Topical 2?-Hydroxyflavanone for Cutaneous Melanoma
Abstract
:1. Introduction
2. Results
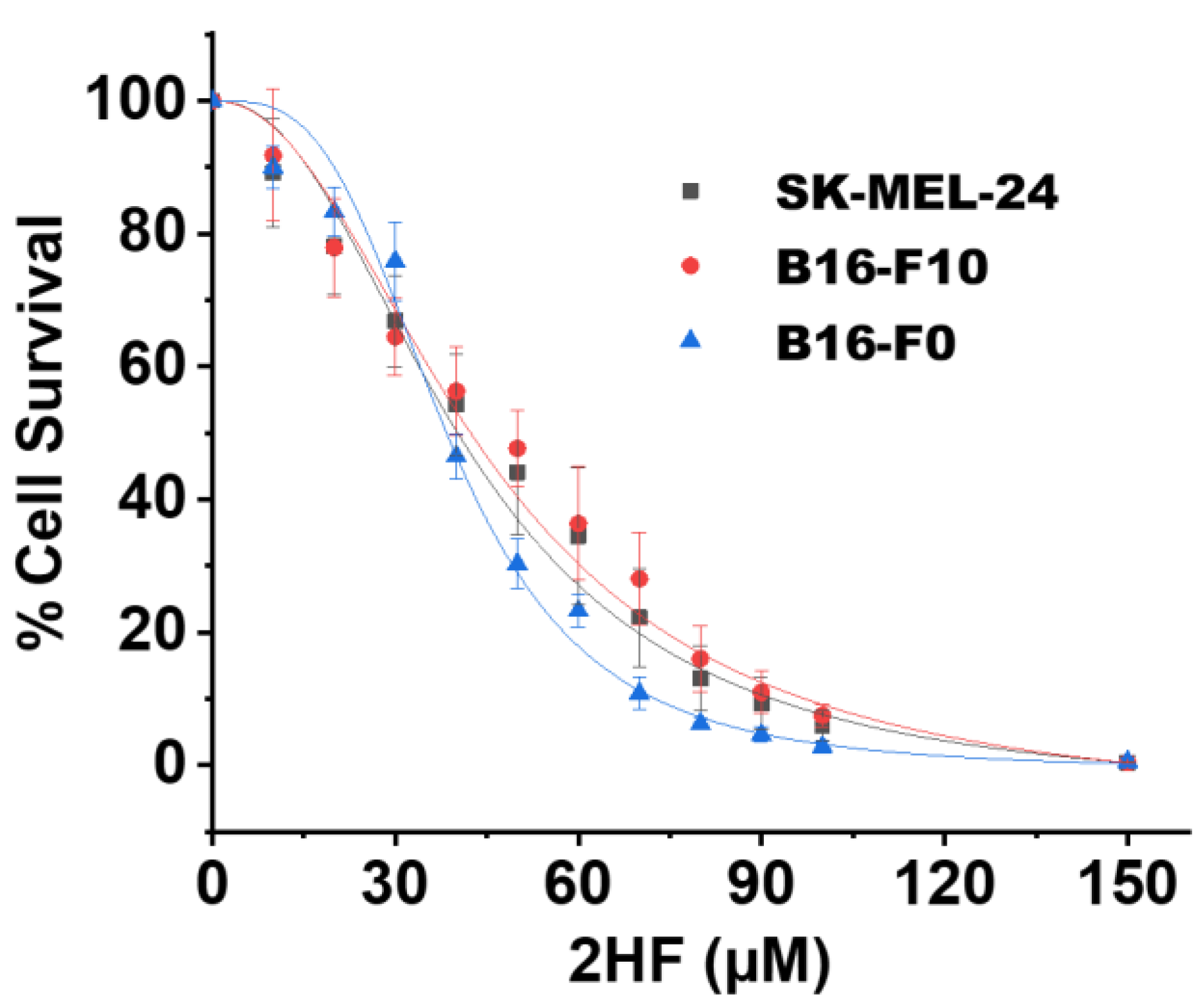
2.1. 2HF Inhibited the Growth/Survival of Melanoma Cell Lines in Vitro
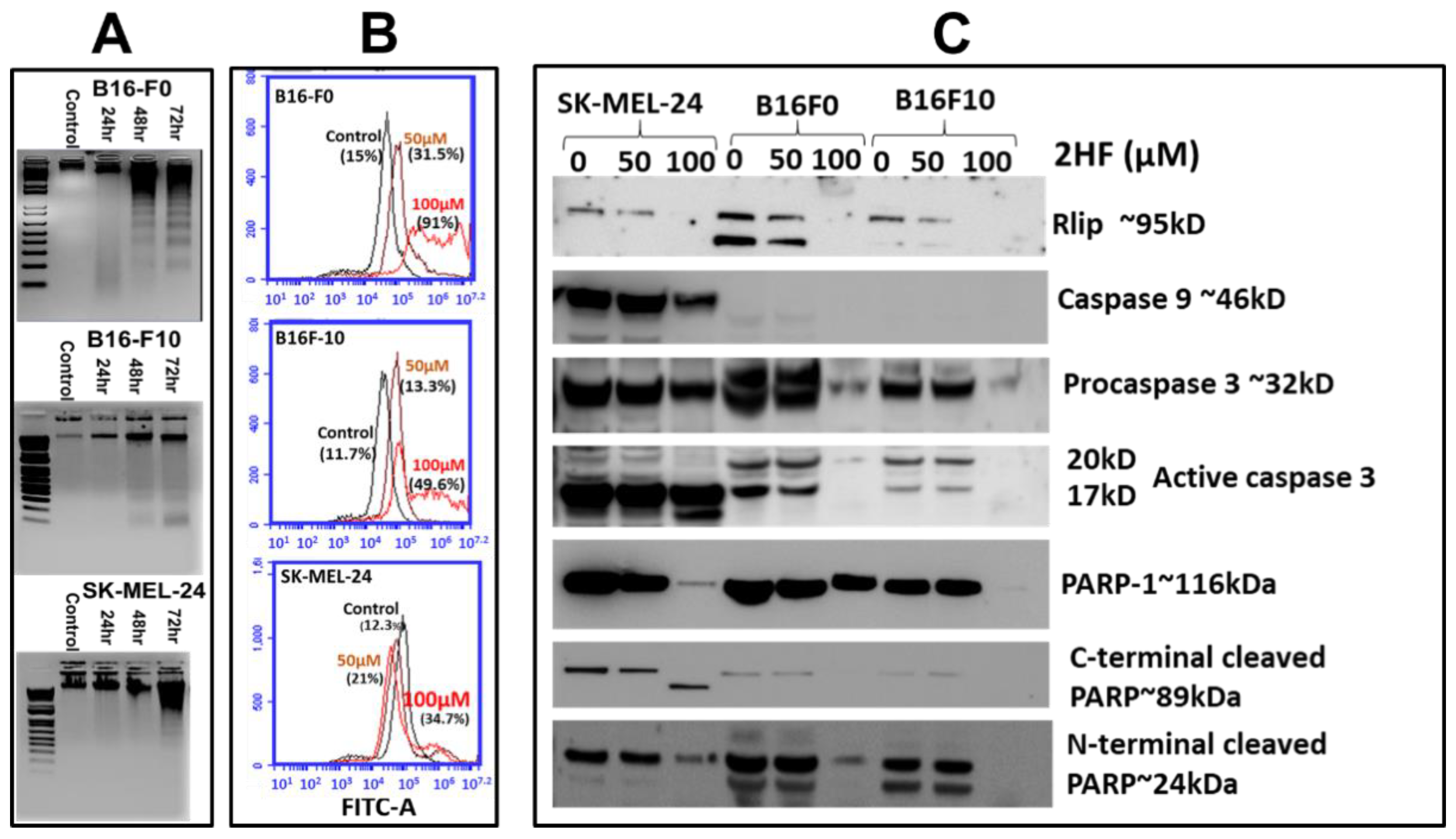
2.2. 2HF-Induced Apoptosis in Melanoma Cell Lines in Vitro
2.3. 2HF Depletes Rlip and PARP1 and Influences the Cytotoxic Effects of PARP1 Inhibitor AZD 2461
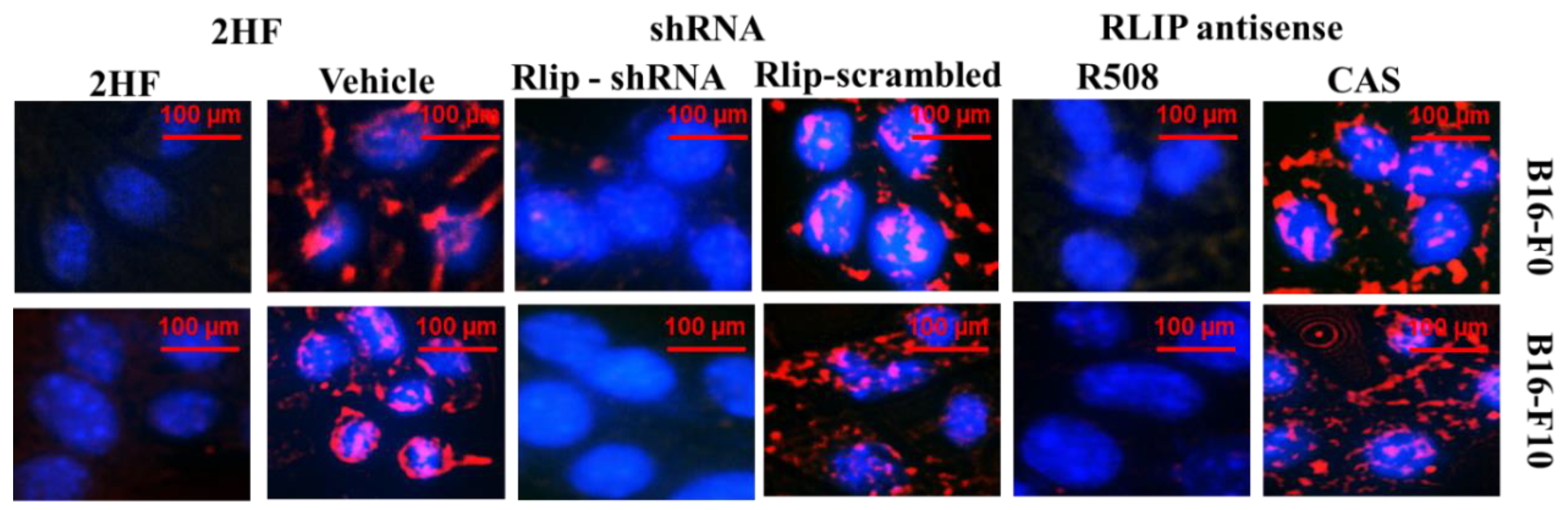
2.4. 2HF Inhibits Endocytosis as Effectively as Depletion of Rlip
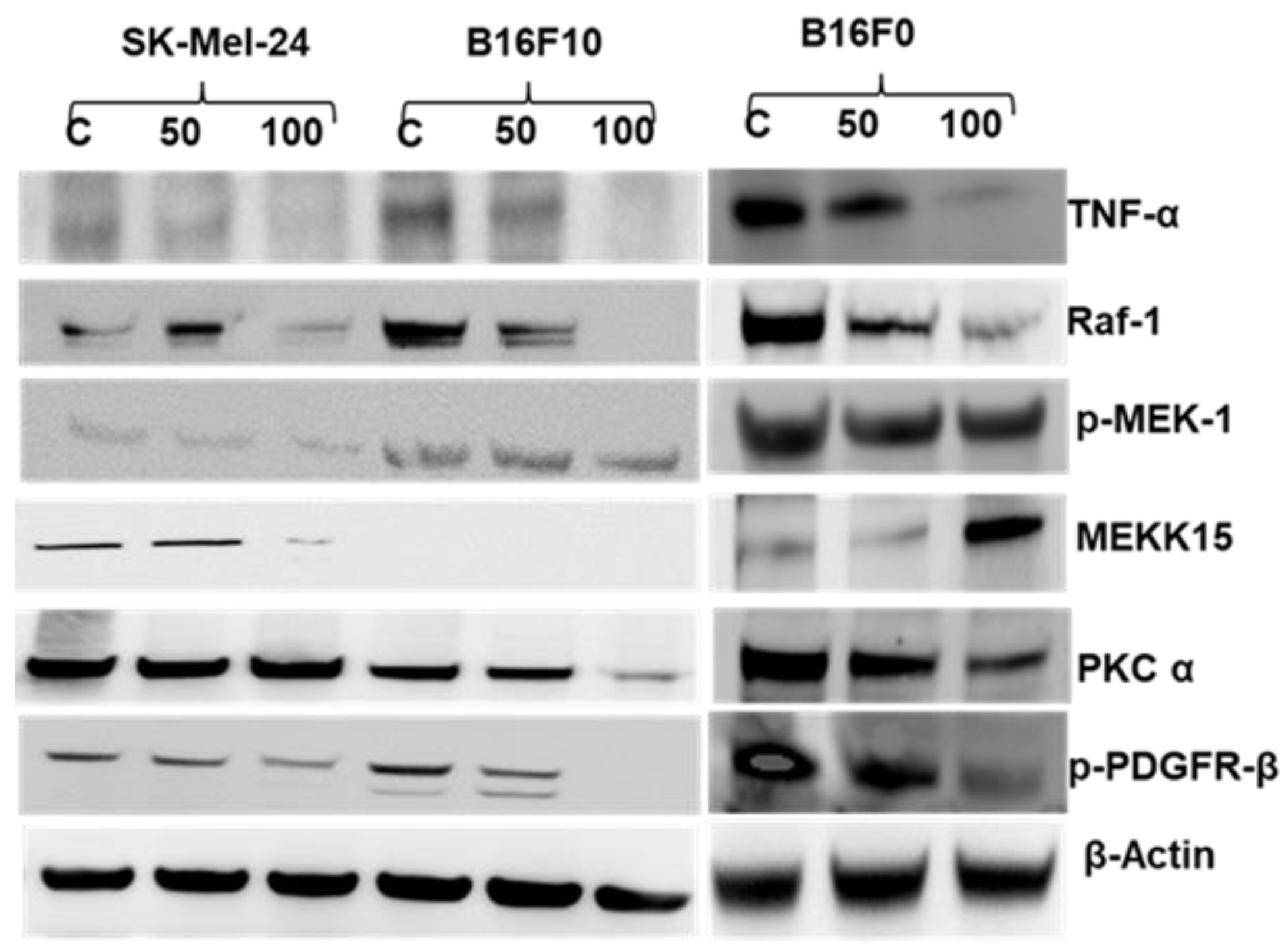
2.5. 2HF Treatment Inhibits Melanoma Signaling in Vitro
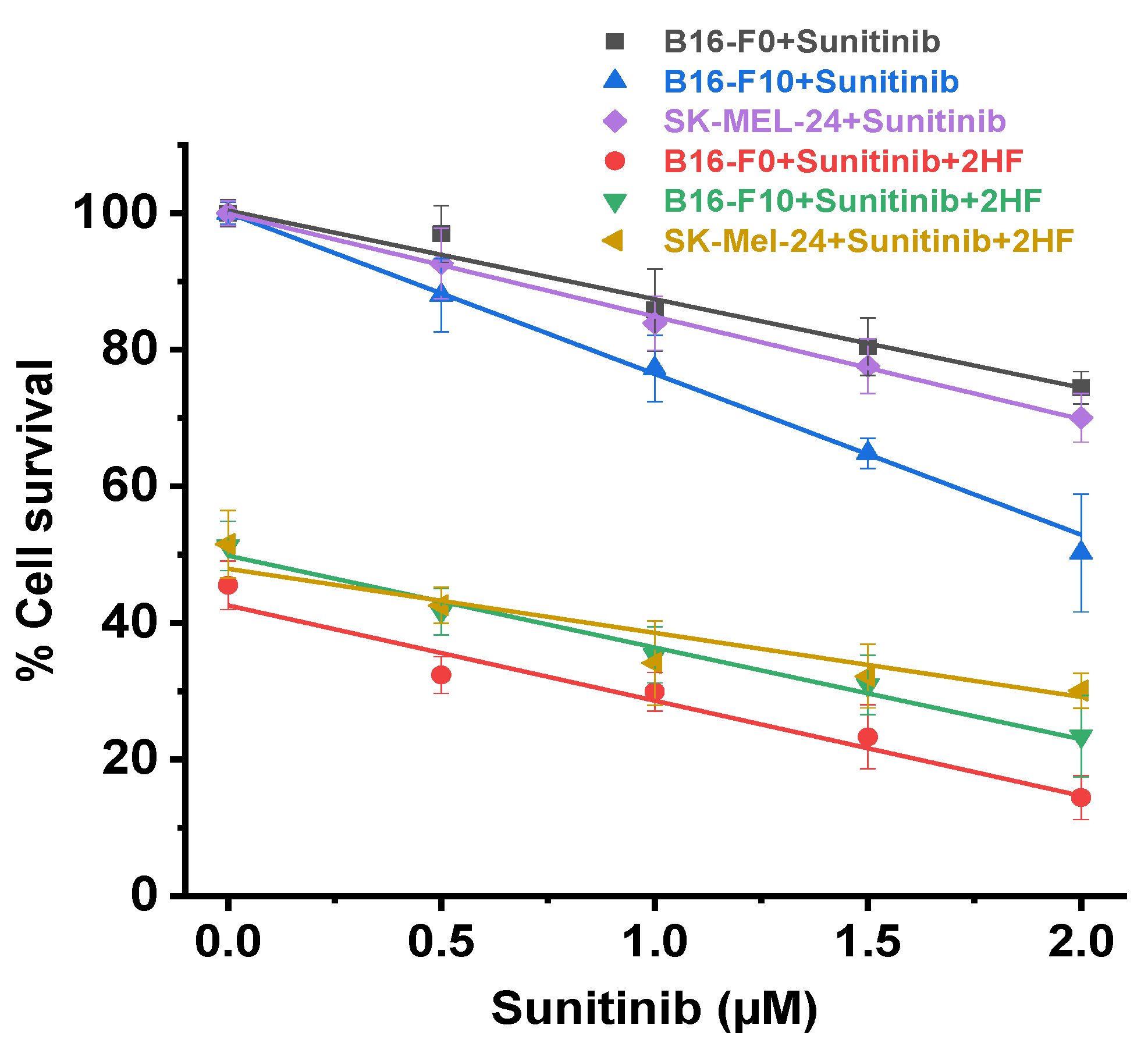
2.6. 2HF and Sunitinib Co-Treatment on Mouse and Human Melanoma Cells in Vitro
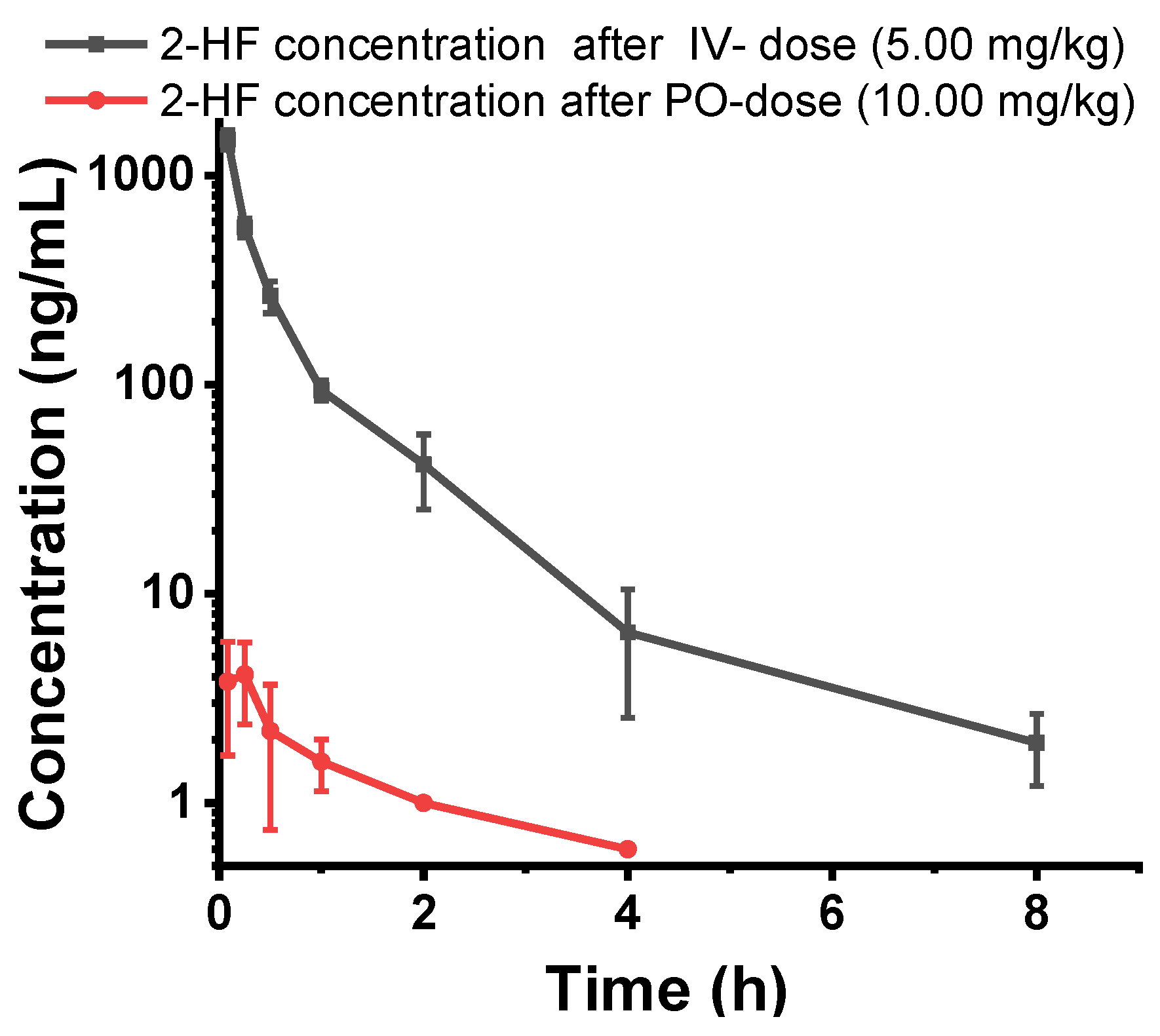
2.7. 2HF is Poorly Orally Absorbed and Rapidly Metabolized after Oral or IV Dosing
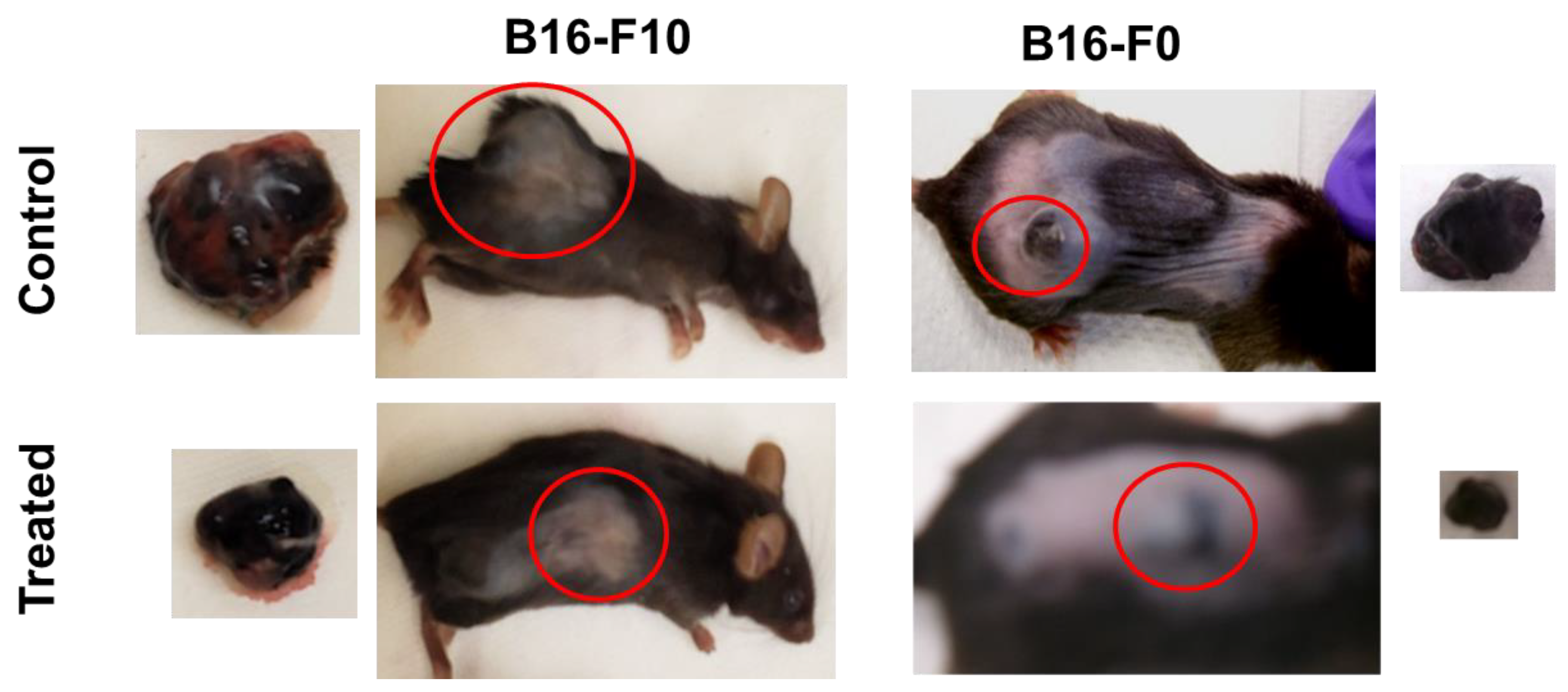
2.8. Topical 2HF Inhibited the Growth of Implants of B16-F0 and B16-F10 Melanoma
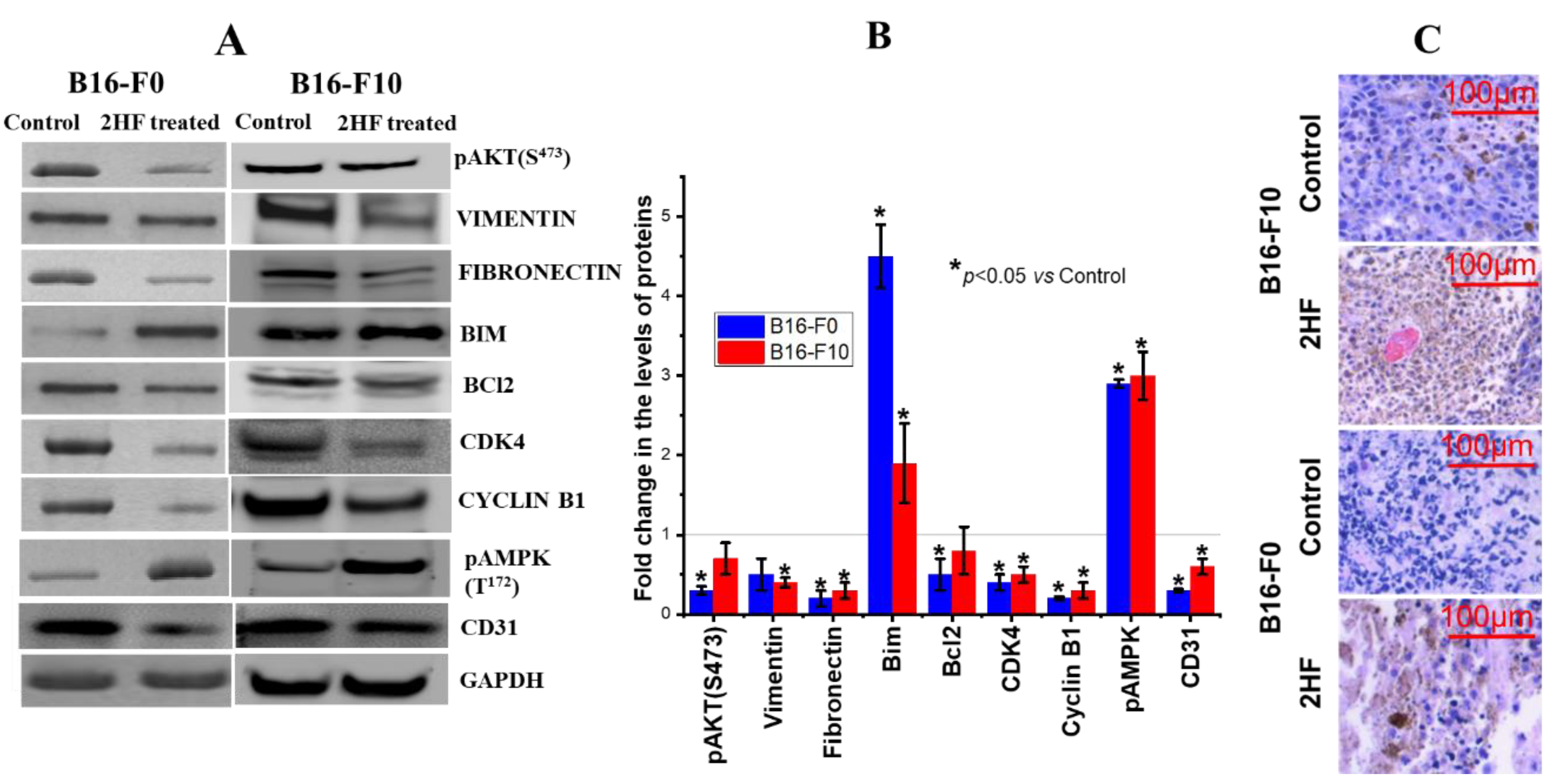
2.9. 2HF Regulates Cancer Signaling in Tumors Induced by B16-F0 and B16-F10 Melanoma Cells
2.10. 2HF Was Absorbed Systemically but Did Not Exhibit any Significant Systemic Toxicity
3. Discussion
4. Materials and Methods
4.1. Reagents and Cell Lines
4.2. Ethics Statement
4.3. IC50 of 2HF on Melanoma Cell Line
4.4. Effect of Sunitinib on 2HF-Mediated B16-F0, B16-F10, and SK-MEL-24 Cell Death
4.5. Effect of 2HF on Key Signaling and Apoptotic Proteins Responsible for Melanoma Cell Growth/Death
4.6. Detection of 2HF-Induced Apoptosis in Mouse and Human Melanoma Cells by DNA Fragmentation
4.7. Detection of 2HF-Induced Apoptosis in Mouse and Human Melanoma Cells by TUNEL Assay
4.8. Effect of RLIP76 Depletion on EGF Binding and Internalization
4.9. Animal Studies
4.10. Assessment of Angiogenesis, Proliferation, and Apoptosis
4.11. LC-MS/MS Analysis of 2HF in Serum
4.12. Single Dose Pharmacokinetic Study of 2HF
4.13. Effect of 2HF on Blood Chemistry of B16-F0 Melanoma-Bearing C57B Mice
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Lookingbill, D.P.; Spangler, N.; Sexton, F.M. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J. Am. Acad. Dermatol. 1990, 22, 19–26. [Google Scholar] [CrossRef]
- De Giorgi, V.; Grazzini, M.; Alfaioli, B.; De Giorgi, V.; Grazzini, M.; Alfaioli, B.; Savarese, I.; Corciova, S.A.; Guerriero, G.; Lotti, T. Cutaneous manifestations of breast carcinoma. Dermatol. Ther. 2010, 23, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Krathen, R.A.; Orengo, I.F.; Rosen, T. Cutaneous metastasis: A Meta-Analysis of data. South Med. J. 2003, 96, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, T.; Brasfield, R. Metastatic melanoma. A clinicopathological study. Cancer 1964, 17, 1323–1339. [Google Scholar] [PubMed]
