DICER1 Syndrome: DICER1 Mutations in Rare Cancers
Abstract
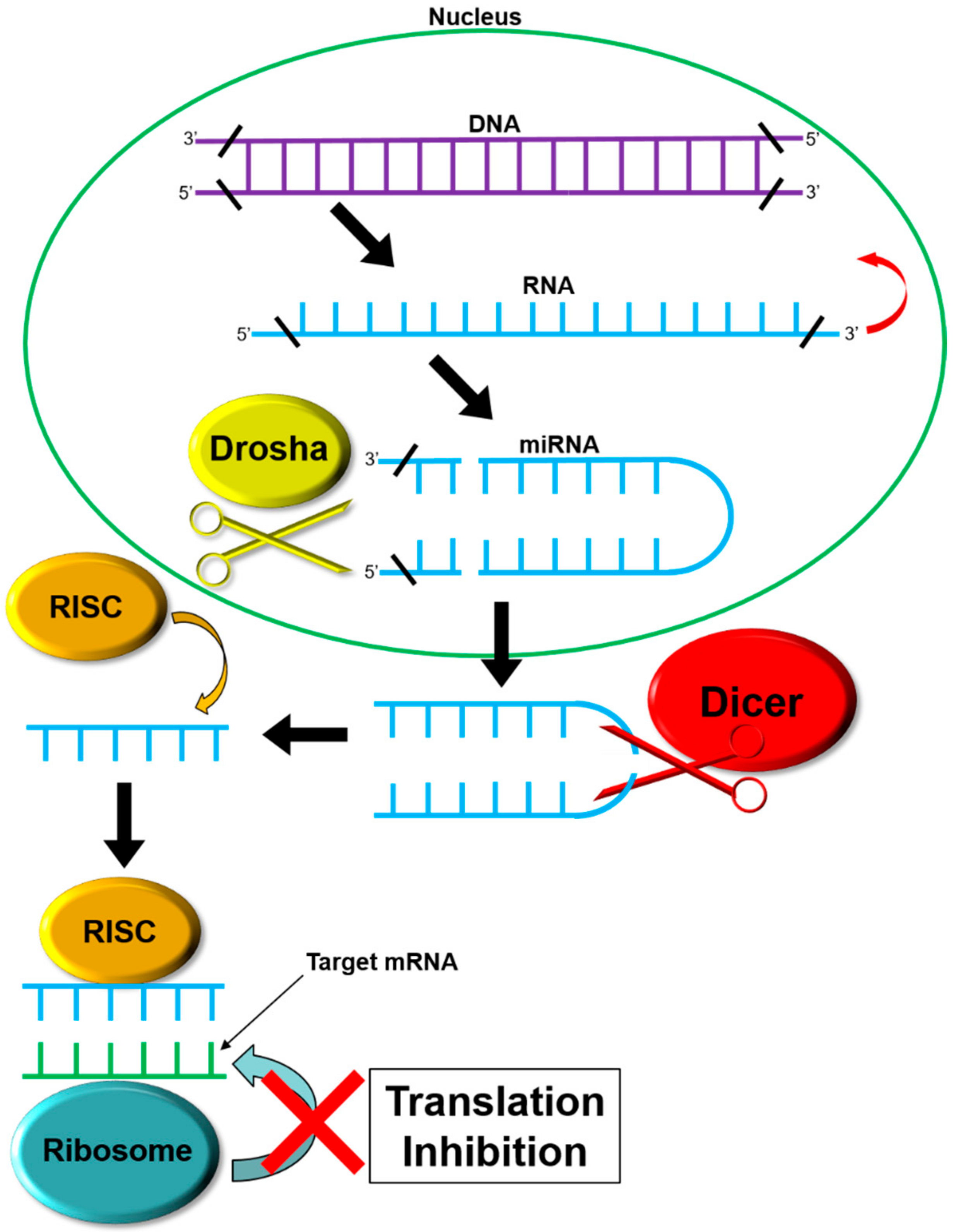
1. Introduction
2. DICER1 Germline Mutations
3. Manifestations of DICER1 Gene Mutations
3.1. Multinodular Goiter (MNG)
3.2. Pleuropulmonary Blastoma
3.3. Cystic Nephroma
3.4. Sertoli–Leydig Cell Tumor
4. Additional Symptoms and Presentations Related to DICER1 Syndrome
4.1. Hodgkin Lymphoma
4.2. Pineoblastoma
4.3. Global Developmental Delay, Lung Cysts, Overgrowth, and Wilms Tumor (GLOW)
4.4. Macrocephaly
5. Molecular Mechanisms of DICER1 Mutations—The Two-Hit Hypothesis
6. Future Directions
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGO2 | argonaute-2 |
| AUF1 | heterogeneous nuclear ribonucleoprotein D |
| DDD | Dicer dimerization domain |
| GLOW | Global developmental delay, Lung cysts, Overgrowth, and Wilms tumor |
| miRNAs | MicroRNA |
| PAZ | Piwi/Argonaute, Zwille domain |
| RISC | RNA-induced silencing complex |
| RLC | RNA-induced silencing complex (RISC) loading complex |
| RNase IIIb | ribonuclease IIIb |
| rRNA | ribosomal RNA |
| TARBP2 | trans-activation responsive RNA binding protein 2 |
References
- Hill, D.A.; Ivanovich, J.; Priest, J.R.; Gurnett, C.A.; Dehner, L.P.; Desruisseau, D.; Jarzembowski, J.A.; Wikenheiser-Brokamp, K.A.; Suarez, B.K.; Whelan, A.J.; et al. DICER1 mutations in familial pleuropulmonary blastoma. Science 2009, 325, 965. [Google Scholar] [CrossRef] [PubMed]
- Orphanet: Pleuropulmonary Blastoma Familial Tumor Susceptibility Syndrome. Available online: http://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=en&Expert=64742 (accessed on 19 April 2017).
- DICER1 Syndrome|Children’s Hospital of Philadelphia. Available online: http://www.chop.edu/conditions-diseases/dicer1-syndrome (accessed on 19 April 2017).
- Solarski, M.; Rotondo, F.; Foulkes, W.D.; Priest, J.R.; Syro, L.V.; Butz, H.; Cusimano, M.D.; Kovacs, K. DICER1 gene mutations in endocrine tumors. Endocr. Relat. Cancer 2018, 25, R197–R208. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, M.; Riba-Wolman, R.; Orsey, A.D.; Balarezo, F.; Finck, C. DICER1 syndrome and thyroid disease. J. Pediatr. Surg. Case Rep. 2016, 11, 31–34. [Google Scholar] [CrossRef][Green Version]
- DICER1-Related Pleuropulmonary Blastoma Cancer Predisposition Syndrome|Genetic and Rare Diseases Information Center (GARD)—An NCATS Program. Available online: https://rarediseases.info.nih.gov/diseases/10734/dicer1-related-pleuropulmonary-blastoma-cancer-predisposition-syndrome (accessed on 19 April 2017).
