Revising PTEN in the Era of Immunotherapy: New Perspectives for an Old Story
Abstract
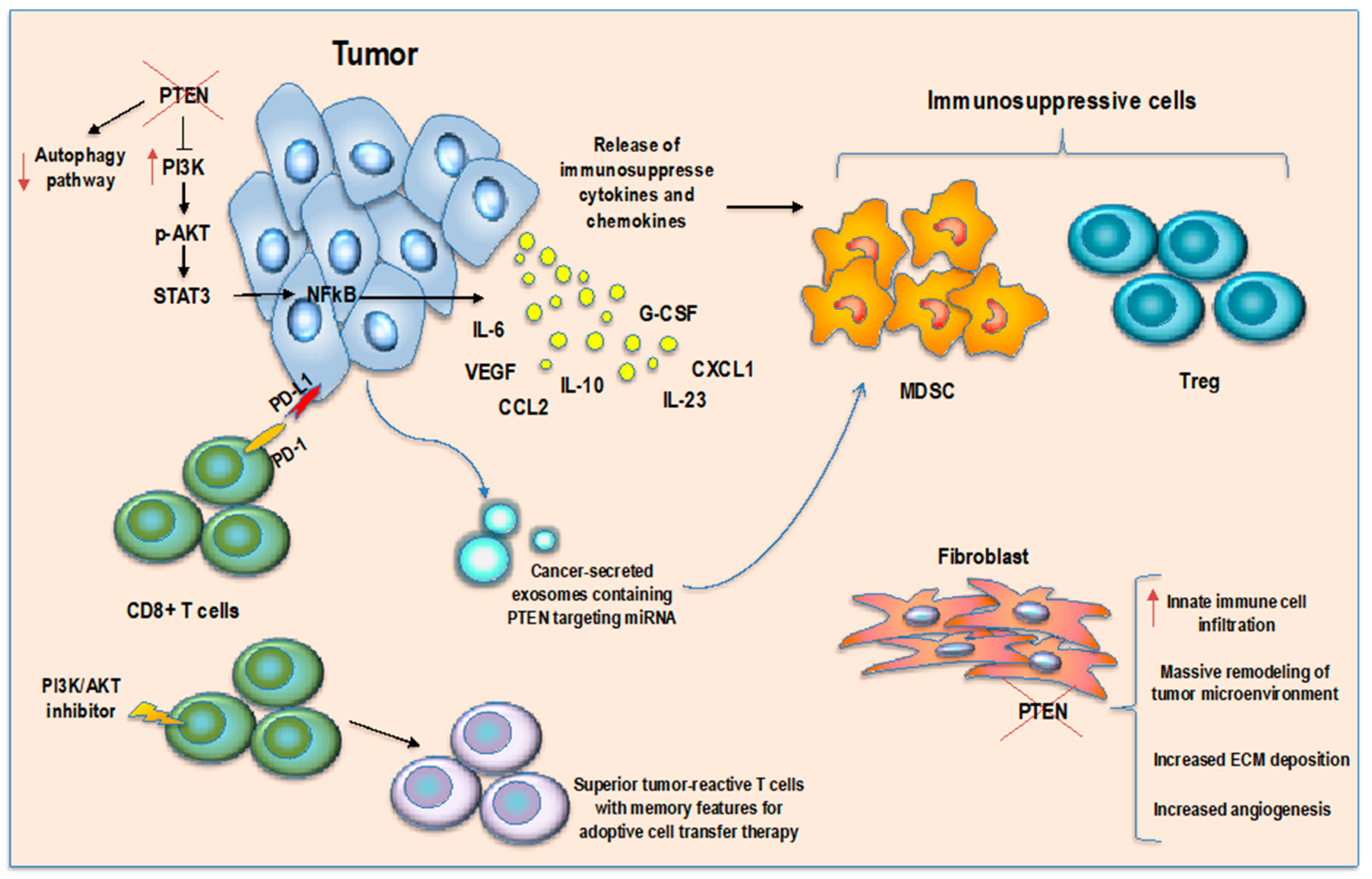
:1. Introduction
2. PTEN Function in Tumor-Immune Microenvironment
3. PTEN Pathway in Regulatory T Cells: A Controversial Role
4. PTEN-Modulating Strategies
4.1. Inhibition of PTEN Function
4.2. Reactivation of PTEN Pathway
5. PTEN Role in Immunotherapy Response
5.1. Immunotherapy
5.2. PTEN and Immunotherapy Resistance
5.3. AKT Pathway and Adoptive Cell Transfer Therapy
6. Ongoing Clinical Trials
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Milella, M.; Falcone, I.; Conciatori, F.; Cesta Incani, U.; Del Curatolo, A.; Inzerilli, N.; Nuzzo, C.M.; Vaccaro, V.; Vari, S.; Cognetti, F.; et al. PTEN: Multiple Functions in Human Malignant Tumors. Front. Oncol. 2015, 5, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alimonti, A.; Carracedo, A.; Clohessy, J.G.; Trotman, L.C.; Nardella, C.; Egia, A.; Salmena, L.; Sampieri, K.; Haveman, W.J.; Brogi, E.; et al. Subtle variations in Pten dose determine cancer susceptibility. Nat. Genet. 2010, 42, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Salmena, L.; Carracedo, A.; Pandolfi, P.P. Tenets of PTEN tumor suppression. Cell 2008, 133, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Podsypanina, K.; Ellenson, L.H.; Nemes, A.; Gu, J.; Tamura, M.; Yamada, K.M.; Cordon-Cardo, C.; Catoretti, G.; Fisher, P.E.; Parsons, R. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl. Acad. Sci. USA 1999, 96, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Di Cristofano, A.; Pesce, B.; Cordon-Cardo, C.; Pandolfi, P.P. Pten is essential for embryonic development and tumour suppression. Nat. Genet. 1998, 19, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Trotman, L.C.; Niki, M.; Dotan, Z.A.; Koutcher, J.A.; Di Cristofano, A.; Xiao, A.; Khoo, A.S.; Roy-Burman, P.; Greenberg, N.M.; Van Dyke, T.; et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003, 1, E59. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Karikomi, M.; Naidu, S.; Rajmohan, R.; Caserta, E.; Chen, H.Z.; Rawahneh, M.; Moffitt, J.; Stephens, J.A.; Fernandez, S.A.; et al. Allele-specific tumor spectrum in pten knockin mice. Proc. Natl. Acad. Sci. USA 2010, 107, 5142–5147. [Google Scholar] [CrossRef]
- Newton, R.H.; Lu, Y.; Papa, A.; Whitcher, G.H.; Kang, Y.J.; Yan, C.; Pandolfi, P.P.; Turka, L.A. Suppression of T-cell lymphomagenesis in mice requires PTEN phosphatase activity. Blood 2015, 125, 852–855. [Google Scholar] [CrossRef] [Green Version]
- Papa, A.; Wan, L.; Bonora, M.; Salmena, L.; Song, M.S.; Hobbs, R.M.; Lunardi, A.; Webster, K.; Ng, C.; Newton, R.H.; et al. Cancer-associated PTEN mutants act in a dominant-negative manner to suppress PTEN protein function. Cell 2014, 157, 595–610. [Google Scholar] [CrossRef]
- Garcia-Cao, I.; Song, M.S.; Hobbs, R.M.; Laurent, G.; Giorgi, C.; de Boer, V.C.; Anastasiou, D.; Ito, K.; Sasaki, A.T.; Rameh, L.; et al. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell 2012, 149, 49–62. [Google Scholar] [CrossRef]
- Bazzichetto, C.; Conciatori, F.; Pallocca, M.; Falcone, I.; Fanciulli, M.; Cognetti, F.; Milella, M.; Ciuffreda, L. PTEN as a Prognostic/Predictive Biomarker in Cancer: An Unfulfilled Promise? Cancers 2019, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Salmena, L.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Boil. 2012, 13, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Gkountakos, A.; Sartori, G.; Falcone, I.; Piro, G.; Ciuffreda, L.; Carbone, C.; Tortora, G.; Scarpa, A.; Bria, E.; Milella, M.; et al. PTEN in Lung Cancer: Dealing with the Problem, Building on New Knowledge and Turning the Game Around. Cancers 2019, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Yao, Y.; Xia, W.; Setijono, S.R.; Kim, J.H.; Vila, I.K.; Chiu, H.H.; Wu, Y.; Billalabeitia, E.G.; Lee, M.G.; et al. PTEN self-regulates through USP11 via the PI3K-FOXO pathway to stabilize tumor suppression. Nat. Commun. 2019, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Staveley-O’Carroll, K.; Sotomayor, E.; Montgomery, J.; Borrello, I.; Hwang, L.; Fein, S.; Pardoll, D.; Levitsky, H. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc. Natl. Acad. Sci. USA 1998, 95, 1178–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuenca, A.; Cheng, F.; Wang, H.; Brayer, J.; Horna, P.; Gu, L.; Bien, H.; Borrello, I.M.; Levitsky, H.I.; Sotomayor, E.M. Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: Dominant role of cross-tolerance to tumor antigens. Cancer Res. 2003, 63, 9007–9015. [Google Scholar] [PubMed]