- Galeone, M.; Bassi, A.; Scarfì, F.; Arunachalam, M.; Difonzo, E.M. Multiple skin metastases of malignant melanoma. QJM Int. J. Med. 2013, 107, 161. [Google Scholar] [CrossRef] [PubMed]
- Padmavathy, L.; Rao, L.L.; Ethirajan, N.; Swamy, B.K. Malignant Melanoma-Cutaneous metastases. Indian J. Dermatol. 2008, 53, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Tas, F. Metastatic Behavior in Melanoma: Timing, Pattern, Survival, and Influencing Factors. J. Oncol. 2012, 2012. [Google Scholar] [CrossRef]
- Cho, J.; Park, Y.; Lee, J.-C.; Jung, W.J.; Lee, S. Case series of different onset of skin metastasis according to the breast cancer subtypes. Cancer Res. Treat. 2014, 46, 194–199. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Frankel, P.; Ruel, C.; Ernstoff, M.S.; Kuzel, T.M.; Logan, T.F.; Khushalani, N.I.; Tawbi, H.A.; Margolin, K.A.; Awasthi, S.; et al. NCI 8628: A randomized phase 2 study of Ziv-Aflibercept and High-Dose interleukin 2 or High-Dose interleukin 2 alone for inoperable stage III or IV melanoma. Cancer 2018, 124, 4332–4341. [Google Scholar] [CrossRef]
- Ariyan, S.; Poo, W.J.; Bolognia, J. Regional isolated perfusion of extremities for melanoma: A 20-year experience with drugs other than L-phenylalanine mustard. Plast. Reconstr. Surg. 1997, 99, 1023–1029. [Google Scholar] [CrossRef]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Reddy, A.B.M.; Majeti, N.; Singhal, S.S. Therapeutic Potential of Natural Antioxidants. Oxid. Med. Cell. Longev. 2018, 2018, 9471051. [Google Scholar] [CrossRef] [PubMed]
- Singhal, J.; Chikara, S.; Horne, D.; Salgia, R.; Awasthi, S.Z.; Singhal, S.S. 2′-Hydroxyflavanone inhibits in vitro and in vivo growth of breast cancer cells by targeting RLIP76. Mol. Carcinog. 2018, 57, 1751–1762. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Singhal, S.S.; Singhal, J.; Nagaprashantha, L.; Li, H.; Yuan, Y.-C.; Liu, Z.; Berz, D.; Igid, H.; Green, W.C.; et al. Anticancer activity of 2′-Hydroxyflavanone towards lung cancer. Oncotarget 2018, 9, 36202–36219. [Google Scholar] [CrossRef]
- Singhal, S.S.; Singhal, J.; Figarola, J.L.; Riggs, A.; Horne, D.; Awasthi, S. 2′-Hydroxyflavanone: A promising molecule for kidney cancer prevention. Biochem. Pharmacol. 2015, 96, 151–158. [Google Scholar] [CrossRef]
