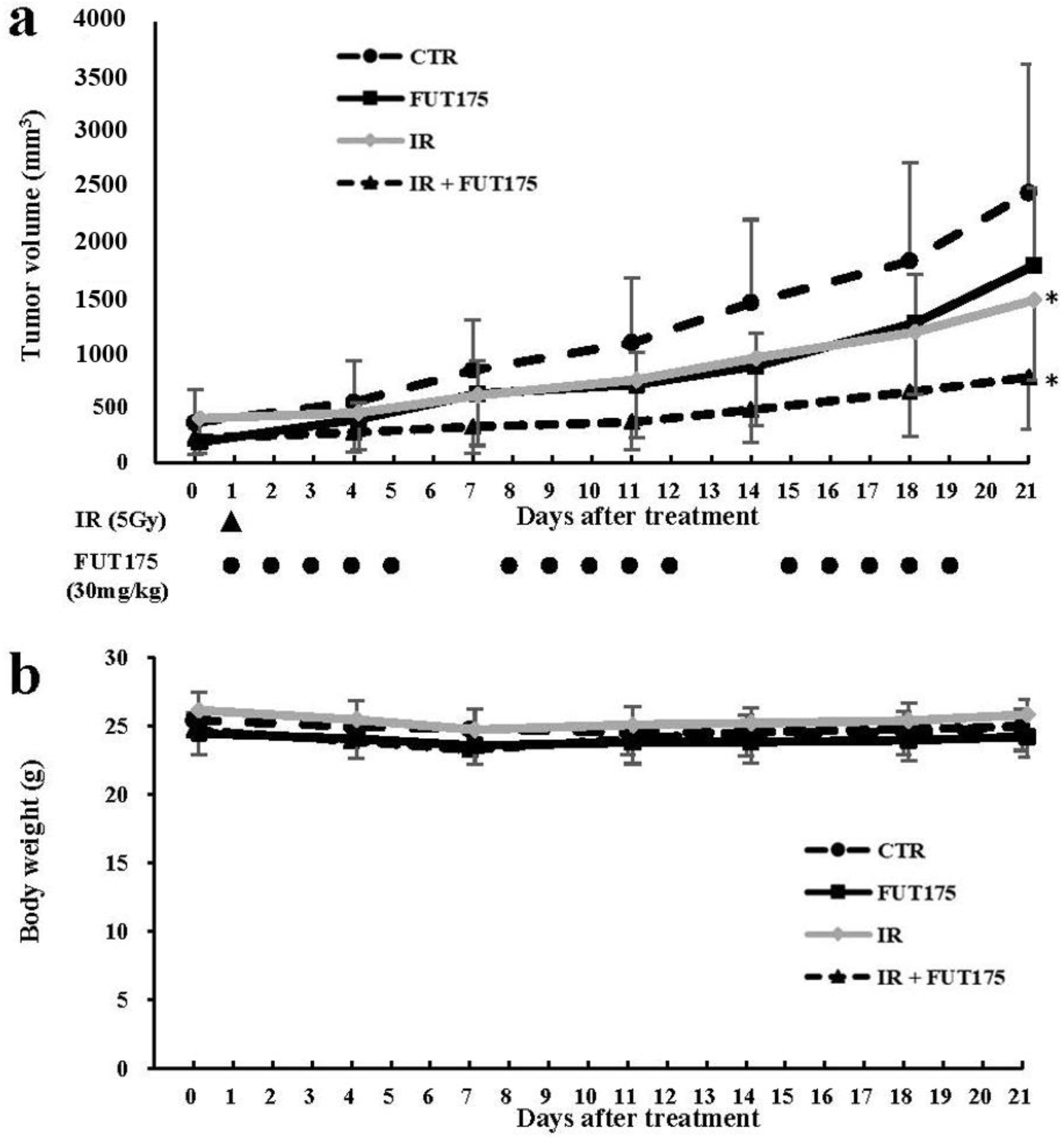
2.2. FUT175 Enhances the Anti-Tumor Effect of Radiotherapy In Vivo
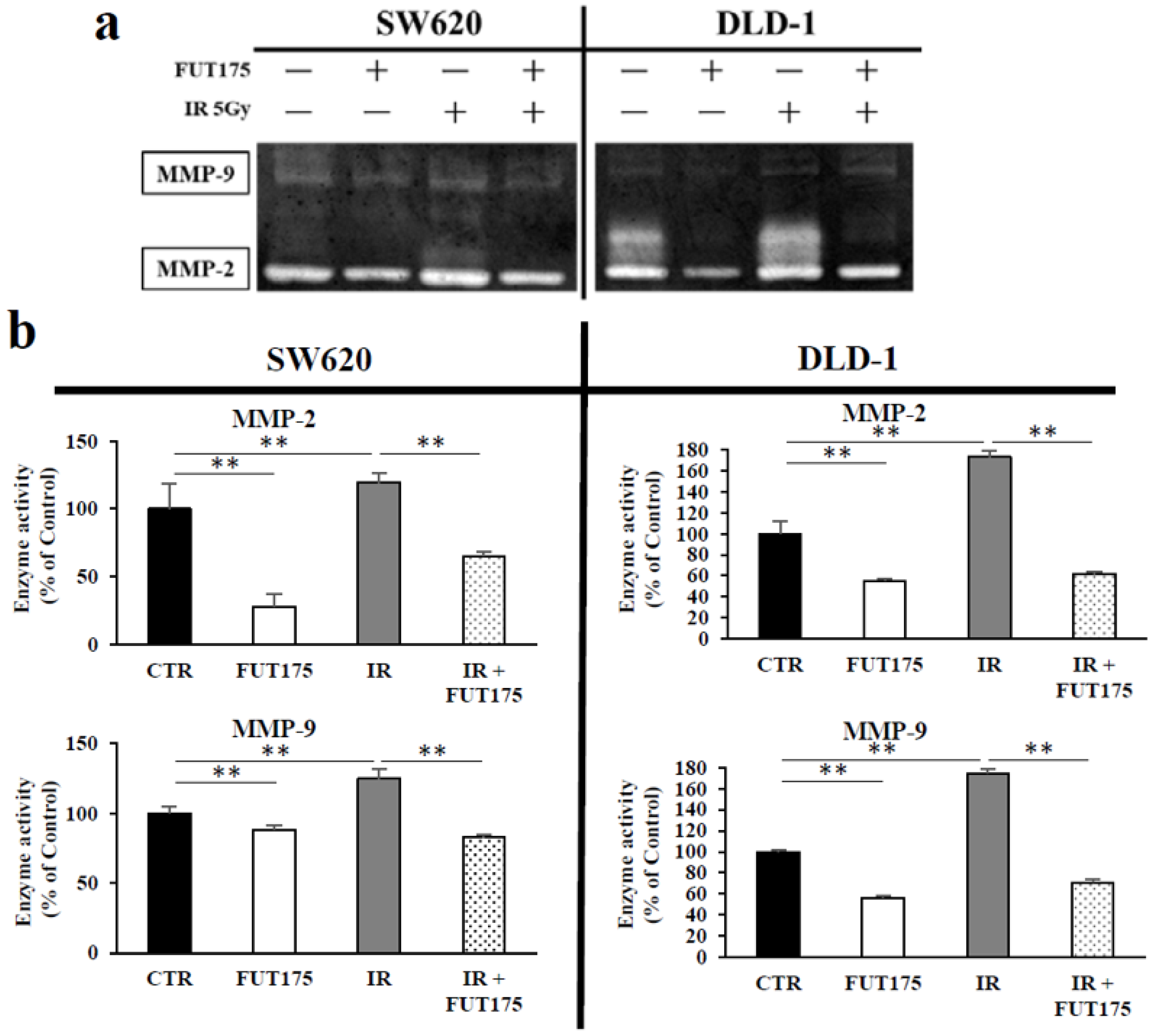
2.3. FUT175 Prevents the Ionizing Radiation (IR)-Induced Invasiveness of CRC Cells through Inhibition of MMP-2 and -9
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents
4.3. Ionizing Radiation
4.4. Antibodies
4.5. In Vitro Experiments’ Treatment Groups
4.6. Cell Proliferation Assays
4.7. Apoptosis Analysis by Flow Cytometry
4.8. Western Blot Analysis
4.9. Quantitative Analysis of NF-κB Activity
4.10. Wound-Healing Assays
4.11. Invasion Assays
4.12. Gelatin Zymography
4.13. Matrix Metalloproteinase (MMP) Activity Assays
4.14. Animal Models
4.15. Immunohistochemical Staining and TdT-Mediated dUTP Nick-End Labeling (TUNEL) Assays
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; World Health Organization: Geneva, Switzerland, 2014; p. 618. [Google Scholar]
- Watanabe, T.; Muro, K.; Ajioka, Y.; Hashiguchi, Y.; Ito, Y.; Saito, Y.; Hamaguchi, T.; Ishida, H.; Ishiguro, M.; Ishihara, S.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2018, 23, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Bosset, J.F.; Collette, L.; Calais, G.; Mineur, L.; Maingon, P.; Radosevic-Jelic, L.; Daban, A.; Bardet, E.; Beny, A.; Ollier, J.C.; et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006, 355, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Von Essen, C.F. Radiation enhancement of metastasis: A review. Clin. Exp. Metastasis 1991, 9, 77–104. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Baltimore, D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Signaling to NF-κB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear factor-κB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Morisaki, T.; Hashizume, K.; Yao, T.; Tsuneyoshi, M.; Noshiro, H.; Nakamura, K.; Yamanaka, T.; Uchiyama, A.; Tanaka, M.; et al. Nuclear factor-κB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin. Cancer Res. 2001, 7, 4136–4142. [Google Scholar] [PubMed]
- Greten, F.R.; Weber, C.K.; Greten, T.F.; Schneider, G.; Wagner, M.; Adler, G.; Schmid, R.M. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology 2002, 123, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Z.; Wang, L.; Zhang, X. NF-κB signaling pathway, inflammation and colorectal cancer. Cell. Mol. Immunol. 2009, 6, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Deorukhkar, A.; Krishnan, S. Targeting inflammatory pathways for tumor radiosensitization. Biochem. Pharmacol. 