Successful Targeting of the Warburg Effect in Prostate Cancer by Glucose-Conjugated 1,4-Naphthoquinones
Abstract
:1. Introduction
2. Results
2.1. Activity of 2-Methoxy-1,4-Naphthoquinones in Human Drug-Resistant Prostate Cancer Cells
2.2. Synthesis of the 6-O-(1,4-Naphthoquinone-2-yl)-D-Glucose Conjugates
2.3. Screening for Cytotoxicity and Selectivity to Human Prostate Cancer Cells
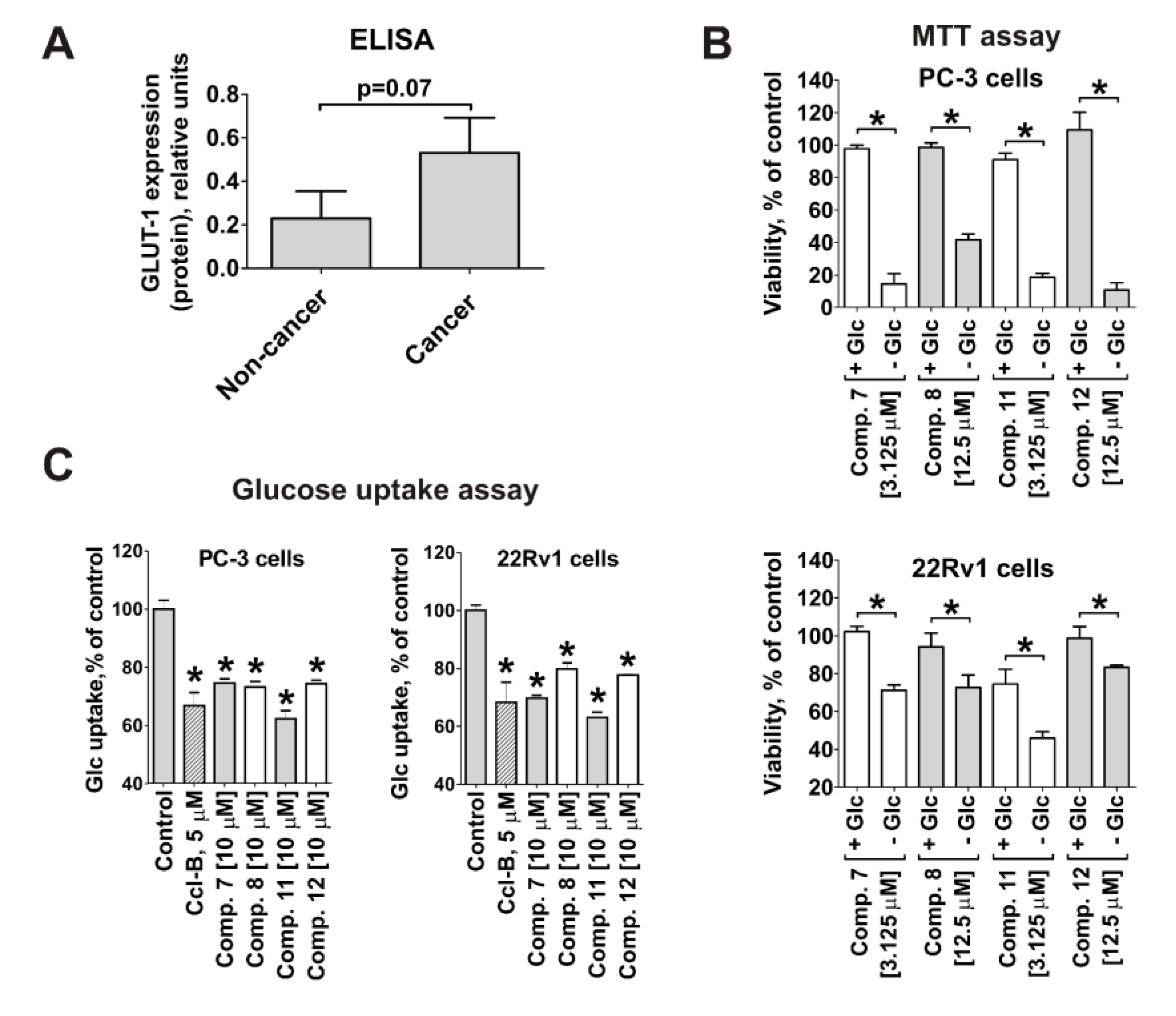
2.4. Cytotoxicity of the Synthesized Compounds Correlates with GLUT-1 Expression and Glucose Uptake Rate in Cancer Cells
2.5. Effect on Prostate Cancer Cell Proliferation, Colony Formation and Viability
2.6. Effect on Resistance Mediating Autophagy and Androgen Receptor Splice Variant-7 Expression
2.7. Analysis of Changes in Proteome of Cancer Cells under Treatment With Synthesized Compounds
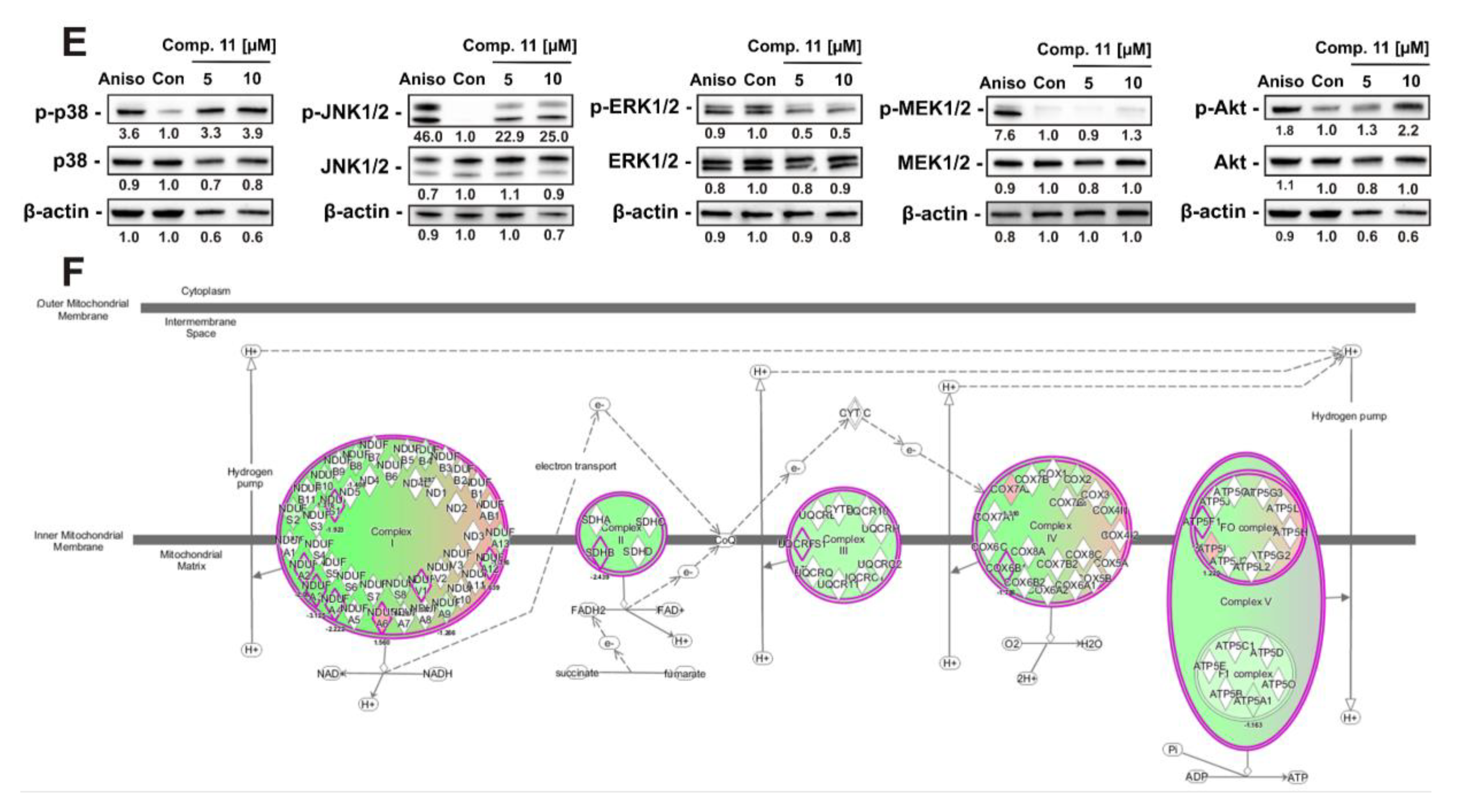
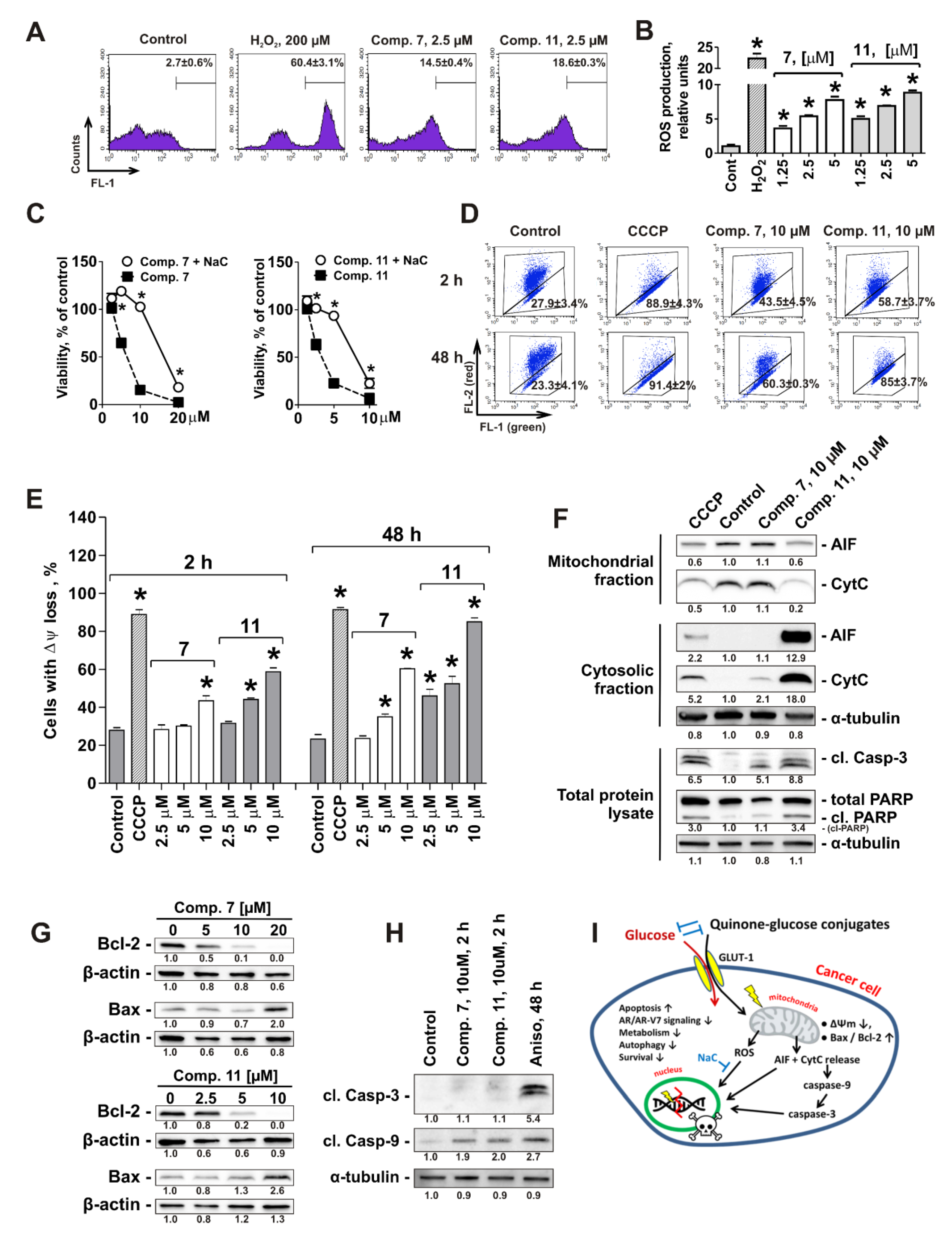
2.8. Mitochondria Are a Primary Target of Quinone-Glucose Conjugates 7 and 11 in Prostate Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Lines and Culture Conditions
4.3. In Vitro MTT-Based Drug Sensitivity Assay
4.4. In Vitro Trypan Blue-Based Viability Assay
4.5. ELISA
4.6. Glucose Uptake Assay
4.7. Colony Formation Assay
4.8. Cell Cycle and DNA Fragmentation Analysis
4.9. Detection of Apoptotic Cells by Annexin-V-FITC/PI Double Staining
4.10. Protein Preparation and Western Blotting
4.11. Proteomic Analysis of the Differentially Expressed Proteins
4.12. Bioinformatics Analysis of Differentially Expressed Proteins
4.13. Evaluation of Intracellular ROS
4.14. Mitochondrial Membrane Potential (Δψ) Assay
4.15. Cell Fractionation
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-NBDG | 2-deoxy-2-((7-nitro-2,1,3-benzoxadiazol-4-yl)amino)-D-glucose |
| ΔΨm | mitochondrial membrane potential (MMP) |
| AIF | apoptosis inducing factor |
| Apig | apigenin |
| Aniso | anisomycin |
| AR | androgen receptor |
| AR-V7 | androgen receptor splice variant 7 |
| CCCP | carbonyl cyanide 3-chlorophenylhydrazone |
| Ccl-B | cytochalasin B |
| CRPC | castration-resistant prostate cancer |
| Glc | glucose |