- Nagaprashantha, L.D.; Vatsyayan, R.; Singhal, J.; Nagaprashantha, L.D.; Vatsyayan, R.; Singhal, J.; Lelsani, P.; Prokai, L.; Awasthi, S.; Singhal, S.S. 2′-Hydroxyflavanone inhibits proliferation, tumor vascularization and promotes normal differentiation in VHL-Mutant renal cell carcinoma. Carcinogenesis 2011, 32, 568–575. [Google Scholar] [CrossRef]
- Singhal, S.S.; Wickramarachchi, D.; Yadav, S.; Singhal, J.; Leake, K.; Vatsyayan, R.; Chaudhary, P.; Lelsani, P.; Suzuki, S.; Yang, S.; et al. Glutathione-Conjugate transport by RLIP76 is required for Clathrin-Dependent endocytosis and chemical carcinogenesis. Mol. Cancer Ther. 2011, 10, 16–28. [Google Scholar] [CrossRef]
- Awasthi, S.; Singhal, S.S.; Yadav, S.; Awasthi, S.; Singhal, S.S.; Yadav, S.; Singhal, J.; Drake, K.; Nadkar, A.; Zajac, E.; et al. RLIP76 is a major determinant of radiation sensitivity. Cancer Res. 2005, 65, 6022–6028. [Google Scholar] [CrossRef]
- Singhal, J.; Singhal, S.S.; Yadav, S.; Suzuki, S.; Warnke, M.M.; Yacoub, A.; Dent, P.; Bae, S.; Sharma, R.; Awasthi, Y.C.; et al. RLIP76 in defense of radiation poisoning. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 553–561. [Google Scholar] [CrossRef]
- Vatsyayan, R.; Lelsani, P.C.; Awasthi, S.; Singhal, S.S. RLIP76: A versatile transporter and an emerging target for cancer therapy. Biochem. Pharmacol. 2010, 79, 1699–1705. [Google Scholar] [CrossRef] [Green Version]
- Singhal, S.S.; Singhal, J.; Yadav, S.; Sahu, M.; Awasthi, Y.C.; Awasthi, S. RLIP76: A target for kidney cancer therapy. Cancer Res. 2009, 69, 4244–4251. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.S.; Singhal, J.; Yadav, S.; Dwivedi, S.; Boor, P.J.; Awasthi, Y.C.; Awasthi, S. Regression of lung and colon cancer xenografts by depleting or inhibiting RLIP76 (Ral-binding protein 1). Cancer Res. 2007, 67, 4382–4389. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.S.; Sehrawat, A.; Sahu, M.; Singhal, P.; Vatsyayan, R.; Lelsani, P.C.R.; Yadav, S.; Awasthi, S. Rlip76 transports sunitinib and sorafenib and mediates drug resistance in kidney cancer. Int. J. Cancer 2010, 126, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.S.; Roth, C.; Leake, K.; Singhal, J.; Yadav, S.; Awasthi, S. Regression of prostate cancer xenografts by RLIP76 depletion. Biochem. Pharmacol. 2009, 77, 1074–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, J.; Nagaprashantha, L.; Chikara, S.; Awasthi, S.; Horne, D.; Singhal, S.S. 2′-Hydroxyflavanone: A novel strategy for targeting breast cancer. Oncotarget 2017, 8, 75025–75037. [Google Scholar] [CrossRef] [PubMed]
