Targeting Negative and Positive Immune Checkpoints with Monoclonal Antibodies in Therapy of Cancer
Abstract
:1. Introduction
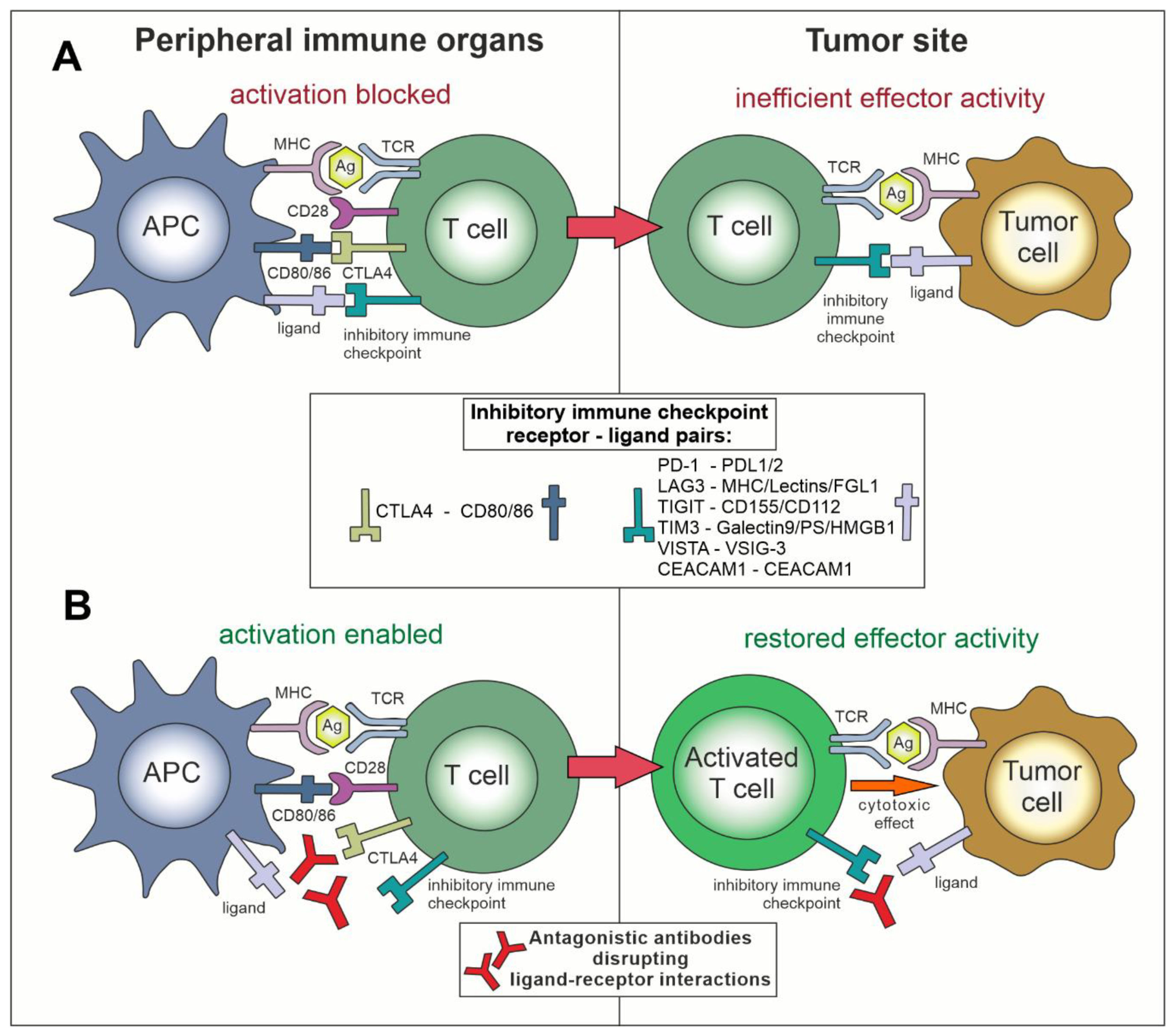
2. Immune Checkpoints
3. Inhibition of Negative Immune Checkpoints in Cancer
3.1. Role of “Classical” Immune Checkpoints—CTLA-4 and PD-L1/PD-1 in Cancer—Early Studies
3.2. Formats of the Anti-Immune Checkpoint Antibodies
3.3. Clinical Application of Anti-CTLA-4, Anti-PD-1 and Anti-PD-L1 Antibodies
3.4. Recent Developments in the Application of Anti-CTLA-4 Antibodies
3.5. Adverse Effects of Immune Checkpoint Inhibition
3.6. Combination Therapies Using Checkpoint Inhibitors
3.7. Antibodies Against Novel Negative Checkpoints
4. Application of Agonistic Compounds towards Positive Immune Checkpoints in Cancer
5. Perspectives in Immune Checkpoint Targeting—Bispecific Antibodies
- Redirectors of cytotoxic effector cells—these bsAb bind to the tumor-associated antigen (a checkpoint molecule in this case) and the molecule responsible for activation of the effector cells (e.g., CD3 on T/NKT cells or CD16 on NK/NKT cells). Such bsAb are also referred to as bi-specific T cell engager (BiTE) or bi-specific killer cell engager (BiKE);
- Dual immunomodulators—the principle of action of these bsAb is to bind two checkpoint molecules simultaneously, usually on the same cell.