| GLUT-1 | glucose transporter-1 |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NaC | N-acetyl-L-cysteine |
| Plt | phloretin |
| ROS | reactive oxygen species |
| RT | room temperature |
| SI | selectivity index |
References
- Caffo, O.; De Giorgi, U.; Fratino, L.; Alesini, D.; Zagonel, V.; Facchini, G.; Gasparro, D.; Ortega, C.; Tucci, M.; Verderame, F.; et al. Clinical outcomes of castration-resistant prostate cancer treatments administered as third or fourth line following failure of docetaxel and other second-line treatment: Results of an italian multicentre study. Eur. Urol. 2015, 68, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, D.; Annala, M.; Finch, D.L.; Oja, C.D.; Vergidis, J.; Zulfiqar, M.; Sunderland, K.; Beja, K.; Vandekerkhove, G.R.; Gleave, M.; et al. Phase 2 randomized cross-over trial of abiraterone + prednisone (abi + p) vs enzalutamide (enz) for patients (pts) with metastatic castration resistant prostate cancer (mcprc): Results for 2nd-line therapy. J. Clin. Oncol. 2018, 36, 5015. [Google Scholar] [CrossRef]
- Anderson, J.; Van Poppel, H.; Bellmunt, J.; Miller, K.; Droz, J.P.; Fitzpatrick, J.M. Chemotherapy for older patients with prostate cancer. BJU Int. 2007, 99, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.; Andreana, P.R. Developments in carbohydrate-based cancer therapeutics. Pharmaceuticals (Basel) 2019, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.J.; Bokemeyer, C.; Hellmann, T.; Schmoll, H.J. Targeting of neoglycoprotein-drug conjugates to cultured human embryonal carcinoma cells. J. Cancer Res. Clin. Oncol. 1987, 113, 126–130. [Google Scholar] [CrossRef]
- Pohl, J.; Bertram, B.; Hilgard, P.; Nowrousian, M.R.; Stüben, J.; Wießler, M. D-19575—a sugar-linked isophosphoramide mustard derivative exploiting transmembrane glucose transport. Cancer Chemother. Pharmacol. 1995, 35, 364–370. [Google Scholar] [CrossRef]
- Briasoulis, E.; Judson, I.; Pavlidis, N.; Beale, P.; Wanders, J.; Groot, Y.; Veerman, G.; Schuessler, M.; Niebch, G.; Siamopoulos, K.; et al. Phase i trial of 6-hour infusion of glufosfamide, a new alkylating agent with potentially enhanced selectivity for tumors that overexpress transmembrane glucose transporters: A study of the european organization for research and treatment of cancer early clinical studies group. J. Clin. Oncol. 2000, 18, 3535–3544. [Google Scholar]