- Slade, I.; Bacchelli, C.; Davies, H.; Murray, A.; Abbaszadeh, F.; Hanks, S.; Barfoot, R.; Burke, A.; Chisholm, J.; Hewitt, M.; et al. DICER1 syndrome: Clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J. Med. Genet. 2011, 48, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Rio Frio, T.; Bahubeshi, A.; Kanellopoulou, C.; Hamel, N.; Niedziela, M.; Sabbaghian, N.; Pouchet, C.; Gilbert, L.; O’Brien, P.K.; Serfas, K.; et al. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA 2011, 305, 68–77. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Rivera, B.; Revil, T.; Thorner, P.; Goudie, C.; Bouron-Dal Soglio, D.; Choong, C.S.; Priest, J.R.; van Diest, P.J.; Tanboon, J.; et al. Sequencing of DICER1 in sarcomas identifies biallelic somatic DICER1 mutations in an adult-onset embryonal rhabdomyosarcoma. Br. J. Cancer 2017, 116, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Saskin, A.; de Kock, L.; Sabbaghian, N.; Apellaniz-Ruiz, M.; Bozkurt, C.; Bouron-Dal Soglio, D.; Foulkes, W.D. A case of neuroblastoma in DICER1 syndrome: Chance finding or noncanonical causation? Pediatr. Blood Cancer 2018, 65, e26715. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Bailey, S.; Raby, K.L.; Saini, H.K.; de Kock, L.; Burke, G.A.A.; Foulkes, W.D.; Enright, A.J.; Coleman, N.; Tischkowitz, M. Serum levels of mature microRNAs in DICER1-mutated pleuropulmonary blastoma. Oncogenesis 2014, 3, e87. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Hammond, S.M. Dicing and slicing. FEBS Lett. 2005, 579, 5822–5829. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed]
- Jansson, M.D.; Lund, A.H. MicroRNA and cancer. Mol. Oncol. 2012, 6, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Kian, R.; Moradi, S.; Ghorbian, S. Role of components of microRNA machinery in carcinogenesis. Exp. Oncol. 2018, 40, 2–9. [Google Scholar] [PubMed]
- Song, M.; Rossi, J.J. Molecular mechanisms of DICER: Endonuclease and enzymatic activity. Biochem. J. 2017, 474, 1603–1618. [Google Scholar] [CrossRef] [PubMed]
- Matskevich, A.A.; Moelling, K. Stimuli-dependent cleavage of DICER during apoptosis. Biochem. J. 2008, 412, 527–534. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Wang, Y.C.; Revil, T.; Badescu, D.; Rivera, B.; Sabbaghian, N.; Wu, M.; Weber, E.; Sandoval, C.; Hopman, S.M.J.; et al. High-sensitivity sequencing reveals multi-organ somatic mosaicism causing DICER1 syndrome. J. Med. Genet. 2016, 53, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Home-PubMed-NCBI. Available online: https://www.ncbi.nlm.nih.gov/pubmed (accessed on 19 April 2017).
- Khan, N.E.; Bauer, A.J.; Schultz, K.A.P.; Doros, L.; Decastro, R.M.; Ling, A.; Lodish, M.B.; Harney, L.A.; Kase, R.G.; Carr, A.G.; et al. Quantification of Thyroid Cancer and Multinodular Goiter Risk in the DICER1 Syndrome: A Family-Based Cohort Study. J. Clin. Endocrinol. Metab. 2017, 102, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Apellaniz-Ruiz, M.; de Kock, L.; Sabbaghian, N.; Guaraldi, F.; Ghizzoni, L.; Beccuti, G.; Foulkes, W.D. Familial multinodular goiter and Sertoli-Leydig cell tumors associated with a large intragenic in-frame DICER1 deletion. Eur. J. Endocrinol. 2018, 178, K11–K19. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Thyroid—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/thyro.html (accessed on 19 April 2017).
- Zarkesh, M.; Zadeh-Vakili, A.; Azizi, F.; Foroughi, F.; Akhavan, M.M.; Hedayati, M. Altered Epigenetic Mechanisms in Thyroid Cancer Subtypes. Mol. Diagn. Ther. 2017, 22, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.; Laurberg, P.; Perrild, H.; Bülow, I.; Ovesen, L.; Jørgensen, T. Risk Factors for Goiter and Thyroid Nodules. Thyroid 2002, 12, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Darrat, I.; Bedoyan, J.K.; Chen, M.; Schuette, J.L.; Lesperance, M.M. Novel DICER1 mutation as cause of multinodular goiter in children. Head Neck 2013. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Bah, I.; Brunet, J.; Druker, H.; Astigarraga, I.; Bosch-Barrera, J.; Soglio, D.B.-D.; Nguyen, V.-H.; Malkin, D.; Priest, J.R.; et al. Somatic DICER1 mutations in adult-onset pulmonary blastoma. Eur. Respir. J. 2016, 47, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Dishop, M.K.; Kuruvilla, S. Primary and metastatic lung tumors in the pediatric population: A review and 25-year experience at a large children’s hospital. Arch. Pathol. Lab. Med. 2008, 132, 1079–1103. [Google Scholar] [PubMed]
- Manivel, J.C.; Priest, J.R.; Watterson, J.; Steiner, M.; Woods, W.G.; Wick, M.R.; Dehner, L.P. Pleuropulmonary blastoma. The so-called pulmonary blastoma of childhood. Cancer 1988, 62, 1516–1526. [Google Scholar] [CrossRef]
- Fosdal, M.B. Pleuropulmonary Blastoma. J. Pediatr. Oncol. Nurs. 2008, 25, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Priest, J.R.; Magnuson, J.; Williams, G.M.; Abromowitch, M.; Byrd, R.; Sprinz, P.; Finkelstein, M.; Moertel, C.L.; Hill, D.A. Cerebral metastasis and other central nervous system complications of pleuropulmonary blastoma. Pediatr. Blood Cancer 2007, 49, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.A.P.; Harris, A.; Messinger, Y.; Sencer, S.; Baldinger, S.; Dehner, L.P.; Hill, D.A. Ovarian tumors related to intronic mutations in DICER1: A report from the international ovarian and testicular stromal tumor registry. Fam. Cancer 2016, 15, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Doros, L.; Yang, J.; Dehner, L.; Rossi, C.T.; Skiver, K.; Jarzembowski, J.A.; Messinger, Y.; Schultz, K.A.; Williams, G.; André, N.; Hill, D.A. DICER1 Mutations in embryonal rhabdomyosarcomas from children with and without familial PPB-tumor predisposition syndrome. Pediatr. Blood Cancer 2012, 59, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.R.; Messinger, Y.; Williams, G.M.; Yang, J.; Field, A.; Schultz, K.A.P.; Harney, L.A.; Doros, L.A.; Dehner, L.P.; Hill, D.A. Nasal chondromesenchymal hamartomas arise secondary to germline and somatic mutations of DICER1 in the pleuropulmonary blastoma tumor predisposition disorder. Hum. Genet. 2014, 133, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- de Kock, L.; Sabbaghian, N.; Soglio, D.B.D.; Guillerman, R.P.; Park, B.K.; Chami, R.; Deal, C.L.; Priest, J.R.; Foulkes, W.D. Exploring the association between DICER1 mutations and differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2014, 99, E1072-7. [Google Scholar] [CrossRef] [PubMed]
