Abstract
The acidic vesicles of the endolysosomal (EL) system are emerging as an intracellular Ca2+ store implicated in the regulation of multiple cellular functions. The EL Ca2+ store releases Ca2+ through a variety of Ca2+-permeable channels, including Transient Receptor Potential (TRP) Mucolipin 1-3 (TRPML1-3) and two-pore channels 1-2 (TPC1-2), whereas EL Ca2+ refilling is sustained by the proton gradient across the EL membrane and/or by the endoplasmic reticulum (ER). EL Ca2+ signals may be either spatially restricted to control vesicle trafficking, autophagy and membrane repair or may be amplified into a global Ca2+ signal through the Ca2+-dependent recruitment of ER-embedded channels. Emerging evidence suggested that nicotinic acid adenine dinucleotide phosphate (NAADP)-gated TPCs sustain multiple cancer hallmarks, such as migration, invasiveness and angiogenesis. Herein, we first survey the EL Ca2+ refilling and release mechanisms and then focus on the oncogenic role of EL Ca2+ signaling. While the evidence in favor of TRPML1 involvement in neoplastic transformation is yet to be clearly provided, TPCs are emerging as an alternative target for anticancer therapies.
Keywords:
endolysosomal Ca2+ signaling; cancer; TRPML1; TPCs; NAADP; proliferation; metastasis; angiogenesis 1. Introduction
Intracellular Ca2+ signals regulate a plethora of cellular functions, including exocytosis, proliferation, migration, differentiation, gene transcription, bioenergetics and survival [1,2]. The resting intracellular Ca2+ concentration ([Ca2+]i) is maintained at ≈100 nM by the activity of a number of Ca2+ transporting systems which extrude Ca2+ across the plasma membrane (PM), such as the Plasma Membrane Ca2+-ATPase (PMCA) and the Na+/Ca2+ exchanger (NCX), or sequester Ca2+ within the lumen of endogenous organelles, such as the Sarco-Endoplasmic Reticulum Ca2+-ATPase (SERCA), the mitochondrial Ca2+ uniporter (MCU) and the Secretory Pathway Ca2+-ATPase (SPCA), which sequesters Ca2+ into the Golgi lumen [1,2,3,4]. An increase in intracellular Ca2+ concentration ([Ca2+]i) is produced by the opening of Ca2+-permeable channels, which are located either on the PM or on the membrane of endogenous organelles, in response to extracellular stimulation. Typically, extracellular agonists, including growth factors, cytokines, neurotransmitters and hormones, bind to their specific tyrosine kinase receptors (TKRs) or Gq-protein coupled receptors (GPCRs), thereby recruiting, respectively, phospholipase Cγ (PLCγ) and PLCβ, which in turn cleave phosphatidylinositol-4,5-bisphosphate (PIP2) into inositol-1,4,5-trisphosphate (InsP3) and diacyl-glycerol (DAG) [1]. Subsequently, InsP3 primes immobile InsP3 receptors (InsP3Rs), which reside at juxtaposed endoplasmic reticulum (ER)-PM junctions, to respond to cytosolic Ca2+ according to the process of Ca2+-induced Ca2+ release (CICR), which enables to spatially restricted Ca2+ signals to spread across the cytoplasm as a regenerative Ca2+ wave [5,6]. Moreover, InsP3-induced Ca2+ release can be further amplified by the recruitment of adjoining ryanodine receptors (RyRs), which are also sensitive to CICR [7]. Additionally, InsP3-induced Ca2+ release causes a large drop in the ER Ca2+ concentration ([Ca2+]ER) which triggers a signaling cascade leading to the opening of a Ca2+-permeable pathway on the PM, known as store-operated Ca2+ entry (SOCE) [8,9]. SOCE is activated when ER Ca2+ dissociates from the luminal EF hands domain of STIM1 (Stromal Interaction Molecule 1), the sensor of [Ca2+]ER, which undergoes a dramatic conformational change and sub-cellular repositioning. The depletion of ER Ca2+ induces STIM1 to oligomerize and translocate from its reticular location in the bulk ER to specialized ER-PM junctions (≈20 nm) where it forms STIM1 puncta and interacts with the pore-forming subunit of store-operated channels (SOCs), Orai1 [8]. The dynamic interplay between InsP3-induced ER Ca2+ release and SOCE gives raise to distinct spatio-temporally complex Ca2+ signatures, ranging from local Ca2+ puffs to repetitive global Ca2+ oscillations, that selectively regulate different cellular functions [2,10,11].
The ER is by far the largest and best studied intracellular Ca2+ reservoir engaged by extracellular stimuli, possessing a complete repertoire of Ca2+ pumps, i.e., SERCA, to effect filling, luminal Ca2+-binding proteins, e.g., calnexin and calreticulin, to effect buffering and Ca2+-permeable channels, i.e., InsP3Rs and RyRs, to effect release [7,12]. Nevertheless, the ER is not the sole endogenous Ca2+ store in mammalian cells. It has been shown that also the acidic vesicles of the endo-lysosomal (EL) system serve as an additional Ca2+ reservoir that is recruited by extracellular ligands through the generation of the novel Ca2+-releasing second messenger, nicotinic acid adenine dinucleotide phosphate (NAADP) [12,13,14,15]. The battery of Ca2+ refilling and release mechanisms endowed to acidic EL vesicles is emerging as a crucial signalling network to finely tune local processes, such as membrane fusion and individualized vesicular movements [16]. In addition, the establishment of EL-ER membrane contact sites (MCSs) enables EL Ca2+ release to trigger cytosolic Ca2+ waves by recruiting ER-resident InsP3Rs and RyRs through the CICR process [17,18].
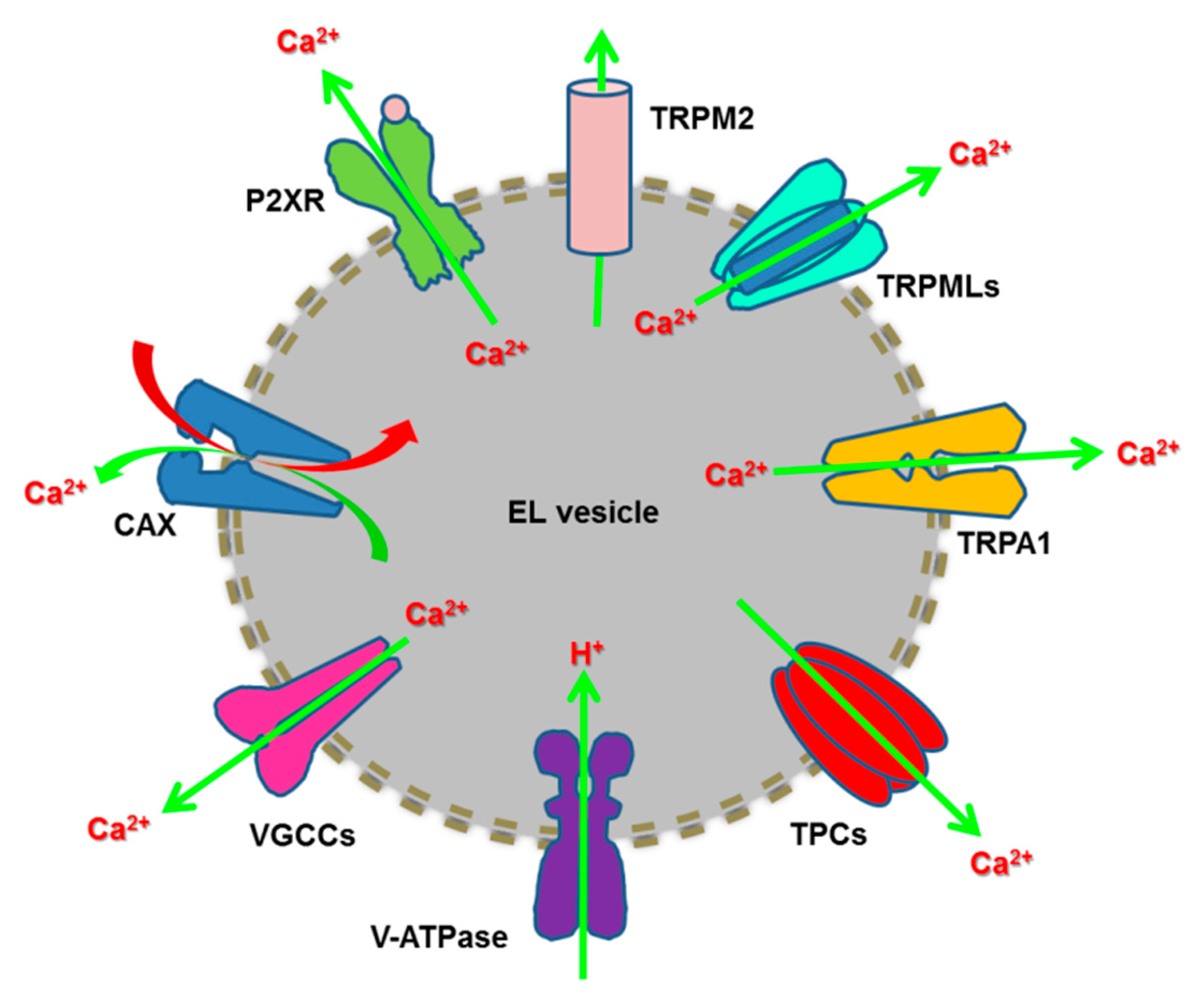
Herein, we discuss the emerging role of EL Ca2+ signalling in tumorigenesis [19,20,21]. We first illustrate the basic mechanisms of EL Ca2+ refilling and release by focusing on the wealth of Ca2+-permeable channels that effect EL Ca2+ mobilization, i.e., Transient Receptor Potential (TRP) Mucolipin 1-3 (TRPML1-3), two-pore channels 1-2 (TPC1-2), TRP Melastatin 2 (TRPM2), TRP Ankyrin 1 (TRPA1), P2X4 receptors and voltage-gated Ca2+ channels (Figure 1). Then, we describe how TPCs control several cancer hallmarks, including migration, invasiveness and angiogenesis, and the potential tumorigenic function of TRPML1. Finally, we speculate about the possibility to interfere with the EL Ca2+ signalling machinery to design alternative anticancer treatments, by focusing on TPCs as emerging molecular targets.
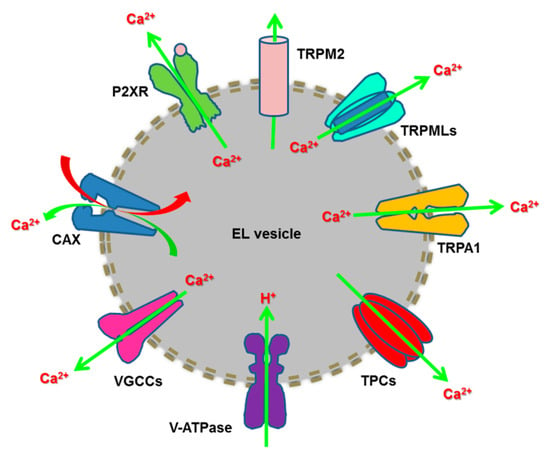
Figure 1.
The endolysosomal Ca2+ toolkit. Refilling of the EL Ca2+ store is driven by the H+ gradient established through the v-ATPase and effected by a Ca2+/H+ exchanger (CAX) in nonplacental mammalian cells. EL Ca2+ release may occur through multiple Ca2+-permeable channels, such as such as TRPML1-3, TRPM2, TRP Ankyrin 1 (TRPA1), TPC1-2, ATP-gated ionotropic P2X4 receptors and voltage-gated Ca2+ channels (VGCCs).
2. The Endolysosomal Ca2+ Store: Functions and Mechanisms of Ca2+ Refilling and Release
2.1. The Function of Endosomes and Lysosomes: Their Emerging Role in Ca2+ Signaling
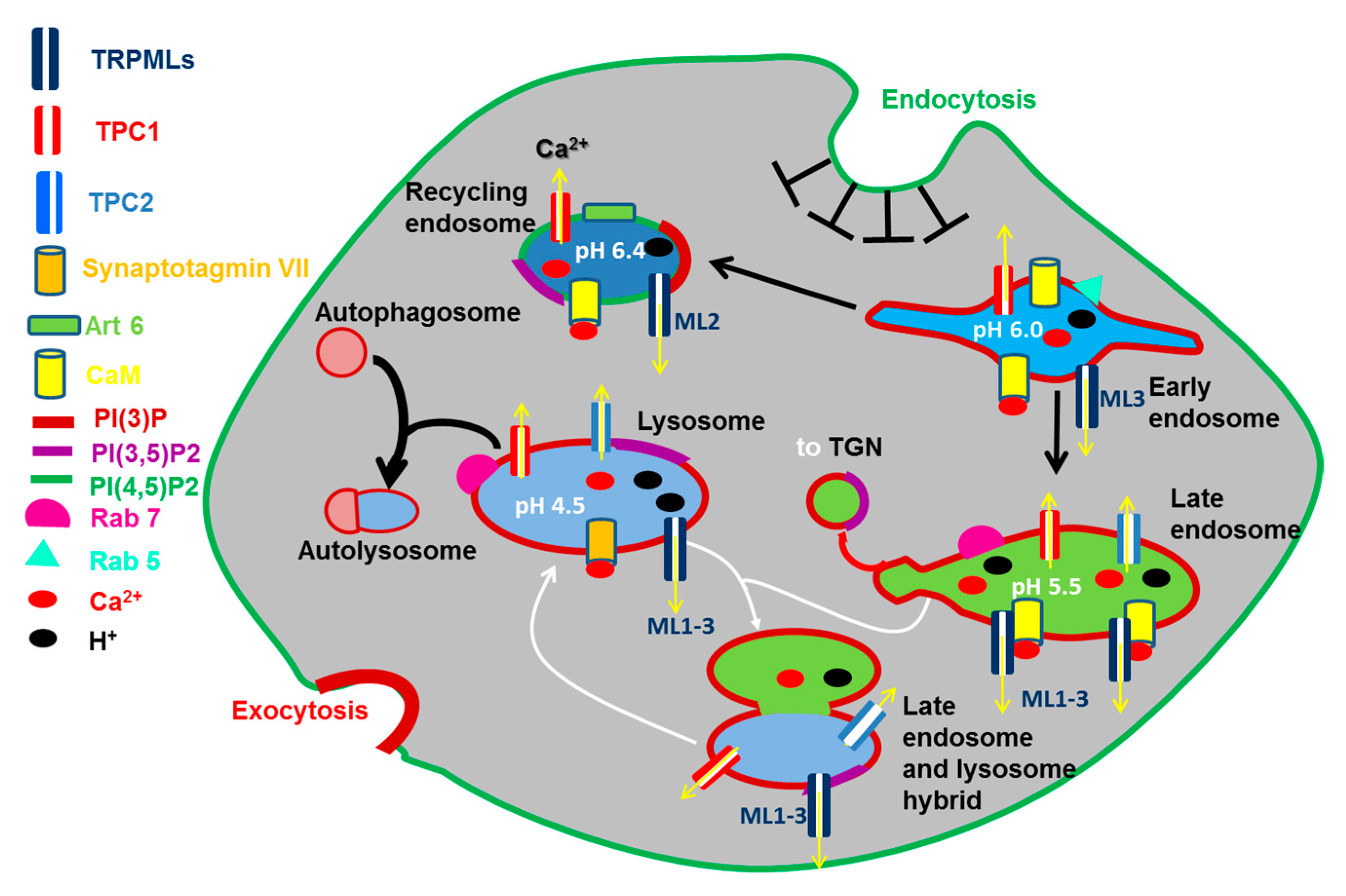
The EL Ca2+ store belongs to the emerging family of acidic Ca2+ stores (Figure 2), which is widespread across the phylogenetic tree and includes acidocalcisomes, vacuoles, lysosome-related organelles, secretory granules [12,15], and the Golgi complex, that has been recently reviewed [22,23]. Endocytosis is the mechanism whereby extracellular solutes, macromolecules, and PM components are internalized and redirected towards the lysosomes through PM invagination followed by the formation of vesicles and vacuoles through membrane fission [24] (Figure 2). Once formed at the PM, early endosomes (EEs) are weakly acidic (pH 6.8–5.9) and, although they were supposed to be on par with the extracellular Ca2+ concentration [12], they contain a relatively low amount of Ca2+ (≈2–3 μM) [25,26] (Figure 2). Nevertheless, their intraluminal Ca2+ concentration is still at least an order of magnitude higher than that of resting [Ca2+]i [12] and, therefore, EEs may serve as endogenous Ca2+ reservoir. While the majority of EEs is recycled back to the PM within about 10–15 min [24], a small fraction of EEs progressively acidify (pH 4.8–6.0) due to the activity of a vacuolar type H+-ATPase (v-ATPase) and gradually mature into late endosomes (LEs) (Figure 2), also known as multivesicular bodies (MVBs), which undergo a remarkable increase in their intraluminal Ca2+ concentration before fusing with lysosomes [24,26,27]. Endosomes occupy only a small percentage of the total cell volume, i.e., (0.65–2%) [24], although they are highly mobile (0.2–0.5 μm/sec) [28]: therefore, they are nicely suited to trigger local increases in [Ca2+]i [12,26], although their intimate association with ER cisternae could result in global intracellular Ca2+ waves (see the Section 2.4.2.1).
