The Potential for Cancer Immunotherapy in Targeting Surgery-Induced Natural Killer Cell Dysfunction
Abstract
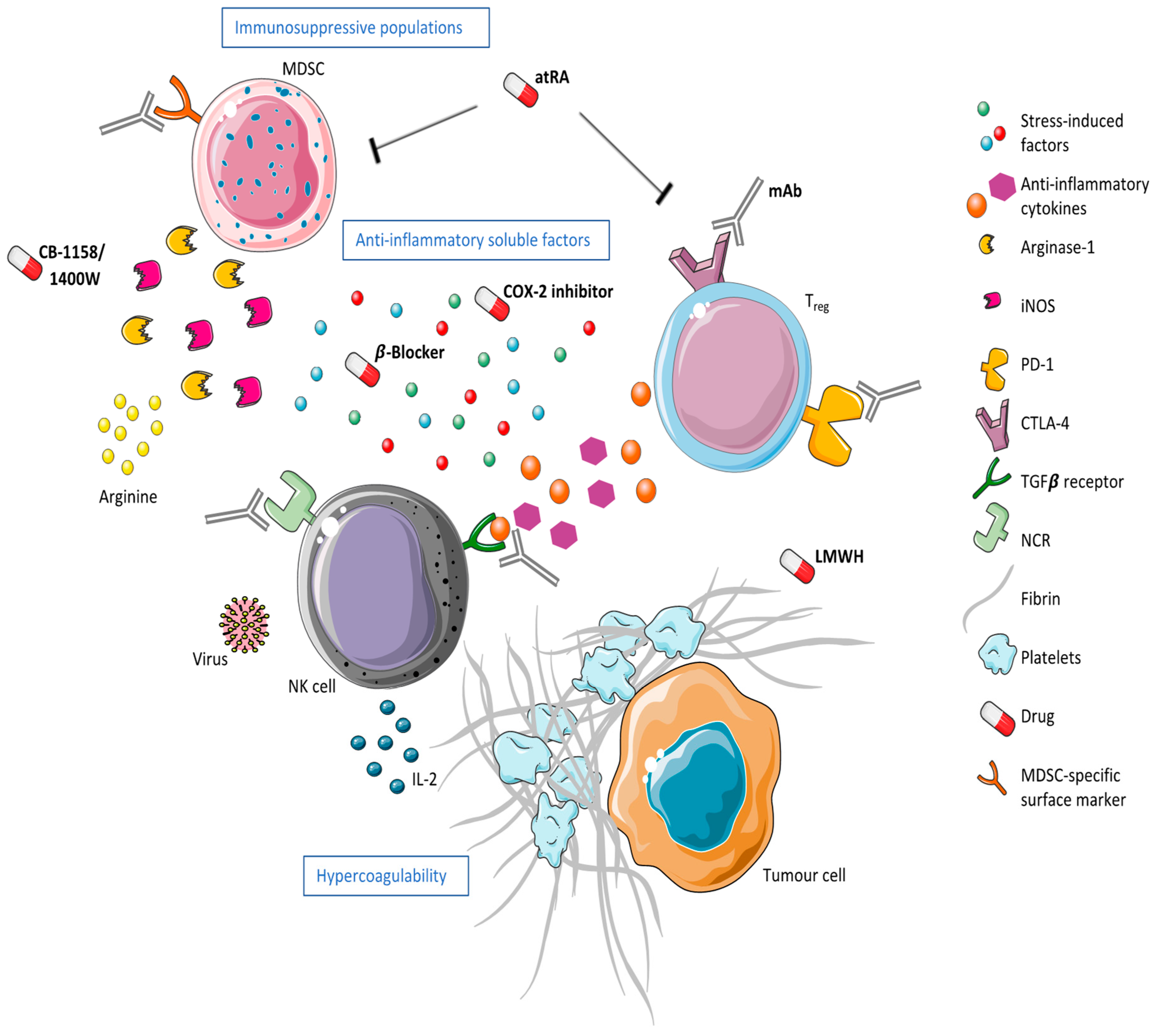
:1. Natural Killer Cells
2. Dysfunctional NK Cells Mediate Metastasis in the Postoperative Period
3. The Postoperative Environment and Potential Therapeutics
3.1. The Hypercoagulable State
3.2. The Release of Anti-Inflammatory Soluble Factors
3.3. The Expansion of Immunosuppressive Populations
3.3.1. Myeloid-Derived Suppressor Cells
3.3.2. Regulatory T Cells
4. Surgical Stress and NK Cell Biology
4.1. Targeting NK Cell Receptor Expression
4.2. Promoters of NK Cell Function
4.3. The Suppressive NK Cell
4.4. The Unresponsive NK Cell
5. Summary and Where to Go from Here
Author Contributions
Funding
Conflicts of Interest
References
- Baginska, J.; Viry, E.; Paggetti, J.; Medves, S.; Berchem, G.; Moussay, E.; Janji, B. The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Front. Immunol. 2013, 4, 490. [Google Scholar] [CrossRef] [PubMed]
- Chester, C.; Fritsch, K.; Kohrt, H.E. Natural Killer Cell Immunomodulation: Targeting Activating, Inhibitory, and Co-stimulatory Receptor Signaling for Cancer Immunotherapy. Front. Immunol. 2015, 6, 601. [Google Scholar] [CrossRef] [PubMed]
- Maghazachi, A.A. Compartmentalization of human natural killer cells. Mol. Immunol. 2005, 42, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Vivier, E.; Ugolini, S. Regulatory natural killer cells: New players in the IL-10 anti-inflammatory response. Cell Host Microbe 2009, 6, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Waldhauer, I.; Steinle, A. NK cells and cancer immunosurveillance. Oncogene 2008, 27, 5932–5943. [Google Scholar] [CrossRef] [Green Version]
- Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef]
- Bubeník, J. Tumour MHC class I downregulation and immunotherapy (Review). Oncol. Rep. 2003, 10, 2005–2008. [Google Scholar] [CrossRef]
- Diefenbach, A.; Raulet, D.H. Strategies for target cell recognition by natural killer cells. Immunol. Rev. 2001, 181, 170–184. [Google Scholar] [CrossRef] [Green Version]
- Raulet, D.H. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat. Immunol. 2004, 5, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Groh, V.; Wu, J.; Steinle, A.; Phillips, J.H.; Lanier, L.L.; Spies, T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999, 285, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Long, E.O. Tumor cell recognition by natural killer cells. Semin. Cancer Biol. 2002, 12, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Old, L.J.; Schreiber, R.D. The roles of IFNγ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002, 13, 95–109. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kimura, T.; Ueta, E.; Tatemoto, Y.; Osaki, T. Characteristic cytokine generation patterns in cancer cells and infiltrating lymphocytes in oral squamous cell carcinomas and the influence of chemoradiation combined with immunotherapy on these patterns. Oncology 2003, 64, 407–415. [Google Scholar] [CrossRef]
- Michel, T.; Hentges, F.; Zimmer, J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front. Immunol. 2012, 3, 403. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Koh, C.Y.; Wilder, J.A. Interactions between B lymphocytes and NK cells. FASEB J. 1994, 8, 1012–1018. [Google Scholar] [CrossRef]
- Fung, M.M.; Rohwer, F.; McGuire, K.L. IL-2 activation of a PI3K-dependent STAT3 serine phosphorylation pathway in primary human T cells. Cell Signal. 2003, 15, 625–636. [Google Scholar] [CrossRef]
- Marçais, A.; Cherfils-Vicini, J.; Viant, C.; Degouve, S.; Viel, S.; Fenis, A.; Rabilloud, J.; Mayol, K.; Tavares, A.; Bienvenu, J.; et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 2014, 15, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Zamai, L.; Ahmad, M.; Bennett, I.M.; Azzoni, L.; Alnemri, E.S.; Perussia, B. Natural killer (NK) cell-mediated cytotoxicity: Differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J. Exp. Med. 1998, 188, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Langers, I.; Renoux, V.M.; Thiry, M.; Delvenne, P.; Jacobs, N. Natural killer cells: Role in local tumor growth and metastasis. Biologics 2012, 6, 73–82. [Google Scholar] [PubMed]
- Terunuma, H.; Deng, X.; Dewan, Z.; Fujimoto, S.; Yamamoto, N. Potential Role of NK Cells in the Induction of Immune Responses: Implications for NK Cell–Based Immunotherapy for Cancers and Viral Infections. Int. Rev. Immunol. 2008, 27, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Blazar, B.A.; Rodrick, M.L.; O’Mahony, J.B.; Wood, J.J.; Bessey, P.Q.; Wilmore, D.W.; Mannick, J.A. Suppression of natural killer-cell function in humans following thermal and traumatic injury. J. Clin. Immunol. 1986, 6, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Iversen, P.O.; Hjeltnes, N.; Holm, B.; Flatebo, T.; Strom-Gundersen, I.; Ronning, W.; Stanghelle, J.; Benestad, H.B. Depressed immunity and impaired proliferation of hematopoietic progenitor cells in patients with complete spinal cord injury. Blood 2000, 96, 2081–2083. [Google Scholar] [PubMed]