- Palculict, T.B.; Ruteshouser, E.C.; Fan, Y.; Wang, W.; Strong, L.; Huff, V. Identification of germline DICER1 mutations and loss of heterozygosity in familial Wilms tumour. J. Med. Genet. 2015, 53, 1–4. [Google Scholar]
- Bahubeshi, A.; Bal, N.; Rio Frio, T.; Hamel, N.; Pouchet, C.; Yilmaz, A.; Bouron-Dal Soglio, D.; Williams, G.M.; Tischkowitz, M.; Priest, J.R.; et al. Germline DICER1 mutations and familial cystic nephroma. J. Med. Genet. 2010, 47, 863–866. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Plourde, F.; Carter, M.T.; Hamel, N.; Srivastava, A.; Meyn, M.S.; Arseneau, J.; Soglio, D.B.-D.; Foulkes, W.D. Germ-line and somatic DICER1 mutations in a pleuropulmonary blastoma. Pediatr. Blood Cancer 2013, 60, 2091–2092. [Google Scholar] [CrossRef] [PubMed]
- Kuhlen, M.; Hönscheid, A.; Schemme, J.; Merz, H.; Mauz-Körholz, C.; Borkhardt, A.; Troeger, A. Hodgkin lymphoma as a novel presentation of familial DICER1 syndrome. Eur. J. Pediatr. 2016, 175, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.R.; Bartley, A.; Charles, A.; Powers, N.; Baynam, G.; Jones, T.; Priest, J.R.; Foulkes, W.D.; Choong, C.S.Y. Multinodular Goiter in children: An important pointer to a germline DICER1 mutation. J. Clin. Endocrinol. Metab. 2014, 99, 1947–1948. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, L.; Villegas, J.A.; Santamaría, Í.; Pitiot, A.S.; Alvarado, M.G.; Fernández, S.; Torres, H.; Paredes, Á.; Blay, P.; Balbín, M. Identification of somatic and germ-line DICER1 mutations in pleuropulmonary blastoma, cystic nephroma and rhabdomyosarcoma tumors within a DICER1 syndrome pedigree. BMC Cancer 2017, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Doros, L.A.; Rossi, C.T.; Yang, J.; Field, A.; Williams, G.M.; Messinger, Y.; Cajaiba, M.M.; Perlman, E.J.; Schultz, K.A.; Cathro, H.P.; et al. DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Mod. Pathol. 2014, 27, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Boman, F.; Hill, D.A.; Williams, G.M.; Chauvenet, A.; Fournet, J.-C.; Soglio, D.B.-D.; Messinger, Y.; Priest, J.R. Familial association of pleuropulmonary blastoma with cystic nephroma and other renal tumors: A report from the International Pleuropulmonary Blastoma Registry. J. Pediatr. 2006, 149, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhao, W.; Nie, X.; Abbas, A.; Fu, L.; Bihi, S.; Feng, G.; Liu, T.; Lv, Y.; Ma, X.; et al. Multimorbidity and Genetic Characteristics of DICER1 Syndrome Based on Systematic Review. J. Pediatr. Hematol. Oncol. 2016, 39, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Turbiner, J.; Amin, M.B.; Humphrey, P.A.; Srigley, J.R.; De Leval, L.; Radhakrishnan, A.; Oliva, E. Cystic nephroma and mixed epithelial and stromal tumor of kidney: A detailed clinicopathologic analysis of 34 cases and proposal for renal epithelial and stromal tumor (REST) as a unifying term. Am. J. Surg. Pathol. 2007, 31, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Bal, N.; Kayaselçuk, F.; Polat, A.; Bolat, F.; Yilmaz, Z.; Tuncer, I. Familial cystic nephroma in two siblings with pleuropulmonary blastoma. Pathol. Oncol. Res. 2005, 11, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Kuzgunbay, B.; Turunc, T.; Bolat, F.; Kilinc, F. Adult cystic nephroma: A case report and a review of the literature. Urol. Oncol. 2009, 27, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Paal, E.; Thompson, L.D.; Heffess, C.S. A clinicopathologic and immunohistochemical study of ten pancreatic lymphangiomas and a review of the literature. Cancer 1998, 82, 2150–2158. [Google Scholar] [CrossRef]
- Bastian, P.J.; Kuhlmann, R.; Vogel, J.; Bastian, H.-P. Local recurrence of a unilateral cystic nephroma. Int. J. Urol. 2004, 11, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.K.; Cotter, M.B.; Pears, J.; McDermott, M.B.; Fabian, M.R.; Foulkes, W.D.; O’Sullivan, M.J. Tumor progression in DICER1-mutated cystic nephroma-witnessing the genesis of anaplastic sarcoma of the kidney. Hum. Pathol. 2016, 53, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.K.; Vujanic, G.M.; Fahiminiya, S.; Watanabe, N.; Thorner, P.S.; O’Sullivan, M.J.; Fabian, M.R.; Foulkes, W.D. Anaplastic sarcomas of the kidney are characterized by DICER1 mutations. Mod. Pathol. 2018, 31, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Croce, S.; de Kock, L.; Boshari, T.; Hostein, I.; Velasco, V.; Foulkes, W.D.; McCluggage, W.G. Uterine Tumor Resembling Ovarian Sex Cord Tumor (UTROSCT) Commonly Exhibits Positivity With Sex Cord Markers FOXL2 and SF-1 but Lacks FOXL2 and DICER1 Mutations. Int. J. Gynecol. Pathol. 2016, 35, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Oliva, E. Ovarian sex cord-stromal tumours: An update in recent molecular advances. Pathology 2018, 50, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Nwogu, L.C.; Showalter, J.A.; Roy, S.; Deavers, M.T.; Zhao, B. Retiform Sertoli-Leydig Cell Tumor in a 38-Year-Old Woman: A Case Report, Retrospective Review, and Review of Current Literature. Case Rep. Pathol. 2017, 2017, 3421832. [Google Scholar] [CrossRef] [PubMed]