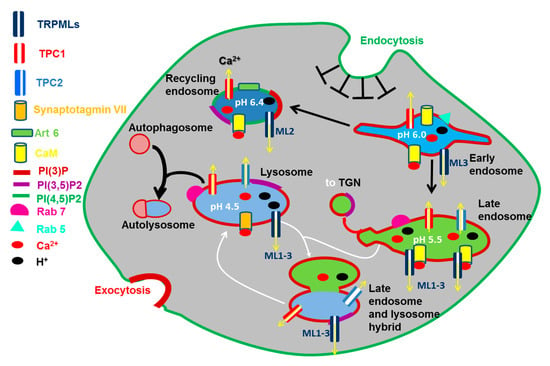
Figure 2.
TRPML1-3 and TPC1-2 in the endocytic pathway. Endosomal vesicles undergo cargo-dependent maturation (black arrows), membrane fusion (white arrows), and fission/budding (red arrow). The molecular identity of each intracellular compartment is defined by specific recruitment of small G proteins (Rab and Arf GTPases) and the composition of phosphoinositides (PIPs). Endolysosomal compartments are featured by a weak-to-highly acidic pH, which has therefore been indicated for each organelle. Many steps of the endocytic pathway are Ca2+-dependent due to the involvement of specific Ca2+-dependent decoders, such as calmodulin (CaM) and synaptotagmin VII (Syt), and Arf6. All the abbreviations are indicated on the left, with the exception of TGN (Trans Golgi Network).
Lysosomes are dynamic membrane-bound organelles that constitute up to 5% of the intracellular volume and show remarkable heterogeneity in size (100–500 nm in diameter), morphology and distribution [29]. Lysosomes were long regarded as mere waste bags by serving as the terminal degradative compartment recycling extracellular particles, cellular macromolecules and damaged organelles delivered via endocytosis, phagocytosis and autophagy [29,30] (Figure 2). Lysosomal function requires the establishment of a highly acidic pH (4.5–5.0), which is generated by an increase in v-ATPase activity on the lysosomal membrane and is supported by a resting lysosomal membrane potential (ΔΨ) ranging between −20 mV and −40 mV (lumen positive) [29,31]. This acidic pH is indispensable to support the activity of more than 60 hydrolases, such as nucleases, glycosidases, phosphatases, sulfatases, lipases and proteases, which are primarily involved in the digestion of proteins, polysaccharides, and complex lipids, into their building-block constituents: respectively, amino acids (AAs), monosaccharides, and free fatty acids [29,30]. Nevertheless, the last ten years witnessed a tremendous increase in our knowledge about the effective role of lysosomes in driving cellular fate and behavior. It is now acknowledged that lysosomes also contain 25 transmembrane proteins that enable these organelles to serve as a sophisticated regulatory hub which integrates multiple signals to finely tune cell metabolism, division and growth [32,33]. The lysosomal membrane plays a crucial role in nutrient sensing and metabolic regulation by hosting the mechanistic target of rapamycin complex 1 (mTORC1) and the energy-sensing kinase, AMP-activated protein kinase (AMPK) [32,34], as well as a number of proteins involved in mTORC1 regulation, including the Rag GTPases, and a pentameric complex termed Ragulator or late endosomal/lysosomal adaptor, mitogen-activated protein kinases (MAPK) and mTOR activator 1 (LAMTOR) [34]. In addition, lysosomes may establish a bidirectional Ca2+ exchange with the ER, thereby regulating pleoiotropic functions across the phylogenetic tree [18,35,36].
Accordingly, lysosomal lumen contains a high amount of Ca2+, ≈600 μM [37,38], that is within the same range as that reported in the ER, is mobilized by the second messenger, NAADP [13], and may sustain both local and global elevations in [Ca2+]i [12,13]. The presence of releasable Ca2+ within the lysosomes has been demonstrated by treating cells with the lysosomotropic agent, Gly-Phe-naphthylamide (GPN), a substrate of cathepsin C which is selectively expressed in lysosomes, but not in prelysosomal endocytic vacuoles [12]. GPN is degraded by cathepsin C, thereby causing osmotic swelling of the lysosomes and inducing a discernible increase in [Ca2+]i in a wide number of cells types [12,39,40,41]. Local lysosomal Ca2+ signals regulate normal trafficking, recycling, and vesicular fusion events with autophagosomes and LEs [16,29]. It has been suggested that the ubiquitously expressed C2-domain containing synaptotagmin isoform VII (Syt VII) and the EF-hands domain containing calmodulin (CaM) and apoptosis-linked gene-2 (ALG-2) function as the Ca2+-sensors that, respectively, mediate lysosomal exocytosis and LE-lysosomes fusion [29,42]. In addition, spatially-restricted lysosomal Ca2+ release is required to promote the starvation-induced nuclear translocation of TFEB [43], as described in the next paragraph.
2.2. Lysosomal Ca2+ Signaling Controls Lysosome Biogenesis and Autophagy
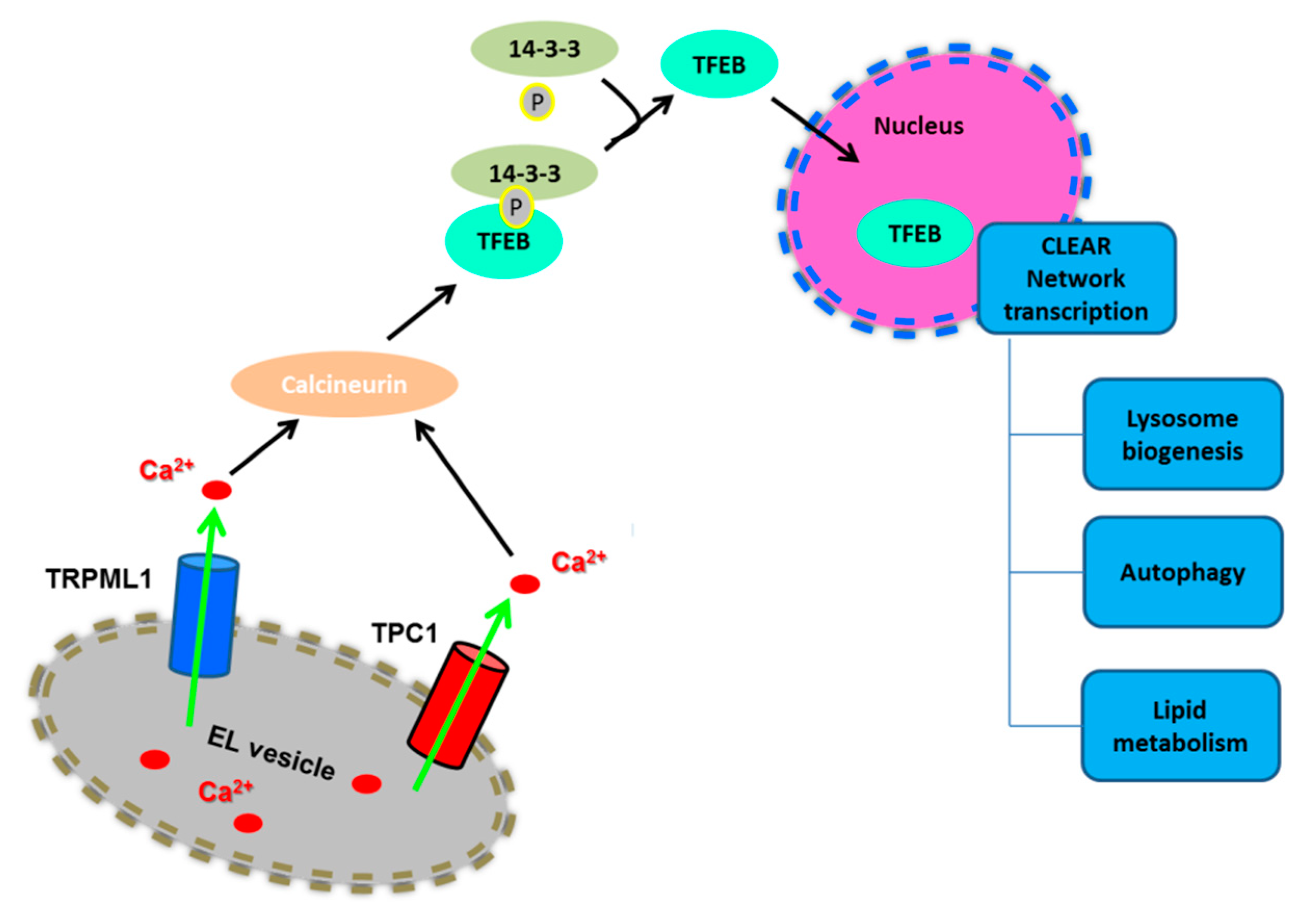
The master nutrient-responsive kinase, mTORC1, controls the cellular response to nutrient deficiency/availability by perceiving signals arising from growth factors, nutrients, and energy [30,44,45]. In the presence of nutrients, such as amino acids, lysosomes are positioned beneath the PM and mTORC1 is easily recruited to the lysosomal surface by the physical interaction with some of their transmembrane proteins, including Rag GTPases and the Ragulator complex and the v-ATPase. Herein, mTORC1 is activated by the small GTPase Rheb and phosphorylates its downstream targets, thereby stimulating cell growth and metabolism through the activation of biosynthetic pathways (including protein, lipid and nucleotide synthesis) and the inhibition of catabolic processes [44,45,46]. In particular, mTORC1 phosphorylates S6K and 4E-BP1 to promote protein synthesis, while it phosphorylates ULK1 and the transcription factor EB (TFEB) to inhibit autophagosome formation and lysosomal biogenesis, respectively [33,45,46]. Accordingly, phosphorylated TFEB is retained in the cytoplasm and cannot activate the catabolic transcriptional program that drives autophagy [47]. In contrast, starvation causes perinuclear clustering of lysosomes, which is caused by an increase in intracellular pH, and inactivates mTORC1 through multiple mechanisms, including the depletion of nutrients and a phosphorylation cascade triggered by AMPK. mTORC1 inactivation, in turn, interrupts the biosynthetic pathways and leads to ULK1 and TFEB dephosphorylation in a lysosomal Ca2+-dependent manner [44,45,46] (Figure 3). As a consequence, TFEB is now free to translocate into the nucleus, where it physically binds to the CLEAR (coordinated lysosomal expression and regulation gene network) elements, thereby inducing the transcription of a gene network involved in lysosomal biogenesis and autophagy [47]. In particular, the CLEAR element is targeted by a family of basic helix-loop-helix transcription factors, known as the MiT/TFE proteins, which include TFEB, TFEC, TFE3, and MITF [47,48,49]. TFEB, as well as MITF and TFE3, directly bind to CLEAR elements, thereby inducing the expression of target genes [33]. For instance, TFEB elicits a remarkable expansion of the lysosomal compartment, in terms of size, number, and protein content of lysosomal vesicles [47]. Furthermore, TFEB stimulates the expression of multiple autophagic proteins, including autophagosomal initiation (NRBF2 and BECN1), elongation (ATG9b, GABARAP, WIPI2), substrate capture (SQSTM1), and autophagosomal trafficking and fusion with lysosomes (UVRAG) [48,50].
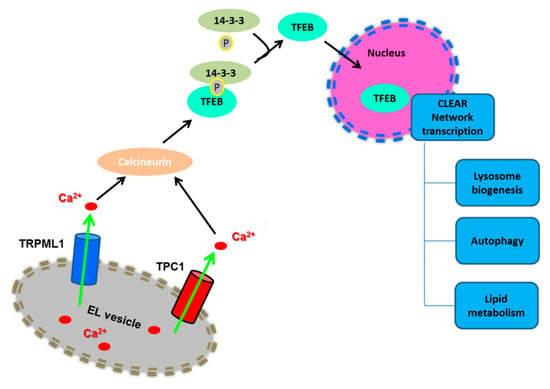
Figure 3.
Lysosomal Ca2+ signaling induces the nuclear translocation of TFEB. Lysosomal Ca2+ release through TRPML1 or TPC1 stimulates the nuclear translocation of TFEB to activate the CLEAR network transcription. Please, see the text for further details.
2.3. Ca2+ Uptake Mechanisms in the Lysosomes
As discussed in [14], endocytosis contributes to only (≈2–3 μM) of the luminal free Ca2+ concentration within the lysosomes [26,37]. It turns out that lysosomes require a Ca2+ transporter or exchanger to sequester cytosolic Ca2+ into their lumen just as SERCA fills the ER with Ca2+ [14,51]. It is largely accepted that lysosomal Ca2+ refilling is driven by the proton-motive force as manoeuvres that dissipate the H+ gradient deplete this Ca2+ store or prevent its refilling [14]. Lysosomal Ca2+ storage may be impaired by direct alkalization of the lumen with NH4Cl or with protonophores, such as monensin and nigericin, that abolish the H+ gradient [14,37]. Additionally, lysosomal Ca2+ uptake can be impaired by targeting the v-ATPase with selective inhibitors, such as bafilomycin A1 and concanamycin [14,40,52,53], provided that sufficient lysosomal Ca2+ leak is available [14,52]. A Ca2+/H+ exchanger (CAX) was first identified in vacuoles, which serve as acidic Ca2+ stores in yeast and plants [14], and, more recently, in nonplacental mammalian cells [54] (Figure 1). Nevertheless, the molecular identification of the CAX in placental mammals remains still elusive [16]. Alternately, lysosomal Ca2+ refilling could occur through a Na+/H+ exchange coupled in series with a Na+/Ca2+ exchanger (yet to be demonstrated) or through a P-type Ca2+-ATPase, which countertransports H+ and would, therefore, be sensitive to the acidic lysosomal lumen [14,55]. However, this latter mechanism has been unequivocally shown only in human platelets, where SERCA3 is primarily responsible for Ca2+ accumulation within acidic organelles [56].
Remarkably, a recent series of studies disclosed that lysosomes may sequester ER Ca2+ released through InsP3Rs in response to physiological stimuli [57,58]. Moreover, it has been suggested that the basal production of InsP3 could drive lysosomal Ca2+ refilling in an InsP3Rs-dependent, but pH-independent, manner in HEK293 cells [59]. It should, however, be pointed out that a previous investigation demonstrated that the H+ gradient was necessary for lysosomes to curb InsP3Rs-mediated Ca2+ signals in the same cell type [57,58]. As mentioned earlier, v-ATPase inhibitors may reliably deplete the lysosomal Ca2+ store only in the presence of an adequate Ca2+ leak. If Ca2+ uptake is effectively driven by a pH-dependent mechanism, but the resting lysosomal Ca2+ permeability is low, bafilomycin A1 and/or concanamycin will require longer time to cause a robust drop in the intraluminal free Ca2+ concentration, as elegantly discussed in [14]. Notably, HEK293 cells were preincubated in the presence of bafilomycin A1 for 1 h in [57,58] but only for less than 10 min in [59]. Of note, an independent study disclosed that lysosomal Ca2+ refilling requires an intact ER Ca2+ pool and is impaired by collapsing the H+ gradient in the widely employed cell line, HeLa cells [40]. An additional Ca2+ source for lysosomal refilling could be provided by SOCE, which replenishes their intraluminal Ca2+ content in peripheral lysosomes upon TFEB overexpression [60]. Collectively, these studies strongly hint at the H+ gradient as the main driver of lysosomal Ca2+ uptake, but the molecular identify of the transporter(s) responsible for lysosomal Ca2+ refilling in mammalian cells is still far from being elucidated. The role played by InsP3Rs in lysosomal Ca2+ refilling also deserves further investigations.
2.4. Endoysosomal Ca2+ Release Channels
The EL Ca2+ store express a variety of Ca2+-permeable channels that may result in both local and global intracellular Ca2+ signals. These include several members of the TRP superfamily of non-selective cation channels, such as TRPML1-3, TRPM2, and TRPA1, NAADP-gated TPC1-2, ATP-gated ionotropic P2X4 receptors and voltage-gated Ca2+ channels [12,14,15,16,19,21,42] (Figure 1). Moreover, the EL Ca2+ store contains a variety of Ca2+-impermeable channels that regulate Ca2+ homeostasis by modulating the H+ gradient and/or the lysosomal ΔΨ. These include ClC chloride channels (Cl−/H+ exchanger), big conductance Ca2+-activated K+ channels (BK, KCa1.1, MaxiK) and TMEM175 [42,61]. Notably, BK channels modulate ΔΨ, thereby controlling InsP3-dependent lysosomal Ca2+ refilling [62] and TRPML1-mediated Ca2+ release [63].
2.4.1. TRPML1-3 Channels
The mucolipin subfamily of TRP proteins comprises three EL non-selective cation channels: TRPML1, TRPML2 and TRPML3 [64,65]. TRPML1, the founding member of this subfamily, was named after the channelopathy, mucolipidosis type IV (MLIV), caused by mutations in the TRPML1 gene (MCOLN1) [66]. Similar to all TRP channels [67], each TRPML is composed of 4 subunits which possess 6 transmembrane (TM) spanning domains with cytosolic NH2- and COOH-terminal tails; moreover, the structure of TRPML proteins presents a pore-forming re-entrant loop between TM5 and TM6, a large intraluminal loop between TM1 and TM2 and various cytosolic regulatory domains [64,65,66]. TRPML channels localize most exclusively to the EL compartment due to the presence of EL targeting sequences (D/EXXXLL/I) in their NH2- and COOH-terminal tails, although these dileucine motifs are absent in TRPML3 [15,66]. TRPML1 is widely distributed within the later vesicles of the endocytic pathway, such as LEs and lysosomes, TRPML2 resides at recycling endosomes, LE/lysosomes and PM, and TRPML3 mainly localizes to the EL system and PM [12,66] (Figure 2). However, it has been proposed that TRPML3 is retained within the acidic vesicles by the interaction with TRPML1 [66,68]. Moreover, TRPML1 may also reach the PM either upon delivery from the Golgi apparatus through the biosynthetic route or upon lysosomal exocytosis [64,69,70].