- Chou, A.; Krukowski, K.; Morganti, J.M.; Riparip, L.-K.; Rosi, S. Persistent Infiltration and Impaired Response of Peripherally-Derived Monocytes after Traumatic Brain Injury in the Aged Brain. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.-H.; Zhang, J.; Scott, K.J.; de Souza, C.T.; Alkayyal, A.A.; Ananth, A.A.; Sahi, S.; Adair, R.A.; Mahmoud, A.B.; Sad, S.; et al. Perioperative influenza vaccination reduces postoperative metastatic disease by reversing surgery-induced dysfunction in natural killer cells. Clin. Cancer Res. 2013, 19, 5104–5115. [Google Scholar] [CrossRef] [PubMed]
- Pollock, R.E.; Lotzová, E.; Stanford, S.D. Mechanism of surgical stress impairment of human perioperative natural killer cell cytotoxicity. Arch. Surg. 1991, 126, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, R.; Pohlmann, S.; Kleinertz, H.; Hepner-Schefczyk, M.; Paul, A.; Flohé, S.B. Invasive Surgery Impairs the Regulatory Function of Human CD56 bright Natural Killer Cells in Response to Staphylococcus aureus. Suppression of Interferon-γ Synthesis. PLoS ONE 2015, 10, e0130155. [Google Scholar] [CrossRef] [PubMed]
- Angka, L.; Martel, A.B.; Kilgour, M.; Jeong, A.; Sadiq, M.; de Souza, C.T.; Baker, L.; Kennedy, M.A.; Kekre, N.; Auer, R.C. Natural Killer Cell IFNγ Secretion is Profoundly Suppressed Following Colorectal Cancer Surgery. Ann. Surg. Oncol. 2018, 25, 3747–3754. [Google Scholar] [CrossRef] [PubMed]
- Baxevanis, C.N.; Papilas, K.; Dedoussis, G.V.; Pavlis, T.; Papamichail, M. Abnormal cytokine serum levels correlate with impaired cellular immune responses after surgery. Clin. Immunol. Immunopathol. 1994, 71, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, M.; Jeong, H.; Chae, J.S.; Kim, Y.S.; Lee, J.G.; Cho, Y.; Lee, J.H. Hyporesponsiveness of natural killer cells and impaired inflammatory responses in critically ill patients. BMC Immunol. 2017, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Van der Bij, G.J.; Oosterling, S.J.; Beelen, R.H.J.; Meijer, S.; Coffey, J.C.; van Egmond, M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann. Surg. 2009, 249, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, R.; Retsky, M.W.; Hrushesky, W.J.M.; Baum, M.; Gukas, I.D. The effects of surgery on tumor growth: A century of investigations. Ann. Oncol. 2008, 19, 1821–1828. [Google Scholar] [CrossRef]
- Baum, M.; Demicheli, R.; Hrushesky, W.; Retsky, M. Does surgery unfavourably perturb the “natural history” of early breast cancer by accelerating the appearance of distant metastases? Eur. J. Cancer 2005, 41, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.F.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef]
- Tyzzer, E.E. Factors in the Production and Growth of tumor Metastases. J. Med. Res. 1913, 28, 309–332. [Google Scholar]
- Decker, D.; Schondorf, M.; Bidlingmaier, F.; Hirner, A.; von Ruecker, A.A. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery 1996, 119, 316–325. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Sawada, S.; Yoshioka, I.; Ohashi, Y.; Matsuo, M.; Harimaya, Y.; Tsukada, K.; Saiki, I. Increased surgical stress promotes tumor metastasis. Surgery 2003, 133, 547–555. [Google Scholar] [CrossRef]
- Eberhardt, J.M.; Kiran, R.P.; Lavery, I.C. The impact of anastomotic leak and intra-abdominal abscess on cancer-related outcomes after resection for colorectal cancer: A case control study. Dis. Colon Rectum 2009, 52, 380–386. [Google Scholar] [CrossRef]
- Tai, L.-H.; de Souza, C.T.; Bélanger, S.; Ly, L.; Alkayyal, A.A.; Zhang, J.; Rintoul, J.L.; Ananth, A.A.; Lam, T.; Breitbach, C.J.; et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. 2013, 73, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.H.; Ramsden, C.W.; Ward, U.; Trejdosiewicz, L.K.; Ambrose, N.S.; Primrose, J.N. Peri-operative modulation of cellular immunity in patients with colorectal cancer. Clin. Exp. Immunol. 1993, 94, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.H.; Ramsden, C.W.; Ward, U.; Sedman, P.C.; Primrose, J.N. Perioperative immunotherapy with recombinant interleukin 2 in patients undergoing surgery for colorectal cancer. Cancer Res. 1992, 52, 5765–5769. [Google Scholar] [PubMed]
- Deehan, D.J.; Heys, S.D.; Ashby, J.; Eremin, O. Interleukin-2 (IL-2) augments host cellular immune reactivity in the perioperative period in patients with malignant disease. Eur. J. Surg. Oncol. 1995, 21, 16–22. [Google Scholar] [CrossRef]
- Houvenaeghel, G.; Bladou, F.; Blache, J.L.; Olive, D.; Monges, G.; Jacquemier, J.; Chaudet, H.; Delpero, J.R.; Guérinel, G. Tolerance and feasibility of perioperative treatment with interferon-alpha 2a in advanced cancers. Int. Surg. 1997, 82, 165–169. [Google Scholar] [PubMed]
- Jain, A.; Slansky, J.E.; Matey, L.C.; Allen, H.E.; Pardoll, D.M.; Schulick, R.D. Synergistic effect of a granulocyte-macrophage colony-stimulating factor-transduced tumor vaccine and systemic interleukin-2 in the treatment of murine colorectal cancer hepatic metastases. Ann. Surg. Oncol. 2003, 10, 810–820. [Google Scholar] [CrossRef]
- Oosterling, S.J.; van der Bij, G.J.; Mels, A.K.; Beelen, R.H.J.; Meijer, S.; van Egmond, M.; van Leeuwen, P.A.M. Perioperative IFN-alpha to avoid surgically induced immune suppression in colorectal cancer patients. Histol. Histopathol. 2006, 21, 753–760. [Google Scholar]