- Tumor-associated antigen-targeted immunomodulators—these bsAb bind to the tumor-associated antigen (on cancer cell) and a checkpoint molecule (e.g., a positive immune checkpoint on the effector cell). The difference between these bsAb and redirectors of cytotoxic effector cells is that they would not discriminate between the effector cell type, as long as the positive immune checkpoint molecule is expressed.
6. Summary and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Pento, J.T. Monoclonal Antibodies for the Treatment of Cancer. Anticancer Res. 2017, 37, 5935–5939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natoli, G.; Ostuni, R. Adaptation and memory in immune responses. Nat. Immunol. 2019, 20, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Zinkernagel, R.M.; Doherty, P.C. The discovery of MHC restriction. Immunol. Today 1997, 18, 14–17. [Google Scholar] [CrossRef]
- Somoza, C.; Lanier, L.L. T-cell costimulation via CD28-CD80/CD86 and CD40-CD40 ligand interactions. Res. Immunol. 1995, 146, 171–176. [Google Scholar] [CrossRef]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010, 22, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Ceeraz, S.; Nowak, E.C.; Burns, C.M.; Noelle, R.J. Immune checkpoint receptors in regulating immune reactivity in rheumatic disease. Arthritis Res. Ther. 2014, 16, 469. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [Green Version]
- Zarour, H.M. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin. Cancer Res. 2016, 22, 1856–1864. [Google Scholar] [CrossRef] [Green Version]
- Donini, C.; D’Ambrosio, L.; Grignani, G.; Aglietta, M.; Sangiolo, D. Next generation immune-checkpoints for cancer therapy. J. Thorac. Dis. 2018, 10, S1581–S1601. [Google Scholar] [CrossRef]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Khattri, R.; Auger, J.A.; Griffin, M.D.; Sharpe, A.H.; Bluestone, J.A. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J. Immunol. 1999, 162, 5784–5791. [Google Scholar] [PubMed]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Ribas, A. Anti-CTLA4 Antibody Clinical Trials in Melanoma. Update Cancer Ther. 2007, 2, 133–139. [Google Scholar] [CrossRef]
- Nishimura, H.; Honjo, T.; Minato, N. Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J. Exp. Med. 2000, 191, 891–898. [Google Scholar] [CrossRef]
- Guleria, I.; Khosroshahi, A.; Ansari, M.J.; Habicht, A.; Azuma, M.; Yagita, H.; Noelle, R.J.; Coyle, A.; Mellor, A.L.; Khoury, S.J.; et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 2005, 202, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef]
- Ikeda, S.; Okamoto, T.; Okano, S.; Umemoto, Y.; Tagawa, T.; Morodomi, Y.; Kohno, M.; Shimamatsu, S.; Kitahara, H.; Suzuki, Y.; et al. PD-L1 Is Upregulated by Simultaneous Amplification of the PD-L1 and JAK2 Genes in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Shiraishi, Y.; Takeda, Y.; Sakata, S.; Matsumoto, M.; Nagano, S.; Maeda, T.; Nagata, Y.; Kitanaka, A.; Mizuno, S.; et al. Aberrant PD-L1 expression through 3’-UTR disruption in multiple cancers. Nature 2016, 534, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Atefi, M.; Avramis, E.; Lassen, A.; Wong, D.J.; Robert, L.; Foulad, D.; Cerniglia, M.; Titz, B.; Chodon, T.; Graeber, T.G.; et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin. Cancer Res. 2014, 20, 3446–3457. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.G.; Jiang, X.N.; Sheng, D.; Sun, C.B.; Lee, J.; Zhou, X.Y.; Li, X.Q. PD-L1 over-expression is driven by B-cell receptor signaling in diffuse large B-cell lymphoma. Lab. Invest. 2019, 99, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhang, J.; Hong, S.; Zhan, J.; Chen, N.; Qin, T.; Tang, Y.; Zhang, Y.; Kang, S.; Zhou, T.; et al. EBV-driven LMP1 and IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget 2014, 5, 12189–12202. [Google Scholar] [CrossRef] [PubMed]
- Mazanet, M.M.; Hughes, C.C. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J. Immunol. 2002, 169, 3581–3588. [Google Scholar] [CrossRef] [PubMed]
- Timeline of Anti-PD-1/L1 Antibody Approvals by the FDA. Available online: https://public.tableau.com/profile/jia.yu7083#!/vizhome/2018-12-07PD-1approvaltimeline/PD-1approvallandscape (accessed on 30 September 2019).