- Willimsky, G.; Blankenstein, T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature 2005, 437, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Boil. 2018, 19, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Di Cristofano, A.; Kotsi, P.; Peng, Y.F.; Cordon-Cardo, C.; Elkon, K.B.; Pandolfi, P.P. Impaired Fas response and autoimmunity in Pten+/- mice. Science 1999, 285, 2122–2125. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamaguchi, M.T.; Ohteki, T.; Sasaki, T.; Kaisho, T.; Kimura, Y.; Yoshida, R.; Wakeham, A.; Higuchi, T.; Fukumoto, M.; et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 2001, 14, 523–534. [Google Scholar] [CrossRef]
- Kral, J.B.; Kuttke, M.; Schrottmaier, W.C.; Birnecker, B.; Warszawska, J.; Wernig, C.; Paar, H.; Salzmann, M.; Sahin, E.; Brunner, J.S.; et al. Sustained PI3K Activation exacerbates BLM-induced Lung Fibrosis via activation of pro-inflammatory and pro-fibrotic pathways. Sci. Rep. 2016, 6, 23034. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, Y.; Pichavant, M.; Loison, F.; Sarraj, B.; Kasorn, A.; You, J.; Robson, B.E.; Umetsu, D.T.; Mizgerd, J.P.; et al. Targeted deletion of tumor suppressor PTEN augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood 2009, 113, 4930–4941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, K.K.; Jia, Y.; Zhu, D.; Simms, B.T.; Jo, H.; Hattori, H.; You, J.; Mizgerd, J.P.; Luo, H.R. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood 2007, 109, 4028–4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anzelon, A.N.; Wu, H.; Rickert, R.C. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat. Immunol. 2003, 4, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Dragomir, A.C.; Kocabayoglu, P.; Rahman, A.H.; Chow, A.; Hashimoto, D.; Leboeuf, M.; Kraus, T.; Moran, T.; Carrasco-Avino, G.; et al. Central role of conventional dendritic cells in regulation of bone marrow release and survival of neutrophils. J. Immunol. 2014, 192, 3374–3382. [Google Scholar] [CrossRef] [PubMed]
- Soond, D.R.; Garcon, F.; Patton, D.T.; Rolf, J.; Turner, M.; Scudamore, C.; Garden, O.A.; Okkenhaug, K. Pten loss in CD4 T cells enhances their helper function but does not lead to autoimmunity or lymphoma. J. Immunol. 2012, 188, 5935–5943. [Google Scholar] [CrossRef]
- Dong, Y.; Richards, J.A.; Gupta, R.; Aung, P.P.; Emley, A.; Kluger, Y.; Dogra, S.K.; Mahalingam, M.; Wajapeyee, N. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene 2014, 33, 4632–4642. [Google Scholar] [CrossRef]
- Sharma, M.D.; Shinde, R.; McGaha, T.L.; Huang, L.; Holmgaard, R.B.; Wolchok, J.D.; Mautino, M.R.; Celis, E.; Sharpe, A.H.; Francisco, L.M.; et al. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci. Adv. 2015, 1, e1500845. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef]
- Toso, A.; Revandkar, A.; Di Mitri, D.; Guccini, I.; Proietti, M.; Sarti, M.; Pinton, S.; Zhang, J.; Kalathur, M.; Civenni, G.; et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 2014, 9, 75–89. [Google Scholar] [CrossRef]
- Di Mitri, D.; Toso, A.; Chen, J.J.; Sarti, M.; Pinton, S.; Jost, T.R.; D’Antuono, R.; Montani, E.; Garcia-Escudero, R.; Guccini, I.; et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 2014, 515, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Bezzi, M.; Seitzer, N.; Ishikawa, T.; Reschke, M.; Chen, M.; Wang, G.; Mitchell, C.; Ng, C.; Katon, J.; Lunardi, A.; et al. Diverse genetic-driven immune landscapes dictate tumor progression through distinct mechanisms. Nat. Med. 2018, 24, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Elpek, K.G.; Vinjamoori, A.; Zimmerman, S.M.; Chu, G.C.; Yan, H.; Fletcher-Sananikone, E.; Zhang, H.; Liu, Y.; Wang, W.; et al. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-kappaB-cytokine network. Cancer Discov. 2011, 1, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015, 527, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, A.J.; Cantemir-Stone, C.Z.; Li, F.; Wallace, J.A.; Merchant, A.; Creasap, N.; Thompson, J.C.; Caserta, E.; Wang, H.; Chong, J.L.; et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 2009, 461, 1084–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronisz, A.; Godlewski, J.; Wallace, J.A.; Merchant, A.S.; Nowicki, M.O.; Mathsyaraja, H.; Srinivasan, R.; Trimboli, A.J.; Martin, C.K.; Li, F.; et al. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat. Cell Biol. 2011, 14, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Sloot, Y.J.E.; Rabold, K.; Netea, M.G.; Smit, J.W.A.; Hoogerbrugge, N.; Netea-Maier, R.T. Effect of PTEN inactivating germline mutations on innate immune cell function and thyroid cancer-induced macrophages in patients with PTEN hamartoma tumor syndrome. Oncogene 2019, 38, 3743–3755. [Google Scholar] [CrossRef]