- Mels, A.K.; Statius Muller, M.G.; van Leeuwen, P.A.M.; von Blomberg, B.M.E.; Scheper, R.J.; Cuesta, M.A.; Beelen, R.H.J.; Meijer, S. Immune-stimulating effects of low-dose perioperative recombinant granulocyte-macrophage colony-stimulating factor in patients operated on for primary colorectal carcinoma. Br. J. Surg. 2001, 88, 539–544. [Google Scholar] [CrossRef]
- Sturm, J.W.; Magdeburg, R.; Berger, K.; Petruch, B.; Samel, S.; Bönninghoff, R.; Keese, M.; Hafner, M.; Post, S. Influence of TNFA on the formation of liver metastases in a syngenic mouse model. Int. J. Cancer 2003, 107, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Webb, N.J.; Bottomley, M.J.; Watson, C.J.; Brenchley, P.E. Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: Implications for measurement of circulating VEGF levels in clinical disease. Clin. Sci. 1998, 94, 395–404. [Google Scholar] [CrossRef]
- Palumbo, J.S.; Kombrinck, K.W.; Drew, A.F.; Grimes, T.S.; Kiser, J.H.; Degen, J.L.; Bugge, T.H. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 2000, 96, 3302–3309. [Google Scholar] [PubMed]
- Palumbo, J.S.; Potter, J.M.; Kaplan, L.S.; Talmage, K.; Jackson, D.G.; Degen, J.L. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002, 62, 6966–6972. [Google Scholar] [PubMed]
- Palumbo, J.S.; Talmage, K.E.; Liu, H.; La Jeunesse, C.M.; Witte, D.P.; Degen, J.L. Plasminogen supports tumor growth through a fibrinogen-dependent mechanism linked to vascular patency. Blood 2003, 102, 2819–2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, D.E.; Weitz, I.C.; Liebman, H.A. Thromboembolic complications of cancer: Epidemiology, pathogenesis, diagnosis, and treatment. Am. J. Hematol. 2003, 72, 43–52. [Google Scholar] [CrossRef]
- Rickles, F.R.; Patierno, S.; Fernandez, P.M. Tissue factor, thrombin, and cancer. Chest 2003, 124, 58S–68S. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.T.; Kakkar, A.K. Coagulation proteases and human cancer. Biochem. Soc. Trans. 2002, 30, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Tai, L.-H.; Falls, T.; de Souza, C.T.; Bell, J.C.; Carrier, M.; Atkins, H.; Boushey, R.; Auer, R.A. Surgical stress promotes the development of cancer metastases by a coagulation-dependent mechanism involving natural killer cells in a murine model. Ann. Surg. 2013, 258, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Lison, S.; Weiss, G.; Spannagl, M.; Heindl, B. Postoperative changes in procoagulant factors after major surgery. Blood Coagul. Fibrinolysis 2011, 22, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ulrych, J.; Kvasnicka, T.; Fryba, V.; Komarc, M.; Malikova, I.; Burget, F.; Brzezkova, R.; Kvasnicka, J., Jr.; Krska, Z.; Kvasnicka, J. 28 day post-operative persisted hypercoagulability after surgery for benign diseases: A prospective cohort study. BMC Surg. 2016, 16, 16. [Google Scholar] [CrossRef]
- Qi, C.; Li, B.; Guo, S.; Wei, B.; Shao, C.; Li, J.; Yang, Y.; Zhang, Q.; Li, J.; He, X.; et al. P-Selectin-Mediated Adhesion between Platelets and Tumor Cells Promotes Intestinal Tumorigenesis in Apc(Min/+) Mice. Int. J. Biol. Sci. 2015, 11, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.L.; Varki, A.; Borsig, L. Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb. Res. 2007, 120 (Suppl. 2), S107–S111. [Google Scholar] [CrossRef]
- Varki, A.; Varki, N.M. P-selectin, carcinoma metastasis and heparin: Novel mechanistic connections with therapeutic implications. Braz. J. Med. Biol. Res. 2001, 34, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Borsig, L.; Wong, R.; Feramisco, J.; Nadeau, D.R.; Varki, N.M.; Varki, A. Heparin and cancer revisited: Mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc. Natl. Acad. Sci. USA 2001, 98, 3352–3357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.; Tai, G.; Gao, Y.; Li, N.; Huang, B.; Zhou, Y.; Hao, S.; Zeng, X. Modified heparin inhibits P-selectin-mediated cell adhesion of human colon carcinoma cells to immobilized platelets under dynamic flow conditions. J. Biol. Chem. 2004, 279, 29202–29210. [Google Scholar] [CrossRef] [PubMed]
- Lazo-Langner, A.; Goss, G.D.; Spaans, J.N.; Rodger, M.A. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J. Thromb. Haemost. 2007, 5, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Kingston, R.D.; Fielding, J.W.; Palmer, M.K. Peri-operative heparin: A possible adjuvant to surgery in colo-rectal cancer? Int. J. Colorectal Dis. 1993, 8, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Von Tempelhoff, G.F.; Harenberg, J.; Niemann, F.; Hommel, G.; Kirkpatrick, C.J.; Heilmann, L. Effect of low molecular weight heparin (Certoparin) versus unfractionated heparin on cancer survival following breast and pelvic cancer surgery: A prospective randomized double-blind trial. Int. J. Oncol. 2000, 16, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Auer, R.C. NCT01455831: A Multicentre Randomized Controlled Trial of the Use of Extended Peri-Operative Low Molecular Weight Heparin to Improve Cancer Specific Survival Following Surgical Resection of Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01455831 (accessed on 26 October 2018).