- Kellner, C.; Otte, A.; Cappuzzello, E.; Klausz, K.; Peipp, M. Modulating Cytotoxic Effector Functions by Fc Engineering to Improve Cancer Therapy. Transfus. Med. Hemother. 2017, 44, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Julia, E.P.; Amante, A.; Pampena, M.B.; Mordoh, J.; Levy, E.M. Avelumab, an IgG1 anti-PD-L1 Immune Checkpoint Inhibitor, Triggers NK Cell-Mediated Cytotoxicity and Cytokine Production Against Triple Negative Breast Cancer Cells. Front. Immunol. 2018, 9, 2140. [Google Scholar] [CrossRef]
- Dahlen, E.; Veitonmaki, N.; Norlen, P. Bispecific antibodies in cancer immunotherapy. Ther. Adv. Vaccines Immunother. 2018, 6, 3–17. [Google Scholar] [CrossRef]
- Sau, S.; Petrovici, A.; Alsaab, H.O.; Bhise, K.; Iyer, A.K. PDL-1 Antibody Drug Conjugate for Selective Chemo-Guided Immune Modulation of Cancer. Cancers 2019, 11, 232. [Google Scholar] [CrossRef]
- Tunger, A.; Sommer, U.; Wehner, R.; Kubasch, A.S.; Grimm, M.O.; Bachmann, M.P.; Platzbecker, U.; Bornhauser, M.; Baretton, G.; Schmitz, M. The Evolving Landscape of Biomarkers for Anti-PD-1 or Anti-PD-L1 Therapy. J. Clin. Med. 2019, 8, 1534. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Chen, Z.; Wang, Y.; Yang, W.; Hu, Y.; Han, W.; Zeng, H.; Ma, H.; Dai, J.; et al. PD-L1 expression and tumor mutational burden status for prediction of response to chemotherapy and targeted therapy in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2019, 38, 193. [Google Scholar] [CrossRef] [PubMed]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef] [PubMed]
- Pai, C.S.; Simons, D.M.; Lu, X.; Evans, M.; Wei, J.; Wang, Y.H.; Chen, M.; Huang, J.; Park, C.; Chang, A.; et al. Tumor-conditional anti-CTLA4 uncouples antitumor efficacy from immunotherapy-related toxicity. J. Clin. Investig. 2019, 129, 349–363. [Google Scholar] [CrossRef]
- Savoia, P.; Astrua, C.; Fava, P. Ipilimumab (Anti-Ctla-4 Mab) in the treatment of metastatic melanoma: Effectiveness and toxicity management. Hum. Vaccin. Immunother. 2016, 12, 1092–1101. [Google Scholar] [CrossRef] [Green Version]
- Vogrig, A.; Fouret, M.; Joubert, B.; Picard, G.; Rogemond, V.; Pinto, A.L.; Muniz-Castrillo, S.; Roger, M.; Raimbourg, J.; Dayen, C.; et al. Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6. [Google Scholar] [CrossRef]
- Champion, S.N.; Stone, J.R. Immune checkpoint inhibitor associated myocarditis occurs in both high-grade and low-grade forms. Mod. Pathol. 2019. [Google Scholar] [CrossRef]
- Pauken, K.E.; Dougan, M.; Rose, N.R.; Lichtman, A.H.; Sharpe, A.H. Adverse Events Following Cancer Immunotherapy: Obstacles and Opportunities. Trends Immunol. 2019, 40, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.F.; Proverbs-Singh, T.A.; Postow, M.A. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncol. 2016, 2, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Maloney, N.J.; Worswick, S. Neutrophilic dermatoses as adverse effects of checkpoint inhibitors: A review. Derm. Ther. 2019, e13074. [Google Scholar] [CrossRef] [PubMed]
- Bobrowicz, M.; Zagozdzon, R.; Domagala, J.; Vasconcelos-Berg, R.; Guenova, E.; Winiarska, M. Monoclonal Antibodies in Dermatooncology-State of the Art and Future Perspectives. Cancers 2019, 11, 1420. [Google Scholar] [CrossRef]