- Conlon, N.; Schultheis, A.M.; Piscuoglio, S.; Silva, A.; Guerra, E.; Tornos, C.; Reuter, V.E.; Soslow, R.A.; Young, R.H.; Oliva, E.; et al. A survey of DICER1 hotspot mutations in ovarian and testicular sex cord-stromal tumors. Mod. Pathol. 2015, 28, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Terzic, T.; McCluggage, W.G.; Stewart, C.J.R.; Shaw, P.; Foulkes, W.D.; Clarke, B.A. DICER1 Mutations Are Consistently Present in Moderately and Poorly Differentiated Sertoli-Leydig Cell Tumors. Am. J. Surg. Pathol. 2017, 41, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Sigismondi, C.; Gadducci, A.; Lorusso, D.; Candiani, M.; Breda, E.; Raspagliesi, F.; Cormio, G.; Marinaccio, M.; Mangili, G. Ovarian Sertoli-Leydig cell tumors. A retrospective MITO study. Gynecol. Oncol. 2012, 125, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Mooney, E.E.; Nogales, F.F.; Bergeron, C.; Tavassoli, F.A. Retiform Sertoli-Leydig cell tumours: Clinical, morphological and immunohistochemical findings. Histopathology 2002, 41, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bellfield, E.J.; Alemzadeh, R. Recurrent ovarian Sertoli-Leydig cell tumor in a child with Peutz-Jeghers syndrome. Oxford Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Durieux, E.; Descotes, F.; Mauduit, C.; Decaussin, M.; Guyetant, S.; Devouassoux-Shisheboran, M. The co-occurrence of an ovarian Sertoli-Leydig cell tumor with a thyroid carcinoma is highly suggestive of a DICER1 syndrome. Virchows Arch. 2016, 468, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Oost, E.E.; Charles, A.; Choong, C.S.; Leung, Y.C.; Salfinger, S.; Sonnendecker, H.; Tan, J.; Townshend, S.; Witkowski, L.; Foulkes, W.D.; et al. Ovarian sex cord-stromal tumors in patients with probable or confirmed germline DICER1 mutations. Int. J. Gynecol. Pathol. 2015, 34, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Sabbaghian, N.; Srivastava, A.; Hamel, N.; Plourde, F.; Gajtko-Metera, M.; Niedziela, M.; Foulkes, W.D. Germ-line deletion in DICER1 revealed by a novel MLPA assay using synthetic oligonucleotides. Eur. J. Hum. Genet. 2014, 22, 564–567. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Bah, I.; Wu, Y.; Xie, M.; Priest, J.R.; Foulkes, W.D. Germline and somatic DICER1 mutations in a well-differentiated fetal adenocarcinoma of the lung. J. Thorac. Oncol. 2016, 11, e31–e33. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, D.; Li, Y.; Bian, L.; Ma, T.; Xie, M. DICER1 mutations in a patient with an ovarian Sertoli-Leydig tumor, well-differentiated fetal adenocarcinoma of the lung, and familial multinodular goiter. Eur. J. Med. Genet. 2014, 57, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Rossing, M.; Gerdes, A.-M.; Juul, A.; Rechnitzer, C.; Rudnicki, M.; Nielsen, F.C.; Vo Hansen, T. A novel DICER1 mutation identified in a female with ovarian Sertoli-Leydig cell tumor and multinodular goiter: A case report. J. Med. Case Rep. 2014, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Bahubeshi, A.; Hamel, N.; Pasini, B.; Asioli, S.; Baynam, G.; Choong, C.S.; Charles, A.; Frieder, R.P.; Dishop, M.K.; et al. Extending the phenotypes associated with DICER1 mutations. Hum. Mutat. 2011, 32, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Sabbaghian, N.; Druker, H.; Weber, E.; Hamel, N.; Miller, S.; Choong, C.S.; Gottardo, N.G.; Kees, U.R.; Rednam, S.P.; et al. Germ-line and somatic DICER1 mutations in pineoblastoma. Acta Neuropathol. 2014, 128, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Sabbaghian, N.; Hamel, N.; Srivastava, A.; Albrecht, S.; Priest, J.R.; Foulkes, W.D. Germline DICER1 mutation and associated loss of heterozygosity in a pineoblastoma. J. Med. Genet. 2012, 49, 417–419. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Druker, H.; Weber, E.; Hamel, N.; Traubici, J.; Malkin, D.; Arseneau, J.; Stewart, C.J.R.; Bouron-Dal Soglio, D.; Priest, J.R.; et al. Ovarian embryonal rhabdomyosarcoma is a rare manifestation of the DICER1 syndrome. Hum. Pathol. 2015, 46, 917–922. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Sabbaghian, N.; Plourde, F.; Srivastava, A.; Weber, E.; Bouron-Dal Soglio, D.; Hamel, N.; Choi, J.H.; Park, S.-H.; Deal, C.L.; et al. Pituitary blastoma: A pathognomonic feature of germ-line DICER1 mutations. Acta Neuropathol. 2014, 128, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Mehraein, Y.; Schmid, I.; Eggert, M.; Kohlhase, J.; Steinlein, O.K. DICER1 syndrome can mimic different genetic tumor predispositions. Cancer Lett. 2016, 370, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.K.; Goudie, C.; Druker, H.; Thorner, P.; Traubici, J.; Grant, R.; Albrecht, S.; Weber, E.; Charles, A.; Priest, J.R.; et al. Evolution of Renal Cysts to Anaplastic Sarcoma of Kidney in a Child With DICER1 Syndrome. Pediatr. Blood Cancer 2016, 63, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.; Calderaro, J.; Letouzé, E.; Nault, J.-C.; Couchy, G.; Boulais, A.; Luciani, A.; Zafrani, E.-S.; Bioulac-Sage, P.; Seror, O.; et al. Germline and somatic DICER1 mutations in familial and sporadic liver tumors. J. Hepatol. 2016, 66, 734–742. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Boshari, T.; Martinelli, F.; Wojcik, E.; Niedziela, M.; Foulkes, W.D. Adult-Onset Cervical Embryonal Rhabdomyosarcoma and DICER1 Mutations. J. Low. Genit. Tract Dis. 2016, 20, e8–e10. [Google Scholar] [CrossRef] [PubMed]