Excellent reviews have recently covered the molecular architecture, biophysical properties and role of TRPML channels [19,21,61,66]. Herein, we briefly recall that TRPML1 is permeable to Ca2+, Na+, Fe2+, Mg2+, and K+ and displays an inwardly-rectifying I/V relationship, which indicates that it facilitates the efflux of EL cations into the cytosol [21,71,72]. TRPML1 is not permeable to H+ [71], although the genetic loss of TRPML1 may cause a dramatic loss in intraluminal pH [73] and TRPML1 is finely tuned by lysosomal pH [21,74]. Elucidation of its crystal structure revealed that the large intraluminal loop between TM1 and TM2 contains 12 aspartate residues that can be protonated at low pH, thereby favoring channel activity [75]. Conversely, at neutral pH (7.4) these aspartate reside retain their negative charge and cause the so-called Ca2+ block [75], as also demonstrated in [76]. TRPML1 may be physiologically gated by phosphatidylinositol-3,5-bisphosphate (PI(3,5)P2), a phosphoinositide which is particularly abundant in LEs and lysosomes and is able to bind to the NH2-terminal tail of the channel [77,78]. Conversely, TRPML1 is inhibited by phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2), which is likely to displace PI(3,5)P2 from its binding sites at the NH2-tail, but is more enriched at PM [78]. In addition, TRPML1 may be activated by reactive oxygen species (ROS), thereby serving as the lysosomal sensor of oxidative stress [79]. TRPML1-mediated Ca2+ release regulates a variety of Ca2+-dependent processes, including LE-lysosome fusion, lysosomal exocytosis, and membrane repair [16,29,80,81]. Furthermore, TRPML1-mediated lysosomal Ca2+ signals finely control autophagy by modulating mTORC1 activity TFEB localization, and autophagome-lysosome fusion [64]. It has been proposed that, when mTORC1 translocates onto the lysosomal surface in full-nutrient conditions, TRPML1-mediated local Ca2+ release supports Rheb-mediated mTORC1 activation to suppress autophagy in a calmodulin (CaM)-dependent manner [82]. As mentioned earlier, once activated, mTORC1 directly phosphorylates TFEB on two critical serine residues (Serine 142 and Serine 211), thereby causing its binding to the scaffold 14-3-3 proteins and its retention into the cytoplasm [33,83].
Conversely, in conditions of nutrient starvation, mTORC1 is inactivated and dissociates from the lysosomal surface [33,45,46]. Moreover, starvation causes a spatially-restricted increase in [Ca2+]i concentration by further mobilizing lysosomal Ca2+ through TRPML1 [43]: these TRPML1-mediated local Ca2+ signals recruit calcineurin (CaN) to dephosphorylate TFEB, which dissociates from 14-3-3 proteins and shuttles into the nucleus to trigger the transcriptional program driving lysosomal biogenesis and autophagy [47,50,70] (Figure 3).
Notably, TRPML1 is also under the transcriptional control of TFEB, which results in the establishment of a feed-forward loop that ensures lysosomal adaptation to prolonged stress and lack of nutrients [84]. It should, however, be pointed out that starvation-induced TRPML1-mediated Ca2+ release could also restore mTORC1 activity during prolonged starvation as an adaptive mechanism to maintain protein synthesis [85]. While starvation causes a reduction in lysosomal PI(3,5)P2 levels [64], it stimulates ROS production [81], which could deliver the gating signal to TRPML1 and induce lysosomal-to-nucleus communication through CaN-dependent TFEB dephosphorylation [79,82]. Moreover, starvation induces the PI 5-phosphatase, OCRL, to translocate from endosomes to lysosomes and dephosphorylate PI(4,5)P2 to PI4P, thereby sustaining TRPML1 activation and the progression of the autophagic process [64,86,87]. Finally, it has recently been shown that PI(4,5)P2-induced TRPML1 activation following autophagy induction stimulates the Ca2+-dependent centripetal repositioning of lysosomes towards the perinuclear region, where autophagosomes accumulate [88]. TRPML1-induced Ca2+ release recruits the EF-hand-containing Ca2+ sensor, ALG-2, to physically associate with the minus-end-directed dynactin-dynein motor, which is the crucial step to ensure lysosomal relocalization [88].
Similar to TRPML1, TRPML2 is permeable to Na+, K+, Ca2+ and Fe2+, displays an inwardly-rectifying I/V relationship and is gated by H+ and PI(3,5)P2 [71,77,89]. It has been suggested that TRPML2 is recycled from PM to the endosome via clathrin-mediated endocytosis [68]. Thereafter, TRPML2 promotes the activation of the small GTPase, ADP-ribosylation factor 6 (Arf6), thereby regulating recycling of glycosylphosphatidylinositol-anchored proteins (GPI-APs), including CD59 [90]. In addition, TRPML2 may associate into functional heteromultimers either with TRPML1 or TRPML3, which present distinct biophysical properties compared to homomeric channels and could control lysosomal integrity and starvation-induced autophagy [91,92,93].
TRPML3 is a Ca2+ channel that is permeable also to monovalent cations (Na+ > K+ ≫ Cs+), but is inhibited by high cytosolic Na+, and shows an inwardly-rectifying I/V relationship [94,95,96,97]. Similar to TRPML1, TRPML3 is gated by PI(3,5)P2 while it is not clear whether it is inhibited by PI(4,5)P2 [98]. Nevertheless, TRPML3 is blocked, rather than potentiated, by lysosomal acidification due to the protonation of a string of three histidine residues (H252, H273, H283) within the large intraluminal loop between TM1 and TM2 [96]. As TRPML3 is blocked by Na+ and H+, which are more abundant in the lysosomal lumen [29], it has been suggested that it mediates Ca2+ release from recycling endosomes and EEs, but not from LEs/lysosomes [21,99]. However, TRPML3 could become activated by lysosomal damage, stress, or pathogen infection, which dissipate the H+ gradient and could lead to TRPML1 closure [21,61,100]. As mentioned earlier, TRPML3 is not targeted to the EL system by the classical dileucine motifs at the NH2- and COOH-terminal tails described above, but it could be redirected to EL vesicle by an EXXLL motif in the NH2 terminus [99]. A number of recent studies hinted at a role for TRPML3 in endocytosis, membrane trafficking, and autophagy [80]. For instance, TRPML3 drives both constitutive (i.e., transferrin) and regulated (i.e., epidermal growth factor [EGF] and EGF receptor [EGFR]) endocytosis most likely though a local increase in [Ca2+]i [101]. Moreover, starvation and cellular stress favor TRPML3 recruitment to autophagosomes, thereby exacerbating the autophagic response [101]. Subsequent work revealed that TRPML3 interacts with GATE16, a mammalian ATG8 homologue, at the autophagosome and extra-autophagosomal organellar membranes to regulate the later phases of autophagosome biogenesis, which include fusion [102]. In this view, a recent investigation suggested that distinct TRPML channels could play different roles in lysosomal biogenesis showing that TRPML1-dependent Ca2+ release stimulates lysosomal fission and reformation in a CaM-dependent manner [80,102].
2.4.2. TPC1-2 Channels: Structure, Gating Mechanisms and Controversies
Two-pore channels (TPCs) belong to the superfamily of voltage-gated ion channels [103]. Voltage-gated ion channels present a modular structure consisting of combinations of pore-forming regions with two TM α-helices and voltage sensors with four TM α-helices, which may be concatenated to form six TM domains [104]. TPCs were originally identified in rats [105] and Arabidopsis thaliana [106] based with their high sequence homology with the α subunit of voltage-gated Na+ (NaV) and Ca2+ (CaV) channels. TPCs are so-named because each subunit is modularly organized into two domains, each containing 6 TM α-elices with the putative pore loops between TM5-TM6 and TM11-TM12, a large cytosolic linker between the two domains and cytosolic NH2- and COOH-termini [107,108,109]. Two TPC subunits dimerize into homo- or heterodimers to form a functional Ca2+-releasing channel. Therefore, TPCs represent the molecular intermediate in the evolutionary transition from tetrameric 1-domain (6 TM) channels, such as the α subunit of voltage-gated K+ channels (KV) or TRP channels, to monomeric 4-domain (4 × 6TM) channels, such as NaV and CaV [103,110,111].
Three isoforms exist of this family: TPC1, TPC2 and TPC3, which are encoded by TPCN1, TPCN2 and TPCN3, respectively. However, TPC3 is not present in mice, rats and primates, although it is expressed in other mammals, such as cats, dogs and chickens [103,112,113]. Two groups recently reported about the crystal structure of TPC1 from Arabidopsis thaliana (AtTPC1), which confirmed the membrane topology predicted on the basis of its amino acid sequence [114,115]. For instance, the X-ray structure of AtTPC1 obtained at 2.87 Å resolution revealed that each subunit comprises two asymmetrical Shaker-like 6-TM domains connected by a large cytosolic EF-hands domain. These subunits assemble into a central quasi-tetrameric channel with each polypeptide displaying two voltage-sensing domains, two-pore domains and activation gates [114]. Subsequently, the electron cryo-microscopy structure of mouse TPC1 (3.4 Å resolution) did not reveal any major difference with the atomic structure of AtTPC1, although Ca2+ cannot bind the EF-hands domain located within the cytosolic linker as this lacks the acidic residues responsible for this interaction. Moreover, the COOH-terminal tail is longer and adopts a horseshoe-shaped arrangement with four α-helices and two β-strands and physically associates with the EF-hands domain, thereby providing a remarkable steric hindrance [116].
TPC1 is widely distributed across the EL system, i.e., recycling endosomes, EEs, LEs and lysosomes, while TPC2 mainly resides at LEs and lysosomes [117,118,119] (Figure 2). The EL distribution of TPC1-2 depends on a dileucin motif at their NH2-terminal tails [120]. Accordingly, it has been demonstrated that TPCs finely tune EL membrane traffic and autophagy through local Ca2+ signals [80,103]. Furthermore, TPCs may trigger global, often repetitive, elevations in [Ca2+]i that drive a variety of cellular processes, ranging from fertilization to muscle contraction [13,52,121,122]. The gating mechanisms of TPCs and their permeability properties have engendered a durable controversy that is yet to be fully resolved [103,123,124,125,126]. TPC1-2 were first identified as the long-sought target for the intracellular second messenger, NAADP, which is synthesized in response to multiple extracellular stimuli by the ADP-ribosyl cyclase, CD38 [127]. A wealth of robust evidences clearly converged on this evidence. First, overexpression of TPCs potentiated NAADP-induced Ca2+ signals in several cellular models [109,117,118,119,128]. Second, gene silencing, gene knockdown or dominant negative TPC mutants suppressed NAADP-induced Ca2+ signals and downstream signalling events [117,120,128,129,130,131]. Third, direct electrophysiological recordings carried out by using the patch-clamp technique on enlarged lysosomes or by exploiting the planar lipid bilayer technology confirmed that NAADP was able to gate TPCs [124,132,133,134,135,136]. Notably, electrophysiological analysis confirmed that TPC1-2 were both permeable to Ca2+ although at a different extent [124,126]. TPC1 is more permeable to monovalent cations, the highest permeability being for H+: H+ >> K+ > Na+ ≥ Ca2+ [133]. This feature suggests that TPC1 may provide a pathway for intraluminal H+ efflux, thereby resulting in alkalization of acidic organelles [18]. Conversely, TPC2 is slightly more selective for Ca2+ over monovalent cations and shows a Ca2+/K+ permeability ratio (PCa2+/PK+) of 2.6 [132]. It should, however, be pointed out that the ion selectivity and single-channel conductance of TPC1-2 may vary depending on the experimental conditions, although it is nowadays accepted that they mediate Ca2+ release [13,124,126].
The discovery that TPCs are Ca2+-permeable EL channels targeted by NAADP was subsequently challenged by two studies from the same group, which exploited a lysosomal patch-clamp method to propose that TPCs were rather Na+-selective channels with poor Ca2+ permeability activated by PI(3,5)P2 [137,138]. Several excellent reviews recently addressed this discrepancy that has a remarkable impact on our knowledge of EL Ca2+ signaling and associated Ca2+-dependent processes. [103,123,125,126,139]. Briefly, by using the same methodology, Jha and coworkers confirmed that TPC2 was permeable to Na+ and activated by PI(3,5)P2 [135]. Nevertheless, TPC2-mediated currents could also be triggered by NAADP and were sensitive to Mg2+: cytosolic Mg2+ fully inhibited NAADP-induced currents, while lysosomal Mg2+ reduced them by about 20% [135]. Notably, unlike previous reports [137], this study confirmed that TPC2 bears a measurable Ca2+ permeability and effectively mediates intracellular Ca2+ release in a Mg2+-dependent manner [135]. Thus, different cytosolic/lysosomal Mg2+ levels could account for the different NAADP-sensitivity displayed by TPC2 in independent studies [123,125]. Accordingly, the studies which neglect the role of TPCs in NAADP-induced Ca2+ release routinely used 2 mM Mg2+ in their cytosolic solutions [137,138], which is predicted to fully abolish NAADP-mediated lysosomal currents [135]. Moreover, two additional investigations evaluated the Na+/Ca2+ permeability (PNa+/PCa2+) ratio of TPCs-mediated currents and found that it was very close to the unity, ranging between 0.86 [129] and 1.1 [133]. Additionally, NAADP does not directly bind to TPCs, but requires the interposition of auxiliary cytosolic proteins [140] that could be diluted or washed away under different in vitro conditions, such as channel reconstitution in planar lipid bilayers or lysosome isolation. To further support the role of TPCs as truly NAADP-gated channels, several concerns were also raised as regard to the validity of the knockout models that were generated to demonstrate that the Ca2+ response to NAADP was still present upon genetic deletion of TPCs [123,125,139]. While lack of mRNA or protein expression was not demonstrated in the earlier Tpcn1−/−/Tpcn2−/− mice [137], Ruas and coworkers revealed that NAADP did not induce Ca2+ signals in embryonic fibroblasts deriving from mice demonstrably lacking TPC1, TPC2 or both [129]. Moreover, the Ca2+ response to NAADP was rescued by re-expressing wild-type, but not pore-mutant, TPCs [129]. Intriguingly, Ruas and coworkers showed that NH2-terminal truncated forms of TPC1 and TPC2 were expressed in the mouse line formerly presented as TPCs-null and were able to rescue the Ca2+ response to NAADP in their truly knocked out TPCs model [129,141].
Based on these observations, it is now acknowledged that TPC1-2 serve as NAADP-gated EL Ca2+-releasing channels that are co-regulated by PI(3,5)P2 and also permeable to monovalent cations, including H+ [103,124]. Moreover, TPCs are able to integrate multiple signals emanated from both the cytosol and the EL luminal although TPC1 and TPC2 show different sensitivity to diverse modulators. For instance, TPC1 (as well as TPC3) was reported to be voltage-activated and to control the electrical excitability of EL vesicles [142], which is in agreement with the presence of two voltage-sensors in TM4 and TM10 in the channel protein. A subsequent study argued that membrane-depolarization is not required for channel activation but increases the affinity of TPC1 for NAADP [134], while TPC2 is voltage-insensitive [132,142]. As mentioned earlier, TPC2 is inhibited by Mg2+ [135], whereas there are no information available regarding TPC1 [125]. The regulation by intraluminal pH is more complex. As intuitively expected, some studies revealed that TPC1-2 were both activated by the acidic intraluminal pH [133,134,136]. Nevertheless, TPC1-2 were also shown to be inhibited [132,142] or unaffected by acidic pH [135,137]. Similar to InsP3Rs and RyRs, TPC1, but not TPC2 [132], is also regulated by cytosolic Ca2+ [133] and could be stimulated by ER-released Ca2+ [18]. Luminal EL Ca2+, in turn, increases TPC2 sensitivity to NAADP [132], while its stimulatory effect on TPC1 is still matter of controversy [133,134]. Finally, TPC2 activity is increased by p38 and JNK kinases and is finely tuned by EL PI(3,5)P2 levels [135]. Likewise, TPCs are stimulated by leucine-rich repeat kinase 2 (LRKK2) [143]. TPC modulation by mTOR will be illustrated in the following paragraph.