- Calvaresi, E.C.; Hergenrother, P.J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci. 2013, 4, 2319–2333. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Wellington, K.W. Understanding cancer and the anticancer activities of naphthoquinones–a review. RSC Advances 2015, 5, 20309–20338. [Google Scholar] [CrossRef]
- Lu, J.J.; Bao, J.L.; Wu, G.S.; Xu, W.S.; Huang, M.Q.; Chen, X.P.; Wang, Y.T. Quinones derived from plant secondary metabolites as anti-cancer agents. Anticancer Agents Med. Chem. 2013, 13, 456–463. [Google Scholar] [PubMed]
- Leopold, W.R.; Shillis, J.L.; Mertus, A.E.; Nelson, J.M.; Roberts, B.J.; Jackson, R.C. Anticancer activity of the structurally novel antibiotic ci-920 and its analogues. Cancer Res. 1984, 44, 1928–1932. [Google Scholar] [PubMed]
- Tewey, K.M.; Chen, G.L.; Nelson, E.M.; Liu, L.F. Intercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase ii. J. Biol. Chem. 1984, 259, 9182–9187. [Google Scholar]
- Lown, J.W.; Sim, S.K.; Majumdar, K.C.; Chang, R.Y. Strand scission of DNA by bound adriamycin and daunorubicin in the presence of reducing agents. Biochem. Biophys. Res. Commun. 1977, 76, 705–710. [Google Scholar] [CrossRef]
- Pelageev, D.N.; Dyshlovoy, S.A.; Pokhilo, N.D.; Denisenko, V.A.; Borisova, K.L.; Keller-von Amsberg, G.; Bokemeyer, C.; Fedorov, S.N.; Honecker, F.; Anufriev, V.P. Quinone-carbohydrate nonglucoside conjugates as a new type of cytotoxic agents: Synthesis and determination of in vitro activity. Eur J. Med. Chem. 2014, 77, 139–144. [Google Scholar] [CrossRef]
- Pokhilo, N.D.; Denisenko, V.A.; Anufriev, V.F. Chemistry of naphthazarin derivatives. Transetherification selectivity of naphthazarin methoxy derivatives. Russ. J. Org. Chem. 2014, 50, 647–653. [Google Scholar] [CrossRef]