- Sahakitrungruang, T.; Srichomthong, C.; Pornkunwilai, S.; Amornfa, J.; Shuangshoti, S.; Kulawonganunchai, S.; Suphapeetiporn, K.; Shotelersuk, V. Germline and somatic DICER1 mutations in a pituitary blastoma causing infantile-onset Cushing’s disease. J. Clin. Endocrinol. Metab. 2014, 99, E1487-92. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, G.; Zhao, X.; Ye, S.; Shen, P.; Wang, W.; Zheng, S. A novel WRN frameshift mutation identified by multiplex genetic testing in a family with multiple cases of cancer. PLoS ONE 2015, 10, e0133020. [Google Scholar] [CrossRef] [PubMed]
- Fremerey, J.; Balzer, S.; Brozou, T.; Schaper, J.; Borkhardt, A.; Kuhlen, M. Embryonal rhabdomyosarcoma in a patient with a heterozygous frameshift variant in the DICER1 gene and additional manifestations of the DICER1 syndrome. Fam. Cancer 2017, 16, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Tomiak, E.; de Kock, L.; Grynspan, D.; Ramphal, R.; Foulkes, W.D. DICER1 mutations in an adolescent with cervical embryonal rhabdomyosarcoma (cERMS). Pediatr. Blood Cancer 2014, 61, 568–569. [Google Scholar] [CrossRef] [PubMed]
- Bardón-Cancho, E.J.; Haro-Díaz, A.; Alonso-García-de la Rosa, F.J.; Huerta-Aragonés, J.; García-Morín, M.; González-Martínez, F.; Garrido-Colino, C. DICER1 mutation and tumors associated with a familial tumor predisposition syndrome: Practical considerations. Fam. Cancer 2016, 16, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M. Metachronous anaplastic sarcoma of the kidney and thyroid follicular carcinoma as manifestations of DICER1 abnormalities. Endocr. Relat. Cancer 2016, 23, L1–L5. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.K.; de Kock, L.; Conwell, L.S.; Stewart, C.J.R.; King, B.R.; Choong, C.S.; Hussain, K.; Sabbaghian, N.; MacRae, I.J.; Fabian, M.R.; et al. Functional characterization of multiple DICER1 mutations in an adolescent. Endocr. Relat. Cancer 2016, 23, L1–L5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klein, S.; Lee, H.; Ghahremani, S.; Kempert, P.; Ischander, M.; Teitell, M.A.; Nelson, S.F.; Martinez-Agosto, J.A. Expanding the phenotype of mutations in DICER1: Mosaic missense mutations in the RNase IIIb domain of DICER1 cause GLOW syndrome. J. Med. Genet. 2014, 51, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.E.; Bauer, A.J.; Doros, L.; Schultz, K.A.P.; Decastro, R.M.; Harney, L.A.; Kase, R.G.; Carr, A.G.; Harris, A.K.; Williams, G.M.; et al. Macrocephaly associated with the DICER1 syndrome. Genet. Med. 2017, 19, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Nordling, C.O. A new theory on cancer-inducing mechanism. Br. J. Cancer 1953, 7, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Eyre, H.; Jacka, F.N.; Dodd, S.; Dean, O.; McEwen, S.; Debnath, M.; McGrath, J.; Maes, M.; Amminger, P.; et al. A review of vulnerability and risks for schizophrenia: Beyond the two hit hypothesis. Proc. Natl. Acad. Sci. USA 2016, 65, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Berthon, A.; Faucz, F.; Bertherat, J.; Stratakis, C.A. Analysis of ARMC5 expression in human tissues. Mol. Cell. Endocrinol. 2017, 441, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.K.; Sabbaghian, N.; Xu, B.; Addidou-Kalucki, S.; Bernard, C.; Zou, D.; Reeve, A.E.; Eccles, M.R.; Cole, C.; Choong, C.S.; et al. Biallelic DICER1 mutations occur in Wilms tumours. J. Pathol. 2013, 230, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Anglesio, M.; Wang, Y.; Yang, W.; Senz, J.; Wan, A.; Heravi-Moussavi, A.; Salamanca, C.; Maines-Bandiera, S.; Huntsman, D.; Morin, G. Cancer-associated somatic DICER1 hotspot mutations cause defective miRNA processing and reverse-strand expression bias to predominantly mature 3p strands through loss of 5p strand cleavage. J. Pathol. 2013, 229, 400–409. [Google Scholar] [CrossRef] [PubMed]
- De Kock, L.; Bah, I.; Revil, T.; Bérubé, P.; Wu, M.K.; Sabbaghian, N.; Priest, J.R.; Ragoussis, J.; Foulkes, W.D. Deep Sequencing Reveals Spatially Distributed Distinct Hot Spot Mutations in DICER1-Related Multinodular Goiter. J. Clin. Endocrinol. Metab. 2016, 101, 3637–3645. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Yoshida, K.; Shiraishi, Y.; Shimamura, T.; Sato, Y.; Nishimura, R.; Okuno, Y.; Chiba, K.; Tanaka, H.; Kato, K.; et al. Biallelic DICER1 mutations in sporadic pleuropulmonary blastoma. Cancer Res. 2014, 74, 2742–2749. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Zajgla, J.; Mercado-Celis, G.E.; Gaytan-Cervantes, J.; Torres, A.; Gabiño, N.B.; Zapata-Tarres, M.; Juarez-Villegas, L.E.; Lezama, P.; Maldonado, V.; Ruiz-Monroy, K.; et al. Genomics of a pediatric ovarian fibrosarcoma. Association with the DICER1 syndrome. Sci. Rep. 2018, 8, 3252. [Google Scholar] [CrossRef] [PubMed]
- Brenneman, M.; Field, A.; Yang, J.; Williams, G.; Doros, L.; Rossi, C.; Schultz, K.A.; Rosenberg, A.; Ivanovich, J.; Turner, J.; et al. Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in DICER1 syndrome: A unique variant of the two-hit tumor suppression model. F1000Research 2015, 4, 214. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; McMonechy, M.K.; Anglesio, M.S.; Yang, W.; Senz, J.; Maines-Bandiera, S.; Rosner, J.; Trigo-Gonzalez, G.; Grace Cheng, S.W.; et al. Recurrent DICER1 hotspot mutations in endometrial tumours and their impact on microRNA biogenesis. J. Pathol. 2015, 237, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G.; Valerio, M.; Cioce, M.; Mori, F.; Casadei, L.; Pulito, C.; Sacconi, A.; Biagioni, F.; Cortese, G.; Galanti, S.; et al. Metformin elicits anticancer effects through the sequential modulation of DICER and c-MYC. Nat. Commun. 2012, 3, 865. [Google Scholar] [CrossRef] [PubMed]