2.4.2.1. The Physiological Role of TPCs: Local vs. Global Ca2+ Signals
Emerging evidence supports the notion that TPCs may regulate cellular fate and behavior through both local and global intracellular Ca2+ signals [13,80,103,123]. TPCs control EL membrane trafficking associated with exocytosis, endocytosis, autophagy and viral infection through an elevation in surrounding Ca2+ levels [103,144,145]. For instance, local Ca2+ release drives homo- and heterotypic fusion between LEs and lysosomes in fibroblasts isolated from patients affected by Parkinson’s disease (PD) [146]. Lysosomes are enlarged and aggregated in these cells, a morphological defect that can be restored by genetic or pharmacological inhibition of TPC2 and by buffering local Ca2+ signals with BAPTA, but not with the slower Ca2+ chelator, EGTA [146]. Likewise, TPC2 mediates the fusion events underlying the trafficking to the acidic compartment of several cargoes, including those containing Ebola virus [147], cholera toxin [119], LDL-cholesterol, and EGF/EGF receptor [148]. Notably, local Ca2+ release through TPC1 was subsequently shown to promote LEs/lysosomes fusion in normal fibroblasts and HeLa cells [149]. As more widely discussed in [80,103], spatially-restricted Ca2+ signals could be generated by TPCs also to mediate retrograde trafficking from endosomes to Golgi [119,150] and secretion of lysosome-related organelles, such as cytolytic granules in cytotoxic T lymphocytes [130]. Two proteomic screens revealed that TPCs may physically interact with a variety of proteins implicated in EL vesicles trafficking and fusion events, including several Rab GTPases, e.g., Rab7A, and syntaxins, e.g., syntaxin 12 and syntaxin 13 [148,151]. Rab7 GTPase in turn boosts NAADP-induced Ca2+ release through TPC2 to promote lysosomal enlargement [146,151], whereas syntaxins mediate Ca2+-dependent membrane fusion events [152], although it is not known whether syntaxin 12 and syntaxin 13 are per se Ca2+-sensitive.
In addition, TPCs may regulate autophagy. Unlike TRPML1, mTOR is part of TPC interactome [151] and, under nutrients-rich conditions, it inhibits both the PI(3,5)P2-induced TPCs-mediated Na+-selective current [138] and NAADP-induced Ca2+ release through TPC2 [153]. As a consequence, TPCs are likely to be, partially or fully, inhibited by nutrient supply and, to further support this notion, they are also blocked by ATP [138]. Notably, TPC1-induced Ca2+ release is also able to promote the nuclear translocation of the autophagic transcription factor, TFEB [154] (Figure 3). This pathway has recently been shown to protect against lipopolysaccharide-induced liver injury [155]. Several independent studies confirmed that the NAADP/TPCs axis was involved in autophagy, although its role could be more complex than envisaged by earlier work. For instance, NAADP-induced TPC2 activation caused an increase in the number of acidic vesicular organelles and in the levels of the autophagic markers, beclin-1 and LC3II, in rat astrocytes [156], whereas NAADP-evoked Ca2+ signals through TPC1 and TPC2 sustained glutamate-induced autophagy in SHSY5Y neuroblastoma cells [157]. Likewise, TPC1 and TPC2 were required for basal and starvation-induced autophagy also in mouse cardiac myocytes [158]. Furthermore, it has been reported that LRKK2 can induce EL Ca2+ release through TPCs, thereby recruiting the Ca2+/CaM-dependent protein kinase kinase β (CaMKKβ), which initiates the autophagic flux by phosphorylating and activating AMPK [143]. Conversely, TPCs may inhibit autophagy in response to other cellular stressors and/or in other cellular models. For instance, autophagy was exacerbated in starving myoblasts isolated from TPCN2−/− mice and treated with colchicine to inhibit lysosomal trafficking [159]. It has been proposed that TPC2 negatively modulates autophagy by inducing lysosomal alkalization and reducing protease activity [159].
In addition to local Ca2+ signals, NAADP may stimulate TPCs to cause global elevations in [Ca2+]i [13,14]. Accordingly, EL Ca2+ release can provide the Ca2+ trigger necessary to recruit InsP3Rs and RyRs, located at the MCSs between EL vesicles and ER cisternae, through the CICR process [35,40,121,122,160,161,162,163]. ER-dependent Ca2+ mobilization, in turn, could stimulate NAADP-induced Ca2+ release through either local NAADP production or TPC sensitization by cytosolic/intraluminal Ca2+ (please, note that only TPC1 is sensitive to intraluminal Ca2+) [18]. Ultrastructural analysis revealed that the EL system can be closely (<30 nm) apposed to the ER network [163,164]. As reviewed elsewhere, endosomes-ER MCSs control lipid exchange, cargo sorting, endosome positioning, trafficking and fission [164,165]. Notably, TPC1 favors the formation of these junctional microdomains, thereby promoting the internalization of EGF receptors and curtailing EGF signalling [149,166]. MCSs can be established also between lysosomes and ER to regulate lysosomal positioning [39,167] and mathematical modeling confirmed that these lysosomal-ER microdomains are able to drive the global Ca2+ response to the lysosomotropic agent, GPN [39]. Therefore, as NAADP synthesis may occur downstream of GPCRs and TKRs, TPC1-2 may be physiologically engaged to interact with InsP3Rs and/or RyRs and control a number of processes [13,51]. These include fertilization [52], neurotransmitter release [168], exocytosis of lytic granules in T cells [130], neurite outgrowth [169], cardiac [35] and vascular smooth muscle [131] contraction, angiogenesis [170], vasculogenesis [171], and nitric oxide release [172,173].
2.4.3. Other endolysosomal Ca2+ releasing channels
As mentioned earlier, Ca2+ release from the EL store can also be effected by other members of the TRP superfamily, such as TRPA1 and TRPM2, ATP-gated ionotropic P2X4 receptors and voltage-gated Ca2+ channels (Figure 1).
TRPM2 is a Ca2+-permeable, non-selective cation channel which primarily resides on the PM, is gated by ADP-ribose (ADPr) and hydrogen peroxide (H2O2), and positively modulated by NAADP and cyclic ADP-ribose [174]. Additionally, TRPM2 may serve as an ADPr-gated lysosomal Ca2+ release channels in mouse pancreatic β cells, in which it mediates H2O2–induced apoptosis [175], and in mouse dendritic cells, in which it controls maturation and migration [176].
TRPA1 belongs to a distinct sub-family of TRP channels and mediates a Ca2+-permeable, non-selective cation current that is activated by noxious cold temperatures and mechanical stimuli and by a wealth of irritant compounds in peripheral somatosensory neurons [177]. Herein, TRPA1 is also located within the EL system, thereby mediating local Ca2+ sparks that stimulate vesicle exocytosis and calcitonin gene-related peptide release. It has, therefore, been proposed that EL TRPA1 further increases the excitability of dorsal root ganglia [178].
P2X receptors are homo- or heterotrimers which serve as ATP-gated ionotropic, Ca2+-permeable, non-selective channels [179]. Seven subunits (P2X1-7) were described in vertebrates, each comprising two TM domains connected by a large extracellular domain, which contains the ATP-binding site, and with cytosolic NH2- and COOH-terminal tails [179]. Earlier work revealed that P2X4 receptors were mainly located to the lysosomal vesicles of several cell types, including rat microglia, vascular endothelial cells and freshly isolated peritoneal macrophages, through a dileucine type motif within their NH2-terminus [180]. Nevertheless, P2X4 receptors can be recruited to the PM or to the phagosome surface in response to different cellular stimuli [180]. A subsequent report demonstrated that lysosomal P2X4 receptors may be gated by intraluminal ATP in a pH-dependent manner: P2X4 activity was increased by lumen alkalization, while it was inhibited by a reduction in intraluminal pH [181]. Notably, the activation of P2X4 receptors stimulated EL membrane fusion in a CaM-dependent manner [182].
Voltage-gated Ca2+ channels mediate extracellular Ca2+ entry in response to membrane depolarization to regulate multiple functions, including muscle contraction and gene expression (CaV1), neurotransmitter release (CaV2), and repetitive firing in sino-atrial cells and thalamic neurons (CaV3) [183]. Surprisingly, a recent study reported that CaV2.1, which mediates voltage-gated P/Q-type Ca2+ currents at cerebellar terminal synapses and is encoded by the CACNA1A gene [183], is also localized to the lysosomes in mouse cultured granule cells [184]. Mutations in CaV2.1 prevented autophagy by inhibiting the fusion between lysosomes and endosomes and/or autophagosomes [184]. Although lysosomal voltage-gated P/Q-type Ca2+ currents were not measured, this finding raises the possibility that lysosomal Ca2+ release could effectively occur in response to a positive shift in ΔΨ [61].
3. The Role of Lysosomal Ca2+ Signals in Cancer: Two-Pore Channels Drive Metastasis and Angiogenesis
3.1. Lysosomes Contribute to Cancer Hallmarks
The lysosomal membrane serves as a signaling platform that regulates multiple functions, including endocytic traffic, autophagy, and lysosomal function, thereby impacting on the metabolism and fate of the entire cell [33,185]. Therefore, any alteration in lysosomal function or structure may impair vesicular trafficking and ultimately lead to life-threatening diseases. For instance, mutations in many EL genes or defects in the acidic Ca2+ content drive or are tightly involved in the development of neurodegenerative disorders, such as MLIV, Parkinson’s disease, Alzheimer’s disease, and Niemann Pick disease, type C [20,33,38,146,186]. In addition, it is now recognized that lysosomes sustain several cancer hallmarks, including aberrant proliferation, metastasis and angiogenesis [185,187,188]. Briefly, lysosomes sustain excessive proliferation by supplying cancer cells with the energy and metabolic precursors necessary for rapid division and mass accumulation, thereby overcoming the limited delivery of nutrients and oxygen to the harsh microenvironment of growing tumors [188,189]. In neoplastic cells, lysosomes heavily recycle exogenous macromolecules and endogenous proteins/organelles to fuel cell growth and, not surprisingly, autophagy plays a crucial role in malignant transformation and cancer progression [188]. Notably, malignant transformation repositions lysosomes from the juxtanuclear pool towards the cell periphery, increases lysosomal volume and ultimately leads to an increase in total protease activity [185,190]. As a consequence, the cathepsins secreted via exocytosis of peripheral lysosomes contribute to pave the way for cancer cell migration and tumor angiogenesis. Accordingly, lysosomal-derived cathepsins may degrade the extracellular matrix components either directly or indirectly by stimulating matrix metalloproteins, which supports primary cancer migration, invasion and metastatic dissemination [185,188]. In parallel, such remodeling of the extracellular matrix is also essential for capillary sprouting and the initiation of angiogenesis, which is indispensable to nourish cancer cells and to let them colonize distant organs [191,192]. Cathepsins D, B, S, K, and L are the main hydrolases supporting the local invasion of primary tumor cells into surrounding tissues and their subsequent intravasation into the circulatory system [185,188]. It has been envisioned that lysosomal exocytosis results in the acidification of local cancer microenvironment, which is necessary for cathepsins to effectively degrade the extracellular matrix [185]. This hypothesis has been supported by the observation that several v-ATPase subunits are up-regulated and expressed on the PM of cancer cells, where they participate in the creation of the acidic extracellular environment which supports a more invasive phenotype [193]. Finally, lysosomes may mediate drug resistance to several chemotherapeutics by exploiting multiple mechanisms, such as passive ion-trapping based sequestration and clearance into the extracellular milieu via exocytosis [194]. It should, however, be pointed out that lysosomes could play an oncosuppressive role during the early stages of the tumorigenic process. We refer the reader to some excellent reviews covering this intriguing issue [190,195].
3.2. The Role of Endolysosomal Ca2+ Signaling in Cancer: Are Two-Pore Channels the Guilty Ones?
As aforementioned, mutations in EL Ca2+-permeable channels, i.e., TRPML1, or defects in the acidic Ca2+ store underlie severe neurodegenerative disorders, such as MLIV and Niemann Pick disease, type C, respectively [38,186]. Moreover, rewiring of the intracellular Ca2+ toolkit supports many cancer hallmarks. For instance, the reduction in ER Ca2+ concentration is responsible for the increased resistance to pro-apoptotic stimuli [196,197] and anti-angiogenic treatments [198,199]. Likewise, the up-regulation of STIM and/or Orai1 proteins, as well as of multiple TRP isoforms, supports uncontrolled proliferation [200,201], tissue invasion and migration [201,202,203], and sustained angiogenesis [204,205]. Therefore, recent studies assessed whether also the EL Ca2+ machinery was implicated in malignant transformation [19,20,21]. No evidence has been presented about the involvement of EL TRPML1, TRPM2, P2X4 receptors and voltage-gated Ca2+ channels in malignant transformation [19,21]. A recent report showed that lysosomal biogenesis and function were increased in pancreatic ductal adenocarcinoma (PAD) and associated to constitutive nuclear localization of TFEB [206]. Surprisingly, TFEB activity was independent on metabolic pathways in PAD and sustained autophagy-lysosome activation even when mTORC1 was not inhibited [206]. This observation suggests that TFEB could be functionally decoupled by TRPML1 in cancer cells. Enhanced TFEB expression and/or activity also induced autophagy, migration and metastasis in non-small lung cell carcinoma [207] and renal cellular carcinoma [208]. These studies, however, did not assess the Ca2+-dependence of the subcellular localization of TFEB in cancer cells. TRPML2 is the only member of this sub-family to have been clearly associated to malignant transformation. A recent investigation revealed that TRPML2 transcript and protein were highly expressed in glioma tissues and that their expression increased during tumor progression [209]. Gene silencing experiments conducted in glioma cell lines showed that TRPML2 regulates proliferation through Akt and ERL 1/2 phosphorylation and prevents caspase-3 activation, thereby protecting glioma cells from apoptosis [209]. The role of Ca2+ signalling in the oncogenic effect of TRPML2 was not explored. Conversely, emerging evidence clearly hints at TPCs as main drivers of multiple cancer hallmarks in a growing number of solid malignancies (Figure 3). These evidences are described in the next paragraph.
3.3. Two-Pore Channels 1 and 2 Support Cancer Cell Migration and Promote Tumor Vascularization
Earlier work revealed that TPC1 transcripts were ~3–8 fold higher than TPC2 in SKBR3 cells, a human breast cancer cell line, and in PC12 cells, a model for rat pheochromocytoma [128]. Subsequently, it was found that TPC1 and TPC2 transcripts were similarly expressed in a different set of human breast cancer cells, although only genetic silencing of TPC2 inhibited EGF-induced vimentin, but not E-cadherin, expression in the highly aggressive MDA-MB-468 cell line. On the other hand, silencing of either TPC isoform did not affect the rate of cell proliferation [210]. TPC2 silencing reduced the amount of Ca2+ released upon pharmacological depletion of the ER under 0 Ca2+ conditions and the amplitude of SOCE recorded upon restoration of extracellular Ca2+ levels [210]. Notably, TPC2 may interact with STIM1 and Orai1 in response to ER Ca2+ depletion in the megakaryoblastic cell line MEG01 [211]. More recently, TPC1 and TPC2 transcripts were identified in several cancer cell lines established from liver (HUH7), bladder (T24) and blood (Jurkat) as compared to a human breast cancer cell line (MDA-MB-231) [212]. Overall, TPC1 transcripts were more abundant than TPC2 in T24 and HUH7 cells, whereas immunocytochemistry revealed that TPC2 protein was up-regulated in liver carcinoma as compared to adjacent healthy tissue [212]. Electrophysiological recordings confirmed that EL currents were activated by PI(3,5)P2 and inhibited by tetrandrine, a rather selective TPC inhibitor [212]. Genetic silencing and pharmacological blockade of TPC1 and TPC2 with tetrandrine or NED-19, another widely employed TPC inhibitor, blocked adhesion and migration in invasive cancer cells (T24 and HUH7) [205]. Furthermore, genetic silencing of TPC2 and pharmacological blockade of TPCs with tetrandrine or NED-19 prevented lung metastasis in a mouse model of mammary carcinoma [212]. The same investigation disclosed that TPCs inhibition caused β1-integrin accumulation within EEs and impaired its trafficking towards the PM, thereby halting lamellipodia formation and interfering with the migration process [212]. Migrating cells extend lamellipodia in the direction of migration, which need to be stabilized through integrins bound to the actin cytoskeleton [205]. Genetic and pharmacological inhibition of TPCs prevented the formation of β1-integrin-, pFAK-, pSrc-, and vinculin-positive polarized lamellipodia [212]. As pointed out in [20], deletion of the recently identified nonplacental mammalian CAX in neural crest cells impaired spreading and focal adhesion formation in vitro, thereby impairing migration in vivo [54]. Notably, this study suggested that local, rather than global, cytosolic Ca2+ signals were required to sustain motility as the same effect was phenocopied by BAPTA, while EGTA was ineffective [54]. Therefore, EL Ca2+ signals could effectively modulate migration in both normal and neoplastic cells. Conversely, TPC2 negatively modulates EL trafficking in 4T1 mouse breast cancer cells and HeLa mouse cervical cancer cells by preventing the recruitment of Rab7 from lysosomes to autophagosomes and causing an increase in lysosomal pH [213]. As mentioned earlier, autophagy tends to suppress the early stages of many solid tumors. One could envisage that, under these circumstances, TPC overexpression could favor tumor progression.
The first report about the pro-tumorigenic role of TPCs was, however, provided by Favia and coworkers [170]. This study revealed that NAADP-induced Ca2+ signals promoted tumor development in mice xenografted with B16 melanoma cells [214]. Accordingly, the pharmacological blockade of TPCs with NED-19 halted melanoma growth and vascularization and significantly reduced lung metastasis [214]. A more insightful analysis disclosed that NAADP stimulated melanoma B16 cells to migrate and proliferate through an increase in [Ca2+]i that was sustained by the EL Ca2+ store [170]. NAADP-evoked Ca2+ signals induced Go/G1 progression and the activation of the focal adhesion kinase (FAK), which is indispensable for cell migration [170].