- D’Errico, G.; Machado, H.L.; Sainz, B., Jr. A current perspective on cancer immune therapy: Step-by-step approach to constructing the magic bullet. Clin. Transl. Med. 2017, 6, 3. [Google Scholar] [CrossRef]
- Elias, A.W.; Kasi, P.M.; Stauffer, J.A.; Thiel, D.D.; Colibaseanu, D.T.; Mody, K.; Joseph, R.W.; Bagaria, S.P. The Feasibility and Safety of Surgery in Patients Receiving Immune Checkpoint Inhibitors: A Retrospective Study. Front. Oncol. 2017, 7, 121. [Google Scholar] [CrossRef]
- Lamichhane, P.; Amin, N.P.; Agarwal, M.; Lamichhane, N. Checkpoint Inhibition: Will Combination with Radiotherapy and Nanoparticle-Mediated Delivery Improve Efficacy? Medicines 2018, 5, 114. [Google Scholar] [CrossRef]
- Wang, C.; Kulkarni, P.; Salgia, R. Combined Checkpoint Inhibition and Chemotherapy: New Era of 1(st)-Line Treatment for Non-Small-Cell Lung Cancer. Mol. Ther. Oncolytics 2019, 13, 1–6. [Google Scholar] [CrossRef]
- Adderley, H.; Blackhall, F.H.; Lindsay, C.R. KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine 2019, 41, 711–716. [Google Scholar] [CrossRef] [Green Version]
- Geynisman, D.M.; Chien, C.R.; Smieliauskas, F.; Shen, C.; Shih, Y.C. Economic evaluation of therapeutic cancer vaccines and immunotherapy: A systematic review. Hum. Vaccin. Immunother. 2014, 10, 3415–3424. [Google Scholar] [CrossRef] [Green Version]
- Barroso-Sousa, R.; Ott, P.A. Transformation of Old Concepts for a New Era of Cancer Immunotherapy: Cytokine Therapy and Cancer Vaccines as Combination Partners of PD1/PD-L1 Inhibitors. Curr. Oncol. Rep. 2018, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Lai, X. Combination therapy for cancer with oncolytic virus and checkpoint inhibitor: A mathematical model. PLoS ONE 2018, 13, e0192449. [Google Scholar] [CrossRef] [PubMed]
- Mullinax, J.E.; Hall, M.; Prabhakaran, S.; Weber, J.; Khushalani, N.; Eroglu, Z.; Brohl, A.S.; Markowitz, J.; Royster, E.; Richards, A.; et al. Combination of Ipilimumab and Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes for Patients with Metastatic Melanoma. Front. Oncol. 2018, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Zych, A.O.; Bajor, M.; Zagozdzon, R. Application of Genome Editing Techniques in Immunology. Arch. Immunol. Ther. Exp. (Warsz.) 2018, 66, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Serganova, I.; Moroz, E.; Cohen, I.; Moroz, M.; Mane, M.; Zurita, J.; Shenker, L.; Ponomarev, V.; Blasberg, R. Enhancement of PSMA-Directed CAR Adoptive Immunotherapy by PD-1/PD-L1 Blockade. Mol. Ther. Oncolytics 2017, 4, 41–54. [Google Scholar] [CrossRef]
- Tundo, G.R.; Sbardella, D.; Lacal, P.M.; Graziani, G.; Marini, S. On the Horizon: Targeting Next-Generation Immune Checkpoints for Cancer Treatment. Chemotherapy 2019, 1–19. [Google Scholar] [CrossRef]
- Long, L.; Zhang, X.; Chen, F.; Pan, Q.; Phiphatwatchara, P.; Zeng, Y.; Chen, H. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer 2018, 9, 176–189. [Google Scholar] [CrossRef]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019, 176, 334–347.e312. [Google Scholar] [CrossRef]
- Zelba, H.; Bedke, J.; Hennenlotter, J.; Mostbock, S.; Zettl, M.; Zichner, T.; Chandran, P.A.; Stenzl, A.; Rammensee, H.G.; Gouttefangeas, C. PD-1 and LAG-3 dominate checkpoint receptor-mediated T cell inhibition in renal cell carcinoma. Cancer Immunol. Res. 2019. [Google Scholar] [CrossRef]
- Woo, S.R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef]