- Noren Hooten, N.; Martin-Montalvo, A.; Dluzen, D.F.; Zhang, Y.; Bernier, M.; Zonderman, A.B.; Becker, K.G.; Gorospe, M.; de Cabo, R.; Evans, M.K. Metformin-mediated increase in DICER1 regulates microRNA expression and cellular senescence. Aging Cell 2016, 15, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, Y.; Huang, Y.; Chen, Y.; Wang, T.; Wu, S.; Tong, L.; Wang, Y.; Lin, L.; Hao, M.; et al. RNA-binding protein AUF1 suppresses miR-122 biogenesis by down-regulating DICER1 in hepatocellular carcinoma. Oncotarget 2018, 9, 14815–14827. [Google Scholar] [CrossRef] [PubMed]
- Oxford, A.E.; Jorcyk, C.L.; Oxford, J.T. Neuropathies of Stüve-Wiedemann Syndrome due to mutations in leukemia inhibitory factor receptor (LIFR) gene. J. Neurol. Neuromedicine 2016, 1, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Welch, E.M.; Barton, E.R.; Zhuo, J.; Tomizawa, Y.; Friesen, W.J.; Trifillis, P.; Paushkin, S.; Patel, M.; Trotta, C.R.; Hwang, S.; et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature 2007, 447, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Pibiri, I.; Lentini, L.; Melfi, R.; Gallucci, G.; Pace, A.; Spinello, A.; Barone, G.; Di Leonardo, A. Enhancement of premature stop codon readthrough in the CFTR gene by Ataluren (PTC124) derivatives. Eur. J. Med. Chem. 2015, 101, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Verrier, F.; Dubois d’Enghien, C.; Gauthier-Villars, M.; Bonadona, V.; Faure-Conter, C.; Dijoud, F.; Stoppa-Lyonnet, D.; Houdayer, C.; Golmard, L. Mutiple DICER1-related lesions associated with a germline deep intronic mutation. Pediatr. Blood Cancer 2018, e27005. [Google Scholar] [CrossRef] [PubMed]
- Diets, I.J.; Waanders, E.; Ligtenberg, M.J.; van Bladel, D.A.G.; Kamping, E.J.; Hoogerbrugge, P.M.; Hopman, S.; Olderode-Berends, M.J.; Gerkes, E.H.; Koolen, D.A.; et al. High Yield of Pathogenic Germline Mutations Causative or Likely Causative of the Cancer Phenotype in Selected Children with Cancer. Clin. Cancer Res. 2018, 24, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Mercurio, S.; Wald, A.I.; Barbi de Moura, M.; Callenberg, K.; Santana-Santos, L.; Gooding, W.E.; Yip, L.; Ferris, R.L.; Nikiforov, Y.E. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.A.P.; Williams, G.M.; Kamihara, J.; Stewart, D.R.; Harris, A.K.; Bauer, A.J.; Turner, J.; Shah, R.; Schneider, K.; Schneider, K.W.; et al. DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, J.D.; Sabbaghian, N.; Fahiminiya, S.; Chami, R.; Mete, O.; Acker, M.; Wu, M.K.; Shlien, A.; de Kock, L.; Foulkes, W.D. DICER1 mutations are frequent in adolescent-onset papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2018, 103, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Van Engelen, K.; Villani, A.; Wasserman, J.D.; Aronoff, L.; Greer, M.-L.C.; Tijerin Bueno, M.; Gallinger, B.; Kim, R.H.; Grant, R.; Meyn, M.S.; et al. DICER1 syndrome: Approach to testing and management at a large pediatric tertiary care center. Pediatr. Blood Cancer 2018, 65, e26720. [Google Scholar] [CrossRef] [PubMed]


| Mutation Type | Chromosomal Mutation | Protein Change | Clinical Manifestation | Reference |
|---|---|---|---|---|
| dup | c.1196_1197dupAG | p.Trp400Serfs*59 | 4-year old, pleuropulmonary blastoma. | Slade, 2011 [7] |
| tran | c.1376+1G>A | p.splice | 13-year old female, peritoneal cysts of right & left round ligaments, nasal polyps, Sertoli–Leydig cell tumor. History: 5 years, type II pleuropulmonary blastoma, 8 years, thyroid nodules. | Schultz, 2016 [34] |
| tran | c.1507G>T | p.Glu503* | pleuropulmonary blastoma. | Hill, 2009 [1] |
| del | c.1684_1685delAT | p.Met562Valfs*11 | pleuropulmonary blastoma. | Hill, 2009 [1] |
| del | c.1716delT | p.Phe572Leufs*15 | 0.8-year old, pleuropulmonary blastoma. | Slade, 2011 [7] |
| dup | c.1910dupA | p.Tyr637* | 5-month old female, pleuropulmonary blastoma, and cervical embryonal rhabdomyosarcoma; pleuropulmonary blastoma & embryonal rhabdomyosarcoma. | Hill, 2009 [1]; Doros, 2012 [35] |
| tran | c.1966C>T | p.Arg656* | pleuropulmonary blastoma; 7-year old, pleuropulmonary blastoma. | Slade, 2011 [7] Hill, 2009 [1] |
| tran | c.2040+1G>T | p.splice | 10-year female, nasal chondromesenchymal hamartoma. History: Pleuropulmonary blastoma. | Stewart, 2014 [36] |
| dup | c.2245_2248dupTACC | p.Pro750Leufs*12 | pleuropulmonary blastoma. | Hill, 2009 [1] |
| tran | c.2247C>A | p.Tyr749* | pleuropulmonary blastoma. | Hill, 2009 [1] |
| del | c.2268_2271delTTTG | p.Cys756* | 0.9-year old, pleuropulmonary blastoma. | Slade, 2011 [7] |
| tran | c.2379T>G | p.Tyr793* | 11.5-years male, bilateral papillary thyroid carcinoma in follicular adenoma. History: 32 months, type II pleuropulmonary blastoma and cystic nephroma. | de Kock, 2014 [37] |
| dup | c.2392dupA | p.Thr798Asnfs*33 | pleuropulmonary blastoma. | Hill, 2009 [1] |
| del | c.2399delG | p.Arg800fs*5 | 3.5-year old and 13-year old, Wilms’ tumor. | Palculict, 2016 [38] |
| tran | c.2830C>T | p.Arg944* | pleuropulmonary blastoma. | Hill, 2009 [1] |
| del | c.2863delA | p.Thr955fs | 7-year old male, nasal chondromesenchymal hamartoma. History: pleuropulmonary blastoma. | Stewart, 2014 [36] |
| tran | c.3019C>T | p.Gln1007* | 27-year old woman, nasal chondromesenchymal hamartoma and pleuropulmonary blastoma. History: multinodular goiter. | Stewart, 2014 [36] |
| del | c.3505delT | p.Ser1169Glnfs*23 | 3-year-old, Pleuropulmonary blastoma. | Slade, 2011 [7] |
| dup | c.3505dupT | p.Ser1169Phefs*8 | 7-year old female, thyroid goiter, multiple nodules on both lobes. History: 4.3 years, pleuropulmonary blastoma in left back musculature. 23 months, type II pleuropulmonary blastoma. | de Kock, 2014 [37] |