- Leake, K.; Singhal, J.; Nagaprashantha, L.; Awasthi, S.; Singhal, S.S. RLIP76 Regulates PI3K/Akt Signaling and Chemo-Radiotherapy Resistance in Pancreatic Cancer. PLoS ONE 2012, 7, e34582. [Google Scholar] [CrossRef]
- Kumar, S.; Kokate, R.A.; Sahu, M.; Chaudhary, P.; Sharma, R.; Awasthi, S.; Awasthi, Y.C. Inhibition of mercapturic acid Pathway-Mediated disposal of 4-Hydroxynonenal causes complete and sustained remission of human cancer xenografts in nude mice. Indian J. Exp. Biol. 2011, 49, 817–825. [Google Scholar]
- Singhal, J.; Nagaprashantha, L.; Vatsyayan, R.; Awasthi, S.; Singhal, S.S. RLIP76, a Glutathione-Conjugate transporter, plays a major role in the pathogenesis of metabolic syndrome. PLoS ONE 2011, 6, e24688. [Google Scholar] [CrossRef]
- Singhal, S.S.; Figarola, J.; Singhal, J.; Reddy, M.A.; Liu, X.; Berz, D.; Natarajan, R.; Awasthi, S. RLIP76 protein knockdown attenuates obesity due to a High-Fat diet. J. Biol. Chem. 2013, 288, 23394–23406. [Google Scholar] [CrossRef]
- Awasthi, S.; Tompkins, J.; Singhal, J.; Riggs, A.D.; Yadav, S.; Wu, X.; Singh, S.; Warden, C.; Liu, Z.; Wang, J.; et al. Rlip depletion prevents spontaneous neoplasia in TP53 null mice. Proc. Natl. Acad. Sci. USA 2018, 115, 3918–3923. [Google Scholar] [CrossRef] [Green Version]
- Singhal, S.S.; Awasthi, Y.C.; Awasthi, S. Regression of melanoma in a murine model by RLIP76 depletion. Cancer Res. 2006, 66, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yoshikawa, N.; Yamaguchi, Y.; Kagota, S.; Shinozuka, K.; Kunitomo, M. Characterization of mouse melanoma cell lines by their mortal malignancy using an experimental metastatic model. Life Sci. 2002, 70, 791–798. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Arozena, A.A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raineri, A.; Prodomini, S.; Fasoli, S.; Gotte, G.; Menegazzi, M. Influence of onconase in the therapeutic potential of PARP inhibitors in A375 malignant melanoma cells. Biochem. Pharmacol. 2019, 167, 173–181. [Google Scholar] [CrossRef] [PubMed]
- de Koning, L.; Decaudin, D.; El Botty, R.; de Koning, L.; Decaudin, D.; El Botty, R.; Nicolas, A.; Carita, G.; Schuller, M.; Ouine, B.; et al. PARP Inhibition Increases the Response to Chemotherapy in Uveal Melanoma. Cancers 2019, 11, 751. [Google Scholar] [CrossRef]