- Puhr, H.C.; Ilhan-Mutlu, A. New emerging targets in cancer immunotherapy: The role of LAG3. ESMO Open 2019, 4, e000482. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Melero, I.; Bhatia, S.; Bono, P.; Sanborn, R.E.; Lipson, E.J.; Callahan, M.K.; Gajewski, T.; Gomez-Roca, C.A.; Hodi, F.S.; et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. J. Clin. Oncol. 2017, 35, 9520. [Google Scholar] [CrossRef]
- Manieri, N.A.; Chiang, E.Y.; Grogan, J.L. TIGIT: A Key Inhibitor of the Cancer Immunity Cycle. Trends Immunol. 2017, 38, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.L.; Garrido-Laguna, I. TIGIT: A novel immunotherapy target moving from bench to bedside. Cancer Immunol. Immunother. 2018, 67, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef]
- Hung, A.L.; Maxwell, R.; Theodros, D.; Belcaid, Z.; Mathios, D.; Luksik, A.S.; Kim, E.; Wu, A.; Xia, Y.; Garzon-Muvdi, T.; et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology 2018, 7, e1466769. [Google Scholar] [CrossRef]
- Friedlaender, A.; Addeo, A.; Banna, G. New emerging targets in cancer immunotherapy: The role of TIM3. ESMO Open 2019, 4, e000497. [Google Scholar] [CrossRef]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Sakuishi, K.; Ngiow, S.F.; Sullivan, J.M.; Teng, M.W.; Kuchroo, V.K.; Smyth, M.J.; Anderson, A.C. TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology 2013, 2, e23849. [Google Scholar] [CrossRef]
- Zhou, G.; Sprengers, D.; Boor, P.P.C.; Doukas, M.; Schutz, H.; Mancham, S.; Pedroza-Gonzalez, A.; Polak, W.G.; de Jonge, J.; Gaspersz, M.; et al. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology 2017, 153, 1107–1119 e1110. [Google Scholar] [CrossRef]
- Liu, J.F.; Ma, S.R.; Mao, L.; Bu, L.L.; Yu, G.T.; Li, Y.C.; Huang, C.F.; Deng, W.W.; Kulkarni, A.B.; Zhang, W.F.; et al. T-cell immunoglobulin mucin 3 blockade drives an antitumor immune response in head and neck cancer. Mol. Oncol. 2017, 11, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Sabatos-Peyton, C.A.; Nevin, J.; Brock, A.; Venable, J.D.; Tan, D.J.; Kassam, N.; Xu, F.; Taraszka, J.; Wesemann, L.; Pertel, T.; et al. Blockade of Tim-3 binding to phosphatidylserine and CEACAM1 is a shared feature of anti-Tim-3 antibodies that have functional efficacy. Oncoimmunology 2018, 7, e1385690. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef] [PubMed]
- Lines, J.L.; Pantazi, E.; Mak, J.; Sempere, L.F.; Wang, L.; O’Connell, S.; Ceeraz, S.; Suriawinata, A.A.; Yan, S.; Ernstoff, M.S.; et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014, 74, 1924–1932. [Google Scholar] [CrossRef]
- Nowak, E.C.; Lines, J.L.; Varn, F.S.; Deng, J.; Sarde, A.; Mabaera, R.; Kuta, A.; Le Mercier, I.; Cheng, C.; Noelle, R.J. Immunoregulatory functions of VISTA. Immunol. Rev. 2017, 276, 66–79. [Google Scholar] [CrossRef]
- Mulati, K.; Hamanishi, J.; Matsumura, N.; Chamoto, K.; Mise, N.; Abiko, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Murakami, R.; et al. VISTA expressed in tumour cells regulates T cell function. Br. J. Cancer 2019, 120, 115–127. [Google Scholar] [CrossRef]
- Blando, J.; Sharma, A.; Higa, M.G.; Zhao, H.; Vence, L.; Yadav, S.S.; Kim, J.; Sepulveda, A.M.; Sharp, M.; Maitra, A.; et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 1692–1697. [Google Scholar] [CrossRef] [Green Version]