2010, 80, 1904–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, A.; Yokoe, T.; Tanaka, K.; Saigusa, S.; Toiyama, Y.; Yasuda, H.; Inoue, Y.; Miki, C.; Kusunoki, M. Radiation induces epithelial-mesenchymal transition in colorectal cancer cells. Oncol. Rep. 2012, 27, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; He, Y.; Ji, J.; Yao, Y.; Shen, W.; Luo, J.; Zhu, W.; Cao, H.; Geng, Y.; Xu, J.; et al. Hypoxia-inducible factor 1alpha (HIF-1alpha) and reactive oxygen species (ROS) mediates radiation-induced invasiveness through the SDF-1alpha/CXCR4 pathway in non-small cell lung carcinoma cells. Oncotarget 2015, 6, 10893–10907. [Google Scholar] [CrossRef] [PubMed]
- Wild-Bode, C.; Weller, M.; Rimner, A.; Dichgans, J.; Wick, W. Sublethal irradiation promotes migration and invasiveness of glioma cells: Implications for radiotherapy of human glioblastoma. Cancer Res. 2001, 61, 2744–2750. [Google Scholar] [PubMed]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Stark, A.; Johnson, C.D.; Daniel, F.; Carmichael, J.; Buckels, J.; Imrie, C.W.; Brown, P.; Neoptolemos, J.P. A phase II trial of marimastat in advanced pancreatic cancer. Br. J. Cancer 2001, 85, 1865–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.D.; Saphner, T.J.; Waterhouse, D.M.; Chen, T.T.; Rush-Taylor, A.; Sparano, J.A.; Wolff, A.C.; Cobleigh, M.A.; Galbraith, S.; Sledge, G.W. A randomized phase II feasibility trial of BMS-275291 in patients with early stage breast cancer. Clin. Cancer Res. 2004, 10, 1971–1975. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Hitomi, Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim. Biophys. Acta 1981, 661, 342–345. [Google Scholar] [CrossRef]
- Iwaki, M.; Ino, Y.; Motoyoshi, A.; Ozeki, M.; Sato, T.; Kurumi, M.; Aoyama, T. Pharmacological studies of FUT-175, nafamostat mesilate. V. Effects on the pancreatic enzymes and experimental acute pancreatitis in rats. Jpn. J. Pharmacol. 1986, 41, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Terao, T.; Maki, M.; Ikenoue, T. Diagnosis and management of acute obstetrical DIC. Semin. Thromb. Hemost. 2001, 27, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Uwagawa, T.; Li, Z.; Chang, Z.; Xia, Q.; Peng, B.; Sclabas, G.M.; Ishiyama, S.; Hung, M.C.; Evans, D.B.; Abbruzzese, J.L.; et al. Mechanisms of synthetic serine protease inhibitor (FUT-175)-mediated cell death. Cancer 2007, 109, 2142–2153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uwagawa, T.; Chiao, P.J.; Gocho, T.; Hirohara, S.; Misawa, T.; Yanaga, K. Combination chemotherapy of nafamostat mesilate with gemcitabine for pancreatic cancer targeting NF-κB activation. Anticancer Res. 2009, 29, 3173–3178. [Google Scholar] [PubMed]