- Cseh, A.M.; Fabian, Z.; Quintana-Cabrera, R.; Szabo, A.; Eros, K.; Soriano, M.E.; Gallyas, F.; Scorrano, L.; Sumegi, B. PARP Inhibitor PJ34 Protects Mitochondria and Induces DNA-Damage Mediated Apoptosis in Combination with Cisplatin or Temozolomide in B16F10 Melanoma Cells. Front. Physiol. 2019, 10, 538. [Google Scholar] [CrossRef]
- Shih, Y.L.; Chou, J.; Yeh, M.-Y.; Chou, H.-M.; Chou, H.-C.; Lu, H.-F.; Shang, H.-S.; Chueh, F.-S.; Chu, Y.-L.; Hsueh, S.-C.; et al. Casticin induces DNA damage and inhibits DNA Repair-Associated protein expression in B16F10 mouse melanoma cancer cells. Oncol. Rep. 2016, 36, 2094–2100. [Google Scholar] [CrossRef]
- Broustas, C.G.; Lieberman, H.B. DNA damage response genes and the development of cancer metastasis. Radiat. Res. 2014, 181, 111–130. [Google Scholar] [CrossRef]
- Rodríguez, M.I.; Peralta-Leal, A.; O’Valle, F.; Rodriguez-Vargas, J.M.; González-Flores, A.; Majuelos-Melguizo, J.; López, L.; Serrano, S.; De Herreros, A.G.; Rodríguez-Manzaneque, J.C.; et al. PARP-1 regulates metastatic melanoma through modulation of Vimentin-Induced malignant transformation. PLoS Genet. 2013, 9, e1003531. [Google Scholar]
- Singhal, S.S.; Figarola, J.; Singhal, J.; Leake, K.; Nagaprashantha, L.; Lincoln, C.; Gugiu, B.G.; Horne, D.; Jove, R.; Awasthi, S.; et al. 1,3-Bis(3,5-Dichlorophenyl) urea compound ‘COH-SR4’ inhibits proliferation and activates apoptosis in melanoma. Biochem. Pharmacol. 2012, 84, 1419–1427. [Google Scholar] [CrossRef]
- Fillatre, J.; Delacour, D.; Van Hove, L.; Bagarre, T.; Houssin, N.; Soulika, M.; Veitia, R.A.; Moreau, J. Dynamics of the subcellular localization of RalBP1/RLIP through the cell cycle: The role of targeting signals and of protein-protein interactions. FASEB J. 2012, 26, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Boissel, L.; Houssin, N.; Chikh, A.; Rynditch, A.; Van Hove, L.; Moreau, J. Recruitment of Cdc42 through the GAP domain of RLIP participates in remodeling of the actin cytoskeleton and is involved in Xenopus gastrulation. Dev. Biol. 2007, 312, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Nagaprashantha, L.D.; Talamantes, T.; Singhal, J.; Nagaprashantha, L.D.; Talamantes, T.; Singhal, J.; Guo, J.; Vatsyayan, R.; Rauniyar, N.; Awasthi, S.; et al. Proteomic analysis of signaling network regulation in renal cell carcinomas with differential Hypoxia-Inducible Factor-2alpha expression. PLoS ONE 2013, 8, e71654. [Google Scholar] [CrossRef]