- Parsa, A.T.; Waldron, J.S.; Panner, A.; Crane, C.A.; Parney, I.F.; Barry, J.J.; Cachola, K.E.; Murray, J.C.; Tihan, T.; Jensen, M.C.; et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007, 13, 84–88. [Google Scholar] [CrossRef]
- Waldron, J.S.; Yang, I.; Han, S.; Tihan, T.; Sughrue, M.E.; Mills, S.A.; Pieper, R.O.; Parsa, A.T. Implications for immunotherapy of tumor-mediated T-cell apoptosis associated with loss of the tumor suppressor PTEN in glioblastoma. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Aust. 2010, 17, 1543–1547. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Zhang, X.; Li, W.; Feng, Q.; Feng, H.; Tong, Y.; Rong, H.; Wang, W.; Zhang, D.; Zhang, Z.; et al. Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer. Cancer Manag. Res. 2019, 11, 4023–4040. [Google Scholar] [CrossRef]
- Eissing, M.; Ripken, L.; Schreibelt, G.; Westdorp, H.; Ligtenberg, M.; Netea-Maier, R.; Netea, M.G.; de Vries, I.J.M.; Hoogerbrugge, N. PTEN Hamartoma Tumor Syndrome and Immune Dysregulation. Transl. Oncol. 2019, 12, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.S.; Lee, H.M.; Lio, C.W. Selection of regulatory T cells in the thymus. Nat. Rev. Immunol. 2012, 12, 157–167. [Google Scholar] [CrossRef]
- Huynh, A.; DuPage, M.; Priyadharshini, B.; Sage, P.T.; Quiros, J.; Borges, C.M.; Townamchai, N.; Gerriets, V.A.; Rathmell, J.C.; Sharpe, A.H.; et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat. Immunol. 2015, 16, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Li, L.; Sari, D.; Petkova, V.; Boussiotis, V.A. PD-1 increases PTEN phosphatase activity while decreasing PTEN protein stability by inhibiting casein kinase 2. Mol. Cell. Biol. 2013, 33, 3091–3098. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Woo, S.R.; Turnis, M.E.; Gravano, D.M.; Guy, C.; Overacre, A.E.; Bettini, M.L.; Vogel, P.; Finkelstein, D.; Bonnevier, J.; et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 2013, 501, 252–256. [Google Scholar] [CrossRef]
- Vacchelli, E.; Aranda, F.; Eggermont, A.; Sautes-Fridman, C.; Tartour, E.; Kennedy, E.P.; Platten, M.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology 2014, 3, e957994. [Google Scholar] [CrossRef]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Conway, S.J.; Marshall, B.; Brown, C.; Mellor, A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998, 281, 1191–1193. [Google Scholar] [CrossRef]
- Uyttenhove, C.; Pilotte, L.; Theate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van den Eynde, B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef]
- Muller, A.J.; Sharma, M.D.; Chandler, P.R.; Duhadaway, J.B.; Everhart, M.E.; Johnson, B.A., 3rd; Kahler, D.J.; Pihkala, J.; Soler, A.P.; Munn, D.H.; et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc. Natl. Acad. Sci. USA 2008, 105, 17073–17078. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.; Chang, M.Y.; Parker, K.H.; Beury, D.W.; DuHadaway, J.B.; Flick, H.E.; Boulden, J.; Sutanto-Ward, E.; Soler, A.P.; Laury-Kleintop, L.D.; et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012, 2, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Abe, B.T.; Macian, F. Uncovering the mechanisms that regulate tumor-induced T-cell anergy. Oncoimmunology 2013, 2, e22679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favre, D.; Mold, J.; Hunt, P.W.; Kanwar, B.; Loke, P.; Seu, L.; Barbour, J.D.; Lowe, M.M.; Jayawardene, A.; Aweeka, F.; et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Science Transl. Med. 2010, 2, 32ra36. [Google Scholar] [CrossRef] [PubMed]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Blache, C.A.; Manuel, E.R.; Kaltcheva, T.I.; Wong, A.N.; Ellenhorn, J.D.; Blazar, B.R.; Diamond, D.J. Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res. 2012, 72, 6447–6456. [Google Scholar] [CrossRef] [PubMed]