| tran | c.3540C>A | p.Tyr1180* | pleuropulmonary blastoma. | Hill, 2009 [1] |
| del | c.3583_3584delGA | N/A | 6-year-old, intraocular medulloepithelioma. History: pleuropulmonary blastoma. | Slade, 2011 [7] |
| del | c.3665delT | p.Leu1222Tyrfs*17 | 4.2-year old, pleuropulmonary blastoma. | Slade, 2011 [7] |
| tran | c.3726C>A | p.Tyr1242* | 4-year old, pleuropulmonary blastoma; 27-year old female, pleuropulmonary blastoma. History: 13 years, Sertoli–Leydig cell tumor and multinodular goiter, 21 years, nasal chondromesenchymal hamartoma. | Slade, 2011 [7]; Stewart, 2014 [36] |
| del | c.4309_4312delGACT | p.Asp1437Metfs*16 | 8-year old female, embryonal rhabdomyosarcoma. History: 4 years, pleuropulmonary blastoma; Median Age 34 months, female, cystic nephroma, pleuropulmonary blastoma. | Doros, 2012 [35]; Bahubeshi, 2010 [39] |
| del | c.4403_4406delCTCT | p.Ser1468Phefs*21 | 1.5-year old, pleuropulmonary blastoma. | Slade, 2011 [7] |
| del | c.4407_4410delTTCT | p.Leu1469fs | 11-year old male, nasal chondromesenchymal hamartoma. History: pleuropulmonary blastoma. | Stewart, 2014 [36] |
| del | c.4555delG | p.Glu1519Lysfs*41 | 3-year old female, Polish, type II pleuropulmonary blastoma. | de Kock, 2013 [40] |
| tran | c.4616C>T | p.Thr1539Met | 11-year-old male, Hodgkin lymphoma, pleuropulmonary blastoma Type I. History: thyroid cysts, syringomyelia. | Kuhlen, 2016 [41] |
| tran | c.4748T>G | p.Leu1583Arg | pleuropulmonary blastoma. | Hill, 2009 [1] |
| tran | c.5104C>T | p.Gln1702* | 9-year old female, pleuropulmonary blastoma & ERMS. | Doros, 2012 [35] |
| del | c.5221_5232delAACAACACCATC | p.Asn1741_1744del | 9-year old male, multinodular goiter, pleuropulmonary blastoma. History: 20 months, cystic nephroma. | Rath, 2014 [42] |
| del | c.5299delC | premature stop in exon 24 | 11-year-old male, Hodgkin lymphoma, pleuropulmonary blastoma Type I. History: thyroid cysts, syringomyelia. | Kuhlen, 2016 [41] |
| tran | c.5387C>T | p.Gln1783* | 14-month old female, type I pleuropulmonary blastoma. History: cystic nephroma. | Fernandez-Martinez, 2017 [43] |
| tran | c.5465A>T | p.Asp1822Val | 1.8-year-old, pleuropulmonary blastoma. | Slade, 2011 [7] |
| tran | c.5477C>A | p.Ser1826* | Median Age 34 months, female, cystic nephroma, pleuropulmonary blastoma. | Bahubeshi, 2010 [39] |
| Mutation Type | Chromosomal Mutation | Protein Change | Clinical Manifestation | Reference |
|---|---|---|---|---|
| tran | c.325C>T | p.Gln109* | 11-year old female, multinodular goiter. History: Sertoli–Leydig cell tumor. | Canfarotta M, 2016 [5] |
| del | c.876_879delAAAG | p.Arg293Ilefs*4 | 18-year old female, Sertoli–Leydig cell tumor. History: 16 years, multinodular goiter. | Rio Frio, 2011 [8] |
| tran | c.1376+1G>A | p.splice | 13-year old female, peritoneal cysts of right & left round ligaments, nasal polyps, Sertoli–Leydig cell tumor. History: 5 years, type II pleuropulmonary blastoma, 8 years, thyroid nodules. | Schultz, 2016 [34] |
| del | c.1532_1533delAT | N/A | 28-year old female, Sertoli–Leydig cell tumor. History: None | 16-year-old, Sertoli–Leydig cell tumor. | Oost, 2015 [63] |
| tran | c.2457C>G | p.Ile813_Tyr819del | 32-year old female, Sertoli–Leydig cell tumor. History: 18 years, multinodular goiter. | Rio Frio, 2011 [8] |
| del/ins | c.3270-6_4051—1280delinsG | p.Tyr1091Ser*28 | 14-year old female, multinodular goiter. History: Sertoli–Leydig cell tumor, primitive neuroectodermal tumor. | Sabbaghian, 2013 [64] |
| tran | c.3540C>A | p.Tyr1180* | 16-year old female, Sertoli–Leydig cell tumor. History: 14 years, bilateral multinodular goiter. 16-year old female, ovarian Sertoli–Leydig cell tumor, and lung lesion. History: 14 years, multinodular goiter. | de Kock, 2016 [65] Wu, 2014 [66] |
| tran | c.3647C>A | p.Ser1216* | 13-year old female, Danish, multinodular goiter and Sertoli–Leydig cell tumor. | Rossing, 2014 [67] |
| tran | c.3649T>A | p.Tyr1217Asn | 13-year old female, Danish, multinodular goiter and Sertoli–Leydig cell tumor. | Rossing, 2014 [67] |
| tran | c.3726C>A | p.Tyr1242* | 27-year old female, pleuropulmonary blastoma. History: 13 years, Sertoli–Leydig cell tumor and multinodular goiter, 21 years, nasal chondromesenchymal hamartoma. | Stewart, 2014 [36] |
| del | c.4050+1delG | p.Val351Valfs*11 | 20-year old female, primitive neuroectodermal tumor & multinodular goiter. History: 9 years, Sertoli–Leydig cell tumor. | Foulkes, 2011 [68] |
| del | c.5018_5021delTCAA | p.Ile1673Thrfs*31 | 32-year old female, Sertoli–Leydig cell tumor. History: 18 years, multinodular goiter. | Rio Frio, 2011 [8] |
| del | c.5122_5128delGGAGATG | p.Gly1708Argfs*7 | 21-year old, Sertoli–Leydig cell tumor. History: 17 years, Sertoli–Leydig cell tumor. | Slade, 2011 [7] |
| Mutation Type | Chromosomal Mutation | Protein Change | Clinical Manifestation | Reference |
|---|---|---|---|---|
| dup | c.328_338dupGTGTCAGCTGT | p.Arg114Cysfs*18 | 3-year old, cystic nephroma. | Slade, 2011 [7] |
| dup | c.912_919dupAGACTGTC | p.Arg307Glnfs*8 | 4-year old male, Wilms’ tumor. | Foulkes, 2011 [68] |
| del | c.1128_1132delAGTAA | p.Lys376Asnfs*11 | Pineoblastomas. | Sabbaghian, 2012 [70] |
| del | c.1153delC | p.Arg385Alafs*73 | 13-year old, Medulloblastoma/infratentorial primitive neuroectodermal tumor. | Slade, 2011 [7] |
| dup | c.1196_1197dupAG | p.Trp400Serfs*59 | 16-year old female, Ashkenazi Jewish/Anglo-Saxon, fibroadenoma of the breast. History: 6 years, ovarian embryonal rhabdomyosarcoma, 11 years, radiologic focal nodular liver hyperplasia, 12 years, cystic nephroma, 13 years, multinodular goiter. | de Kock, 2015 [71] |