- Tamaki, H.; Harashima, N.; Hiraki, M.; Arichi, N.; Nishimura, N.; Shiina, H.; Naora, K.; Harada, M. Bcl-2 family inhibition sensitizes human prostate cancer cells to docetaxel and promotes unexpected apoptosis under caspase-9 inhibition. Oncotarget 2014, 5, 11399–11412. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhu, Y.; Lou, W.; Nadiminty, N.; Chen, X.; Zhou, Q.; Shi, X.B.; deVere White, R.W.; Gao, A.C. Functional p53 determines docetaxel sensitivity in prostate cancer cells. Prostate 2013, 73, 418–427. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Otte, K.; Tabakmakher, K.M.; Hauschild, J.; Makarieva, T.N.; Shubina, L.K.; Fedorov, S.N.; Bokemeyer, C.; Stonik, V.A.; von Amsberg, G. Synthesis and anticancer activity of the derivatives of marine compound rhizochalin in castration resistant prostate cancer. Oncotarget 2018, 9, 16962–16973. [Google Scholar] [CrossRef]
- Kupcsik, L. Cell viability analysis using trypan blue: Manual and automated methods. In Mammalian Cell Viability: Methods and Protocols; Stoddart, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 13–19. [Google Scholar]
- Louis, K.S.; Siegel, A.C. Cell viability analysis using trypan blue: Manual and automated methods. In Mammalian Cell Viability: Methods and Protocols; Stoddart, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 7–12. [Google Scholar]
- Kung, H.-J.; Changou, C.; Nguyen, H.G.; Yang, J.C.; Evans, C.P.; Bold, R.J.; Chuang, F. Autophagy and prostate cancer therapeutics. In Prostate Cancer; Tindal, D.J., Ed.; Springer: New York, NY, USA, 2013; Volume 16, pp. 497–518. [Google Scholar]
- Farrow, J.M.; Yang, J.C.; Evans, C.P. Autophagy as a modulator and target in prostate cancer. Nat. Rev. Urol. 2014, 11, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, P.S. Targeting the androgen receptor in prostate cancer—a resilient foe. New Engl. J. Med. 2014, 371, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Fedor, H.L.; Lotan, T.L.; et al. Ar-v7 and resistance to enzalutamide and abiraterone in prostate cancer. New Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- Stephens, P.; Hunter, C.; Bignell, G.; Edkins, S.; Davies, H.; Teague, J.; Stevens, C.; O’Meara, S.; Smith, R.; Parker, A.; et al. Lung cancer: Intragenic erbb2 kinase mutations in tumours. Nature 2004, 431, 525–526. [Google Scholar] [CrossRef]