- Manuel, E.R.; Diamond, D.J. A road less traveled paved by IDO silencing: Harnessing the antitumor activity of neutrophils. Oncoimmunology 2013, 2, e23322. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.J.; DuHadaway, J.B.; Donover, P.S.; Sutanto-Ward, E.; Prendergast, G.C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005, 11, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Sharma, M.D.; Johnson, T.S.; Rodriguez, P. IDO, PTEN-expressing Tregs and control of antigen-presentation in the murine tumor microenvironment. Cancer Immunol. Immunother. 2017, 66, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.D.; Baban, B.; Chandler, P.; Hou, D.Y.; Singh, N.; Yagita, H.; Azuma, M.; Blazar, B.R.; Mellor, A.L.; Munn, D.H. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Investig. 2007, 117, 2570–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munn, D.H.; Sharma, M.D.; Johnson, T.S. Treg Destabilization and Reprogramming: Implications for Cancer Immunotherapy. Cancer Res. 2018, 78, 5191–5199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensinger, S.J.; Walsh, P.T.; Zhang, J.; Carroll, M.; Parsons, R.; Rathmell, J.C.; Thompson, C.B.; Burchill, M.A.; Farrar, M.A.; Turka, L.A. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J. Immunol. 2004, 172, 5287–5296. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Yang, K.; Guy, C.; Vogel, P.; Neale, G.; Chi, H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol. 2015, 16, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, P.T.; Buckler, J.L.; Zhang, J.; Gelman, A.E.; Dalton, N.M.; Taylor, D.K.; Bensinger, S.J.; Hancock, W.W.; Turka, L.A. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J. Clin. Investig. 2006, 116, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Soond, D.R.; Pineiro, R.; Hagemann, T.; Pearce, W.; Lim, E.L.; Bouabe, H.; Scudamore, C.L.; Hancox, T.; Maecker, H.; et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature 2014, 510, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Mak, L.H.; Vilar, R.; Woscholski, R. Characterisation of the PTEN inhibitor VO-OHpic. J. Chem. Biol. 2010, 3, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.R.; Chen, M.; Lee, J.D.; Zhang, J.; Lin, S.Y.; Fu, T.M.; Chen, H.; Ishikawa, T.; Chiang, S.Y.; Katon, J.; et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science 2019, 364, eaau0159. [Google Scholar] [CrossRef]
- Ahmad, A.; Sakr, W.A.; Rahman, K.M. Anticancer properties of indole compounds: Mechanism of apoptosis induction and role in chemotherapy. Curr. Drug Targ. 2010, 11, 652–666. [Google Scholar] [CrossRef]
- Lavictoire, S.J.; Gont, A.; Julian, L.M.; Stanford, W.L.; Vlasschaert, C.; Gray, D.A.; Jomaa, D.; Lorimer, I.A.J. Engineering PTEN-L for Cell-Mediated Delivery. Mol. Ther. Methods Clin. Dev. 2018, 9, 12–22. [Google Scholar] [CrossRef]
- Islam, M.A.; Xu, Y.; Tao, W.; Ubellacker, J.M.; Lim, M.; Aum, D.; Lee, G.Y.; Zhou, K.; Zope, H.; Yu, M.; et al. Restoration of tumour-growth suppression in vivo via systemic nanoparticle-mediated delivery of PTEN mRNA. Nat. Biomed. Eng. 2018, 2, 850–864. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buque, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508. [Google Scholar] [CrossRef]
- Krummel, M.F.; Allison, J.P. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 1996, 183, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Tagami, T.; Yamazaki, S.; Uede, T.; Shimizu, J.; Sakaguchi, N.; Mak, T.W.; Sakaguchi, S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000, 192, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, O.S.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.M.; Baker, J.; Jeffery, L.E.; Kaur, S.; Briggs, Z.; et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science 2011, 332, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Bennett, F.; Luxenberg, D.; Ling, V.; Wang, I.M.; Marquette, K.; Lowe, D.; Khan, N.; Veldman, G.; Jacobs, K.A.; Valge-Archer, V.E.; et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: Attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J. Immunol. 2003, 170, 711–718. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Nixon, N.A.; Blais, N.; Ernst, S.; Kollmannsberger, C.; Bebb, G.; Butler, M.; Smylie, M.; Verma, S. Current landscape of immunotherapy in the treatment of solid tumours, with future opportunities and challenges. Curr. Oncol. 2018, 25, e373–e384. [Google Scholar] [CrossRef]
- Song, M.; Chen, D.; Lu, B.; Wang, C.; Zhang, J.; Huang, L.; Wang, X.; Timmons, C.L.; Hu, J.; Liu, B.; et al. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS ONE 2013, 8, e65821. [Google Scholar] [CrossRef]
- Konishi, J.; Yamazaki, K.; Azuma, M.; Kinoshita, I.; Dosaka-Akita, H.; Nishimura, M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin. Cancer Res. 2004, 10, 5094–5100. [Google Scholar] [CrossRef]