- Haruki, K.; Shiba, H.; Fujiwara, Y.; Furukawa, K.; Iwase, R.; Uwagawa, T.; Misawa, T.; Ohashi, T.; Yanaga, K. Inhibition of nuclear factor-κB enhances the antitumor effect of paclitaxel against gastric cancer with peritoneal dissemination in mice. Dig. Dis. Sci. 2013, 58, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Iwase, R.; Haruki, K.; Fujiwara, Y.; Furukawa, K.; Shiba, H.; Uwagawa, T.; Misawa, T.; Ohashi, T.; Yanaga, K. Combination chemotherapy of nafamostat mesylate with gemcitabine for gallbladder cancer targeting nuclear factor-κB activation. J. Surg. Res. 2013, 184, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Shiba, H.; Iwase, R.; Haruki, K.; Fujiwara, Y.; Furukawa, K.; Uwagawa, T.; Ohashi, T.; Yanaga, K. Dual inhibition of nuclear factor kappa-B and Mdm2 enhance the antitumor effect of radiation therapy for pancreatic cancer. Cancer Lett. 2016, 370, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Didelot, C.; Barberi-Heyob, M.; Bianchi, A.; Becuwe, P.; Mirjolet, J.F.; Dauca, M.; Merlin, J.L. Constitutive NF-κB activity influences basal apoptosis and radiosensitivity of head-and-neck carcinoma cell lines. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 1354–1360. [Google Scholar] [CrossRef]
- Mukogawa, T.; Koyama, F.; Tachibana, M.; Takayanagi, A.; Shimizu, N.; Fujii, H.; Ueno, M.; Matsumoto, H.; Takeuchi, T.; Nakajima, Y. Adenovirus-mediated gene transduction of truncated I kappa B alpha enhances radiosensitivity in human colon cancer cells. Cancer Sci. 2003, 94, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.M.; Tepper, J.E.; Baldwin, A.S., Jr.; Liu, R.; Adams, J.; Elliott, P.; Cusack, J.C., Jr. Enhancement of radiosensitivity by proteasome inhibition: Implications for a role of NF-κB. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 183–193. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lin, W.C.; Chiang, I.T.; Chang, Y.F.; Chen, C.W.; Su, S.H.; Chen, C.L.; Hwang, J.J. Sorafenib sensitizes human colorectal carcinoma to radiation via suppression of NF-κB expression in vitro and in vivo. Biomed. Pharmacother. 2012, 66, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Diagaradjane, P.; Guha, S.; Deorukhkar, A.; Shentu, S.; Aggarwal, B.B.; Krishnan, S. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-κB-regulated gene products. Clin. Cancer Res. 2008, 14, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Raffoul, J.J.; Banerjee, S.; Che, M.; Knoll, Z.E.; Doerge, D.R.; Abrams, J.; Kucuk, O.; Sarkar, F.H.; Hillman, G.G. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int. J. Cancer 2007, 120, 2491–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uwagawa, T.; Misawa, T.; Sakamoto, T.; Ito, R.; Gocho, T.; Shiba, H.; Wakiyama, S.; Hirohara, S.; Sadaoka, S.; Yanaga, K. A phase I study of full-dose gemcitabine and regional arterial infusion of nafamostat mesilate for advanced pancreatic cancer. Ann. Oncol. 