- Hagen, J.; Muniz, V.P.; Falls, K.C.; Reed, S.M.; Taghiyev, A.F.; Quelle, F.W.; Gourronc, F.A.; Klingelhutz, A.J.; Major, H.J.; Askeland, R.W.; et al. Rabl6a promotes g1-s phase progression and pancreatic neuroendocrine tumor cell proliferation in an rb1-dependent manner. Cancer Res. 2014, 74, 6661–6670. [Google Scholar] [CrossRef]
- Hartman, M.L.; Czyz, M. Mitf in melanoma: Mechanisms behind its expression and activity. Cell. Mol. Life Sci. 2015, 72, 1249–1260. [Google Scholar] [CrossRef]
- Lee, D.K.; Chang, C. Expression and degradation of androgen receptor: Mechanism and clinical implication. J. Clin. Endocrinol. Metab. 2003, 88, 4043–4054. [Google Scholar] [CrossRef]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef]
- Dan, N.; Setua, S.; Kashyap, V.K.; Khan, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Antibody-drug conjugates for cancer therapy: Chemistry to clinical implications. Pharmaceuticals (Basel) 2018, 11, 32. [Google Scholar] [CrossRef]
- Vlahov, I.R.; Leamon, C.P. Engineering folate–drug conjugates to target cancer: From chemistry to clinic. Bioconjug. Chem. 2012, 23, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials. U.S. National Library of Medicine, National Institutes of Health. 2019. Available online: https://clinicaltrials.gov/ct2/results?cond=glufosfamide&term=&cntry=&state=&city=&dist= (accessed on 2 October 2019).
- Cutruzzola, F.; Giardina, G.; Marani, M.; Macone, A.; Paiardini, A.; Rinaldo, S.; Paone, A. Glucose metabolism in the progression of prostate cancer. Front. Physiol. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M.; Makepeace, C. Model of the exofacial substrate-binding site and helical folding of the human glut1 glucose transporter based on scanning mutagenesis. Biochemistry (Mosc). 2009, 48, 5934–5942. [Google Scholar] [CrossRef]