- Thompson, R.H.; Gillett, M.D.; Cheville, J.C.; Lohse, C.M.; Dong, H.; Webster, W.S.; Chen, L.; Zincke, H.; Blute, M.L.; Leibovich, B.C.; et al. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer 2005, 104, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- Crane, C.A.; Panner, A.; Murray, J.C.; Wilson, S.P.; Xu, H.; Chen, L.; Simko, J.P.; Waldman, F.M.; Pieper, R.O.; Parsa, A.T. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene 2009, 28, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Roh, W.; Chen, P.L.; Reuben, A.; Spencer, C.N.; Prieto, P.A.; Miller, J.P.; Gopalakrishnan, V.; Wang, F.; Cooper, Z.A.; Reddy, S.M.; et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci. Transl. Med. 2017, 9, eaah3560. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Miao, D.; Demetri, G.D.; Adeegbe, D.; Rodig, S.J.; Shukla, S.; Lipschitz, M.; Amin-Mansour, A.; Raut, C.P.; Carter, S.L.; et al. Loss of PTEN Is Associated with Resistance to Anti-PD-1 Checkpoint Blockade Therapy in Metastatic Uterine Leiomyosarcoma. Immunity 2017, 46, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Chen, A.X.; Gartrell, R.D.; Silverman, A.M.; Aparicio, L.; Chu, T.; Bordbar, D.; Shan, D.; Samanamud, J.; Mahajan, A.; et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 2019, 25, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chong, W.; Teng, C.; Yao, Y.; Wang, X.; Li, X. The immune response-related mutational signatures and driver genes in non-small-cell lung cancer. Cancer Sci. 2019, 110, 2348–2356. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.R.; Ali, S.M.; Schrock, A.B.; Albacker, L.A.; Miller, V.A.; Stephens, P.J.; Crilley, P.; Markman, M. Response to rapamycin analogs but not PD-1 inhibitors in PTEN-mutated metastatic non-small-cell lung cancer with high tumor mutational burden. Lung Cancer (Auckl.) 2018, 9, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, A.; Furness, A.; Joshi, K.; Ghorani, E.; Ford, K.; Ward, M.J.; King, E.V.; Lechner, M.; Marafioti, T.; Quezada, S.A.; et al. Pan-cancer deconvolution of tumour composition using DNA methylation. Nat. Commun. 2018, 9, 3220. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautes-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Elliott, L.A.; Doherty, G.A.; Sheahan, K.; Ryan, E.J. Human Tumor-Infiltrating Myeloid Cells: Phenotypic and Functional Diversity. Front. Immunol. 2017, 8, 86. [Google Scholar] [CrossRef]
- Piro, G.; Simionato, F.; Carbone, C.; Frizziero, M.; Malleo, G.; Zanini, S.; Casolino, R.; Santoro, R.; Mina, M.M.; Zecchetto, C.; et al. A circulating TH2 cytokines profile predicts survival in patients with resectable pancreatic adenocarcinoma. Oncoimmunology 2017, 6, e1322242. [Google Scholar] [CrossRef] [PubMed]
- Gartrell, R.D.; Marks, D.K.; Hart, T.D.; Li, G.; Davari, D.R.; Wu, A.; Blake, Z.; Lu, Y.; Askin, K.N.; Monod, A.; et al. Quantitative Analysis of Immune Infiltrates in Primary Melanoma. Cancer Immunol. Res. 2018, 6, 481–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, N.A.; Galvin, K.C.; Corcoran, A.M.; Boon, L.; Higgs, R.; Mills, K.H. Immunotherapy with PI3K inhibitor and Toll-like receptor agonist induces IFN-gamma+IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer Res. 2012, 72, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Mousset, C.M.; Hobo, W.; Ji, Y.; Fredrix, H.; De Giorgi, V.; Allison, R.D.; Kester, M.G.D.; Falkenburg, J.H.F.; Schaap, N.P.M.; Jansen, J.H.; et al. Ex vivo AKT-inhibition facilitates generation of polyfunctional stem cell memory-like CD8(+) T cells for adoptive immunotherapy. Oncoimmunology 2018, 7, e1488565. [Google Scholar] [CrossRef] [PubMed]
- Crompton, J.G.; Sukumar, M.; Roychoudhuri, R.; Clever, D.; Gros, A.; Eil, R.L.; Tran, E.; Hanada, K.; Yu, Z.; Palmer, D.C.; et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015, 75, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Van der Waart, A.B.; van de Weem, N.M.; Maas, F.; Kramer, C.S.; Kester, M.G.; Falkenburg, J.H.; Schaap, N.; Jansen, J.H.; van der Voort, R.; Gattinoni, L.; et al. Inhibition of Akt signaling promotes the generation of superior tumor-reactive T cells for adoptive immunotherapy. Blood 2014, 124, 3490–3500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Eid, R.; Ahmad, S.; Lin, Y.; Webb, M.; Berrong, Z.; Shrimali, R.; Kumai, T.; Ananth, S.; Rodriguez, P.C.; Celis, E.; et al. Enhanced Therapeutic Efficacy and Memory of Tumor-Specific CD8 T Cells by Ex Vivo PI3K-delta Inhibition. Cancer Res. 2017, 77, 4135–4145. [Google Scholar] [CrossRef] [PubMed]