2009, 20, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Uwagawa, T.; Misawa, T.; Tsutsui, N.; Ito, R.; Gocho, T.; Hirohara, S.; Sadaoka, S.; Yanaga, K. Phase II study of gemcitabine in combination with regional arterial infusion of nafamostat mesilate for advanced pancreatic cancer. Am. J. Clin. Oncol. 2013, 36, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Bufill, J.A. Colorectal cancer: Evidence for distinct genetic categories based on proximal or distal tumor location. Ann. Intern. Med. 1990, 113, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitzner, M.; Emons, G.; Kramer, F.; Gaedcke, J.; Rave-Frank, M.; Scharf, J.G.; Burfeind, P.; Becker, H.; Beissbarth, T.; Ghadimi, B.M.; et al. A gene expression signature for chemoradiosensitivity of colorectal cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Chendil, D.; Oakes, R.; Alcock, R.A.; Patel, N.; Mayhew, C.; Mohiuddin, M.; Gallicchio, V.S.; Ahmed, M.M. Low dose fractionated radiation enhances the radiosensitization effect of paclitaxel in colorectal tumor cells with mutant p53. Cancer 2000, 89, 1893–1900. [Google Scholar] [CrossRef] [Green Version]
- Sandur, S.K.; Deorukhkar, A.; Pandey, M.K.; Pabon, A.M.; Shentu, S.; Guha, S.; Aggarwal, B.B.; Krishnan, S. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-κB activity. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Kargiotis, O.; Geka, A.; Rao, J.S.; Kyritsis, A.P. Effects of irradiation on tumor cell survival, invasion and angiogenesis. J. Neurooncol. 2010, 100, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Ra, H.J.; Parks, W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007, 26, 587–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [PubMed]
- Stetler-Stevenson, W.G.; Yu, A.E. Proteases in invasion: Matrix metalloproteinases. Semin. Cancer Biol. 2001, 11, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sier, C.F.; Kubben, F.J.; Ganesh, S.; Heerding, M.M.; Griffioen, G.; Hanemaaijer, R.; van Krieken, J.H.; Lamers, C.B.; Verspaget, H.W. Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are related to the overall survival of patients with gastric carcinoma. Br. J. Cancer 1996, 74, 413–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talvensaari-Mattila, A.; Paakko, P.