- Awasthi, S.; Singhal, S.S.; Yadav, S.; Awasthi, S.; Singhal, S.S.; Yadav, S.; Singhal, J.; Vatsyayan, R.; Zajac, E.; Luchowski, R.; et al. A central role of RLIP76 in regulation of glycemic control. Diabetes 2010, 59, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Nardone, B.; Hammel, J.A.; Raisch, D.W.; Weaver, L.L.; Schneider, D.; West, D.P. Melanoma associated with tumour necrosis Factor-Alpha inhibitors: A Research on Adverse Drug events And Reports (RADAR) project. Br. J. Dermatol. 2014, 170, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, A.A.; Gogas, H.; Kirkwood, J.M. IFN-α in the treatment of melanoma. J. Immunol. 2012, 189, 3789–3793. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, C.R.; Xu, H.; Smith, K.; Etemadmoghadam, D.; Leong, H.S.; Choong, D.Y.; Byrne, D.J.; Iravani, A.; Beck, S.; Mileshkin, L.; et al. Profound MEK inhibitor response in a cutaneous melanoma harboring a GOLGA4-RAF1 fusion. J. Clin. Investig. 2019, 129, 1940–1945. [Google Scholar] [CrossRef] [Green Version]
- Broman, K.K.; Dossett, L.A.; Sun, J.; Eroglu, Z.; Zager, J.S. Update on BRAF and MEK inhibition for treatment of melanoma in metastatic, unresectable, and adjuvant settings. Expert Opin. Drug Saf. 2019, 18, 381–392. [Google Scholar] [CrossRef]
- Denning, M.F. Specifying protein kinase C functions in melanoma. Pigment. Cell Melanoma Res. 2012, 25, 466–476. [Google Scholar] [CrossRef]
- Shi, H.; Kong, X.; Ribas, A.; Lo, R.S. Combinatorial treatments that overcome PDGFRbeta-Driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res. 2011, 71, 5067–5074. [Google Scholar] [CrossRef]
- Kaji, T.; Yoshida, S.; Kawai, K.; Fuchigami, Y.; Watanabe, W.; Kubodera, H.; Kishimoto, T. ASK3, a novel member of the apoptosis Signal-Regulating kinase family, is essential for stress-induced cell death in HeLa cells. Biochem. Biophys. Res. Commun. 2010, 395, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Indsto, J.O.; Nassif, N.T.; Kefford, R.F.; Mann, G.J. Frequent loss of heterozygosity targeting the inactive X chromosome in melanoma. Clin. Cancer Res. 2003, 9, 6476–6482. [Google Scholar] [PubMed]
- Savoia, P.; Fava, P.; Casoni, F.; Cremona, O. Targeting the ERK Signaling Pathway in Melanoma. Int. J. Mol. Sci. 2019, 20, 1483. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; McLaughlin, S.L.; Klinke, D.J. Quantifying spontaneous metastasis in a syngeneic mouse melanoma model using real time PCR. bioRxiv 2017, 136945. [Google Scholar] [CrossRef]
- Livingstone, E.; Zimmer, L.; Vaubel, J.; Schadendorf, D. BRAF, MEK and KIT inhibitors for melanoma: adverse events and their management. Chin. Clin. Oncol. 2014, 3. [Google Scholar]