- Urak, R.; Walter, M.; Lim, L.; Wong, C.W.; Budde, L.E.; Thomas, S.; Forman, S.J.; Wang, X. Ex vivo Akt inhibition promotes the generation of potent CD19CAR T cells for adoptive immunotherapy. J. Immunother. Cancer 2017, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Klebanoff, C.A.; Crompton, J.G.; Leonardi, A.J.; Yamamoto, T.N.; Chandran, S.S.; Eil, R.L.; Sukumar, M.; Vodnala, S.K.; Hu, J.; Ji, Y.; et al. Inhibition of AKT signaling uncouples T cell differentiation from expansion for receptor-engineered adoptive immunotherapy. JCI Insight 2017, 2, e95103. [Google Scholar] [CrossRef]


| Tumor Type | Tumor Model | Consequence for Immune Regulation and Tumor Immune Microenvironment | Mechanism Involved | Reference |
|---|---|---|---|---|
| Melanoma | Patient-derived short-term melanoma cultures that either naturally express or lacked PTEN gene; PTEN knock-down/knock-in melanoma cell lines. | Increase of IL-10, IL-6 and VEGF; reduction of secretion of the pro-inflammatory cytokine IL-12 by monocyte-derived dendritic cells. | In melanoma cells lacking PTEN, STAT3 activated the transcription of immunosuppressive cytokines in a PI3K-dependent manner. Moreover, PD-L1 was upregulated, leading to immune evasion. | Dong et al., Oncogene 2014 [27] |
| Melanoma | Genetically engineered mouse models with specific deletion of PTEN in Tregs (PTENTreg-KO mice). | Intra-tumor increment of activated proinflammatory Ly6c+CD11b+ myeloid dendritic cells, which expressed more CD86 and less PD-L1. Tregs in the tumor lost their suppressive phenotype and converted into proinflammatory helper cells (ex-Tregs). | Genetically modified mice with specific deletion of PTEN in Tregs showed Treg destabilization, slow melanoma tumor growth, high grade of inflammation and were not able to create an immunosuppressive tumor microenvironment. | Sharma et al., Science advances 2015 [28] |
| Melanoma | Mouse model bearing PTEN deleted melanoma tumors. | Decreased of T cell trafficking in tumor bulk in adoptive T cell therapy mouse models. | Loss of PTEN promoted resistance to T cell killing and decresed T cell infiltration by inducing expression of immunomodulatory cytokines, such as CCL2 or VEGF, and inhibiting autophagy pathway. | Peng et al., Cancer discovery 2016 [29] |
| Prostate tumor | Mice bearing PTEN-null senescent prostate tumors. | Increase of tumor infiltration of MDSCs. Reduction of CD4+, CD8+ and natural killer (NK) infiltrates. | In PTEN-null senescent tumors, activation of the JAK2/STAT3 pathway via protein tyrosine phosphatase PTPN11/SHP2 established an immunosuppressive tumor microenvironment with production of MDSC chemoattractant cytokines. | Toso et al., Cell reports 2014 [30] |
| Prostate tumor | Genetically engineered mouse models with specific deletion of PTEN in prostate epithelial cells (Ptenpc−/− mice). | Increase of tumor infiltration of MDSCs. | The massive infiltration of MDSCs induced secretion of IL-1 receptor antagonist (IL-1RA) that hampers senescence response thus sustaining tumor growth. | Di Mitri et al., Nature 2014 [31] |
| Prostate tumor | Genetically engineered mouse models with specific prostate deletion of PTEN (Ptenpc−/− mice), PTEN and Zbtb7a (Ptenpc−/−; Zbtb7apc−/− mice), PTEN and p53 (Ptenpc−/−; Trp53pc−/− mice) and organoid cultures. | Increase of tumor infiltration of MDSCs. | Combined deletion of PTEN and Zbtb7a or PTEN and p53 in prostate tumors promoted tumor progression through MDSC recruitment, NF-κB signalling activation and cytokines secretion. | Bezzi et al., Nature medicine 2018 [32] |
| Pancreatic ductal adenocarcinoma (PDAC) | Genetically engineered mouse models with specific pancreatic deletion of PTEN (Pdx1-Cre, KrasG12D and PtenL mice). | Increase of tumor infiltration of MDSCs, neutrophils, monocytes and Tregs. | PTEN loss induced secretion of chemoattractant cytokines CXCL1, G-CSF, IL-23 via NFkB. | Ying et al., Cancer discovery 2011 [33] |
| Brain metastatic tumor | Co-culture of tumour cells with primary glia (90% astrocytes). Mouse model obtained by intracarotidly injection of syngeneic mouse melanoma B16BL6 cells to form brain metastase with or whitout astrocyte-specific depletion of PTEN-targeting miRNAs. | Recruitment of ionized calcium-binding adapter molecule 1 (IBA1)-expressing myeloid cells. | Astrocyte-derived exosomes mediated an intercellular transfer of PTEN-miRNAs to brain metastatic tumor cells to simulate transient PTEN loss status which in turn induced secretion of CCL2 with recruitment of IBA1-expressing myeloid cells, thus further enhancing metastasis outgrowth. | Zhang et al., Nature 2015 [34] |