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Narita, S.; Tsuchiya, N.; Kumazawa, T.; Maita, S.; Numakura, K.; Obara, T.; Tsuruta, H.; Saito, M.; Inoue, T.; Horikawa, Y.; et al. Comparison of surgical stress in patients undergoing open versus laparoscopic radical prostatectomy by measuring perioperative serum cytokine levels. J. Laparoendosc. Adv. Surg. Tech. A 2013, 23, 33–37. [Google Scholar] [CrossRef]
- Shariat, S.F.; Kattan, M.W.; Traxel, E.; Andrews, B.; Zhu, K.; Wheeler, T.M.; Slawin, K.M. Association of pre- and postoperative plasma levels of transforming growth factor beta(1) and interleukin 6 and its soluble receptor with prostate cancer progression. Clin. Cancer Res. 2004, 10, 1992–1999. [Google Scholar] [CrossRef]
- Gao, J.; Duan, Z.; Zhang, L.; Huang, X.; Long, L.; Tu, J.; Liang, H.; Zhang, Y.; Shen, T.; Lu, F. Failure recovery of circulating NKG2D+CD56dimNK cells in HBV-associated hepatocellular carcinoma after hepatectomy predicts early recurrence. Oncoimmunology 2016, 5, e1048061. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Rossi Del Monte, S.; Salerno, G.; Bocchetti, T.; Angeletti, S.; Malisan, F.; Cardelli, P.; Ziparo, V.; Torrisi, M.R.; Visco, V. Recovery of immunological homeostasis positively correlates both with early stages of right-colorectal cancer and laparoscopic surgery. PLoS ONE 2013, 8, e74455. [Google Scholar] [CrossRef] [PubMed]
- Scheid, C.; Young, R.; McDermott, R.; Fitzsimmons, L.; Scarffe, J.H.; Stern, P.L. Immune function of patients receiving recombinant human interleukin-6 (IL-6) in a phase I clinical study: Induction of C-reactive protein and IgE and inhibition of natural killer and lymphokine-activated killer cell activity. Cancer Immunol. Immunother. 1994, 38, 119–126. [Google Scholar] [PubMed]
- Kang, Y.-J.; Jeung, I.C.; Park, A.; Park, Y.-J.; Jung, H.; Kim, T.-D.; Lee, H.G.; Choi, I.; Yoon, S.R. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 2014, 29, 2176–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cifaldi, L.; Prencipe, G.; Caiello, I.; Bracaglia, C.; Locatelli, F.; De Benedetti, F.; Strippoli, R. Inhibition of natural killer cell cytotoxicity by interleukin-6: Implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 2015, 67, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Vredevoe, D.L.; Widawski, M.; Fonarow, G.C.; Hamilton, M.; Martínez-Maza, O.; Gage, J.R. Interleukin-6 (IL-6) expression and natural killer (NK) cell dysfunction and anergy in heart failure. Am. J. Cardiol. 2004, 93, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Cantoni, C.; Della Chiesa, M.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4120–4125. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Fu, B.; Gao, Y.; Liao, X.; Sun, R.; Tian, Z.; Wei, H. TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012, 8, e1002594. [Google Scholar] [CrossRef]
- Crane, C.A.; Han, S.J.; Barry, J.J.; Ahn, B.J.; Lanier, L.L.; Parsa, A.T. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol. 2010, 12, 7–13. [Google Scholar] [CrossRef]
- Lee, J.-C.; Lee, K.-M.; Kim, D.-W.; Heo, D.S. Elevated TGF-β1 Secretion and Down-Modulation of NKG2D Underlies Impaired NK Cytotoxicity in Cancer Patients. J. Immunol. 2004, 172, 7335–7340. [Google Scholar] [CrossRef]
- Zhang, X.L.; Topley, N.; Ito, T.; Phillips, A. Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling. J. Biol. Chem. 2005, 280, 12239–12245. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Chantry, D.; Feldmann, M. Transforming growth factor beta induces the production of interleukin 6 by human peripheral blood mononuclear cells. Cytokine 1990, 2, 211–216. [Google Scholar] [CrossRef]
- Ulich, T.R.; Yin, S.; Guo, K.; Yi, E.S.; Remick, D.; del Castillo, J. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. Am. J. Pathol. 1991, 138, 1097–1101. [Google Scholar] [PubMed]
- Meron, G.; Tishler, Y.; Shaashua, L.; Rosenne, E.; Levi, B.; Melamed, R.; Gotlieb, N.; Matzner, P.; Sorski, L.; Ben-Eliyahu, S. PGE2 suppresses NK activity in vivo directly and through adrenal hormones: Effects that cannot be reflected by ex vivo assessment of NK cytotoxicity. Brain Behav. Immun. 2013, 28, 128–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenne, E.; Sorski, L.; Shaashua, L.; Neeman, E.; Matzner, P.; Levi, B.; Ben-Eliyahu, S. In vivo suppression of NK cell cytotoxicity by stress and surgery: Glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav. Immun. 2014, 37, 207–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, D.; Ma, X.; Kundu, N.; Fulton, A. Prostaglandin E(2) (PGE (2)) suppresses natural killer cell function primarily through the PGE(2) receptor EP4. Cancer Immunol. Immunother. 2011, 60, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Holt, D.; Kundu, N.; Reader, J.; Goloubeva, O.; Take, Y.; Fulton, A.M. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE2-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology 2013, 2, e22647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choy, C.; Raytis, J.L.; Smith, D.D.; Duenas, M.; Neman, J.; Jandial, R.; Lew, M.W. Inhibition of β2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative β-blockade. Oncol. Rep. 2016, 35, 3135–3142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horowitz, M.; Neeman, E.; Sharon, E.; Ben-Eliyahu, S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat. Rev. Clin. Oncol. 2015, 12, 213–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaashua, L.; Shabat-Simon, M.; Haldar, R.; Matzner, P.; Zmora, O.; Shabtai, M.; Sharon, E.; Allweis, T.; Barshack, I.; Hayman, L.; et al. Perioperative COX-2 and β-Adrenergic Blockade Improves Metastatic Biomarkers in Breast Cancer Patients in a Phase-II Randomized Trial. Clin. Cancer Res. 2017, 23, 4651–4661. [Google Scholar] [CrossRef]
- Benish, M.; Bartal, I.; Goldfarb, Y.; Levi, B.; Avraham, R.; Raz, A.; Ben-Eliyahu, S. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann. Surg. Oncol. 2008, 15, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Zmora, O.; Tulchinsky, H.; Wasserberg, N. NCT00888797: Perioperative ß-Adrenergic Blocker and a COX2 Inhibitor in Patients Undergoing Resection for Primary Colon and Rectal Cancer: Effect on Tumor Recurrence and Postoperative Immune Perturbations. A Multicenter Randomized Prospective Trial. Available online: https://clinicaltrials.gov/ct2/show/record/NCT00888797 (accessed on 26 October 2018).
- Otegbeye, F.; Ojo, E.; Moreton, S.; Mackowski, N.; Lee, D.A.; de Lima, M.; Wald, D.N. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS ONE 2018, 13, e0191358. [Google Scholar]
- Cekic, C.; Day, Y.-J.; Sag, D.; Linden, J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014, 74, 7250–7259. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Sarhan, D.; Steven, A.; Seliger, B.; Kiessling, R.; Lundqvist, A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin. Cancer Res. 2014, 20, 4096–4106. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, D.; Cichocki, F.; Zhang, B.; Yingst, A.; Spellman, S.R.; Cooley, S.; Verneris, M.R.; Blazar, B.R.; Miller, J.S. Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells. Cancer Res. 2016, 76, 5696–5706. [Google Scholar] [CrossRef] [PubMed]
- Elkabets, M.; Ribeiro, V.S.G.; Dinarello, C.A.; Ostrand-Rosenberg, S.; Di Santo, J.P.; Apte, R.N.; Vosshenrich, C.A.J. IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur. J. Immunol. 2010, 40, 3347–3357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, C.C.; Roggerson, K.M.; Lee, H.C. Hepatitis C virus–induced myeloid-derived suppressor cells suppress NK cell IFN-γ production by altering cellular metabolism via arginase-1. J. Immunol. 2016, 196, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Hoechst, B.; Voigtlaender, T.; Ormandy, L.; Gamrekelashvili, J.; Zhao, F.; Wedemeyer, H.; Lehner, F.; Manns, M.P.; Greten, T.F.; Korangy, F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009, 50, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Tai, L.-H.; Alkayyal, A.A.; Leslie, A.L.; Sahi, S.; Bennett, S.; Tanese de Souza, C.; Baxter, K.; Angka, L.; Xu, R.; Kennedy, M.A.; et al. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cell-dependent inhibition of Natural Killer cell cytotoxicity. Oncoimmunology 2018, 7, e1431082. [Google Scholar] [CrossRef]
- Li, H.; Han, Y.; Guo, Q.; Zhang, M.; Cao, X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J. Immunol. 2009, 182, 240–249. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, L.; Yu, L.; Wang, Y.-Y.; Chen, R.; Qian, J.; Hong, Z.-P.; Su, X.-S. Surgery-induced monocytic myeloid-derived suppressor cells expand regulatory T cells in lung cancer. Oncotarget 2017, 8, 17050–17058. [Google Scholar] [CrossRef] [Green Version]
- Manz, M.G.; Boettcher, S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014, 14, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Loftus, T.J.; Mohr, A.M.; Moldawer, L.L. Dysregulated myelopoiesis and hematopoietic function following acute physiologic insult. Curr. Opin. Hematol. 2018, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hüsecken, Y.; Muche, S.; Kustermann, M.; Klingspor, M.; Palmer, A.; Braumüller, S.; Huber-Lang, M.; Debatin, K.-M.; Strauss, G. MDSCs are induced after experimental blunt chest trauma and subsequently alter antigen-specific T cell responses. Sci. Rep. 2017, 7, 12808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; He, H.; Gu, Y.; Wang, Y.; Sun, Z.; Yang, L.; Miao, C. Surgical trauma contributes to progression of colon cancer by downregulating CXCL4 and recruiting MDSCs. Exp. Cell Res. 2018, 370, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Stiff, A.; Trikha, P.; Mundy-Bosse, B.; McMichael, E.; Mace, T.A.; Benner, B.; Kendra, K.; Campbell, A.; Gautam, S.; Abood, D.; et al. Nitric Oxide Production by Myeloid-Derived Suppressor Cells Plays a Role in Impairing Fc Receptor-Mediated Natural Killer Cell Function. Clin. Cancer Res. 2018, 24, 1891–1904. [Google Scholar] [CrossRef]
- Zhu, X.; Herrera, G.; Ochoa, J.B. Immunosupression and infection after major surgery: A nutritional deficiency. Crit. Care Clin. 2010, 26, 491–500. [Google Scholar] [CrossRef]
- Serafini, P.; Meckel, K.; Kelso, M.; Noonan, K.; Califano, J.; Koch, W.; Dolcetti, L.; Bronte, V.; Borrello, I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 2006, 203, 2691–2702. [Google Scholar] [CrossRef] [Green Version]
- Auer, R.C. NCT02998736: PERIOP-04 Phase Ib Trial of Perioperative Tadalafil and Influenza Vaccination in Cancer Patients Undergoing Major Surgical Resection of a Primary Abdominal Malignancy. Available online: https://clinicaltrials.gov/ct2/show/record/NCT02998736 (accessed on 26 October 2018).