- Kugel, C.H., 3rd; Aplin, A.E. Adaptive resistance to RAF inhibitors in melanoma. Pigment. Cell Melanoma Res. 2014, 27, 1032–1038. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.-H.; Liu, J.; Zhang, J.; Wang, Y.; Peng, X.-C.; Wei, Y.-Q.; Jiang, Y. Exogenous norepinephrine attenuates the efficacy of sunitinib in a mouse cancer model. J. Exp. Clin. Cancer Res. 2014, 33, 21. [Google Scholar] [CrossRef]
- Gaustad, J.V.; Simonsen, T.G.; Andersen, L.M.; Rofstad, E.K. The Effect of Sunitinib Treatment in Human Melanoma Xenografts: Associations with Angiogenic Profiles. Transl. Oncol. 2017, 10, 158–167. [Google Scholar] [CrossRef]
- Lee, S.; Wurtzel, J.G.; Singhal, S.S.; Awasthi, S.; Goldfinger, L.E. RALBP1/RLIP76 depletion in mice suppresses tumor growth by inhibiting tumor neovascularization. Cancer Res. 2012, 72, 5165–5173. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Sorkin, A.; von Zastrow, M. Endocytosis and signalling: Intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 2009, 10, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Receptor-Mediated endocytosis: Insights from the lipoprotein receptor system. Proc. Natl. Acad. Sci. USA 1979, 76, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.S.; Salgia, R.; Singhal, S.; Horne, D.; Awasthi, S. RLIP: An existential requirement for breast carcinogenesis. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Nagaprashantha, L.D.; Singhal, J.; Li, H.; Warden, C.; Liu, X.; Horne, D.; Awasthi, S.; Salgia, R.; Singhal, S.S. 2′-Hydroxyflavanone effectively targets RLIP76-mediated drug transport and regulates critical signaling networks in breast cancer. Oncotarget 2018, 9, 18053–18068. [Google Scholar] [CrossRef]
- Singhal, S.S.; Nagaprashantha, L.; Singhal, P.; Singhal, S.; Singhal, J.; Awasthi, S.; Horne, D. RLIP76 Inhibition: A promising developmental therapy for neuroblastoma. Pharm. Res. 2017, 34, 1673–1682. [Google Scholar] [CrossRef]
- Singhal, S.S.; Jain, D.; Singhal, P.; Awasthi, S.; Singhal, J.; Horne, D. Targeting the mercapturic acid pathway and Vicenin-2 for prevention of prostate cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 167–175. [Google Scholar] [CrossRef]
- Pandey, M.; Belgamwar, V.; Gattani, S.; Surana, S.; Tekade, A. Pluronic lecithin organogel as a topical drug delivery system. Drug Deliv. 2010, 17, 38–47. [Google Scholar] [CrossRef]
- Pinilla-Macua, I.; Sorkin, A. Methods to study endocytic trafficking of the EGF receptor. Methods Cell Biol. 2015, 130, 347–367. [Google Scholar] [Green Version]