| Breast cancer | Genetically engineered mouse models with specific inactivation of Pten in stromal fibroblasts of mouse mammary glands. | Massive remodeling of the extra-cellular matrix (ECM), enhanced deposition of collagen, innate immune cell infiltration and increased angiogenesis. | Loss of PTEN in stromal fibroblasts Sustained tumor growth through an Ets2-dependent transcriptional program with induction of MMP9 and CCL3 and VEGF pathway. | Trimboli et al., Nature 2009 [35] |
| Breast cancer | Genetically engineered mouse models with specific delection of PTEN in fibroblast. | Increase of MMP9, MMP2, BMP1, LOXL2 and EMILIN2, increased angiogenesis. | PTEN loss from mammary stromal fibroblasts activates an oncogenic secretome that orchestrates the transcriptional reprogramming of other cell types in the microenvironment. Downregulation of miR-320 and upregulation of one of its direct targets ETS2, are critical events in Pten-deleted stromal fibroblasts responsible for inducing this oncogenic secretome, which in turn promotes tumour angiogenesis and tumour-cell invasion. | Bronisz et al., Nature cell biology 2011 [36] |
| Thyroid cancer | Co-culture of PTEN-deficient thyroid cancer cell line with monocytes derived from PTEN hamartoma tumor syndrome (PHTS) patients. | Innate immune cells from PHTS patients acquired a more proinflammatory phenotype and increased lactate production. | Secretion of proinflammatory factors. | Sloot et al., Oncogene 2019 [37] |
| Glioma | Glioma cell line with genetic deletions in or mutations of PTEN | Increase of immunosuppressive mileu. | Specific loss of PTEN in glioma cells induced reduction of anti-tumor immunity and resistance to tumor-specific T cells lysis with increase of PD-L1 expression through a translational regulation mechanism. | Parsa et al.,Nature medicine 2007 [38] |
| Glioblastoma | Primary human glioblastoma cell lines derived from resected patients and co-cultured with matched autologous T-cells. | High T-cell apoptosis upon contact with PTEN-deficient cancer cells. | PTEN loss confered immunoresistant phenotype through the PI3K/Akt/mTOR pathway. | Waldron et al., Journal of clinical neuroscience 2010 [39] |
| Gastric cancer | Mouse models treated with gastric cancer cell derived exosomes. | Increase of MDSCs activation. | Gastric cancer-secreted exosomes were able to deliver miRNA-107 to the host MDSCs inducing their activation through PTEN-downregulation. Indeed, the release of PI3K pathway induced the expression of ARG1 in MDSCs thus increasing their suppressive function. | Ren et al, Cancer Management and Research 2019 [40] |
| Treatment | Tumor Type | Study Results | n. of Patients | Reference |
|---|---|---|---|---|
| anti-PD-1 pembrolizumab or nivolumab | Melanoma | Analysis of a cohort of 39 metastatic melanoma patients treated with anti-PD-1 antibodies (pembrolizumab and nivolumab) demonstrated that patients with PTEN positive tumors achieved significantly greater reduction of tumor size than patients with PTEN negative tumors (p = 0.029) | Cohort of 39 patients | Peng et al., Cancer discovery 2016 [29] |
| anti CTLA-4 ipilimumab and/or anti-PD-1 pembrolizumab | Melanoma | Analysis of a cohort of longitudinal tissue samples from metastatic melanoma patients treated with sequential immune checkpoint blockade (CTLA-4 blockade followed by PD-1 blockade at time of progression) demonstrated that PTEN loss is associated with CTLA-4 blockade resistance. | Cohort of 56 patients | Roh et al., Science translational medicine 2017 [83] |
| anti-PD-1 pembrolizumab | Uterine leiomyosarcoma | Analysis of primary tumor, the sole treatment-resistant metastasis, and germline tissue identified biallelic PTEN loss as potential clinical mechanism of acquired resistance to immune checkpoint therapy. | Case report | George et al., Immunity 2017 [84] |
| anti PD-1 nivolumab or pembrolizumab | Glioblastoma | Mutations on PTEN were significantly enriched in nonresponders to anti-PD-1 inhibitors. Analysis of matched pre- and post-anti-PD-1 treatment samples showed that PTEN-mutated tumors had a significantly higher level of CD68+HLA-DR− macrophages, which was previously linked to poor survival in melanoma. | Cohort of 76 patients | Zhao et al., Nature medicine 2019 [85] |