- Oberlies, J.; Watzl, C.; Giese, T.; Luckner, C.; Kropf, P.; Müller, I.; Ho, A.D.; Munder, M. Regulation of NK cell function by human granulocyte arginase. J. Immunol. 2009, 182, 5259–5267. [Google Scholar] [CrossRef]
- Garvey, E.P.; Oplinger, J.A.; Furfine, E.S.; Kiff, R.J.; Laszlo, F.; Whittle, B.J.; Knowles, R.G. 1400 W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J. Biol. Chem. 1997, 272, 4959–4963. [Google Scholar] [CrossRef] [PubMed]
- Atochina-Vasserman, E.N.; Beers, M.F.; Kadire, H.; Tomer, Y.; Inch, A.; Scott, P.; Guo, C.J.; Gow, A.J. Selective inhibition of inducible NO synthase activity in vivo reverses inflammatory abnormalities in surfactant protein D-deficient mice. J. Immunol. 2007, 179, 8090–8097. [Google Scholar] [CrossRef] [PubMed]
- Steggerda, S.M.; Bennett, M.K.; Chen, J.; Emberley, E.; Huang, T.; Janes, J.R.; Li, W.; MacKinnon, A.L.; Makkouk, A.; Marguier, G.; et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J. Immunother. Cancer 2017, 5, 101. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Tsai, F.Y.-C.; Bauer, T.M.; Muigai, L.; Liang, Y.; Bennett, M.K.; Orford, K.W.; Fu, S. CX-1158-101: A first-in-human phase 1 study of CB-1158, a small molecule inhibitor of arginase, as monotherapy and in combination with an anti-PD-1 checkpoint inhibitor in patients (pts) with solid tumors. J. Clin. Orthod. 2017, 35, 3005. [Google Scholar] [CrossRef]
- Tsai, F.; Bauer, T.; Naing, A. NCT02903914: Arginase Inhibitor INCB001158 as a Single Agent and in Combination with Immune Checkpoint Therapy in Patients with Advanced/Metastatic Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/record/NCT02903914 (accessed on 26 October 2018).
- Campbell, L.; Saville, C.R.; Murray, P.J.; Cruickshank, S.M.; Hardman, M.J. Local arginase 1 activity is required for cutaneous wound healing. J. Investig. Dermatol. 2013, 133, 2461–2470. [Google Scholar] [CrossRef]
- Gould, A.; Candy, G.P. Arginine metabolism and wound healing: Basic science review. Wound Heal. S. Afr. 2008, 1, 48–50. [Google Scholar]
- Alexander, J.W.; Supp, D.M. Role of Arginine and Omega-3 Fatty Acids in Wound Healing and Infection. Adv. Wound Care 2014, 3, 682–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyland, D.K.; Novak, F.; Drover, J.W.; Jain, M.; Su, X.; Suchner, U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001, 286, 944–953. [Google Scholar] [CrossRef]
- Heine, A.; Flores, C.; Gevensleben, H.; Diehl, L.; Heikenwalder, M.; Ringelhan, M.; Janssen, K.-P.; Nitsche, U.; Garbi, N.; Brossart, P.; et al. Targeting myeloid derived suppressor cells with all-trans retinoic acid is highly time-dependent in therapeutic tumor vaccination. Oncoimmunology 2017, 6, e1338995. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Shimada, M.; Utsunomiya, T.; Morine, Y.; Imura, S.; Ikemoto, T.; Mori, H.; Hanaoka, J.; Iwahashi, S.; Yamada, S.; et al. Regulatory T cells in the blood: A new marker of surgical stress. Surg. Today 2013, 43, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Farhood, B.; Mortezaee, K. Contribution of regulatory T cells to cancer: A review. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ino, Y.; Yamazaki-Itoh, R.; Shimada, K.; Iwasaki, M.; Kosuge, T.; Kanai, Y.; Hiraoka, N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer 2013, 108, 914–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Pan, S.; Lyu, Q.; Wu, P.; Qin, G.; Wang, Q.; He, Z.; He, X.; Wu, M.; Chen, G. Postoperative Regulatory T-Cells and Natural Killer Cells in Stage I Nonsmall Cell Lung Cancer Underwent Video-assisted Thoracoscopic Lobectomy or Thoracotomy. Chin. Med. J. (Engl.) 2015, 128, 1502–1509. [Google Scholar] [PubMed]
- Tang, Y.; Xu, X.; Guo, S.; Zhang, C.; Tang, Y.; Tian, Y.; Ni, B.; Lu, B.; Wang, H. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS ONE 2014, 9, e91551. [Google Scholar] [CrossRef] [PubMed]
- Littwitz-Salomon, E.; Malyshkina, A.; Schimmer, S.; Dittmer, U. The Cytotoxic Activity of Natural Killer Cells Is Suppressed by IL-10+ Regulatory T Cells During Acute Retroviral Infection. Front. Immunol. 2018, 9, 1947. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, D.; Zhang, Y.; Chen, Z.; Zhu, W.; Zhang, B.; Wang, Z.; Le, H. Changes of CD4+CD25+FOXP3+ and CD8+CD28- regulatory T cells in non-small cell lung cancer patients undergoing surgery. Int. Immunopharmacol. 2014, 18, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Y.; Li, X.; Chen, G.; Liang, H.; Wu, Y.; Tong, J.; Ouyang, W. Propranolol Attenuates Surgical Stress-Induced Elevation of the Regulatory T Cell Response in Patients Undergoing Radical Mastectomy. J. Immunol. 2016, 196, 3460–3469. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, L.; Sun, B.; Li, X.; Duan, K.; Wu, Y.; Ouyang, W. Interleukin-2 administration after modified radical mastectomy in breast cancer therapy increases peripheral regulatory T cells. Int. J. Clin. Exp. Med. 2015, 8, 7816–7822. [Google Scholar] [PubMed]