This study further suggested that the Ca2+ response to NAADP stimulated angiogenesis through the paracrine release of growth factors from the cancer cells. In addition, the same group previously reported that NAADP sustains vascular endothelial growth factor (VEGF)-dependent angiogenesis in vitro and neovessel formation in vivo [170]. Accordingly, they found that VEGF-induced intracellular Ca2+ signals were attenuated by NED-19 and v-ATPase inhibition with bafilomycin A1. Likewise, the pharmacological blockade of TPCs with NED-19 blocked cell proliferation, migration and tube formation by suppressing VEGF-induced phosphorylation of ERK1/2, Akt, JNK and endothelial nitric oxide synthase (eNOS) [170] (Figure 4). Finally, the Matrigel plug assay revealed that VEGF-induced angiogenesis in vivo was blocked by NED-19 in wild type mice and in TPCN2−/−, but not TPCN1−/−, mice [170]. These results were further supported by the finding that also the natural flavonoid, naringenin, attenuated VEGF-induced angiogenesis in vitro and neovessel formation in vivo [215]. Accordingly, naringenin blocked TPC1- and TPC2-mediated lysosomal currents, significantly reduced VEGF-induced Ca2+ signals in HUVECs and prevented their assembly into capillary-like bidimensional structures in Matrigel scaffolds [215]. Furthermore, naringenin halted VEGF-induced vascularization of Matrigel plugs subcutaneously implanted in mice [215]. In addition, a functional EL Ca2+ store enriched with TPC1 is also expressed in human endothelial colony forming cell (ECFCs) [41,171], a subset of endothelial progenitor cells that support tumor vascularization by physically engrafting within neovessels and through paracrine release of pro-angiogenic growth factors [198,216]. Notably, liposomal delivery of NAADP promoted ECFC proliferation by likely recruiting eNOS in a Ca2+/CaM-dependent manner [41,171]. Tumor-derived ECFCs are largely insensitive to VEGF [198,204,217,218,219]. This feature raises the intriguing possibility that TPC1 could provide an alternative target to VEGF signalling to prevent or, at least attenuate, ECFC homing to tumor neovessels.
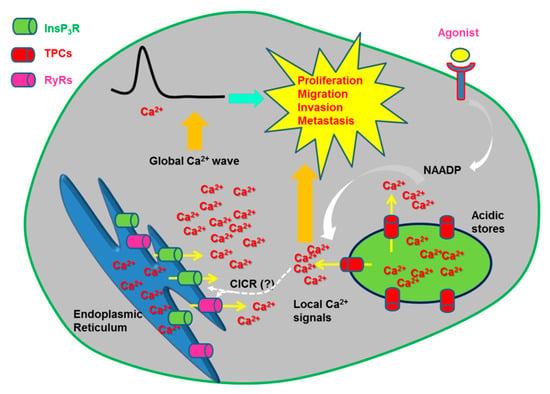
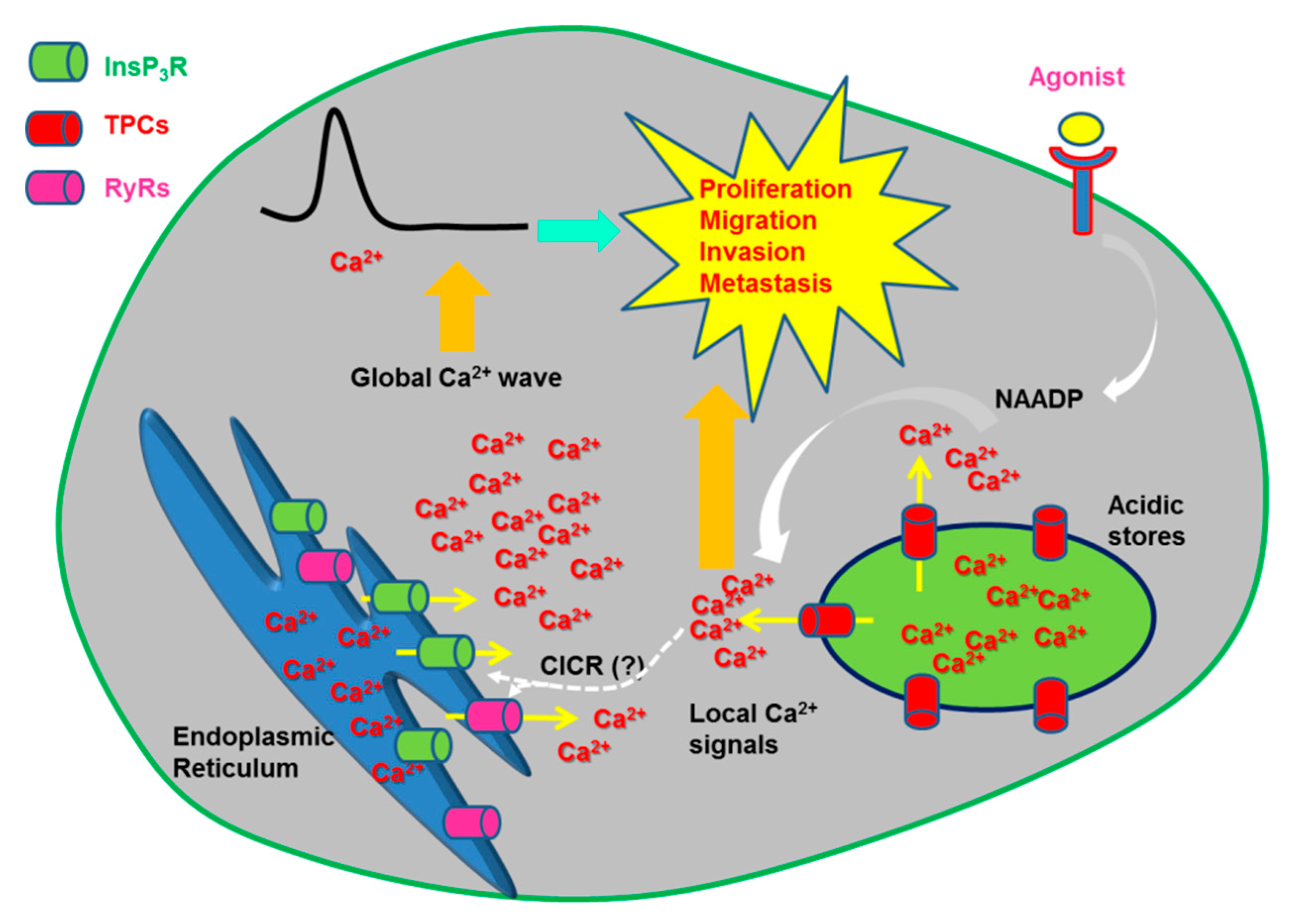
Figure 4.
TPCs mediate oncogenic Ca2+ signals. Emerging evidence indicates that NAADP promotes tumorigenesis by inducing EL Ca2+ release through TPCs. Work carried out on melanoma B16 cells suggest that NAADP is synthesized in response to VEGF stimulation. NAADP-induced Ca2+ signals promote tumorigenesis by stimulating migration, invasion, angiogenesis and, maybe, proliferation. The evidence that NAADP-induced local Ca2+ release through TPCs is amplified in a global elevation in [Ca2+]i through the Ca2+-induced Ca2+ release (CICR) process is yet to be provided.
3.4. TPCs as Therapeutic Target in Cancer
The studies described strongly suggest that TPC1 and TPC2 represent suitable molecular targets to interfere with tumor growth, vascularization, and metastasis. Pharmacological blockade of TPCs could thus be regarded as a promising, alternative anticancer approach [19,21]. Throughout the present article, we have already introduced a number of compounds that are routinely employed to block TPCs and their associated functions. These include NED-19, which is likely to prevent NAADP binding [220], naringenin, tetrandrin and structurally-related derivatives, that are likely to plug the channel pore [147,221]. Moreover, the nucleotide mimetics, PPADS and PPNDS, may also be exploited as competitive antagonists of NAADP receptors [222], i.e., TPC1 and TPC2. Intriguingly, it has long been known that the Ca2+ response to NAADP was inhibited by antagonists of L-type CaV channels, such as verapamil and diltiazem and dihydropyridines (e.g., nifedipine, nisoldipine, nimodipine and nicardipine) [223,224]. Subsequent work revealed that TPCs present a binding site for CaV antagonists within their selectivity filter, which is not surprising when considering the strong molecular similarity between TPCs and CaV [110]. As reviewed elsewhere [225], CaV antagonists, such as nitrendipine, nifedipine and verapamil, are routinely employed to treat life-threatening disorders [9], such as arrhythmia, infarction-induced cardiac failure, hypertension and chronic stable angina, and lack severe off-target effects. This observation suggests that CaV antagonists could also be probed in preclinical trials assessing whether they are able to induce cancer shrinkage by blocking TPCs.
A larger arsenal of drugs is currently under intense scrutiny to design alternative treatments of diseases associated to mutated or dysfunctional TPCs, including neurodegenerative diseases, or requiring a functional EL trafficking, such as viral infections. A recent screening campaign conducted on sea urchin egg homogenates disclosed a novel pharmacopeia of 18 drugs effectively (>80%) inhibiting NAADP-induced Ca2+ release. The most efficient inhibitors were PF-543, a cell permeant blocker of sphingosine kinase 1, SKF96365, a non-specific antagonist of Ca2+-permeable channels that was already known to affect NAADP signalling [226], and racecadotril, a neutral endopeptidase inhibitor [227]. The subsequent validation on a human cell line, i.e., U2OS, confirmed that these drugs were able to inhibit NAADP-induced Ca2+ signals [227] and attenuated the translocation of a Middle East Respiratory Syndrome Coronavirus pseudovirus through the EL system [221]. Parallel work screened a database of ≈1500 Food and Drug Administration (FDA)-approved drugs in search for therapeutically relevant TPC2 inhibitors [228]. This throughout analysis revealed that four dopamine receptor antagonists and five selective estrogen receptor modulators were able to bind to TPC pore. Accordingly, these drugs reduced the mean open probability of TPC2 rerouted to the PM and inhibited lysosomal TPC2 currents [228]. Notably, these drugs inhibited infection of HeLa by Ebola virus [228]. Finally, a battery of small-molecule NAADP analogues were recently prepared by alkylation of nicotinic acid derivatives with a series of bromoacetamides and tested on T cells- and cardiomyocytes-related disorders [229]. One of these agents, termed lead compound 2, inhibited NAADP-induced Ca2+ release in rat Jurkat T-lymphocytes in vitro and was effective in an EAE model of multiple sclerosis in vivo. Another agent obtained by this screening, termed 3 or BZ194 [229], blocked the Ca2+ response to NAADP in vitro [230] and cardiac arrhythmias evoked by β-adrenergic stimulation in vivo [231].
Only NED-19, tetrandrin and naringenin were hitherto probed to halt tumor growth and vascularization. Unfortunately, tetrandrine and naringenin may target multiple signalling pathways and are not approved for human use worldwide [228]. However, the effort to identify novel drugs to effectively treat a number of diseases associated to EL Ca2+-permeable channels, such as TPCs, is now coming of age and is predicted to rapidly enlarge our arsenal of selective TPC inhibitors, which could be also testable for anticancer treatments.
4. Conclusions
EL Ca2+ signalling through TRPML1-3 and TPC1-2 finely regulates cellular fate and behavior by driving the EL trafficking machinery and the autophagic flux, thus enabling the cells to adapt to stressful conditions, such as those imposed by lack of nutrients. In addition, local EL Ca2+ signals control trafficking and correct positioning of signaling proteins involved in cell proliferation and migration, such as integrins and EGF receptors, and the endocytosis of bacteria, toxins and viruses, which may cause cell death. Moreover, TPCs may interact with ER-embedded InsP3Rs and RyRs to generate global Ca2+ signals that further expand the repertoire of cellular functions regulated by the EL Ca2+ store. Herein, we discussed the evidences supporting the role of EL Ca2+ signalling in cancer. While the oncogenic function of TRPML1, the main driver of TFEB activation in healthy cells, remains obscure, TPC1 and TPC2 are standing out as crucial drivers of many cancer hallmarks, including excessive proliferation, aberrant migration and sustained vascularization. Many issues remain to be elucidated. For instance, is TPC expression actually altered in cancer cells? Is NAADP the main ligand of tumor TPCs or is there a role also for PI(3,5)P2? Is the EL Ca2+ store altered by neoplastic transformation, just as it happens for the ER? Which is the main source of Ca2+ responsible for EL Ca2+ refilling in cancer cells: the cytosol, the ER or the extracellular milieu? Does CICR support the Ca2+ response to NAADP also in cancer cells (Figure 3)? With all these questions awaiting an answer, the field is surely going to open its doors to much exciting research.