- Barnett, J.E.; Holman, G.D.; Munday, K.A. Structural requirements for binding to the sugar-transport system of the human erythrocyte. Biochem. J. 1973, 131, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Pokhilo, N.D.; Atopkina, L.N.; Kiseleva, M.I.; Denisenko, V.A.; Anufriev, V.P. Synthesis and cytotoxic evaluation of glucoconjugated ethylmompain derivatives. Nat. Prod. Commun. 2017, 12, 1475–1478. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Gao, A.C. Drug resistance in castration resistant prostate cancer: Resistance mechanisms and emerging treatment strategies. Am. J. Clin. Exp. Urol 2015, 3, 64–76. [Google Scholar]
- Boudadi, K.; Antonarakis, E.S. Resistance to novel antiandrogen therapies in metastatic castration-resistant prostate cancer. Clin. Med. Insights Oncol. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef]
- Battogtokh, G.; Choi, Y.S.; Kang, D.S.; Park, S.J.; Shim, M.S.; Huh, K.M.; Cho, Y.Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: Current strategies and future perspectives. Acta Pharm. Sin. B 2018, 8, 862–880. [Google Scholar] [CrossRef]
- Wang, J.; Fan, X.-Y.; Yang, L.-Y.; He, H.; Huang, R.; Jiang, F.-L.; Liu, Y. Conjugated 5-fluorouracil with mitochondria-targeting lipophilic cation: Design, synthesis and biological evaluation. Med. Chem. Comm. 2016, 7, 2016–2019. [Google Scholar] [CrossRef]
- Chamberlain, G.R.; Tulumello, D.V.; Kelley, S.O. Targeted delivery of doxorubicin to mitochondria. ACS Chem. Biol. 2013, 8, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Yu, M. Generation, function and diagnostic value of mitochondrial DNA copy number alterations in human cancers. Life Sci. 2011, 89, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Tabakmakher, K.M.; Hauschild, J.; Shchekaleva, R.K.; Otte, K.; Guzii, A.G.; Makarieva, T.N.; Kudryashova, E.K.; Fedorov, S.N.; Shubina, L.K.; et al. Guanidine alkaloids from the marine sponge monanchora pulchra show cytotoxic properties and prevent egf-induced neoplastic transformation in vitro. Mar. Drugs 2016, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Menchinskaya, E.S.; Venz, S.; Rast, S.; Amann, K.; Hauschild, J.; Otte, K.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; et al. The marine triterpene glycoside frondoside a exhibits activity in vitro and in vivo in prostate cancer. Int. J. Cancer 2016, 138, 2450–2465. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Fedorov, S.N.; Kalinovsky, A.I.; Shubina, L.K.; Bokemeyer, C.; Stonik, V.A.; Honecker, F. Mycalamide a shows cytotoxic properties and prevents egf-induced neoplastic transformation through inhibition of nuclear factors. Mar. Drugs 2012, 10, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.; Rast, S.; Hauschild, J.; Otte, K.; Alsdorf, W.; Madanchi, R.; Kalinin, V.; Silchenko, A.; Avilov, S.; Dierlamm, J.; et al. Frondoside a induces aif-associated caspase-independent apoptosis in burkitt’s lymphoma cells. Leuk. Lymphoma 2017, 1–11. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyshlovoy, S.A.; Pelageev, D.N.; Hauschild, J.; Borisova, K.L.; Kaune, M.; Krisp, C.; Venz, S.; Sabutskii, Y.E.; Khmelevskaya, E.A.; Busenbender, T.; et al. Successful Targeting of the Warburg Effect in Prostate Cancer by Glucose-Conjugated 1,4-Naphthoquinones. Cancers 2019, 11, 1690. https://doi.org/10.3390/cancers11111690
Dyshlovoy SA, Pelageev DN, Hauschild J, Borisova KL, Kaune M, Krisp C, Venz S, Sabutskii YE, Khmelevskaya EA, Busenbender T, et al. Successful Targeting of the Warburg Effect in Prostate Cancer by Glucose-Conjugated 1,4-Naphthoquinones. Cancers. 2019; 11(11):1690. https://doi.org/10.3390/cancers11111690
Chicago/Turabian StyleDyshlovoy, Sergey A., Dmitry N. Pelageev, Jessica Hauschild, Ksenia L. Borisova, Moritz Kaune, Christoph Krisp, Simone Venz, Yurii E. Sabutskii, Ekaterina A. Khmelevskaya, Tobias Busenbender, and et al. 2019. "Successful Targeting of the Warburg Effect in Prostate Cancer by Glucose-Conjugated 1,4-Naphthoquinones" Cancers 11, no. 11: 1690. https://doi.org/10.3390/cancers11111690
APA StyleDyshlovoy, S. A., Pelageev, D. N., Hauschild, J., Borisova, K. L., Kaune, M., Krisp, C., Venz, S., Sabutskii, Y. E., Khmelevskaya, E. A., Busenbender, T., Denisenko, V. A., Pokhilo, N. D., Atopkina, L. N., Graefen, M., Schlüter, H., Stonik, V. A., Bokemeyer, C., Anufriev, V. P., & von Amsberg, G. (2019). Successful Targeting of the Warburg Effect in Prostate Cancer by Glucose-Conjugated 1,4-Naphthoquinones. Cancers, 11(11), 1690. https://doi.org/10.3390/cancers11111690