- Pedroza-Pacheco, I.; Madrigal, A.; Saudemont, A. Interaction between natural killer cells and regulatory T cells: Perspectives for immunotherapy. Cell. Mol. Immunol. 2013, 10, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Littwitz-Salomon, E.; Akhmetzyanova, I.; Vallet, C.; Francois, S.; Dittmer, U.; Gibbert, K. Activated regulatory T cells suppress effector NK cell responses by an IL-2-mediated mechanism during an acute retroviral infection. Retrovirology 2015, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Kohm, A.P.; Sanders, V.M. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol. Rev. 2001, 53, 487–525. [Google Scholar] [PubMed]
- Madden, K.S. Catecholamines, sympathetic innervation, and immunity. Brain Behav. Immun. 2003, 17 (Suppl. 1), S5–S10. [Google Scholar] [CrossRef]
- Xiang, L.; Marshall, G.D., Jr. Immunomodulatory effects of in vitro stress hormones on FoxP3, Th1/Th2 cytokine and costimulatory molecule mRNA expression in human peripheral blood mononuclear cells. Neuroimmunomodulation 2011, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.H.S.; Britton, G.J.; Hill, E.V.; Verhagen, J.; Burton, B.R.; Wraith, D.C. Regulation of adaptive immunity; the role of interleukin-10. Front. Immunol. 2013, 4, 129. [Google Scholar] [CrossRef] [PubMed]
- Klatte, T.; Ittenson, A.; Röhl, F.-W.; Ecke, M.; Allhoff, E.P.; Böhm, M. Perioperative immunomodulation with interleukin-2 in patients with renal cell carcinoma: Results of a controlled phase II trial. Br. J. Cancer 2006, 95, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Ittenson, A.; Schierbaum, K.F.; Röhl, F.-W.; Ansorge, S.; Allhoff, E.P. Pretreatment with interleukin-2 modulates peri-operative immuno-dysfunction in patients with renal cell carcinoma. Eur. Urol. 2002, 41, 458–467. [Google Scholar] [CrossRef]
- Romano, F.; Garancini, M.; Uggeri, F.; Degrate, L.; Nespoli, L.; Gianotti, L.; Nespoli, A.; Uggeri, F. Surgical treatment of liver metastases of gastric cancer: State of the art. World J. Surg. Oncol. 2012, 10, 157. [Google Scholar] [CrossRef]
- Taylor, N.A.; Vick, S.C.; Iglesia, M.D.; Brickey, W.J.; Midkiff, B.R.; McKinnon, K.P.; Reisdorf, S.; Anders, C.K.; Carey, L.A.; Parker, J.S.; et al. Treg depletion potentiates checkpoint inhibition in claudin-low breast cancer. J. Clin. Investig. 2017, 127, 3472–3483. [Google Scholar] [CrossRef]
- Klages, K.; Mayer, C.T.; Lahl, K.; Loddenkemper, C.; Teng, M.W.L.; Ngiow, S.F.; Smyth, M.J.; Hamann, A.; Huehn, J.; Sparwasser, T. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010, 70, 7788–7799. [Google Scholar] [CrossRef]
- Leao, I.C.; Ganesan, P.; Armstrong, T.D.; Jaffee, E.M. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clin. Transl. Sci. 2008, 1, 228–239. [Google Scholar] [CrossRef]
- Mahnke, K.; Schönfeld, K.; Fondel, S.; Ring, S.; Karakhanova, S.; Wiedemeyer, K.; Bedke, T.; Johnson, T.S.; Storn, V.; Schallenberg, S.; et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: Kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int. J. Cancer 2007, 120, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Bielekova, B. Daclizumab therapy for multiple sclerosis. Neurotherapeutics 2013, 10, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, J.L.; Janik, J.E.; Stewart, D.M.; Jaffe, E.S.; Stetler-Stevenson, M.; Shih, J.H.; Fleisher, T.A.; Turner, M.; Urquhart, N.E.; Wharfe, G.H.; et al. Safety, efficacy, and pharmacokinetics/pharmacodynamics of daclizumab (anti-CD25) in patients with adult T-cell leukemia/lymphoma. Clin. Immunol. 2014, 155, 176–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickson, R. NCT00109161: A Phase II Randomized, Double-Blinded, Placebo-Controlled, Multi-Center Study of Subcutaneous Daclizumab in Patients with Active, Relapsing Forms of Multiple Sclerosis. Available online: https://clinicaltrials.gov/ct2/show/record/NCT0010+161 (accessed on 26 October 2018).
- Shoup, M. NCT00726037: A Pilot Study Evaluating the Efficacy of Regulatory T-Cell (T-Reg) Suppression by Denileukin Diftitox (Ontak) in Metastatic Pancreatic Cancer. Available online: https://clinicaltrials.gov/ct2/show/record/NCT00726037 (accessed on 26 October 2018).
- Huss, D.J.; Mehta, D.S.; Sharma, A.; You, X.; Riester, K.A.; Sheridan, J.P.; Amaravadi, L.S.; Elkins, J.S.; Fontenot, J.D. In vivo maintenance of human regulatory T cells during CD25 blockade. J. Immunol. 2015, 194, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Menard, C.; Puig, P.E.; Ladoire, S.; Roux, S.; Martin, F.; Solary, E.; Le Cesne, A.; Zitvogel, L.; Chauffert, B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007, 56, 641–648. [Google Scholar] [CrossRef]
- Bachireddy, P. NCT03219450: A Pilot Study of a Personalized Neoantigen Cancer Vaccine with and without Low-Dose Cyclophosphamide in Treatment Naïve, Asymptomatic Patients with IGHV Unmutated Chronic Lymphocytic Leukemia. Available online: https://clinicaltrials.gov/ct2/show/record/NCT03219450 (accessed on 26 October 2018).