- Rizzolio, S.; Tamagnone, L. Epidermal Growth Factor (EGF) Receptor Endocytosis Assay in A549 Cells. Bio-Protocol 2013, 3, e847. [Google Scholar] [CrossRef]



| Parameter | B16-F0 | B16-F10 | SK-MEL-24 |
|---|---|---|---|
| 2HF IC50 | 38.59 ± 1.61 µM | 41.95 ± 1.83 µM | 44.92 ± 1.67 µM |
| PARP1i IC50 | 16.52 ± 4.86 µM | 29.88 ± 28.53 µM | 55.00 ± 23.90 µM |
| PARP1i IC50 with 20µM 2HF | 10.31 ± 1.41 µM | 12.86 ± 2.72 µM | 43.02 ± 16.21 µM |
| Intact PARP1 | ++ | + | ++++ |
| Cleaved PARP1 C-terminus | + | ++ | +++ |
| Cleaved PARP1 N-terminus | +++ | ++ | ++ |
| BRCA1 detection by WB | + | - | - |
| BRCA2 detection by WB | - | - | + |
| Parameter | Vehicle (PLO Gel) | 2HF + PLO Gel Treated (Topically Each Day) |
|---|---|---|
| CBC | ||
| RBC (×106/µL) | 8.6 ± 0.3 | 8.7 ± 0.2 |
| WBC (×103/µL) | 9.0 ± 0.7 | 9.4 ± 0.7 |
| Platelets (×103/µL) | 746.0 ± 48 | 689.0 ± 54 |
| Hemoglobin (g/dL) | 13.6 ± 0.3 | 13.7 ± 0.4 |
| Hematocrit (%) | 42.4 ± 0.8 | 41.8 ± 1.1 |
| Plasma/Serum | ||
| Glucose (mg/dL) | 176.0 ± 12.4 | 179.0 ± 15.7 |
| Creatinine (mg/dL) | 0.17 ± 0.0 | 0.19 ± 0.0 |
| Albumin (g/dL) | 2.3 ± 0.1 | 2.8 ± 0.1 |
| ALT(units/L) | 105.0 ± 8.7 | 84.0 ± 5.4 * |
| AST(units/L) | 414.0 ± 14.2 | 311.0 ± 12.0 * |
| ALP(units/L) | 75.7 ± 4.6 | 108.4 ± 7.2 * |
| LDH(units/L) | 6126.0 ± 274.0 | 2150.0 ± 123.0 * |
| Triglycerides(mg/dL) | 166.0 ± 9.7 | 111.0 ± 7.3 * |
| Cholesterol (mg/dL) | 98.0 ± 5.5 | 83.0 ± 2.4 |
| n = 3 mice in each group * p < 0.05 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bose, C.; Singh, S.P.; Igid, H.; Green, W.C.; Singhal, S.S.; Lee, J.; Palade, P.T.; Rajan, A.; Ball, S.; Tonk, V.; et al. Topical 2?-Hydroxyflavanone for Cutaneous Melanoma. Cancers 2019, 11, 1556. https://doi.org/10.3390/cancers11101556
Bose C, Singh SP, Igid H, Green WC, Singhal SS, Lee J, Palade PT, Rajan A, Ball S, Tonk V, et al. Topical 2?-Hydroxyflavanone for Cutaneous Melanoma. Cancers. 2019; 11(10):1556. https://doi.org/10.3390/cancers11101556
Chicago/Turabian StyleBose, Chhanda, Sharda P. Singh, Henry Igid, William C. Green, Sharad S. Singhal, Jihyun Lee, Philip T. Palade, Aditya Rajan, Somedeb Ball, Vijay Tonk, and et al. 2019. "Topical 2?-Hydroxyflavanone for Cutaneous Melanoma" Cancers 11, no. 10: 1556. https://doi.org/10.3390/cancers11101556
APA StyleBose, C., Singh, S. P., Igid, H., Green, W. C., Singhal, S. S., Lee, J., Palade, P. T., Rajan, A., Ball, S., Tonk, V., Hindle, A., Tarbox, M., & Awasthi, S. (2019). Topical 2?-Hydroxyflavanone for Cutaneous Melanoma. Cancers, 11(10), 1556. https://doi.org/10.3390/cancers11101556