- Ortenberg, R.; Sapir, Y.; Raz, L.; Hershkovitz, L.; Ben Arav, A.; Sapoznik, S.; Barshack, I.; Avivi, C.; Berkun, Y.; Besser, M.J.; et al. Novel immunotherapy for malignant melanoma with a monoclonal antibody that blocks CEACAM1 homophilic interactions. Mol. Cancer Ther. 2012, 11, 1300–1310. [Google Scholar] [CrossRef]
- Nichita, L.; Zurac, S.; Bastian, A.; Stinga, P.; Nedelcu, R.; Brinzea, A.; Turcu, G.; Ion, D.; Jilaveanu, L.; Sticlaru, L.; et al. Comparative analysis of CEACAM1 expression in thin melanomas with and without regression. Oncol. Lett. 2019, 17, 4149–4154. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Duan, Y.; Liu, Y.; Wang, H.; Lian, S.; Zhuang, G.; Fan, Y. Combined Blockade of T Cell Immunoglobulin and Mucin Domain 3 and Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 Results in Durable Therapeutic Efficacy in Mice with Intracranial Gliomas. Med. Sci. Monit. 2017, 23, 3593–3602. [Google Scholar] [CrossRef] [Green Version]
- Markel, G.; Sapir, Y.; Mandel, I.; Hakim, M.; Shaked, R.; Meilin, E.; McClanahan, T.; Loboda, A.; Hashmueli, S.; Moshe, T.B. Inhibition of the novel immune checkpoint CEACAM1 to enhance anti-tumor immunological activity. J. Clin. Oncol. 2016, 34, 3044. [Google Scholar] [CrossRef]
- McLeod, R.L.; Angagaw, M.H.; Baral, T.N.; Liu, L.; Moniz, R.J.; Laskey, J.; Hsieh, S.; Lee, M.; Han, J.H.; Issafras, H.; et al. Characterization of murine CEACAM1 in vivo reveals low expression on CD8(+) T cells and no tumor growth modulating activity by anti-CEACAM1 mAb CC1. Oncotarget 2018, 9, 34459–34470. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Pyo, K.-H.; Jung, M.-J.; Hur, M.; Won, J.; Cho, B.C. Abstract 2266: Efficacy of CEACAM1-targeting immunoglobulin in combination with pembrolizumab in lung cancer. Cancer Res. 2019, 79, 2266. [Google Scholar] [CrossRef]
- Helfrich, I.; Singer, B.B. Size Matters: The Functional Role of the CEACAM1 Isoform Signature and Its Impact for NK Cell-Mediated Killing in Melanoma. Cancers 2019, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.E. Revisiting CD28 Superagonist TGN1412 as Potential Therapeutic for Pediatric B Cell Leukemia: A Review. Diseases 2018, 6, 41. [Google Scholar] [CrossRef]
- Suntharalingam, G.; Perry, M.R.; Ward, S.; Brett, S.J.; Castello-Cortes, A.; Brunner, M.D.; Panoskaltsis, N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006, 355, 1018–1028. [Google Scholar] [CrossRef]
- Stebbings, R.; Poole, S.; Thorpe, R. Safety of biologics, lessons learnt from TGN1412. Curr. Opin. Biotechnol. 2009, 20, 673–677. [Google Scholar] [CrossRef]
- Redmond, W.L.; Ruby, C.E.; Weinberg, A.D. The role of OX40-mediated co-stimulation in T-cell activation and survival. Crit. Rev. Immunol. 2009, 29, 187–201. [Google Scholar] [CrossRef]
- Aspeslagh, S.; Postel-Vinay, S.; Rusakiewicz, S.; Soria, J.C.; Zitvogel, L.; Marabelle, A. Rationale for anti-OX40 cancer immunotherapy. Eur. J. Cancer 2016, 52, 50–66. [Google Scholar] [CrossRef]
- Curti, B.D.; Kovacsovics-Bankowski, M.; Morris, N.; Walker, E.; Chisholm, L.; Floyd, K.; Walker, J.; Gonzalez, I.; Meeuwsen, T.; Fox, B.A.; et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013, 73, 7189–7198. [Google Scholar] [CrossRef]
- Sagiv-Barfi, I.; Czerwinski, D.K.; Levy, S.; Alam, I.S.; Mayer, A.T.; Gambhir, S.S.; Levy, R. Eradication of spontaneous malignancy by local immunotherapy. Sci. