IL-15 and a Two-Step Maturation Process Improve Bone Marrow-Derived Dendritic Cell Cancer Vaccine
Abstract
:1. Introduction
2. Results
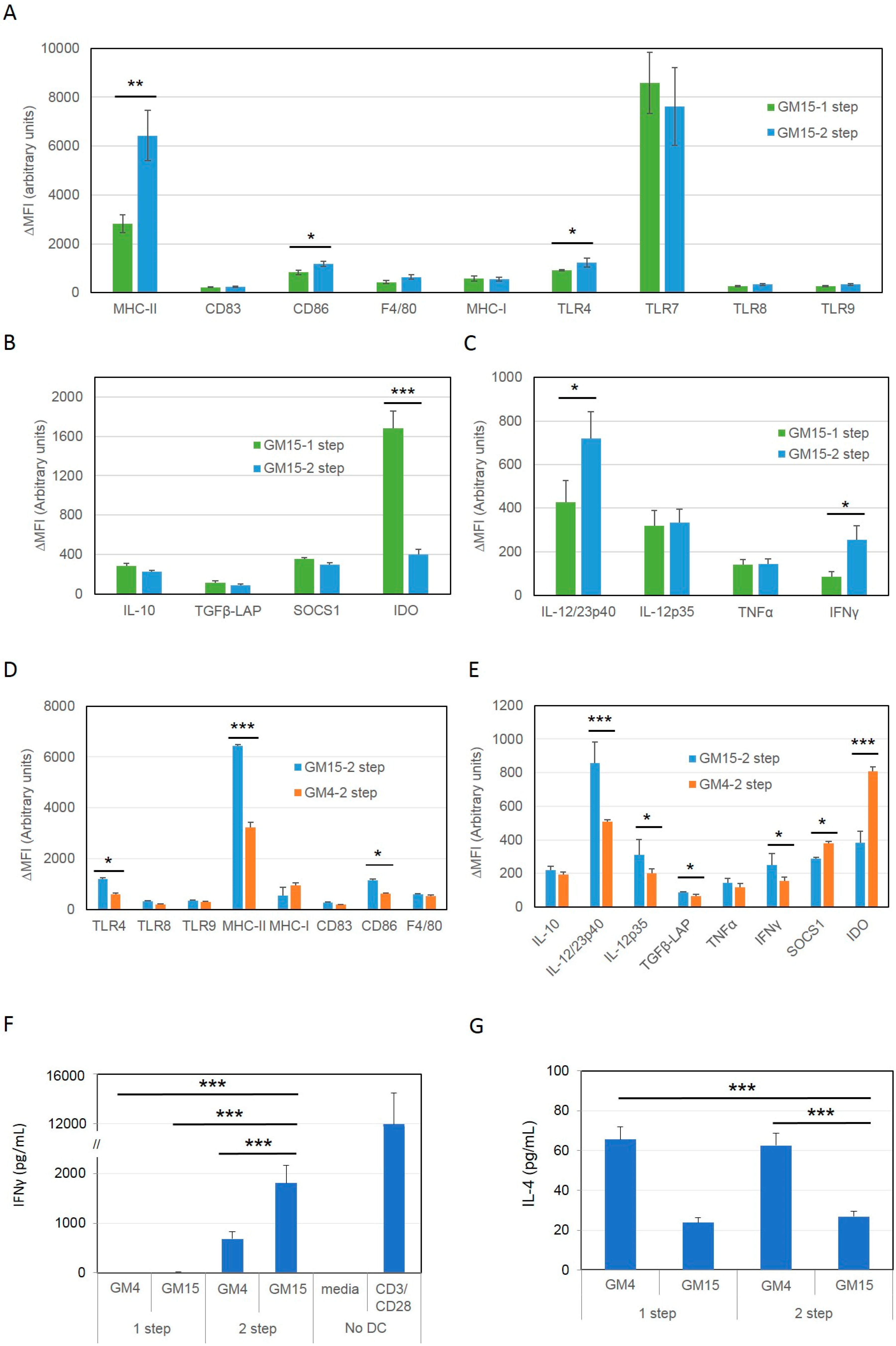
2.1. Differentiation with GM-CSF and IL-15 Yields a More Immunogenic DC Phenotype than Canonical DCs Differentiated with GM-CSF and IL-4
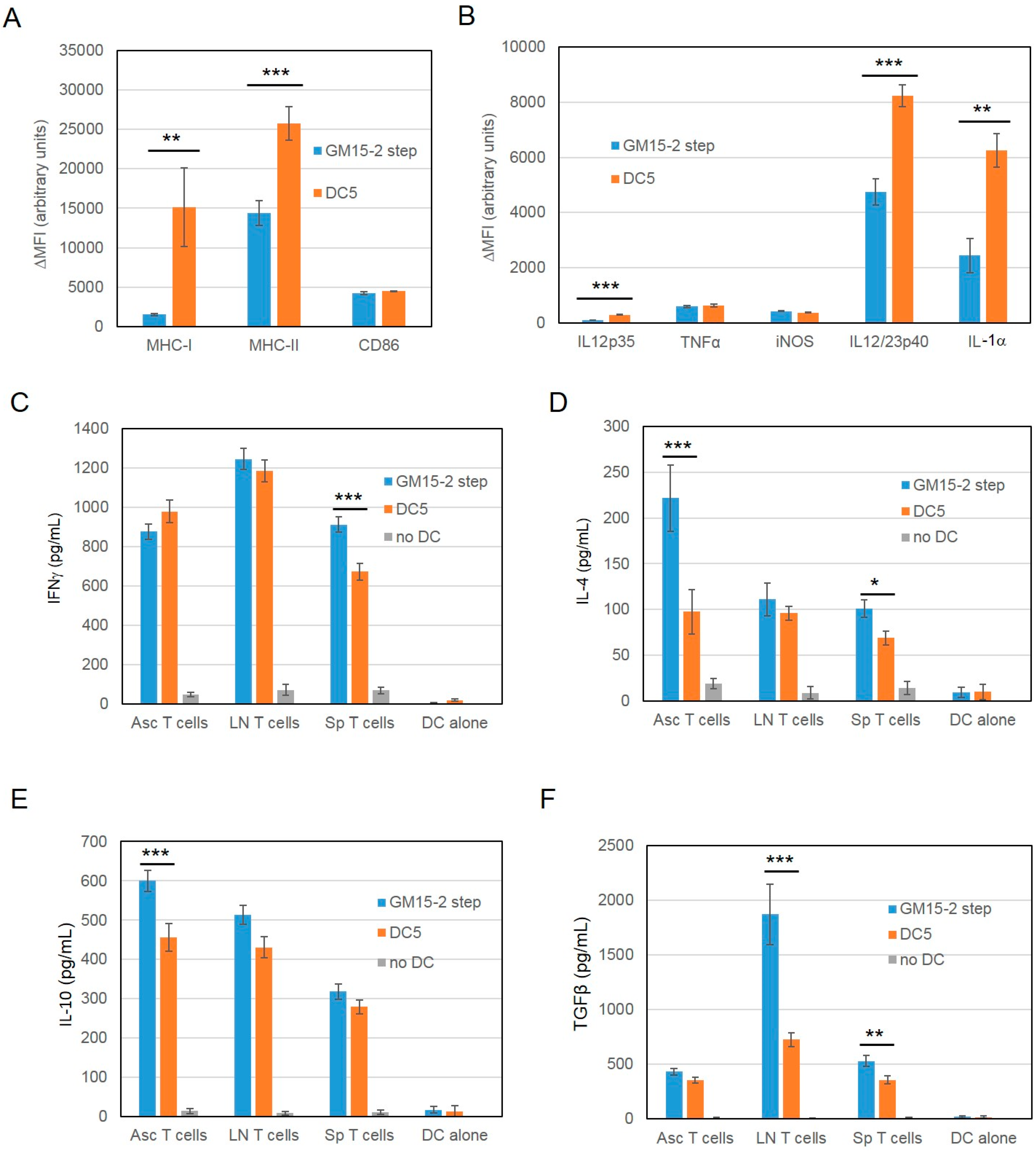
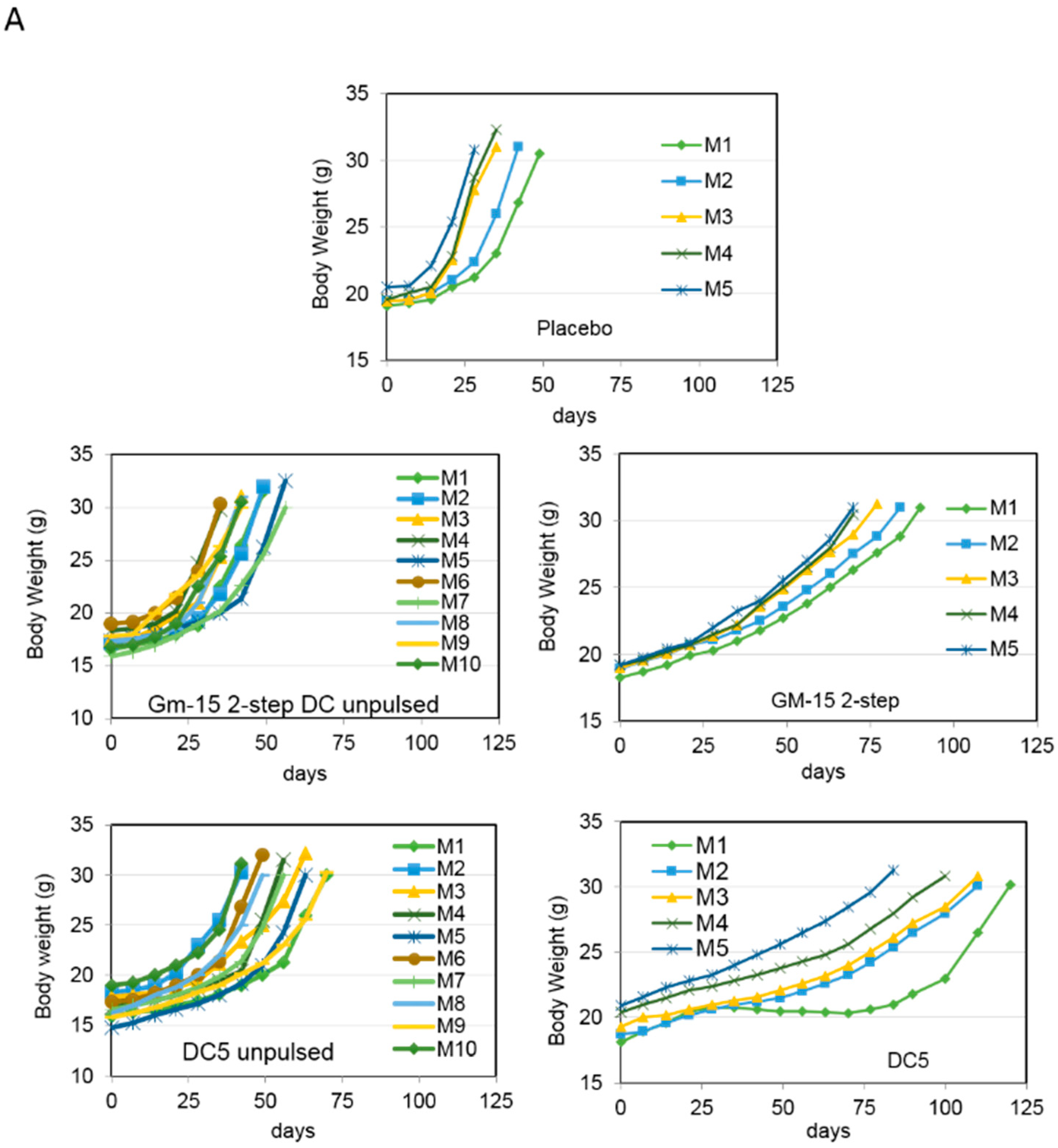
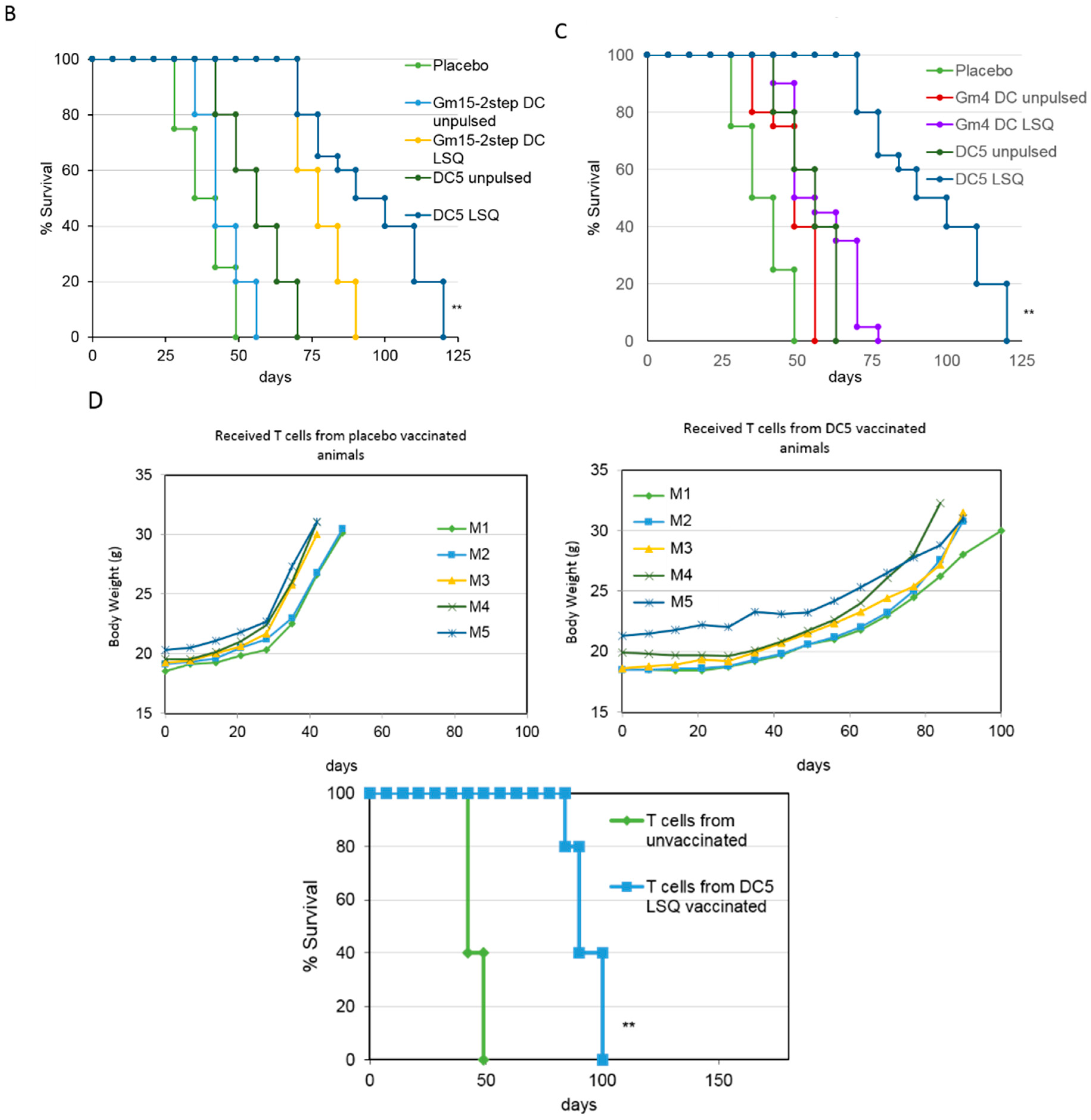
2.2. Improving the Efficacy of GM15-2 Step DCs through Inhibiting the Prostaglandin Pathway
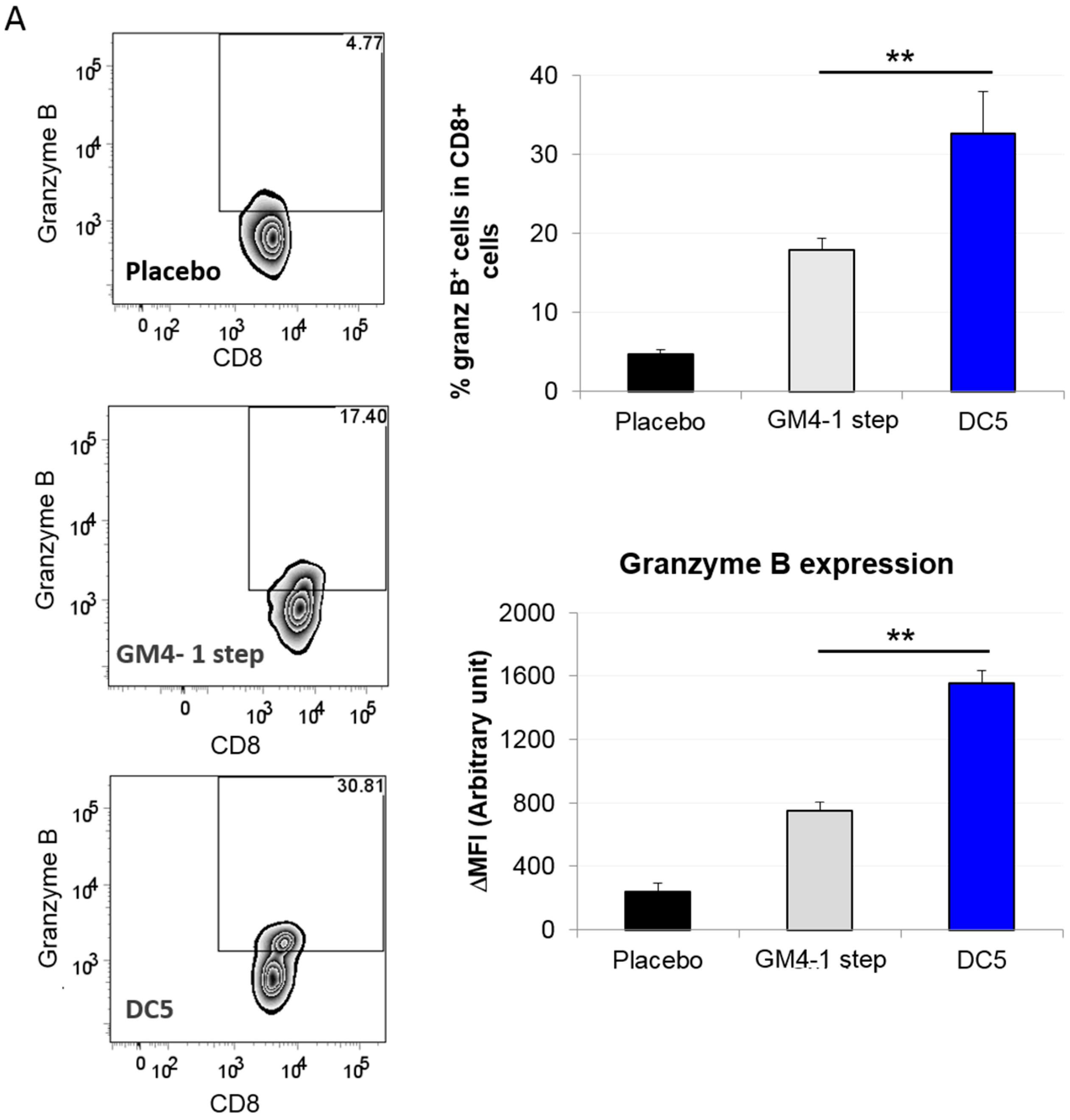
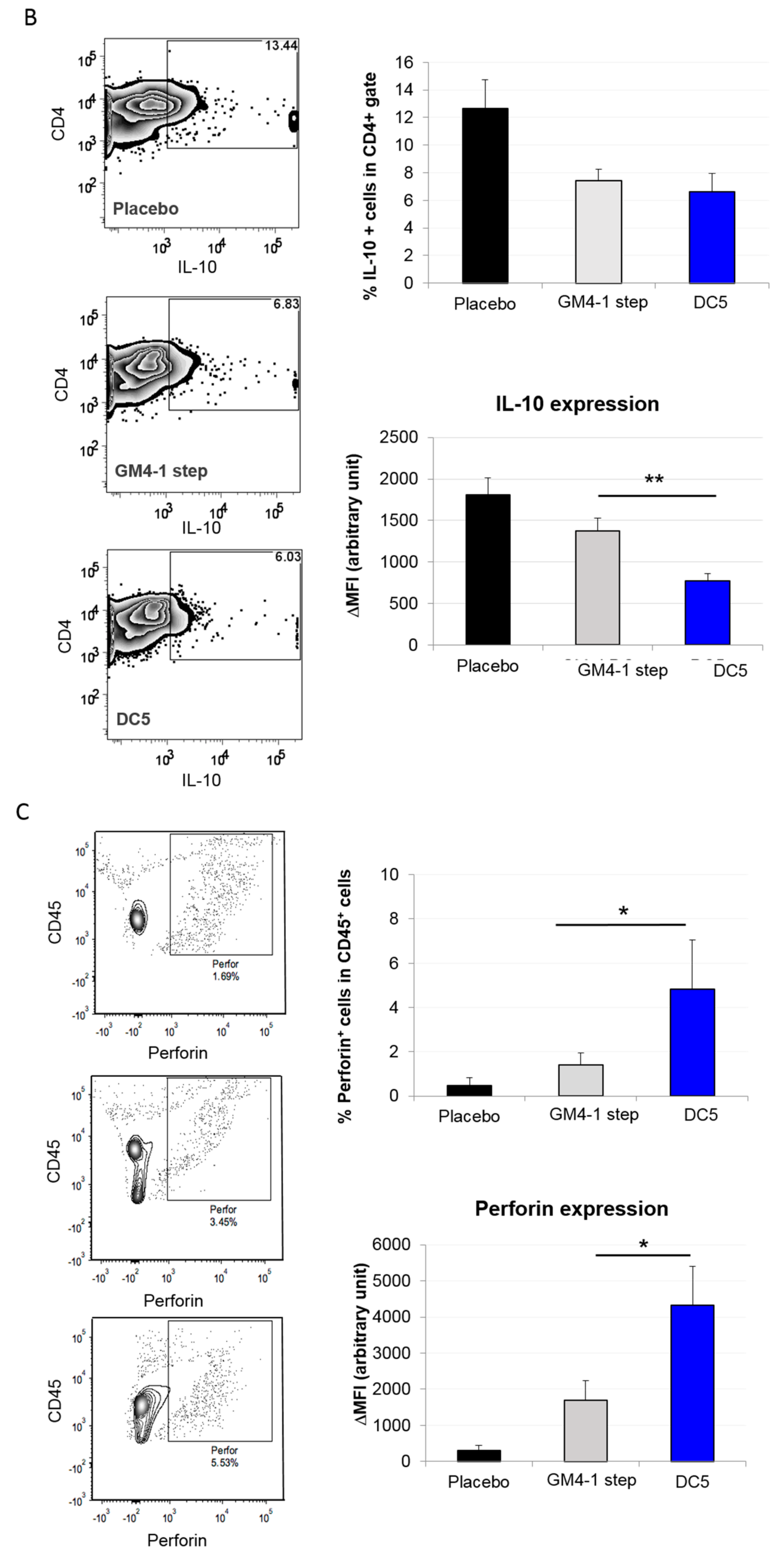
2.3. Characterization of the T Cell Response Induced by Vaccination with DC5 DCs
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines and Animals
4.3. Preparation of Tumor Antigen
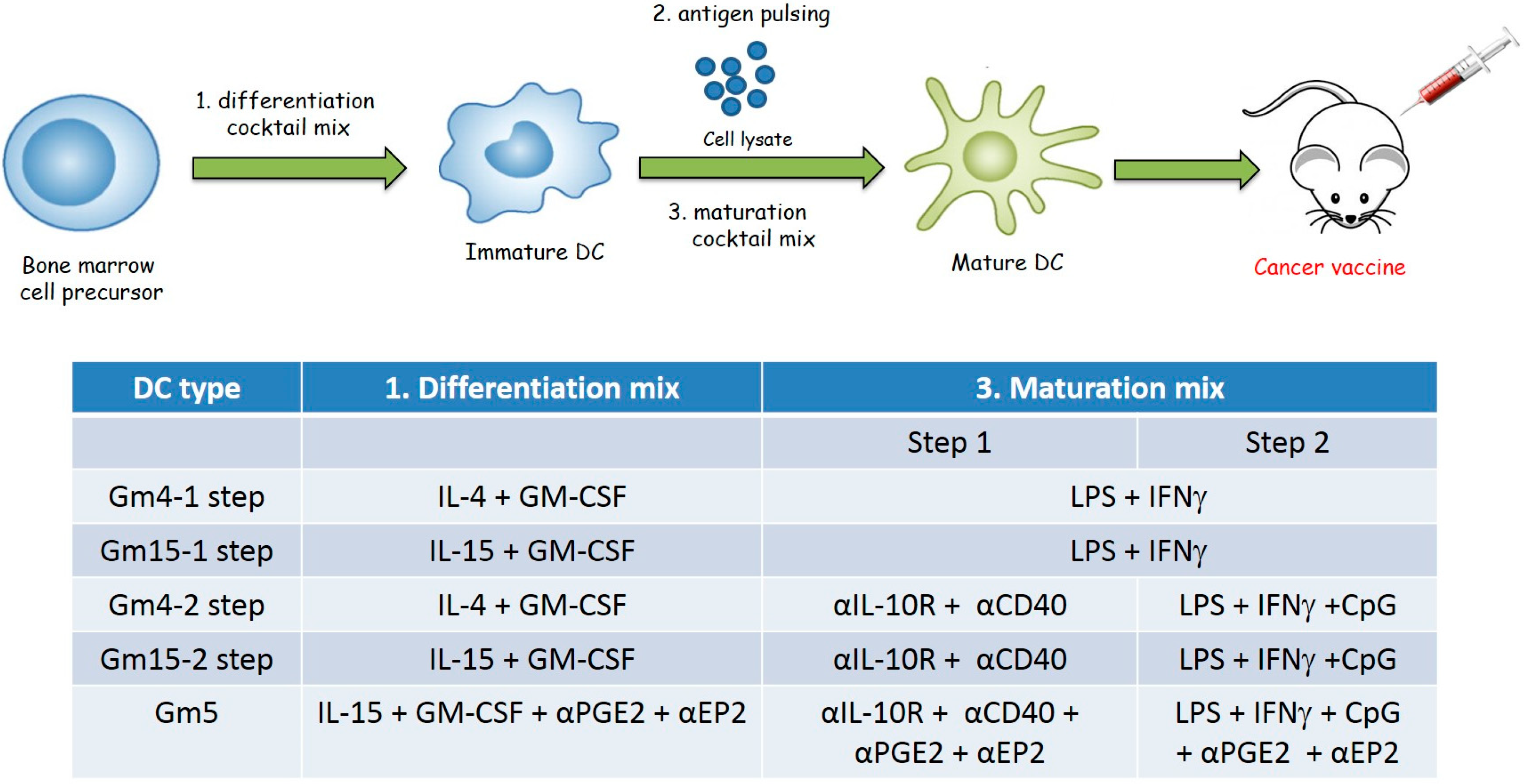
4.4. Generation of Bone Marrow-Derived Mouse DCs
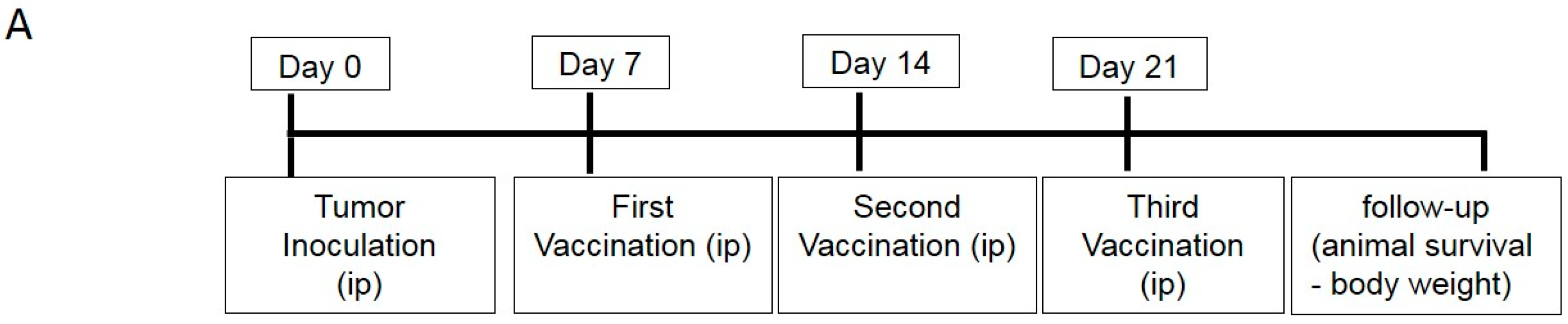
4.5. DC Vaccination
4.6. Adoptive T Cell Transfer Experiments
4.7. DC and T Cell Co-Culture
4.8. Reverse Transcription-Quantitative Polymerase Chain Reaction (RTqPCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Shin, H.-R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T Cells, Recurrence, and Survival in Epithelial Ovarian Cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Hermonat, P.L.; Ravaggi, A.; Bellone, S.; Roman, J.J.; Smith, C.V.; Pecorelli, S.; Radominska-Pandya, A.; Cannon, M.J.; Parham, G.P. Phenotypic and Functional Analysis of Tumor-Infiltrating Lymphocytes Compared with Tumor-Associated Lymphocytes from Ascitic Fluid and Peripheral Blood Lymphocytes in Patients with Advanced Ovarian Cancer. Gynecol. Obstet. Investig. 2001, 51, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Martin Lluesma, S.; Wolfer, A.; Harari, A.; Kandalaft, L. Cancer Vaccines in Ovarian Cancer: How Can We Improve? Biomedicines 2016, 4, 10. [Google Scholar] [CrossRef]
- Schaaf, M.; Garg, A.D.; Perez, M.V.; Schaaf, M.; Agostinis, P. Trial Watch: Dendritic cell-based anticancer immunotherapy Trial Watch: Dendritic cell-based anticancer immunotherapy. Oncoimmunology 2017, 2, e1328341. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Garg, A.D.; Coulie, P.G.; Van den Eynde, B.J.; Agostinis, P. Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape. Trends Immunol. 2017, 38, 577–593. [Google Scholar] [CrossRef]
- Bol, K.F.; Schreibelt, G.; Gerritsen, W.R.; De Vries, I.J.M.; Figdor, C.G. Dendritic cell-based immunotherapy: State of the art and beyond. Clin. Cancer Res. 2016, 22, 1897–1906. [Google Scholar] [CrossRef]
- Saxena, M.; Bhardwaj, N. Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment. Trends Cancer 2018, 4, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.L.; Kandalaft, L.E.; Tanyi, J.; Hagemann, A.R.; Motz, G.T.; Svoronos, N.; Montone, K.; Mantia-Smaldone, G.M.; Smith, L.; Nisenbaum, H.L.; et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: From bench to bedside. Clin. Cancer Res. 2013, 19, 4801–4815. [Google Scholar] [CrossRef] [PubMed]
- Kandalaft, L.E.; Chiang, C.L.; Tanyi, J.; Motz, G.; Balint, K.; Mick, R.; Coukos, G. A Phase I vaccine trial using dendritic cells pulsed with autologous oxidized lysate for recurrent ovarian cancer. J. Transl. Med. 2013, 11, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mookerjee, A.; Graciotti, M.; Kandalaft, L. A cancer vaccine with dendritic cells differentiated with GM-CSF and IFNa and pulsed with a squaric acid treated cell lysate improves T cell priming and tumor growth control in a mouse model. BioImpacts 2018, 8, 211–221. [Google Scholar] [CrossRef]
- Anguille, S.; Smits, E.L.; Bryant, C.; Van Acker, H.H.; Goossens, H.; Lion, E.; Fromm, P.D.; Van Tendeloo, V.F.; Berneman, Z.N. Dendritic Cells as Pharmacological Tools for Cancer Immunotherapy. Pharmacol. Rev. 2015, 67, 731–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anguille, S.; Lion, E.; Van Den Bergh, J.; Van Acker, H.H.; Willemen, Y.; Smits, E.L.; Van Tendeloo, V.F.; Berneman, Z.N. Interleukin-15 dendritic cells as vaccine candidates for cancer immunotherapy. Hum. Vaccines Immunother. 2013, 9, 1956–1961. [Google Scholar] [CrossRef] [Green Version]
- Harris, K.M. Monocytes differentiated with GM-CSF and IL-15 initiate Th17 and Th1 responses that are contact-dependent and mediated by IL-15. J. Leukoc. Biol. 2011, 90, 727–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulendran, B.; Dillon, S.; Joseph, C.; Curiel, T.; Banchereau, J.; Mohamadzadeh, M. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo. Eur. J. Immunol. 2004, 34, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.; Truong, T.; Bickham, K.; Fonteneau, J.F.; Larsson, M.; Da Silva, I.; Somersan, S.; Thomas, E.K.; Bhardwaj, N. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: Implications for immunotherapy. Vaccine 2002, 20, 8–22. [Google Scholar] [CrossRef]
- Martin Lluesma, S.; Graciotti, M.; Chiang, C.; Kandalaft, L. Does the Immunocompetent Status of Cancer Patients Have an Impact on Therapeutic DC Vaccination Strategies? Vaccines 2018, 6, 79. [Google Scholar] [CrossRef]
- Lasek, W.; Zagożdżon, R.; Jakobisiak, M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 2014, 63, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.L.-L.; Maier, D.A.; Kandalaft, L.E.; Brennan, A.L.; Lanitis, E.; Ye, Q.; Levine, B.L.; Czerniecki, B.J.; Powell, D.J., Jr.; Coukos, G. Optimizing parameters for clinical-scale production of high IL-12 secreting dendritic cells pulsed with oxidized whole tumor cell lysate. J. Transl. Med. 2011, 9, 198. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.L.L.; Hagemann, A.R.; Leskowitz, R.; Mick, R.; Garrabrant, T.; Czerniecki, B.J.; Kandalaft, L.E.; Powell, D.J.; Coukos, G. Day-4 myeloid dendritic cells pulsed with whole tumor lysate are highly immunogenic and elicit potent anti-tumor responses. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef]
- Carreno, B.M.; Becker-Hapak, M.; Huang, A.; Chan, M.; Alyasiry, A.; Lie, W.R.; Aft, R.L.; Cornelius, L.A.; Trinkaus, K.M.; Linette, G.P. IL-12p70-producing patient DC vaccine elicits Tc1-polarized immunity. J. Clin. Investig. 2013, 123, 3383–3394. [Google Scholar] [CrossRef]
- Lee, J.-J.; Foon, K.A.; Mailliard, R.B.; Muthuswamy, R.; Kalinski, P. Type 1-polarized dendritic cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemia. J. Leukoc. Biol. 2008, 84, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Smits, E.L.J.M.; Cools, N.; Goossens, H.; Berneman, Z.N.; Van Tendeloo, V.F.I. Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties. J. Transl. Med. 2009, 7, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hira, S.K.; Mondal, I.; Manna, P.P. Combined immunotherapy with whole tumor lysate-pulsed interleukin-15-activated dendritic cells and cucurbitacin I promotes strong CD8+ T-cell responses and cures highly aggressive lymphoma. Cytotherapy 2015, 17, 647–664. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Beretta, O.; Anguille, S.; De Caluwé, L.; Papagna, A.; Van den Bergh, J.M.; Willemen, Y.; Goossens, H.; Berneman, Z.N.; Van Tendeloo, V.F.; et al. Desirable cytolytic immune effector cell recruitment by interleukin-15 dendritic cells. Oncotarget 2017, 8, 13652–13665. [Google Scholar] [CrossRef] [Green Version]
- Würtzen, P.A.; Nissen, M.H.; Claesson, M.H. Maturation of dendritic cells by recombinant human CD40L-trimer leads to a homogeneous cell population with enhanced surface marker expression and increased cytokine production. Scand. J. Immunol. 2001, 53, 579–587. [Google Scholar] [CrossRef]
- Brunekreeft, K.L.; Strohm, C.; Gooden, M.J.; Rybczynska, A.A.; Nijman, H.W.; Grigoleit, G.U.; Helfrich, W.; Bremer, E.; Siegmund, D.; Wajant, H.; et al. Targeted delivery of CD40L promotes restricted activation of antigen-presenting cells and induction of cancer cell death. Mol. Cancer 2014, 13, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Gao, H.-Y.; Zhang, T.-Y.; Lou, J.-X.; Yang, K.; Liu, X.-D.; He, X.-P.; Chen, H.-R. Adenovirus co-expressing CD40 ligand and interleukin (IL)-2 contributes to maturation of dendritic cells and production of IL-12. Biomed. Rep. 2016, 5, 567–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corinti, S.; Albanesi, C.; la Sala, A.; Pastore, S.; Girolomoni, G. Regulatory Activity of Autocrine IL-10 on Dendritic Cell Functions. J. Immunol. 2001, 166, 4312–4318. [Google Scholar] [CrossRef] [Green Version]
- Manuzak, J.; Dillon, S.; Wilson, C. Differential interleukin-10 (IL-10) and IL-23 production by human blood monocytes and dendritic cells in response to commensal enteric bacteria. Clin. Vaccine Immunol. 2012, 19, 1207–1217. [Google Scholar] [CrossRef]
- Murad, Y.M.; Clay, T.M. CpG oligodeoxynucleotides as TLR9 agonists: Therapeutic applications in cancer. BioDrugs 2009, 23, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Kaliński, P.; Hilkens, C.M.; Snijders, A.; Snijdewint, F.G.; Kapsenberg, M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 1997, 159, 28–35. [Google Scholar] [PubMed]
- Sharma, S.; Stolina, M.; Yang, S.-C.; Baratelli, F.; Lin, J.F.; Atianzar, K.; Luo, J.; Zhu, L.; Lin, Y.; Huang, M.; et al. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin. Cancer Res. 2003, 9, 961–968. [Google Scholar] [PubMed]
- Obermajer, N.; Muthuswamy, R.; Lesnock, J.; Edwards, R.P.; Kalinski, P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 2011, 118, 5498–5505. [Google Scholar] [CrossRef] [Green Version]
- Mohamadzadeh, M.; Berard, F.; Essert, G.; Chalouni, C.; Pulendran, B.; Davoust, J.; Bridges, G.; Palucka, A.K.; Banchereau, J. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J. Exp. Med. 2001, 194, 1013–1020. [Google Scholar] [CrossRef]
- Dubsky, P.; Saito, H.; Leogier, M.; Dantin, C.; Connolly, J.E.; Banchereau, J.; Palucka, A.K. IL-15-induced human DC efficiently prime melanomaspecific naive CD8+T cells to differentiate into CTL. Eur. J. Immunol. 2007, 37, 1678–1690. [Google Scholar] [CrossRef]
- Romano, E.; Rossi, M.; Ratzinger, G.; De Cos, M.A.; Chung, D.J.; Panageas, K.S.; Wolchock, J.D.; Houghton, A.N.; Chapman, P.B.; Heller, G.; et al. Peptide-loaded langerhans cells, despite increased IL15 secretion and T-cell activation in vitro, elicit antitumor T-cell responses comparable to peptide-loaded monocyte-derived dendritic cells in vivo. Clin. Cancer Res. 2011, 17, 1984–1997. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Bae, Y.-S. Dendritic cell-based therapeutic cancer vaccines: Past, present and future. Clin. Exp. Vaccine Res. 2014, 3, 113–116. [Google Scholar] [CrossRef]
- Anguille, S.; Smits, E.L.; Lion, E.; Van Tendeloo, V.F.; Berneman, Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014, 15, e257–e267. [Google Scholar] [CrossRef]
- Heath, W.R.; Carbone, F.R. Cross-presentation in viral immunity and self-tolerance. Nat. Rev. Immunol. 2001, 1, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Frleta, D.; Noelle, R.J.; Wade, W.F. CD40-mediated up-regulation of Toll-like receptor 4-MD2 complex on the surface of murine dendritic cells. J. Leukoc. Biol. 2003, 74, 1064–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worzfeld, T.; Pogge von Strandmann, E.; Huber, M.; Adhikary, T.; Wagner, U.; Reinartz, S.; Müller, R. The Unique Molecular and Cellular Microenvironment of Ovarian Cancer. Front. Oncol. 2017, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, A.; Afify, H.; Salih, Z.; Kelly, M.; Said, N. Role of tumor microenvironment in ovarian cancer pathobiology. Oncotarget 2018, 9, 22832–22849. [Google Scholar] [CrossRef] [Green Version]
- Bronger, H.; Singer, J.; Windmüller, C.; Reuning, U.; Zech, D.; Delbridge, C.; Dorn, J.; Kiechle, M.; Schmalfeldt, B.; Schmitt, M.; et al. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br. J. Cancer 2016, 115, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Matsumura, N.; Mandai, M.; Li, K.; Yagi, H.; Baba, T.; Suzuki, A.; Hamanishi, J.; Fukuhara, K.; Konishi, I. Classification using hierarchical clustering of tumor-infiltrating immune cells identifies poor prognostic ovarian cancers with high levels of COX expression. Mod. Pathol. 2009, 22, 373–384. [Google Scholar] [CrossRef]
- Walker, C.; Kristensen, F.; Bettens, F.; deWeck, A.L. Lymphokine regulation of activated (G1) lymphocytes. I. Prostaglandin E2-induced inhibition of interleukin 2 production. J. Immunol. 1983, 130, 1770–1773. [Google Scholar]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Harizi, H.; Grosset, C.; Gualde, N. Prostaglandin E2 modulates dendritic cell function via EP2 and EP4 receptor subtypes. J. Leukoc. Biol. 2003, 73, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Legitimo, A.; Consolini, R.; Failli, A.; Orsini, G.; Spisni, R. Dendritic cell defects in the colorectal cancer. Hum. Vaccines Immunother. 2014, 10, 3224–3235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrilovich, D.I.; Corak, J.; Ciernik, I.F.; Kavanaugh, D.Y.; Carbone, D.P. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin. Cancer Res. 1997, 3, 483–490. [Google Scholar] [PubMed]
- Takahashi, K.; Toyokawa, H.; Takai, S.; Satoi, S.; Yanagimoto, H.; Terakawa, N.; Araki, H.; Kwon, A.H.; Kamiyama, Y. Surgical influence of pancreatectomy on the function and count of circulating dendritic cells in patients with pancreatic cancer. Cancer Immunol. Immunother. 2006, 55, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Della Porta, M.; Danova, M.; Rigolin, G.M.; Brugnatelli, S.; Rovati, B.; Tronconi, C.; Fraulini, C.; Rossi, A.R.; Riccardi, A.; Castoldi, G. Dendritic cells and vascular endothelial growth factor in colorectal cancer: Correlations with clinicobiological findings. Oncology 2005, 68, 276–284. [Google Scholar] [CrossRef]
- Roby, K.F.; Taylor, C.C.; Sweetwood, J.P.; Cheng, Y.; Pace, J.L.; Tawfik, O.; Persons, D.L.; Smith, P.G.; Terranova, P.F. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 2000, 21, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Garrigan, K.; Moroni-Rawson, P.; McMurray, C.; Hermans, I.; Abernethy, N.; Watson, J.; Ronchese, F. Functional comparison of spleen dendritic cells and dendritic cells cultured in vitro from bone marrow precursors. Blood 1996, 88, 3508–3512. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mookerjee, A.; Graciotti, M.; Kandalaft, L.E. IL-15 and a Two-Step Maturation Process Improve Bone Marrow-Derived Dendritic Cell Cancer Vaccine. Cancers 2019, 11, 40. https://doi.org/10.3390/cancers11010040
Mookerjee A, Graciotti M, Kandalaft LE. IL-15 and a Two-Step Maturation Process Improve Bone Marrow-Derived Dendritic Cell Cancer Vaccine. Cancers. 2019; 11(1):40. https://doi.org/10.3390/cancers11010040
Chicago/Turabian StyleMookerjee, Ananda, Michele Graciotti, and Lana E. Kandalaft. 2019. "IL-15 and a Two-Step Maturation Process Improve Bone Marrow-Derived Dendritic Cell Cancer Vaccine" Cancers 11, no. 1: 40. https://doi.org/10.3390/cancers11010040
APA StyleMookerjee, A., Graciotti, M., & Kandalaft, L. E. (2019). IL-15 and a Two-Step Maturation Process Improve Bone Marrow-Derived Dendritic Cell Cancer Vaccine. Cancers, 11(1), 40. https://doi.org/10.3390/cancers11010040