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br. J. Cancer 2003, 89, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Collins, H.M.; Scholefield, J.H.; Watson, S.A. Increased type-IV collagenase (MMP-2 and MMP-9) activity following preoperative radiotherapy in rectal cancer. Br. J. Cancer 2000, 82, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Furukawa, K.; Haruki, K.; Shimada, Y.; Iida, T.; Shiba, H.; Uwagawa, T.; Ohashi, T.; Yanaga, K. Nafamostat mesilate can prevent adhesion, invasion and peritoneal dissemination of pancreatic cancer thorough nuclear factor kappa-B inhibition. J. Hepatobiliary Pancreat. Sci. 2011, 18, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, A.S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J. Clin. Investig. 2001, 107, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, H.; Seiki, M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene 1993, 8, 395–405. [Google Scholar] [PubMed]
- Chen, Y.J.; Chang, W.M.; Liu, Y.W.; Lee, C.Y.; Jang, Y.H.; Kuo, C.D.; Liao, H.F. A small-molecule metastasis inhibitor, norcantharidin, downregulates matrix metalloproteinase-9 expression by inhibiting Sp1 transcriptional activity in colorectal cancer cells. Chem. Biol. Interact. 2009, 181, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Nishimura, E.; Hoshina, H.; Kobayashi, H.; Higuchi, T.; Eto, Y.; Ida, H.; Ohashi, T. Proteasome Inhibitor Bortezomib Enhances the Activity of Multiple Mutant Forms of Lysosomal alpha-Glucosidase in Pompe Disease. JIMD Rep. 2015, 18, 33–39. [Google Scholar] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugano, H.; Shirai, Y.; Horiuchi, T.; Saito, N.; Shimada, Y.; Eto, K.; Uwagawa, T.; Ohashi, T.; Yanaga, K. Nafamostat Mesilate Enhances the Radiosensitivity and Reduces the Radiation-Induced Invasive Ability of Colorectal Cancer Cells. Cancers 2018, 10, 386. https://doi.org/10.3390/cancers10100386
Sugano H, Shirai Y, Horiuchi T, Saito N, Shimada Y, Eto K, Uwagawa T, Ohashi T, Yanaga K. Nafamostat Mesilate Enhances the Radiosensitivity and Reduces the Radiation-Induced Invasive Ability of Colorectal Cancer Cells. Cancers. 2018; 10(10):386. https://doi.org/10.3390/cancers10100386
Chicago/Turabian StyleSugano, Hiroshi, Yoshihiro Shirai, Takashi Horiuchi, Nobuhiro Saito, Yohta Shimada, Ken Eto, Tadashi Uwagawa, Toya Ohashi, and Katsuhiko Yanaga. 2018. "Nafamostat Mesilate Enhances the Radiosensitivity and Reduces the Radiation-Induced Invasive Ability of Colorectal Cancer Cells" Cancers 10, no. 10: 386. https://doi.org/10.3390/cancers10100386
APA StyleSugano, H., Shirai, Y., Horiuchi, T., Saito, N., Shimada, Y., Eto, K., Uwagawa, T., Ohashi, T., & Yanaga, K. (2018). Nafamostat Mesilate Enhances the Radiosensitivity and Reduces the Radiation-Induced Invasive Ability of Colorectal Cancer Cells. Cancers, 10(10), 386. https://doi.org/10.3390/cancers10100386