| del | c.1284delGA | N/A | 23-month old female, pituitary blastoma | de Kock, 2014 [72] |
| dup | c.1306dupT | p.Ser436Phefs*41 | 2-year old male, Wlims’ tumor. | Foulkes, 2011 [68] |
| tran | c.1525C>T | p.Arg509* | 12-year old female, multinodular goiter. History: 6 years, dermoid cyst. | Darrat, 2013 [28] |
| tran | c.1966C>T | p.Arg656* | 15-month old female, Pulmonary sequestration & cystic nephroma. 14-year old female, Belarusian-Serbian, hepatic focal nodular hyperplasia. History: Right Brain ventricle tumor (part teratoma, party embryonic carcinoma) at 8 months old. pilomatrixoma at 3 years, Renal cysts at 4 years, thyroid nodules at 10 years, basal cell carcinoma at 13 years. | Foulkes, 2011 [68] Mehraein, 2016 [73] |
| tran | c.2026C>T | N/A | 17-year old female, pituitary blastoma. | de Kock, 2014 [72] |
| tran | c.2062C>T | p.Arg688* | 8 year, a 9-month-old girl, anaplastic sarcoma of the kidney. History: pneumothorax, left upper lung cyst and left renal cyst at 10 months. cysts multiplied and increased in size over next few years. | Wu, 2016 [74] |
| tran | c.2117-1G>A | p.Gly706Aspsfs*8 | 10-year old female, multinodular goiter. History: 5 years, Wilms’ tumor. | Foulkes, 2011 [68] |
| tran | c.2247C>A | p.Tyr749* | 6-week old male, embryonal rhabdomyosarcoma. | Doros, 2012 [35] |
| del | c.2399delG | p.Arg800fs*5 | 3.5-year old and 13-year old, Wilms’ tumor. | Palculict, 2016 [38] |
| tran | c.2407G>A | p.Gly803Arg | The average age of 44 months, Wilms’ tumor. | Palculict, 2016 [38] |
| del | c.2450delC | p.Pro817Leufs*15 | 7-month old female, Polish, multiseptated cystic mass in abdomen (early anaplastic sarcoma). | Wu, 2016 [52] |
| tran | c.2455T>C | p.Tyr819His | 34 & 32-year old male family members, hepatocellular tumors. | Caruso, 2016 [75] |
| tran | c.2457C>G | p.Ile813_Tyr819del | 53-year-old female, cERMS. History: multinodular goiter. | de Kock, 2015 [76] |
| tran | c.2516C>T | p.Ser839Phe | 15-year old female, multinodular goiter. | Rio Frio, 2011 [8] |
| tran | c.2805-1G>T | p.Tyr936_Arg996del | The patient died at 20 years from alveolar rhabdomyosarcoma. History: multinodular goiter. | Rio Frio, 2011 [8] |
| del | c.3046delA | p.Ser1016Valfs*1065 | 12-month old female, pituitary blastoma. | Sahakitrungruang, 2014 [77] |
| tran | c.2379T>G | N/A | 3-year old male, pituitary blastoma. | de Kock, 2014 [72] |
| del | c.3277_3280delAACT | N/A | 7-year old female, pituitary blastoma. | de Kock, 2014 [72] |
| ins | c.3288_3289insTTTC | p.Gly1097Phefs*8 | 1.5-year old, cystic nephroma. | Slade, 2011 [7] |
| tran | c.3334A>G | p.Asn1112Asp | 55-year old female, endometrial cancer. | Yang, 2015 [78] |
| dup | c.3405dupA | p.Gly1136Arg | 12-year old female, renal cysts & focal nodular hyperplasia of the liver. History: 6 months, eRMS of the bladder and a cystic lesion in the lung at. 3 & 4.5 years, Ciliary body medulloepithelioma. | Fremerey, 2016 [79] |
| del | c.3535_3538delTCTT | p.Ser1179Thrfs*12 | 13-year old female, cervical sarcoma botryoides. | Tomiak, 2014 [80] |
| tran | c.3540C>G | p.Tyr1180 | 2-year old female, a multilocular cyst in left kidney, 2 cystic lesions in the lung, multicystic nephroma extended from left kidney. | Bardon-Cancho, 2016 [81] |
| del | c.3611_3616delACTACAinsT | p.Tyr1204Leufs*29 | 14-year old female, cERMS and thyroid goiter. | Foulkes, 2011 [68] |
| dup | c.3665dupT | p.Leu1222fs*13 | 30–39 year old female, soft tissue sarcoma | de Kock, 2017 [9] |
| del | c.3793delA | p.Thr1265Glnfs*37 | 6-year-old, ovarian sex cord stromal tumour. | Slade, 2011 [7] |
| del | c.3907_3908delCT | p.Leu1303Valfs*4 | 13-year old female, cervical embryonal rhabdomyosarcoma & two small lung cysts. History: 11 years, multinodular goiter. | Foulkes, 2011 [68] |
| del | c.4309_4312delGACT | N/A | Male, deceased 8 months post-surgery, pituitary blastoma. | de Kock, 2014 [72] |
| dup | c.4566_4579dupCTTTG | p.Val1524fs*38 | 14-month old female, neuroblastoma & cystic nephroma, multinodular goiter at age 7. | Saskin, 2017 [10] |
| tran | c.4740G>T | p.Gln1580His | 32-year old, seminoma. | Slade, 2011 [7] |
| tran | c.5096-12G>A | N/A | 10-year old female, undifferentiated sarcoma at ovary. | de Kock, 2017 [9] |
| tran | c.5125G>C(de novo) | N/A | 21-month old male, pituitary blastoma. | de Kock, 2014 [72] |
| del | c.5221_5232delAACAACACCATC | p.Asn1741_1744del | 9-year old male, multinodular goiter, pleuropulmonary blastoma. History: 20 months, cystic nephroma. | Rath, 2014 [42] |
| del/ins | c.5426_5442 del GGGATATTTTTGAGTCGinsCA | p.Gly1809_Ser1814delinsAla | 15-year old female, thyroid follicular carcinoma. History: ASK for 12 years & multiple cystic-appearing thyroid nodules, no malignancy. | Yoshida, 2017 [82] |
| tran | c.5441C>T | p.Ser1814Leu | 12.5-year-old female, ovarian tumor. History: 12 years, multinodular goiter. | Wu, 2016 [83] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robertson, J.C.; Jorcyk, C.L.; Oxford, J.T. DICER1 Syndrome: DICER1 Mutations in Rare Cancers. Cancers 2018, 10, 143. https://doi.org/10.3390/cancers10050143
Robertson JC, Jorcyk CL, Oxford JT. DICER1 Syndrome: DICER1 Mutations in Rare Cancers. Cancers. 2018; 10(5):143. https://doi.org/10.3390/cancers10050143
Chicago/Turabian StyleRobertson, Jake C., Cheryl L. Jorcyk, and Julia Thom Oxford. 2018. "DICER1 Syndrome: DICER1 Mutations in Rare Cancers" Cancers 10, no. 5: 143. https://doi.org/10.3390/cancers10050143
APA StyleRobertson, J. C., Jorcyk, C. L., & Oxford, J. T. (2018). DICER1 Syndrome: DICER1 Mutations in Rare Cancers. Cancers, 10(5), 143. https://doi.org/10.3390/cancers10050143