- Lee, S.-H.; Fragoso, M.F.; Biron, C.A. Cutting edge: A novel mechanism bridging innate and adaptive immunity: IL-12 induction of CD25 to form high-affinity IL-2 receptors on NK cells. J. Immunol. 2012, 189, 2712–2716. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Gowen, B.G.; Zhang, L.; Wang, L.; Lau, S.; Iannello, A.; Xu, J.; Rovis, T.L.; Xiong, N.; Raulet, D.H. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science 2015, 348, 136–139. [Google Scholar] [CrossRef]
- Molfetta, R.; Quatrini, L.; Santoni, A.; Paolini, R. Regulation of NKG2D-Dependent NK Cell Functions: The Yin and the Yang of Receptor Endocytosis. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Gasser, S.; Orsulic, S.; Brown, E.J.; Raulet, D.H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005, 436, 1186–1190. [Google Scholar] [CrossRef] [Green Version]
- Eissmann, P.; Evans, J.H.; Mehrabi, M.; Rose, E.L.; Nedvetzki, S.; Davis, D.M. Multiple mechanisms downstream of TLR-4 stimulation allow expression of NKG2D ligands to facilitate macrophage/NK cell crosstalk. J. Immunol. 2010, 184, 6901–6909. [Google Scholar] [CrossRef] [PubMed]
- Bui, J.D.; Carayannopoulos, L.N.; Lanier, L.L.; Yokoyama, W.M.; Schreiber, R.D. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J. Immunol. 2006, 176, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Valés-Gómez, M.; Chisholm, S.E.; Cassady-Cain, R.L.; Roda-Navarro, P.; Reyburn, H.T. Selective induction of expression of a ligand for the NKG2D receptor by proteasome inhibitors. Cancer Res. 2008, 68, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Skov, S.; Pedersen, M.T.; Andresen, L.; Straten, P.T.; Woetmann, A.; Ødum, N. Cancer Cells Become Susceptible to Natural Killer Cell Killing after Exposure to Histone Deacetylase Inhibitors Due to Glycogen Synthase Kinase-3–Dependent Expression of MHC Class I–Related Chain A and B. Cancer Res. 2005, 65, 11136–11145. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, M.; Rusakiewicz, S.; Minard-Colin, V.; Delahaye, N.F.; Enot, D.; Vély, F.; Marabelle, A.; Papoular, B.; Piperoglou, C.; Ponzoni, M.; et al. Clinical impact of the NKp30/B7-H6 axis in high-risk neuroblastoma patients. Sci. Transl. Med. 2015, 7, 283ra55. [Google Scholar] [CrossRef] [PubMed]
- Garni-Wagner, B.A.; Purohit, A.; Mathew, P.A.; Bennett, M.; Kumar, V. A novel function-associated molecule related to non-MHC-restricted cytotoxicity mediated by activated natural killer cells and T cells. J. Immunol. 1993, 151, 60–70. [Google Scholar] [PubMed]
- Makkouk, A.; Chester, C.; Kohrt, H.E. Rationale for anti-CD137 cancer immunotherapy. Eur. J. Cancer 2016, 54, 112–119. [Google Scholar] [CrossRef]
- Kohrt, H.E.; Houot, R.; Goldstein, M.J.; Weiskopf, K.; Alizadeh, A.A.; Brody, J.; Müller, A.; Pachynski, R.; Czerwinski, D.; Coutre, S.; et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood 2011, 117, 2423–2432. [Google Scholar] [CrossRef]
- Kohrt, H.E.; Colevas, A.D.; Houot, R.; Weiskopf, K.; Goldstein, M.J.; Lund, P.; Mueller, A.; Sagiv-Barfi, I.; Marabelle, A.; Lira, R.; et al. Targeting CD137 enhances the efficacy of cetuximab. J. Clin. Investig. 2014, 124, 2668–2682. [Google Scholar] [CrossRef] [Green Version]
- Vitale, M.; Falco, M.; Castriconi, R.; Parolini, S.; Zambello, R.; Semenzato, G.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Identification of NKp80, a novel triggering molecule expressed by human NK cells. Eur. J. Immunol. 2001, 31, 233–242. [Google Scholar] [CrossRef] [Green Version]
- He, L.-Z.; Prostak, N.; Thomas, L.J.; Vitale, L.; Weidlick, J.; Crocker, A.; Pilsmaker, C.D.; Round, S.M.; Tutt, A.; Glennie, M.J.; et al. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J. Immunol. 2013, 191, 4174–4183. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A.; Infante, J.R.; Ansell, S.M.; Nemunaitis, J.J.; Weiss, G.R.; Villalobos, V.M.; Sikic, B.I.; Taylor, M.H.; Northfelt, D.W.; Carson, W.E., 3rd; et al. Safety and Activity of Varlilumab, a Novel and First-in-Class Agonist Anti-CD27 Antibody, in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2017, 35, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Hofer, E.; Koehl, U. Natural Killer Cell-Based Cancer Immunotherapies: From Immune Evasion to Promising Targeted Cellular Therapies. Front. Immunol. 2017, 8, 745. [Google Scholar] [CrossRef]
- Bristol-MyersSquibb NCT01750580: A Phase 1 Study of BMS-986015, an Anti-KIR Monoclonal Antibody, Administered with Ipilimumab, an Anti-CTLA4 Monoclonal Antibody, in Subjects with Select Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/record/NCT01750580 (accessed on 26 October 2018).
- Bristol-MyersSquibb NCT01714739: A Phase 1/2 Study of the Combination of Lirilumab (Anti-KIR) Plus Nivolumab (Anti-PD-1) or Lirilumab Plus Nivolumab and Ipilimumab in Advanced Refractory Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/record/NCT01714739 (accessed on 26 October 2018).
- Gallois, A.; Silva, I.; Osman, I.; Bhardwaj, N. Reversal of natural killer cell exhaustion by TIM-3 blockade. Oncoimmunology 2014, 3, e946365. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.-C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef] [PubMed]
- Oyer, J.; Gitto, S.B.; Altomare, D.; Igarashi, R.; Copik, A. Effect of PDL1 signaling blockade on NK cell cytolytic activity towards ovarian cancer. J. Clin. Orthod. 2018, 36, 13. [Google Scholar] [CrossRef]
- Benson, D.M., Jr.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K.; et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef]
- Angka, L.; Khan, S.T.; Kilgour, M.K.; Xu, R.; Kennedy, M.A.; Auer, R.C. Dysfunctional Natural Killer Cells in the Aftermath of Cancer Surgery. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Islam, M.N.; Bradley, B.A.; Ceredig, R. Sterile post-traumatic immunosuppression. Clin. Transl. Immunol. 2016, 5, e77. [Google Scholar] [CrossRef] [Green Version]
- Auer, R.C. NCT02987296: A Translational Clinical Trial of Perioperative Immunonutrition in Colorectal Cancer Patients Undergoing Abdominal Surgery: PERIOP-02. Available online: https://clinicaltrials.gov/ct2/show/record/NCT02987296 (accessed on 26 October 2018).