| anti-PD-1 nivolumab and anti-CTLA-4 ipilimumab | Non-small cell lung cancer (NSCLC) | PTEN mutations were significanly associated with resistence to immunocheckpoint inhibitor (p < 0.05). | Cohort of 113 patients | Chen et al., Cancer Sci. 2019 [86] |
| anti PD-1 nivolumab and pembrolizumab | Non-small cell lung cancer (NSCLC) | A metastatic NSCLC case with PTEN mutation, 80% PD-L1 expression and high tumor mutational load showed a durable response to mTORC1 inhibitor but was refractory to treatment with anti-PD-1 antibodies. | Case report | Parikh et al., Lung Cancer 2018 [87] |
| Conditions | Immunotherapy Treatment | Study Description | State | Estimated Enrolment | Study Identifier |
|---|---|---|---|---|---|
| Advanced or metastatic solid tumors | anti PD-L1 durvalumab | This is a Phase I dose-escalation study to evaluate the safety and tolerability of combination treatment of AKT inhibitor AZD5363 + PARP inhibitor olaparib + durvalumab. An exploratory objective is to explore molecular correlates of the relationship between mutations in AKT/PIK3CA/PTEN pathway and treatment response. | Recruiting | 40 participants | NCT03772561 |
| Advanced solid tumors selected for specific molecular alterations, including PTEN mutation | anti PD-L1 durvalumab | This is a Phase Ib study to evaluate effects and best dose of the PI3Kinase inhibitior copanlisib and PARP inhibitor olaparib when given together with durvalumab in patients with molecularly-selected solid tumors including PTEN mutation. | Not yet recruiting | 102 participants | NCT03842228 |
| Metastatic melanoma with PTEN Loss | anti-PD-1 pembrolizumab | This is a Phase I/II study to evaluate objective response rate and overall survival of the selective PI3K-Beta Inhibitor GSK2636771 in combination with pembrolizumab in patients with metastatic melanoma and PTEN Loss. | Recruiting | 41 participants | NCT03131908 |
| Relapsed/refractory mismatch-repair proficient colorectal cancer | anti-PD-1 nivolumab | This is a Phase I/II study to evaluate objective response rate of PI3Kinase inhibitor copanlisib and nivolumab. | Recruiting | 54 participants | NCT03711058 |
| Recurrent/refractory diffuse large B-cell lymphoma or primary mediastinal large B-cell lymphoma | anti-PD-1 nivolumab | This is a Phase II study to evaluate objective response rate of PI3Kinase inhibition copanlisib hydrochloride and nivolumab. | Suspended | 106 participants | NCT03484819 |
| Metastatic triple-negative breast cancer or ovarian cancer | A2aR and A2bR antagonist AB928 | This is a Phase I/Ib study to evaluate safety, tolerability, pharmacokinetic, pharmacodynamic, and clinical activity of immunotherapy combinations. dual adenosine receptor antagonist AB928 in combination with pegylated liposomal doxorubicin with or without PI3kinase-gamma inhibitor IPI-549. | Recruiting | 214 participants | NCT03719326 |
| Non Small Cell Lung Cancer | anti-PD-1 pembrolizumab | This is a Phase Ib/II study to evaluate safety and objective response rate of the standard pembrolizumab in combination with the investigational agent PI3K-delta inhibitor idelalisib. | Recruiting | 40 participants | NCT03257722 |
| HLA-DPB1*04:01 positive adults with advanced cancers | T Cell Receptor Engineered T Cells (KITE-718) | This is a Phase I study to evaluate safety and objective response rate of KITE-718 treated with AKT inhibitor during manufacturing process. | Recruiting | 75 participants | NCT03139370 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piro, G.; Carbone, C.; Carbognin, L.; Pilotto, S.; Ciccarese, C.; Iacovelli, R.; Milella, M.; Bria, E.; Tortora, G. Revising PTEN in the Era of Immunotherapy: New Perspectives for an Old Story. Cancers 2019, 11, 1525. https://doi.org/10.3390/cancers11101525
Piro G, Carbone C, Carbognin L, Pilotto S, Ciccarese C, Iacovelli R, Milella M, Bria E, Tortora G. Revising PTEN in the Era of Immunotherapy: New Perspectives for an Old Story. Cancers. 2019; 11(10):1525. https://doi.org/10.3390/cancers11101525
Chicago/Turabian StylePiro, Geny, Carmine Carbone, Luisa Carbognin, Sara Pilotto, Chiara Ciccarese, Roberto Iacovelli, Michele Milella, Emilio Bria, and Giampaolo Tortora. 2019. "Revising PTEN in the Era of Immunotherapy: New Perspectives for an Old Story" Cancers 11, no. 10: 1525. https://doi.org/10.3390/cancers11101525
APA StylePiro, G., Carbone, C., Carbognin, L., Pilotto, S., Ciccarese, C., Iacovelli, R., Milella, M., Bria, E., & Tortora, G. (2019). Revising PTEN in the Era of Immunotherapy: New Perspectives for an Old Story. Cancers, 11(10), 1525. https://doi.org/10.3390/cancers11101525