Funding
This research was funded by: Italian Ministry of Education, University and Research (MIUR): Dipartimenti di Eccellenza Program (2018–2022)—Dept. of Biology and Biotechnology “L. Spallanzani”, University of Pavia (F.M.), Fondo Ricerca Giovani from the University of Pavia (F.M.), Italian Ministry of Health Grants RF-2010-2316319 (D.M.), and by program “Ricerca Corrente” of the IRCCS Policlinico San Matteo: RC/08059815B (D.M.). PF was supported by MAECI (Ministero degli Affari Esteri e della Cooperazione Internazionale).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, D.; Rizzuto, R.; Pozzan, T. Enjoy the Trip: Calcium in Mitochondria Back and Forth. Annu. Rev. Biochem. 2016, 85, 161–192. [Google Scholar] [CrossRef] [PubMed]
- Missiaen, L.; Dode, L.; Vanoevelen, J.; Raeymaekers, L.; Wuytack, F. Calcium in the Golgi apparatus. Cell Calcium 2007, 41, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Thillaiappan, N.B.; Chavda, A.P.; Tovey, S.C.; Prole, D.L.; Taylor, C.W. Ca2+ signals initiate at immobile IP3 receptors adjacent to ER-plasma membrane junctions. Nat. Commun. 2017, 8, 1505. [Google Scholar] [CrossRef] [PubMed]
- Keebler, M.V.; Taylor, C.W. Endogenous signalling pathways and caged IP3 evoke Ca2+ puffs at the same abundant immobile intracellular sites. J. Cell Sci. 2017, 130, 3728–3739. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. The endoplasmic reticulum: A multifunctional signaling organelle. Cell Calcium 2002, 32, 235–249. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef]
- Moccia, F.; Dragoni, S.; Lodola, F.; Bonetti, E.; Bottino, C.; Guerra, G.; Laforenza, U.; Rosti, V.; Tanzi, F. Store-dependent Ca2+ entry in endothelial progenitor cells as a perspective tool to enhance cell-based therapy and adverse tumour vascularization. Curr. Med. Chem. 2012, 19, 5802–5818. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta 2009, 1793, 933–940. [Google Scholar] [CrossRef]
- Laude, A.J.; Simpson, A.W. Compartmentalized signalling: Ca2+ compartments, microdomains and the many facets of Ca2+ signalling. FEBS J. 2009, 276, 1800–1816. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Docampo, R. Acidic calcium stores open for business: Expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010, 20, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Galione, A. A primer of NAADP-mediated Ca2+ signalling: From sea urchin eggs to mammalian cells. Cell Calcium 2015, 58, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.J.; Platt, F.M.; Lloyd-Evans, E.; Galione, A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem. J. 2011, 439, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Cai, X. Evolution of acidic Ca2+ stores and their resident Ca2+-permeable channels. Cell Calcium 2015, 57, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Z.; Gu, M.; Feng, X.; Xu, H. Release and uptake mechanisms of vesicular Ca2+ stores. Protein Cell 2018. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K.; Galione, A. The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim. Biophys. Acta 2013, 1833, 2542–2559. [Google Scholar] [CrossRef]
- Morgan, A.J.; Davis, L.C.; Wagner, S.K.; Lewis, A.M.; Parrington, J.; Churchill, G.C.; Galione, A. Bidirectional Ca2+ signaling occurs between the endoplasmic reticulum and acidic organelles. J. Cell Biol. 2013, 200, 789–805. [Google Scholar] [CrossRef]
- Grimm, C.; Bartel, K.; Vollmar, A.M.; Biel, M. Endolysosomal Cation Channels and Cancer-A Link with Great Potential. Pharmaceuticals (Basel) 2018, 11, 4. [Google Scholar] [CrossRef]
- Patel, S.; Kilpatrick, B.S. Two-pore channels and disease. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1678–1686. [Google Scholar] [CrossRef]
- Sterea, A.M.; Almasi, S.; El Hiani, Y. The hidden potential of lysosomal ion channels: A new era of oncogenes. Cell Calcium 2018, 72, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, P.; Lissandron, V.; Capitanio, P.; Pozzan, T. Ca2+ signalling in the Golgi apparatus. Cell Calcium 2011, 50, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.K.; Capitanio, P.; Lissandron, V.; Bortolozzi, M.; Pozzan, T.; Pizzo, P. Heterogeneity of Ca2+ handling among and within Golgi compartments. J. Mol. Cell Biol. 2013, 5, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, J.V.; Tepikin, A.V.; Petersen, O.H.; Gerasimenko, O.V. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr. Biol. 1998, 8, 1335–1338. [Google Scholar] [CrossRef]
- Albrecht, T.; Zhao, Y.; Nguyen, T.H.; Campbell, R.E.; Johnson, J.D. Fluorescent biosensors illuminate calcium levels within defined beta-cell endosome subpopulations. Cell Calcium 2015, 57, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.C.; Gruenberg, J. Ion flux and the function of endosomes and lysosomes: pH is just the start: The flux of ions across endosomal membranes influences endosome function not only through regulation of the luminal pH. Bioessays 2011, 33, 103–110. [Google Scholar] [CrossRef]
- Prekeris, R.; Klumperman, J.; Chen, Y.A.; Scheller, R.H. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J. Cell Biol. 1998, 143, 957–971. [Google Scholar] [CrossRef]
- Xu, H.; Ren, D. Lysosomal physiology. Annu. Rev. Physiol. 2015, 77, 57–80. [Google Scholar] [CrossRef]
- Appelqvist, H.; Waster, P.; Kagedal, K.; Ollinger, K. The lysosome: From waste bag to potential therapeutic target. J. Mol. Cell Biol. 2013, 5, 214–226. [Google Scholar] [CrossRef]
- Maxson, M.E.; Grinstein, S. The vacuolar-type H(+)-ATPase at a glance—More than a proton pump. J. Cell Sci. 2014, 127 Pt 23, 4987–4993. [Google Scholar] [CrossRef]
- Hesketh, G.G.; Wartosch, L.; Davis, L.J.; Bright, N.A.; Luzio, J.P. The Lysosome and Intracellular Signalling. Prog. Mol. Subcell. Biol. 2018, 57, 151–180. [Google Scholar] [PubMed]
- Perera, R.M.; Zoncu, R. The Lysosome as a Regulatory Hub. Annu. Rev. Cell Dev. Biol. 2016, 32, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.; Dunlop, E.A. The lysosome: A crucial hub for AMPK and mTORC1 signalling. Biochem. J. 2017, 474, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, A.; Yamasaki, M.; Rakovic, S.; Sanders, L.; Parkesh, R.; Churchill, G.C.; Galione, A.; Terrar, D.A. NAADP controls cross-talk between distinct Ca2+ stores in the heart. J. Biol. Chem. 2007, 282, 15302–15311. [Google Scholar] [CrossRef] [PubMed]
- Atakpa, P.; Thillaiappan, N.B.; Mataragka, S.; Prole, D.L.; Taylor, C.W. IP3 Receptors Preferentially Associate with ER-Lysosome Contact Sites and Selectively Deliver Ca2+ to Lysosomes. Cell Rep. 2018, 25, 3180–3193. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.A.; Myers, J.T.; Swanson, J.A. pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 2002, 115 Pt 3, 599–607. [Google Scholar]
- Lloyd-Evans, E.; Morgan, A.J.; He, X.; Smith, D.A.; Elliot-Smith, E.; Sillence, D.J.; Churchill, G.C.; Schuchman, E.H.; Galione, A.; Platt, F.M. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 2008, 14, 1247–1255. [Google Scholar] [CrossRef]
- Kilpatrick, B.S.; Eden, E.R.; Schapira, A.H.; Futter, C.E.; Patel, S. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J. Cell Sci. 2013, 126 Pt 1, 60–66. [Google Scholar] [CrossRef]
- Ronco, V.; Potenza, D.M.; Denti, F.; Vullo, S.; Gagliano, G.; Tognolina, M.; Guerra, G.; Pinton, P.; Genazzani, A.A.; Mapelli, L.; et al. A novel Ca2+-mediated cross-talk between endoplasmic reticulum and acidic organelles: Implications for NAADP-dependent Ca2+ signalling. Cell Calcium 2015, 57, 89–100. [Google Scholar] [CrossRef]
- Zuccolo, E.; Dragoni, S.; Poletto, V.; Catarsi, P.; Guido, D.; Rappa, A.; Reforgiato, M.; Lodola, F.; Lim, D.; Rosti, V.; et al. Arachidonic acid-evoked Ca2+ signals promote nitric oxide release and proliferation in human endothelial colony forming cells. Vasc. Pharmacol. 2016, 87, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.Z.; Yang, Y.; Sun, X.; Dong, X.P. Methods for monitoring Ca2+ and ion channels in the lysosome. Cell Calcium 2017, 64, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.L.; Di Paola, S.; Peluso, I.; Armani, A.; De Stefani, D.; Venditti, R.; Montefusco, S.; Scotto-Rosato, A.; Prezioso, C.; Forrester, A.; et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015, 17, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Saiki, S.; Lichtenberg, M.; Siddiqi, F.H.; Roberts, E.A.; Imarisio, S.; Jahreiss, L.; Sarkar, S.; Futter, M.; Menzies, F.M.; et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Efeyan, A.; Zoncu, R.; Sabatini, D.M. Amino acids and mTORC1: From lysosomes to disease. Trends Mol. Med. 2012, 18, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Rabanal-Ruiz, Y.; Korolchuk, V.I. mTORC1 and Nutrient Homeostasis: The Central Role of the Lysosome. Int. J. Mol. Sci. 2018, 19, 818. [Google Scholar] [CrossRef] [PubMed]
- Sardiello, M.; Palmieri, M.; di Ronza, A.; Medina, D.L.; Valenza, M.; Gennarino, V.A.; Di Malta, C.; Donaudy, F.; Embrione, V.; Polishchuk, R.S.; et al. A gene network regulating lysosomal biogenesis and function. Science 2009, 325, 473–477. [Google Scholar] [CrossRef]
- Palmieri, M.; Impey, S.; Kang, H.; di Ronza, A.; Pelz, C.; Sardiello, M.; Ballabio, A. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 2011, 20, 3852–3866. [Google Scholar] [CrossRef]
- Settembre, C.; Fraldi, A.; Medina, D.L.; Ballabio, A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 2013, 14, 283–296. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Morgan, A.J. Ca2+ dialogue between acidic vesicles and ER. Biochem. Soc. Trans. 2016, 44, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Churchill, G.C.; Okada, Y.; Thomas, J.M.; Genazzani, A.A.; Patel, S.; Galione, A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 2002, 111, 703–708. [Google Scholar] [CrossRef]
- Srinivas, S.P.; Ong, A.; Goon, L.; Goon, L.; Bonanno, J.A. Lysosomal Ca2+ stores in bovine corneal endothelium. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2341–2350. [Google Scholar]
- Melchionda, M.; Pittman, J.K.; Mayor, R.; Patel, S. Ca2+/H+ exchange by acidic organelles regulates cell migration in vivo. J. Cell Biol. 2016, 212, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Inesi, G.; Tadini-Buoninsegni, F. Ca2+/H+ exchange, lumenal Ca2+ release and Ca (2+)/ATP coupling ratios in the sarcoplasmic reticulum ATPase. J. Cell Commun. Signal. 2014, 8, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.J.; Camello-Almaraz, C.; Pariente, J.A.; Salido, G.M.; Rosado, J.A. Ca2+ accumulation into acidic organelles mediated by Ca2+- and vacuolar H+-ATPases in human platelets. Biochem. J. 2005, 390 Pt 1, 243–252. [Google Scholar] [CrossRef]
- Lopez Sanjurjo, C.I.; Tovey, S.C.; Taylor, C.W. Rapid recycling of Ca2+ between IP3-sensitive stores and lysosomes. PLoS ONE 2014, 9, e111275. [Google Scholar] [CrossRef]
- Lopez-Sanjurjo, C.I.; Tovey, S.C.; Prole, D.L.; Taylor, C.W. Lysosomes shape Ins(1,4,5)P3-evoked Ca2+ signals by selectively sequestering Ca2+ released from the endoplasmic reticulum. J. Cell Sci. 2013, 126 Pt 1, 289–300. [Google Scholar] [CrossRef]
- Garrity, A.G.; Wang, W.; Collier, C.M.; Levey, S.A.; Gao, Q.; Xu, H. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. eLife 2016, 5, e15887. [Google Scholar] [CrossRef]
- Sbano, L.; Bonora, M.; Marchi, S.; Baldassari, F.; Medina, D.L.; Ballabio, A.; Giorgi, C.; Pinton, P. TFEB-mediated increase in peripheral lysosomes regulates store-operated calcium entry. Sci. Rep. 2017, 7, 40797. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, M.X. Regulation of lysosomal ion homeostasis by channels and transporters. Sci. China Life Sci. 2016, 59, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, X.; Gao, Q.; Lawas, M.; Yu, L.; Cheng, X.; Gu, M.; Sahoo, N.; Li, X.; Li, P.; et al. A voltage-dependent K(+) channel in the lysosome is required for refilling lysosomal Ca2+ stores. J. Cell Biol. 2017, 216, 1715–1730. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zhong, X.Z.; Zou, Y.; Zhang, Z.; Toro, L.; Dong, X.P. BK Channels Alleviate Lysosomal Storage Diseases by Providing Positive Feedback Regulation of Lysosomal Ca2+ Release. Dev. Cell 2015, 33, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, S.; Scotto-Rosato, A.; Medina, D.L. TRPML1: The Ca(2+)retaker of the lysosome. Cell Calcium 2018, 69, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Shen, D.; Samie, M.; Xu, H. Mucolipins: Intracellular TRPML1-3 channels. FEBS Lett. 2010, 584, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Hassan, S.; Wahl-Schott, C.; Biel, M. Role of TRPML and two-pore channels in endolysosomal cation homeostasis. J. Pharmacol. Exp. Ther. 2012, 342, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Gees, M.; Colsoul, B.; Nilius, B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a003962. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Hofmann, T.; Montell, C. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J. Biol. Chem. 2006, 281, 17517–17527. [Google Scholar] [CrossRef]
- Pryor, P.R.; Reimann, F.; Gribble, F.M.; Luzio, J.P. Mucolipin-1 is a lysosomal membrane protein required for intracellular lactosylceramide traffic. Traffic 2006, 7, 1388–1398. [Google Scholar] [CrossRef]
- Medina, D.L.; Fraldi, A.; Bouche, V.; Annunziata, F.; Mansueto, G.; Spampanato, C.; Puri, C.; Pignata, A.; Martina, J.A.; Sardiello, M.; et al. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell 2011, 21, 421–430. [Google Scholar] [CrossRef]
- Dong, X.P.; Cheng, X.; Mills, E.; Delling, M.; Wang, F.; Kurz, T.; Xu, H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 2008, 455, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Delling, M.; Li, L.; Dong, X.; Clapham, D.E. Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint-waddler mice. Proc. Natl. Acad. Sci. USA 2007, 104, 18321–18326. [Google Scholar] [CrossRef] [PubMed]
- Miedel, M.T.; Rbaibi, Y.; Guerriero, C.J.; Colletti, G.; Weixel, K.M.; Weisz, O.A.; Kiselyov, K. Membrane traffic and turnover in TRP-ML1-deficient cells: A revised model for mucolipidosis type IV pathogenesis. J. Exp. Med. 2008, 205, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Raychowdhury, M.K.; Gonzalez-Perrett, S.; Montalbetti, N.; Timpanaro, G.A.; Chasan, B.; Goldmann, W.H.; Stahl, S.; Cooney, A.; Goldin, E.; Cantiello, H.F. Molecular pathophysiology of mucolipidosis type IV: pH dysregulation of the mucolipin-1 cation channel. Hum. Mol. Genet. 2004, 13, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, W.K.; Benvin, N.M.; Zhou, X.; Su, D.; Li, H.; Wang, S.; Michailidis, I.E.; Tong, L.; Li, X.; et al. Structural basis of dual Ca2+/pH regulation of the endolysosomal TRPML1 channel. Nat. Struct. Mol. Biol. 2017, 24, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, N.; Zeng, W.; Gao, N.; Yang, M. Cryo-EM structures of the mammalian endo-lysosomal TRPML1 channel elucidate the combined regulation mechanism. Protein Cell 2017, 8, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.P.; Shen, D.; Wang, X.; Dawson, T.; Li, X.; Zhang, Q.; Cheng, X.; Zhang, Y.; Weisman, L.S.; Delling, M.; et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat. Commun. 2010, 1, 38. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Xu, H. Phosphoinositide isoforms determine compartment-specific ion channel activity. Proc. Natl. Acad. Sci. USA 2012, 109, 11384–11389. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, X.; Yu, L.; Yang, J.; Calvo, R.; Patnaik, S.; Hu, X.; Gao, Q.; Yang, M.; Lawas, M.; et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 2016, 7, 12109. [Google Scholar] [CrossRef]
- Grimm, C.; Butz, E.; Chen, C.C.; Wahl-Schott, C.; Biel, M. From mucolipidosis type IV to Ebola: TRPML and two-pore channels at the crossroads of endo-lysosomal trafficking and disease. Cell Calcium 2017, 67, 148–155. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, M.; Yang, Y.; Xu, H. Organellar TRP channels. Nat. Struct. Mol. Biol. 2018, 25, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Xu, J.; Fu, C.; Zhang, J.; Zheng, Y.G.; Jia, H.; Liu, J.O. Regulation of mTORC1 by lysosomal calcium and calmodulin. eLife 2016, 5, e19360. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Zoncu, R.; Medina, D.L.; Vetrini, F.; Erdin, S.; Erdin, S.; Huynh, T.; Ferron, M.; Karsenty, G.; Vellard, M.C.; et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012, 31, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, Q.; Yang, M.; Zhang, X.; Yu, L.; Lawas, M.; Li, X.; Bryant-Genevier, M.; Southall, N.T.; Marugan, J.; et al. Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc. Natl. Acad. Sci. USA 2015, 112, E1373–E1381. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, Y.; Zhong, X.Z.; Cao, Q.; Zhu, X.H.; Zhu, X.; Dong, X.P. A negative feedback regulation of MTORC1 activity by the lysosomal Ca2+ channel MCOLN1 (mucolipin 1) using a CALM (calmodulin)-dependent mechanism. Autophagy 2018, 14, 38–52. [Google Scholar] [CrossRef] [PubMed]
- De Leo, M.G.; Staiano, L.; Vicinanza, M.; Luciani, A.; Carissimo, A.; Mutarelli, M.; Di Campli, A.; Polishchuk, E.; Di Tullio, G.; Morra, V.; et al. Autophagosome-lysosome fusion triggers a lysosomal response mediated by TLR9 and controlled by OCRL. Nat. Cell Biol. 2016, 18, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Anoveros, J.; Wiwatpanit, T. TRPML2 and mucolipin evolution. Handb. Exp. Pharmacol. 2014, 222, 647–658. [Google Scholar] [PubMed]
- Li, X.; Rydzewski, N.; Hider, A.; Zhang, X.; Yang, J.; Wang, W.; Gao, Q.; Cheng, X.; Xu, H. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat. Cell Biol. 2016, 18, 404–417. [Google Scholar] [CrossRef]
- Lev, S.; Zeevi, D.A.; Frumkin, A.; Offen-Glasner, V.; Bach, G.; Minke, B. Constitutive activity of the human TRPML2 channel induces cell degeneration. J. Biol. Chem. 2010, 285, 2771–2782. [Google Scholar] [CrossRef]
- Karacsonyi, C.; Miguel, A.S.; Puertollano, R. Mucolipin-2 localizes to the Arf6-associated pathway and regulates recycling of GPI-APs. Traffic 2007, 8, 1404–1414. [Google Scholar] [CrossRef]
- Curcio-Morelli, C.; Zhang, P.; Venugopal, B.; Charles, F.A.; Browning, M.F.; Cantiello, H.F.; Slaugenhaupt, S.A. Functional multimerization of mucolipin channel proteins. J. Cell. Physiol. 2010, 222, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.A.; Lev, S.; Frumkin, A.; Minke, B.; Bach, G. Heteromultimeric TRPML channel assemblies play a crucial role in the regulation of cell viability models and starvation-induced autophagy. J. Cell Sci. 2010, 123 Pt 18, 3112–3124. [Google Scholar] [CrossRef]
- Zeevi, D.A.; Frumkin, A.; Offen-Glasner, V.; Kogot-Levin, A.; Bach, G. A potentially dynamic lysosomal role for the endogenous TRPML proteins. J. Pathol. 2009, 219, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Li, Q.; Tjon-Kon-Sang, S.; So, I.; Kiselyov, K.; Muallem, S. Gain-of-function mutation in TRPML3 causes the mouse Varitint-Waddler phenotype. J. Biol. Chem. 2007, 282, 36138–36142. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yamaguchi, S.; Li, Q.; So, I.; Muallem, S. Properties of the TRPML3 channel pore and its stable expansion by the Varitint-Waddler-causing mutation. J. Biol. Chem. 2010, 285, 16513–16520. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Li, Q.; Tjon-Kon-Sang, S.; So, I.; Kiselyov, K.; Soyombo, A.A.; Muallem, S. A novel mode of TRPML3 regulation by extracytosolic pH absent in the varitint-waddler phenotype. EMBO J. 2008, 27, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Cuajungco, M.P.; van Aken, A.F.; Schnee, M.; Jors, S.; Kros, C.J.; Ricci, A.J.; Heller, S. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc. Natl. Acad. Sci. USA 2007, 104, 19583–19588. [Google Scholar] [CrossRef]
- Feng, X.; Huang, Y.; Lu, Y.; Xiong, J.; Wong, C.O.; Yang, P.; Xia, J.; Chen, D.; Du, G.; Venkatachalam, K.; et al. Drosophila TRPML forms PI(3,5)P2-activated cation channels in both endolysosomes and plasma membrane. J. Biol. Chem. 2014, 289, 4262–4272. [Google Scholar] [CrossRef]
- Grimm, C.; Barthmes, M.; Wahl-Schott, C. Trpml3. Handb. Exp. Pharmacol. 2014, 222, 659–674. [Google Scholar]
- Miao, Y.; Li, G.; Zhang, X.; Xu, H.; Abraham, S.N. A TRP Channel Senses Lysosome Neutralization by Pathogens to Trigger Their Expulsion. Cell 2015, 161, 1306–1319. [Google Scholar] [CrossRef]
- Kim, H.J.; Soyombo, A.A.; Tjon-Kon-Sang, S.; So, I.; Muallem, S. The Ca2+ channel TRPML3 regulates membrane trafficking and autophagy. Traffic 2009, 10, 1157–1167. [Google Scholar] [CrossRef]
- Choi, S.; Kim, H.J. The Ca2+ channel TRPML3 specifically interacts with the mammalian ATG8 homologue GATE16 to regulate autophagy. Biochem. Biophys. Res. Commun. 2014, 443, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Function and dysfunction of two-pore channels. Sci. Signal. 2015, 8, re7. [Google Scholar] [CrossRef]
- Yu, F.H.; Catterall, W.A. The VGL-chanome: A protein superfamily specialized for electrical signaling and ionic homeostasis. Sci. STKE 2004, 2004, re15. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Suzuki, M.; Imai, M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem. Biophys. Res. Commun. 2000, 270, 370–376. [Google Scholar] [CrossRef]
- Furuichi, T.; Cunningham, K.W.; Muto, S. A putative two pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant Cell Physiol. 2001, 42, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Churamani, D.; Hooper, R.; Brailoiu, E.; Patel, S. Domain assembly of NAADP-gated two-pore channels. Biochem. J. 2012, 441, 317–323. [Google Scholar] [CrossRef]
- Rietdorf, K.; Funnell, T.M.; Ruas, M.; Heinemann, J.; Parrington, J.; Galione, A. Two-pore channels form homo- and heterodimers. J. Biol. Chem. 2011, 286, 37058–37062. [Google Scholar] [CrossRef]
- Hooper, R.; Churamani, D.; Brailoiu, E.; Taylor, C.W.; Patel, S. Membrane topology of NAADP-sensitive two-pore channels and their regulation by N-linked glycosylation. J. Biol. Chem. 2011, 286, 9141–9149. [Google Scholar] [CrossRef]
- Rahman, T.; Cai, X.; Brailoiu, G.C.; Abood, M.E.; Brailoiu, E.; Patel, S. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci. Signal. 2014, 7, ra109. [Google Scholar] [CrossRef]
- Larisch, N.; Kirsch, S.A.; Schambony, A.; Studtrucker, T.; Bockmann, R.A.; Dietrich, P. The function of the two-pore channel TPC1 depends on dimerization of its carboxy-terminal helix. Cell. Mol. Life Sci. 2016, 73, 2565–2581. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Hooper, R.; Cai, X.; Brailoiu, G.C.; Keebler, M.V.; Dun, N.J.; Marchant, J.S.; Patel, S. An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J. Biol. Chem. 2010, 285, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Patel, S. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol. Biol. Evol. 2010, 27, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Kintzer, A.F.; Stroud, R.M. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature 2016, 531, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zeng, W.; Chen, Q.; Lee, C.; Chen, L.; Yang, Y.; Cang, C.; Ren, D.; Jiang, Y. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature 2016, 531, 196–201. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Guo, J.; Chen, Q.; Zeng, W.; Jiang, Y.; Bai, X.C. Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature 2018, 556, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Calcraft, P.J.; Ruas, M.; Pan, Z.; Cheng, X.; Arredouani, A.; Hao, X.; Tang, J.; Rietdorf, K.; Teboul, L.; Chuang, K.T.; et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 2009, 459, 596–600. [Google Scholar] [CrossRef]
- Zong, X.; Schieder, M.; Cuny, H.; Fenske, S.; Gruner, C.; Rotzer, K.; Griesbeck, O.; Harz, H.; Biel, M.; Wahl-Schott, C. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009, 458, 891–899. [Google Scholar] [CrossRef]
- Ruas, M.; Rietdorf, K.; Arredouani, A.; Davis, L.C.; Lloyd-Evans, E.; Koegel, H.; Funnell, T.M.; Morgan, A.J.; Ward, J.A.; Watanabe, K.; et al. Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endolysosomal trafficking. Curr. Biol. 2010, 20, 703–709. [Google Scholar] [CrossRef]
- Brailoiu, E.; Rahman, T.; Churamani, D.; Prole, D.L.; Brailoiu, G.C.; Hooper, R.; Taylor, C.W.; Patel, S. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J. Biol. Chem. 2010, 285, 38511–38516. [Google Scholar] [CrossRef]
- Fameli, N.; Ogunbayo, O.A.; van Breemen, C.; Evans, A.M. Cytoplasmic nanojunctions between lysosomes and sarcoplasmic reticulum are required for specific calcium signaling. F1000Research 2014, 3, 93. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, N.P.; Wyatt, C.N.; Clark, J.H.; Calcraft, P.J.; Fleischer, S.; Jeyakumar, L.H.; Nixon, G.F.; Evans, A.M. Lysosomes co-localize with ryanodine receptor subtype 3 to form a trigger zone for calcium signalling by NAADP in rat pulmonary arterial smooth muscle. Cell Calcium 2008, 44, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Chen, C.C.; Wahl-Schott, C.; Biel, M. Two-Pore Channels: Catalyzers of Endolysosomal Transport and Function. Front. Pharmacol. 2017, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Pitt, S.J.; Reilly-O’Donnell, B.; Sitsapesan, R. Exploring the biophysical evidence that mammalian two-pore channels are NAADP-activated calcium-permeable channels. J. Physiol. 2016, 594, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.J.; Davis, L.C.; Ruas, M.; Galione, A. TPC: The NAADP discovery channel? Biochem. Soc. Trans. 2015, 43, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.J.; Galione, A. Two-pore channels (TPCs): Current controversies. Bioessays 2014, 36, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Guse, A.H.; Diercks, B.P. Integration of nicotinic acid adenine dinucleotide phosphate (NAADP)-dependent calcium signalling. J. Physiol. 2018, 596, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Churamani, D.; Cai, X.; Schrlau, M.G.; Brailoiu, G.C.; Gao, X.; Hooper, R.; Boulware, M.J.; Dun, N.J.; Marchant, J.S.; et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 2009, 186, 201–209. [Google Scholar] [CrossRef]
- Ruas, M.; Davis, L.C.; Chen, C.C.; Morgan, A.J.; Chuang, K.T.; Walseth, T.F.; Grimm, C.; Garnham, C.; Powell, T.; Platt, N.; et al. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015, 34, 1743–1758. [Google Scholar] [CrossRef]
- Davis, L.C.; Morgan, A.J.; Chen, J.L.; Snead, C.M.; Bloor-Young, D.; Shenderov, E.; Stanton-Humphreys, M.N.; Conway, S.J.; Churchill, G.C.; Parrington, J.; et al. NAADP activates two-pore channels on T cell cytolytic granules to stimulate exocytosis and killing. Curr. Biol. 2012, 22, 2331–2337. [Google Scholar] [CrossRef]
- Tugba Durlu-Kandilci, N.; Ruas, M.; Chuang, K.T.; Brading, A.; Parrington, J.; Galione, A. TPC2 proteins mediate nicotinic acid adenine dinucleotide phosphate (NAADP)- and agonist-evoked contractions of smooth muscle. J. Biol. Chem. 2010, 285, 24925–24932. [Google Scholar] [CrossRef] [PubMed]
- Pitt, S.J.; Funnell, T.M.; Sitsapesan, M.; Venturi, E.; Rietdorf, K.; Ruas, M.; Ganesan, A.; Gosain, R.; Churchill, G.C.; Zhu, M.X.; et al. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+. J. Biol. Chem. 2010, 285, 35039–35046. [Google Scholar] [CrossRef] [PubMed]
- Pitt, S.J.; Lam, A.K.; Rietdorf, K.; Galione, A.; Sitsapesan, R. Reconstituted human TPC1 is a proton-permeable ion channel and is activated by NAADP or Ca2+. Sci. Signal. 2014, 7, ra46. [Google Scholar] [CrossRef] [PubMed]
- Rybalchenko, V.; Ahuja, M.; Coblentz, J.; Churamani, D.; Patel, S.; Kiselyov, K.; Muallem, S. Membrane potential regulates nicotinic acid adenine dinucleotide phosphate (NAADP) dependence of the pH- and Ca2+-sensitive organellar two-pore channel TPC1. J. Biol. Chem. 2012, 287, 20407–20416. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Ahuja, M.; Patel, S.; Brailoiu, E.; Muallem, S. Convergent regulation of the lysosomal two-pore channel-2 by Mg(2)(+), NAADP, PI(3,5)P(2) and multiple protein kinases. EMBO J. 2014, 33, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Schieder, M.; Rotzer, K.; Bruggemann, A.; Biel, M.; Wahl-Schott, C.A. Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes. J. Biol. Chem. 2010, 285, 21219–21222. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Dong, X.P.; Samie, M.; Li, X.; Cheng, X.; Goschka, A.; Shen, D.; Zhou, Y.; Harlow, J.; et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell 2012, 151, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Cang, C.; Zhou, Y.; Navarro, B.; Seo, Y.J.; Aranda, K.; Shi, L.; Battaglia-Hsu, S.; Nissim, I.; Clapham, D.E.; Ren, D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell 2013, 152, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Ruas, M.; Galione, A.; Parrington, J. Two-Pore Channels: Lessons from Mutant Mouse Models. Messenger 2015, 4, 4–22. [Google Scholar] [CrossRef]
- Walseth, T.F.; Lin-Moshier, Y.; Jain, P.; Ruas, M.; Parrington, J.; Galione, A.; Marchant, J.S.; Slama, J.T. Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg. J. Biol. Chem. 2012, 287, 2308–2315. [Google Scholar] [CrossRef]
- Lin-Moshier, Y.; Walseth, T.F.; Churamani, D.; Davidson, S.M.; Slama, J.T.; Hooper, R.; Brailoiu, E.; Patel, S.; Marchant, J.S. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 2012, 287, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Cang, C.; Bekele, B.; Ren, D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat. Chem. Biol. 2014, 10, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Suaga, P.; Luzon-Toro, B.; Churamani, D.; Zhang, L.; Bloor-Young, D.; Patel, S.; Woodman, P.G.; Churchill, G.C.; Hilfiker, S. Leucine-rich repeat kinase 2 regulates autophagy through a calcium-dependent pathway involving NAADP. Hum. Mol. Genet. 2012, 21, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Pryor, P.R.; Mullock, B.M.; Bright, N.A.; Gray, S.R.; Luzio, J.P. The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 2000, 149, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Marchant, J.S.; Patel, S. Two-pore channels at the intersection of endolysosomal membrane traffic. Biochem. Soc. Trans. 2015, 43, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Hockey, L.N.; Kilpatrick, B.S.; Eden, E.R.; Lin-Moshier, Y.; Brailoiu, G.C.; Brailoiu, E.; Futter, C.E.; Schapira, A.H.; Marchant, J.S.; Patel, S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J. Cell Sci. 2015, 128, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Kolokoltsov, A.A.; Chen, C.C.; Tidwell, M.W.; Bauta, W.E.; Klugbauer, N.; Grimm, C.; Wahl-Schott, C.; Biel, M.; Davey, R.A. Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 2015, 347, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Holdt, L.M.; Chen, C.C.; Hassan, S.; Muller, C.; Jors, S.; Cuny, H.; Kissing, S.; Schroder, B.; Butz, E.; et al. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat. Commun. 2014, 5, 4699. [Google Scholar] [CrossRef]
- Kilpatrick, B.S.; Eden, E.R.; Hockey, L.N.; Yates, E.; Futter, C.E.; Patel, S. An Endosomal NAADP-Sensitive Two-Pore Ca2+ Channel Regulates ER-Endosome Membrane Contact Sites to Control Growth Factor Signaling. Cell Rep. 2017, 18, 1636–1645. [Google Scholar] [CrossRef]
- Ruas, M.; Chuang, K.T.; Davis, L.C.; Al-Douri, A.; Tynan, P.W.; Tunn, R.; Teboul, L.; Galione, A.; Parrington, J. TPC1 has two variant isoforms, and their removal has different effects on endo-lysosomal functions compared to loss of TPC2. Mol. Cell. Biol. 2014, 34, 3981–3992. [Google Scholar] [CrossRef]
- Lin-Moshier, Y.; Keebler, M.V.; Hooper, R.; Boulware, M.J.; Liu, X.; Churamani, D.; Abood, M.E.; Walseth, T.F.; Brailoiu, E.; Patel, S.; et al. The Two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc. Natl. Acad. Sci. USA 2014, 111, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Atlas, D. Voltage-gated calcium channels function as Ca2+-activated signaling receptors. Trends Biochem. Sci. 2014, 39, 45–52. [Google Scholar] [CrossRef]
- Ogunbayo, O.A.; Duan, J.; Xiong, J.; Wang, Q.; Feng, X.; Ma, J.; Zhu, M.X.; Evans, A.M. mTORC1 controls lysosomal Ca2+ release through the two-pore channel TPC2. Sci. Signal. 2018, 11, eaao5775. [Google Scholar] [CrossRef] [PubMed]
- Hoglinger, D.; Haberkant, P.; Aguilera-Romero, A.; Riezman, H.; Porter, F.D.; Platt, F.M.; Galione, A.; Schultz, C. Intracellular sphingosine releases calcium from lysosomes. eLife 2015, 4, e10616. [Google Scholar] [CrossRef] [PubMed]
- Rah, S.Y.; Lee, Y.H.; Kim, U.H. NAADP-mediated Ca2+ signaling promotes autophagy and protects against LPS-induced liver injury. FASEB J. 2017, 31, 3126–3137. [Google Scholar] [CrossRef]
- Pereira, G.J.; Hirata, H.; Fimia, G.M.; do Carmo, L.G.; Bincoletto, C.; Han, S.W.; Stilhano, R.S.; Ureshino, R.P.; Bloor-Young, D.; Churchill, G.; et al. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J. Biol. Chem. 2011, 286, 27875–27881. [Google Scholar] [CrossRef]
- Pereira, G.J.; Antonioli, M.; Hirata, H.; Ureshino, R.P.; Nascimento, A.R.; Bincoletto, C.; Vescovo, T.; Piacentini, M.; Fimia, G.M.; Smaili, S.S. Glutamate induces autophagy via the two-pore channels in neural cells. Oncotarget 2017, 8, 12730–12740. [Google Scholar] [CrossRef]
- Garcia-Rua, V.; Feijoo-Bandin, S.; Rodriguez-Penas, D.; Mosquera-Leal, A.; Abu-Assi, E.; Beiras, A.; Maria Seoane, L.; Lear, P.; Parrington, J.; Portoles, M.; et al. Endolysosomal two-pore channels regulate autophagy in cardiomyocytes. J. Physiol. 2016, 594, 3061–3077. [Google Scholar] [CrossRef]
- Lin, P.H.; Duann, P.; Komazaki, S.; Park, K.H.; Li, H.; Sun, M.; Sermersheim, M.; Gumpper, K.; Parrington, J.; Galione, A.; et al. Lysosomal two-pore channel subtype 2 (TPC2) regulates skeletal muscle autophagic signaling. J. Biol. Chem. 2015, 290, 3377–3389. [Google Scholar] [CrossRef]
- Cancela, J.M.; Van Coppenolle, F.; Galione, A.; Tepikin, A.V.; Petersen, O.H. Transformation of local Ca2+ spikes to global Ca2+ transients: The combinatorial roles of multiple Ca2+ releasing messengers. EMBO J. 2002, 21, 909–919. [Google Scholar] [CrossRef]
- Churchill, G.C.; Galione, A. NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores. EMBO J. 2001, 20, 2666–2671. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Lin, A.H.; Xia, Y.; Lee, S.; Paudel, O.; Sun, H.; Yang, X.R.; Ran, P.; Sham, J.S. Nicotinic acid adenine dinucleotide phosphate (NAADP) activates global and heterogeneous local Ca2+ signals from NAADP- and ryanodine receptor-gated Ca2+ stores in pulmonary arterial myocytes. J. Biol. Chem. 2013, 288, 10381–10394. [Google Scholar] [CrossRef] [PubMed]
- Penny, C.J.; Kilpatrick, B.S.; Eden, E.R.; Patel, S. Coupling acidic organelles with the ER through Ca2+ microdomains at membrane contact sites. Cell Calcium 2015, 58, 387–396. [Google Scholar] [CrossRef]
- Henne, W.M. Discovery and Roles of ER-Endolysosomal Contact Sites in Disease. Adv. Exp. Med. Biol. 2017, 997, 135–147. [Google Scholar]
- Wu, H.; Carvalho, P.; Voeltz, G.K. Here, there, and everywhere: The importance of ER membrane contact sites. Science 2018, 361, eaan5835. [Google Scholar] [CrossRef]
- Eden, E.R.; White, I.J.; Tsapara, A.; Futter, C.E. Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat. Cell Biol. 2010, 12, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Cabukusta, B.; Neefjes, J. Mechanisms of lysosomal positioning and movement. Traffic 2018, 19, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Miyamoto, M.D.; Dun, N.J. Nicotinic acid adenine dinucleotide phosphate enhances quantal neurosecretion at the frog neuromuscular junction: Possible action on synaptic vesicles in the releasable pool. Mol. Pharmacol. 2001, 60, 718–724. [Google Scholar]
- Brailoiu, E.; Hoard, J.L.; Filipeanu, C.M.; Brailoiu, G.C.; Dun, S.L.; Patel, S.; Dun, N.J. Nicotinic acid adenine dinucleotide phosphate potentiates neurite outgrowth. J. Biol. Chem. 2005, 280, 5646–5650. [Google Scholar] [CrossRef]
- Favia, A.; Desideri, M.; Gambara, G.; D’Alessio, A.; Ruas, M.; Esposito, B.; Del Bufalo, D.; Parrington, J.; Ziparo, E.; Palombi, F.; et al. VEGF-induced neoangiogenesis is mediated by NAADP and two-pore channel-2-dependent Ca2+ signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E4706–E4715. [Google Scholar] [CrossRef]
- Di Nezza, F.; Zuccolo, E.; Poletto, V.; Rosti, V.; De Luca, A.; Moccia, F.; Guerra, G.; Ambrosone, L. Liposomes as a Putative Tool to Investigate NAADP Signaling in Vasculogenesis. J. Cell. Biochem. 2017, 118, 3722–3729. [Google Scholar] [CrossRef] [PubMed]
- Zuccolo, E.; Laforenza, U.; Negri, S.; Botta, L.; Berra-Romani, R.; Faris, P.; Scarpellino, G.; Forcaia, G.; Pellavio, G.; Sancini, G.; et al. Muscarinic M5 receptors trigger acetylcholine-induced Ca2+ signals and nitric oxide release in human brain microvascular endothelial cells. J. Cell. Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zuccolo, E.; Negri, S.; Pellavio, G.; Scarpellino, G.; Laforenza, U.; Sancini, G.; Guerra, G.; Moccia, F. Acetylcholine induces Ca2+ signals and nitric oxide release from human brain microvascular endothelial cells. Vasc. Pharmacol. 2018, 103, 65. [Google Scholar] [CrossRef]
- Faouzi, M.; Penner, R. Trpm2. Handb. Exp. Pharmacol. 2014, 222, 403–426. [Google Scholar] [PubMed]
- Lange, I.; Yamamoto, S.; Partida-Sanchez, S.; Mori, Y.; Fleig, A.; Penner, R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci. Signal. 2009, 2, ra23. [Google Scholar] [CrossRef] [PubMed]
- Sumoza-Toledo, A.; Lange, I.; Cortado, H.; Bhagat, H.; Mori, Y.; Fleig, A.; Penner, R.; Partida-Sanchez, S. Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca2+ release. FASEB J. 2011, 25, 3529–3542. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Hogestatt, E.D. Trpa1. Handb. Exp. Pharmacol. 2014, 222, 583–630. [Google Scholar]
- Shang, S.; Zhu, F.; Liu, B.; Chai, Z.; Wu, Q.; Hu, M.; Wang, Y.; Huang, R.; Zhang, X.; Wu, X.; et al. Intracellular TRPA1 mediates Ca2+ release from lysosomes in dorsal root ganglion neurons. J. Cell Biol. 2016, 215, 369–381. [Google Scholar] [CrossRef]
- North, R.A. P2X receptors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef]
- Qureshi, O.S.; Paramasivam, A.; Yu, J.C.; Murrell-Lagnado, R.D. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J. Cell Sci. 2007, 120 Pt 21, 3838–3849. [Google Scholar] [CrossRef]
- Huang, P.; Zou, Y.; Zhong, X.Z.; Cao, Q.; Zhao, K.; Zhu, M.X.; Murrell-Lagnado, R.; Dong, X.P. P2X4 forms functional ATP-activated cation channels on lysosomal membranes regulated by luminal pH. J. Biol. Chem. 2014, 289, 17658–17667. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zhong, X.Z.; Zou, Y.; Murrell-Lagnado, R.; Zhu, M.X.; Dong, X.P. Calcium release through P2X4 activates calmodulin to promote endolysosomal membrane fusion. J. Cell Biol. 2015, 209, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Gala, U.; Zhang, Y.; Shang, W.; Nagarkar Jaiswal, S.; di Ronza, A.; Jaiswal, M.; Yamamoto, S.; Sandoval, H.; Duraine, L.; et al. A voltage-gated calcium channel regulates lysosomal fusion with endosomes and autophagosomes and is required for neuronal homeostasis. PLoS Biol. 2015, 13, e1002103. [Google Scholar] [CrossRef] [PubMed]
- Hamalisto, S.; Jaattela, M. Lysosomes in cancer-living on the edge (of the cell). Curr. Opin. Cell Biol. 2016, 39, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bargal, R.; Avidan, N.; Ben-Asher, E.; Olender, Z.; Zeigler, M.; Frumkin, A.; Raas-Rothschild, A.; Glusman, G.; Lancet, D.; Bach, G. Identification of the gene causing mucolipidosis type IV. Nat. Genet. 2000, 26, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Amaravadi, R.K. Targeting the lysosome in cancer. Ann. N. Y. Acad. Sci. 2016, 1371, 45–54. [Google Scholar] [CrossRef]
- Davidson, S.M.; Vander Heiden, M.G. Critical Functions of the Lysosome in Cancer Biology. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 481–507. [Google Scholar] [CrossRef]
- Goveia, J.; Stapor, P.; Carmeliet, P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol. Med. 2014, 6, 1105–1120. [Google Scholar] [CrossRef]
- Kirkegaard, T.; Jaattela, M. Lysosomal involvement in cell death and cancer. Biochim. Biophys. Acta 2009, 1793, 746–754. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Carmeliet, P. SnapShot: Tumor angiogenesis. Cell 2012, 149, 1408. [Google Scholar] [CrossRef] [PubMed]
- Stransky, L.; Cotter, K.; Forgac, M. The Function of V-ATPases in Cancer. Physiol. Rev. 2016, 96, 1071–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, B.; Assaraf, Y.G. Lysosomes as mediators of drug resistance in cancer. Drug Resist. Updates 2016, 24, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Pietrocola, F.; Bravo-San Pedro, J.M.; Amaravadi, R.K.; Baehrecke, E.H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.A.; Karantza, V.; et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015, 34, 856–880. [Google Scholar] [CrossRef] [PubMed]
- Pierro, C.; Cook, S.J.; Foets, T.C.; Bootman, M.D.; Roderick, H.L. Oncogenic K-Ras suppresses IP(3)-dependent Ca2+ release through remodelling of the isoform composition of IP(3)Rs and ER luminal Ca2+ levels in colorectal cancer cell lines. J. Cell Sci. 2014, 127 Pt 7, 1607–1619. [Google Scholar] [CrossRef]
- Poletto, V.; Dragoni, S.; Lim, D.; Biggiogera, M.; Aronica, A.; Cinelli, M.; De Luca, A.; Rosti, V.; Porta, C.; Guerra, G.; et al. Endoplasmic Reticulum Ca2+ Handling and Apoptotic Resistance in Tumor-Derived Endothelial Colony Forming Cells. J. Cell. Biochem. 2016, 117, 2260–2271. [Google Scholar] [CrossRef]
- Moccia, F.; Poletto, V. May the remodeling of the Ca2+ toolkit in endothelial progenitor cells derived from cancer patients suggest alternative targets for anti-angiogenic treatment? Biochim. Biophys. Acta 2015, 1853, 1958–1973. [Google Scholar] [CrossRef]
- Moccia, F. Endothelial Ca2+ Signaling and the Resistance to Anticancer Treatments: Partners in Crime. Int. J. Mol. Sci. 2018, 19, 217. [Google Scholar] [CrossRef]
- Dubois, C.; Vanden Abeele, F.; Lehen’kyi, V.; Gkika, D.; Guarmit, B.; Lepage, G.; Slomianny, C.; Borowiec, A.S.; Bidaux, G.; Benahmed, M.; et al. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell 2014, 26, 19–32. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef] [PubMed]
- Fiorio Pla, A.; Gkika, D. Emerging role of TRP channels in cell migration: From tumor vascularization to metastasis. Front. Physiol. 2013, 4, 311. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Zuccolo, E.; Poletto, V.; Turin, I.; Guerra, G.; Pedrazzoli, P.; Rosti, V.; Porta, C.; Montagna, D. Targeting Stim and Orai Proteins as an Alternative Approach in Anticancer Therapy. Curr. Med. Chem. 2016, 23, 3450–3480. [Google Scholar] [CrossRef] [PubMed]
- Lodola, F.; Laforenza, U.; Bonetti, E.; Lim, D.; Dragoni, S.; Bottino, C.; Ong, H.L.; Guerra, G.; Ganini, C.; Massa, M.; et al. Store-operated Ca2+ entry is remodelled and controls in vitro angiogenesis in endothelial progenitor cells isolated from tumoral patients. PLoS ONE 2012, 7, e42541. [Google Scholar] [CrossRef] [PubMed]
- Iamshanova, O.; Fiorio Pla, A.; Prevarskaya, N. Molecular mechanisms of tumour invasion: Regulation by calcium signals. J. Physiol. 2017, 595, 3063–3075. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.M.; Stoykova, S.; Nicolay, B.N.; Ross, K.N.; Fitamant, J.; Boukhali, M.; Lengrand, J.; Deshpande, V.; Selig, M.K.; Ferrone, C.R.; et al. Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature 2015, 524, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Giatromanolaki, A.; Kalamida, D.; Sivridis, E.; Karagounis, I.V.; Gatter, K.C.; Harris, A.L.; Koukourakis, M.I. Increased expression of transcription factor EB (TFEB) is associated with autophagy, migratory phenotype and poor prognosis in non-small cell lung cancer. Lung Cancer 2015, 90, 98–105. [Google Scholar] [CrossRef]
- Calcagni, A.; Kors, L.; Verschuren, E.; De Cegli, R.; Zampelli, N.; Nusco, E.; Confalonieri, S.; Bertalot, G.; Pece, S.; Settembre, C.; et al. Modelling TFE renal cell carcinoma in mice reveals a critical role of WNT signaling. eLife 2016, 5, e17047. [Google Scholar] [CrossRef]
- Morelli, M.B.; Nabissi, M.; Amantini, C.; Tomassoni, D.; Rossi, F.; Cardinali, C.; Santoni, M.; Arcella, A.; Oliva, M.A.; Santoni, A.; et al. Overexpression of transient receptor potential mucolipin-2 ion channels in gliomas: Role in tumor growth and progression. Oncotarget 2016, 7, 43654–43668. [Google Scholar] [CrossRef]
- Jahidin, A.H.; Stewart, T.A.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. Differential effects of two-pore channel protein 1 and 2 silencing in MDA-MB-468 breast cancer cells. Biochem. Biophys. Res. Commun. 2016, 477, 731–736. [Google Scholar] [CrossRef]
- Lopez, J.; Dionisio, N.; Berna-Erro, A.; Galan, C.; Salido, G.M.; Rosado, J.A. Two-pore channel 2 (TPC2) modulates store-operated Ca2+ entry. Biochim. Biophys. Acta 2012, 1823, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, O.N.; Grimm, C.; Schneider, L.S.; Chao, Y.K.; Atzberger, C.; Bartel, K.; Watermann, A.; Ulrich, M.; Mayr, D.; Wahl-Schott, C.; et al. Two-Pore Channel Function Is Crucial for the Migration of Invasive Cancer Cells. Cancer Res. 2017, 77, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yue, J. TPC2 mediates autophagy progression and extracellular vesicle secretion in cancer cells. Exp. Cell Res. 2018, 370, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Favia, A.; Pafumi, I.; Desideri, M.; Padula, F.; Montesano, C.; Passeri, D.; Nicoletti, C.; Orlandi, A.; Del Bufalo, D.; Sergi, M.; et al. NAADP-Dependent Ca2+ Signaling Controls Melanoma Progression, Metastatic Dissemination and Neoangiogenesis. Sci. Rep. 2016, 6, 18925. [Google Scholar] [CrossRef] [PubMed]
- Pafumi, I.; Festa, M.; Papacci, F.; Lagostena, L.; Giunta, C.; Gutla, V.; Cornara, L.; Favia, A.; Palombi, F.; Gambale, F.; et al. Naringenin Impairs Two-Pore Channel 2 Activity And Inhibits VEGF-Induced Angiogenesis. Sci. Rep. 2017, 7, 5121. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Zuccolo, E.; Poletto, V.; Cinelli, M.; Bonetti, E.; Guerra, G.; Rosti, V. Endothelial progenitor cells support tumour growth and metastatisation: Implications for the resistance to anti-angiogenic therapy. Tumour Biol. 2015, 36, 6603–6614. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, S.; Reforgiato, M.; Zuccolo, E.; Poletto, V.; Lodola, F.; Ruffinatti, F.A.; Bonetti, E.; Guerra, G.; Barosi, G.; Rosti, V.; et al. Dysregulation of VEGF-induced proangiogenic Ca2+ oscillations in primary myelofibrosis-derived endothelial colony-forming cells. Exp. Hematol. 2015, 43, 1019–1030. [Google Scholar] [CrossRef]
- Lodola, F.; Laforenza, U.; Cattaneo, F.; Ruffinatti, F.A.; Poletto, V.; Massa, M.; Tancredi, R.; Zuccolo, E.; Khdar, A.D.; Riccardi, A.; et al. VEGF-induced intracellular Ca2+ oscillations are down-regulated and do not stimulate angiogenesis in breast cancer-derived endothelial colony forming cells. Oncotarget 2017, 8, 95223–95246. [Google Scholar] [CrossRef]
- Poletto, V.; Rosti, V.; Biggiogera, M.; Guerra, G.; Moccia, F.; Porta, C. The role of endothelial colony forming cells in kidney cancer’s pathogenesis, and in resistance to anti-VEGFR agents and mTOR inhibitors: A speculative review. Crit. Rev. Oncol. Hematol. 2018, 132, 89–99. [Google Scholar] [CrossRef]
- Naylor, E.; Arredouani, A.; Vasudevan, S.R.; Lewis, A.M.; Parkesh, R.; Mizote, A.; Rosen, D.; Thomas, J.M.; Izumi, M.; Ganesan, A.; et al. Identification of a chemical probe for NAADP by virtual screening. Nat. Chem. Biol. 2009, 5, 220–226. [Google Scholar] [CrossRef]
- Gunaratne, G.S.; Yang, Y.; Li, F.; Walseth, T.F.; Marchant, J.S. NAADP-dependent Ca2+ signaling regulates Middle East respiratory syndrome-coronavirus pseudovirus translocation through the endolysosomal system. Cell Calcium 2018, 75, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Billington, R.A.; Genazzani, A.A. PPADS is a reversible competitive antagonist of the NAADP receptor. Cell Calcium 2007, 41, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Genazzani, A.A.; Mezna, M.; Dickey, D.M.; Michelangeli, F.; Walseth, T.F.; Galione, A. Pharmacological properties of the Ca2+-release mechanism sensitive to NAADP in the sea urchin egg. Br. J. Pharmacol. 1997, 121, 1489–1495. [Google Scholar] [CrossRef]
- Moccia, F.; Lim, D.; Nusco, G.A.; Ercolano, E.; Santella, L. NAADP activates a Ca2+ current that is dependent on F-actin cytoskeleton. FASEB J. 2003, 17, 1907–1909. [Google Scholar] [CrossRef] [PubMed]
- Monteith, G.R.; McAndrew, D.; Faddy, H.M.; Roberts-Thomson, S.J. Calcium and cancer: Targeting Ca2+ transport. Nat. Rev. Cancer 2007, 7, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Lim, D.; Kyozuka, K.; Santella, L. NAADP triggers the fertilization potential in starfish oocytes. Cell Calcium 2004, 36, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, G.S.; Johns, M.E.; Hintz, H.M.; Walseth, T.F.; Marchant, J.S. A screening campaign in sea urchin egg homogenate as a platform for discovering modulators of NAADP-dependent Ca2+ signaling in human cells. Cell Calcium 2018, 75, 42–52. [Google Scholar] [CrossRef]
- Penny, C.J.; Vassileva, K.; Jha, A.; Yuan, Y.; Chee, X.; Yates, E.; Mazzon, M.; Kilpatrick, B.S.; Muallem, S.; Marsh, M.; et al. Mining of Ebola virus entry inhibitors identifies approved drugs as two-pore channel pore blockers. Biochim. Biophys. Acta Mol. Cell Res. 2018. [Google Scholar] [CrossRef]
- Zhang, B.; Watt, J.M.; Cordiglieri, C.; Dammermann, W.; Mahon, M.F.; Flugel, A.; Guse, A.H.; Potter, B.V.L. Small Molecule Antagonists of NAADP-Induced Ca2+ Release in T-Lymphocytes Suggest Potential Therapeutic Agents for Autoimmune Disease. Sci. Rep. 2018, 8, 16775. [Google Scholar] [CrossRef]
- Cordiglieri, C.; Odoardi, F.; Zhang, B.; Nebel, M.; Kawakami, N.; Klinkert, W.E.; Lodygin, D.; Luhder, F.; Breunig, E.; Schild, D.; et al. Nicotinic acid adenine dinucleotide phosphate-mediated calcium signalling in effector T cells regulates autoimmunity of the central nervous system. Brain 2010, 133 Pt 7, 1930–1943. [Google Scholar] [CrossRef]
- Nebel, M.; Schwoerer, A.P.; Warszta, D.; Siebrands, C.C.; Limbrock, A.C.; Swarbrick, J.M.; Fliegert, R.; Weber, K.; Bruhn, S.; Hohenegger, M.; et al. Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling and arrhythmias in the heart evoked by beta-adrenergic stimulation. J. Biol. Chem. 2013, 288, 16017–16030. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).