- Mehrotra, P.T.; Donnelly, R.P.; Wong, S.; Kanegane, H.; Geremew, A.; Mostowski, H.S.; Furuke, K.; Siegel, J.P.; Bloom, E.T. Production of IL-10 by Human Natural Killer Cells Stimulated with IL-2 and/or IL-12. J. Immunol. 1998, 160, 2637–2644. [Google Scholar] [PubMed]
- Jiang, Y.; Yang, M.; Sun, X.; Chen, X.; Ma, M.; Yin, X.; Qian, S.; Zhang, Z.; Fu, Y.; Liu, J.; et al. IL-10+ NK and TGF-β+ NK cells play negative regulatory roles in HIV infection. BMC Infect. Dis. 2018, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Terme, M.; Ullrich, E.; Aymeric, L.; Meinhardt, K.; Coudert, J.D.; Desbois, M.; Ghiringhelli, F.; Viaud, S.; Ryffel, B.; Yagita, H.; et al. Cancer-induced immunosuppression: IL-18-elicited immunoablative NK cells. Cancer Res. 2012, 72, 2757–2767. [Google Scholar] [CrossRef] [PubMed]
- Perona-Wright, G.; Mohrs, K.; Szaba, F.M.; Kummer, L.W.; Madan, R.; Karp, C.L.; Johnson, L.L.; Smiley, S.T.; Mohrs, M. Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host Microbe 2009, 6, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tian, Z.; Wei, H. Developmental and Functional Control of Natural Killer Cells by Cytokines. Front. Immunol. 2017, 8, 930. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Tang, S.Y.; Toh, L.L.; Wang, S. Generation of “Off-the-Shelf” Natural Killer Cells from Peripheral Blood Cell-Derived Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 9, 1796–1812. [Google Scholar] [CrossRef]
- Dahlberg, C.I.M.; Sarhan, D.; Chrobok, M.; Duru, A.D.; Alici, E. Natural Killer Cell-Based Therapies Targeting Cancer: Possible Strategies to Gain and Sustain Anti-Tumor Activity. Front. Immunol. 2015, 6, 605. [Google Scholar] [CrossRef]
- Granzin, M.; Wagner, J.; Köhl, U.; Cerwenka, A.; Huppert, V.; Ullrich, E. Shaping of Natural Killer Cell Antitumor Activity by Ex Vivo Cultivation. Front. Immunol. 2017, 8, 458. [Google Scholar] [CrossRef]
- Sakamoto, N.; Ishikawa, T.; Kokura, S.; Okayama, T.; Oka, K.; Ideno, M.; Sakai, F.; Kato, A.; Tanabe, M.; Enoki, T.; et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J. Transl. Med. 2015, 13, 277. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.S.; Oelkers, S.; Verfaillie, C.; McGlave, P. Role of monocytes in the expansion of human activated natural killer cells. Blood 1992, 80, 2221–2229. [Google Scholar] [PubMed]
- Luhm, J.; Brand, J.-M.; Koritke, P.; Höppner, M.; Kirchner, H.; Frohn, C. Large-scale generation of natural killer lymphocytes for clinical application. J. Hematother. Stem Cell Res. 2002, 11, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Kuentz, M.; Vernant, J.-P.; Dhedin, N.; Bories, D.; Debré, P.; Vieillard, V. Involvement of mature donor T cells in the NK cell reconstitution after haploidentical hematopoietic stem-cell transplantation. Leukemia 2008, 22, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Lundqvist, A.; McCoy, P., Jr.; Samsel, L.; Fan, Y.; Tawab, A.; Childs, R. Clinical-grade ex vivo-expanded human natural killer cells up-regulate activating receptors and death receptor ligands and have enhanced cytolytic activity against tumor cells. Cytotherapy 2009, 11, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Siegler, U.; Meyer-Monard, S.; Jörger, S.; Stern, M.; Tichelli, A.; Gratwohl, A.; Wodnar-Filipowicz, A.; Kalberer, C.P. Good manufacturing practice-compliant cell sorting and large-scale expansion of single KIR-positive alloreactive human natural killer cells for multiple infusions to leukemia patients. Cytotherapy 2010, 12, 750–763. [Google Scholar] [CrossRef]
- Spanholtz, J.; Preijers, F.; Tordoir, M.; Trilsbeek, C.; Paardekooper, J.; de Witte, T.; Schaap, N.; Dolstra, H. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS ONE 2011, 6, e20740. [Google Scholar] [CrossRef] [PubMed]
- Woll, P.S.; Grzywacz, B.; Tian, X.; Marcus, R.K.; Knorr, D.A.; Verneris, M.R.; Kaufman, D.S. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood 2009, 113, 6094–6101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knorr, D.A.; Ni, Z.; Hermanson, D.; Hexum, M.K.; Bendzick, L.; Cooper, L.J.N.; Lee, D.A.; Kaufman, D.S. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl. Med. 2013, 2, 274–283. [Google Scholar] [CrossRef]
- Kruse, V.; Hamann, C.; Monecke, S.; Cyganek, L.; Elsner, L.; Hübscher, D.; Walter, L.; Streckfuss-Bömeke, K.; Guan, K.; Dressel, R. Human Induced Pluripotent Stem Cells Are Targets for Allogeneic and Autologous Natural Killer (NK) Cells and Killing Is Partly Mediated by the Activating NK Receptor DNAM-1. PLoS ONE 2015, 10, e0125544. [Google Scholar] [CrossRef] [PubMed]
- Klingemann, H.; Boissel, L.; Toneguzzo, F. Natural Killer Cells for Immunotherapy—Advantages of the NK-92 Cell Line over Blood NK Cells. Front. Immunol. 2016, 7, 91. [Google Scholar] [CrossRef]
- Eguizabal, C.; Zenarruzabeitia, O.; Monge, J.; Santos, S.; Vesga, M.A.; Maruri, N.; Arrieta, A.; Riñón, M.; Tamayo-Orbegozo, E.; Amo, L.; et al. Natural killer cells for cancer immunotherapy: Pluripotent stem cells-derived NK cells as an immunotherapeutic perspective. Front. Immunol. 2014, 5, 439. [Google Scholar] [CrossRef] [PubMed]
- Boyiadzis, M. NCT00900809: Phase I Study of Adoptive Immunotherapy Using the Natural Killer Cell Line, NeukoplastTM(NK-92), for the Treatment of Refractory or Relapsed Acute Myeloid Leukemia. Available online: https://clinicaltrials.gov/ct2/show/record/NCT00900809 (accessed on 26 October 2018).
- Keating, A. NCT00990717: A Dose Escalation Study of NK-92 Cell Infusions in Patients with Hematological Malignancies in Relapse after Autologous Stem Cell Transplantation. Available online: https://clinicaltrials.gov/ct2/show/record/NCT00990717 (accessed on 26 October 2018).
- NantKwest, Inc. NCT02465957: Phase 2 Study of aNK (Activated NK-92 Natural Killer Cells) Infusions in Combination with ALT-803 (IL-15) in Patients with Stage III (IIIB) or Stage IV Merkel Cell Carcinoma (MCC). Available online: https://clinicaltrials.gov/ct2/show/record/NCT02465957 (accessed on 26 October 2018).
- Cooper, M.A.; Bush, J.E.; Fehniger, T.A.; VanDeusen, J.B.; Waite, R.E.; Liu, Y.; Aguila, H.L.; Caligiuri, M.A. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood 2002, 100, 3633–3638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rham, C.; Ferrari-Lacraz, S.; Jendly, S.; Schneiter, G.; Dayer, J.-M.; Villard, J. The proinflammatory cytokines IL-2, IL-15 and IL-21 modulate the repertoire of mature human natural killer cell receptors. Arthritis Res. Ther. 2007, 9, R125. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Rosenberg, S.A. Modulation of murine tumor major histocompatibility antigens by cytokines in vivo and in vitro. Cancer Res. 1988, 48, 5818–5824. [Google Scholar] [PubMed]
- Nagashima, S.; Mailliard, R.; Kashii, Y.; Reichert, T.E.; Herberman, R.B.; Robbins, P.; Whiteside, T.L. Stable transduction of the interleukin-2 gene into human natural killer cell lines and their phenotypic and functional characterization in vitro and in vivo. Blood 1998, 91, 3850–3861. [Google Scholar]
- Li, Y.; Hermanson, D.L.; Moriarity, B.S.; Kaufman, D.S. Human iPSC-Derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-tumor Activity. Cell Stem Cell 2018, 23, 181–192. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Market, M.; Baxter, K.E.; Angka, L.; Kennedy, M.A.; Auer, R.C. The Potential for Cancer Immunotherapy in Targeting Surgery-Induced Natural Killer Cell Dysfunction. Cancers 2019, 11, 2. https://doi.org/10.3390/cancers11010002
Market M, Baxter KE, Angka L, Kennedy MA, Auer RC. The Potential for Cancer Immunotherapy in Targeting Surgery-Induced Natural Killer Cell Dysfunction. Cancers. 2019; 11(1):2. https://doi.org/10.3390/cancers11010002
Chicago/Turabian StyleMarket, Marisa, Katherine E. Baxter, Leonard Angka, Michael A. Kennedy, and Rebecca C. Auer. 2019. "The Potential for Cancer Immunotherapy in Targeting Surgery-Induced Natural Killer Cell Dysfunction" Cancers 11, no. 1: 2. https://doi.org/10.3390/cancers11010002
APA StyleMarket, M., Baxter, K. E., Angka, L., Kennedy, M. A., & Auer, R. C. (2019). The Potential for Cancer Immunotherapy in Targeting Surgery-Induced Natural Killer Cell Dysfunction. Cancers, 11(1), 2. https://doi.org/10.3390/cancers11010002




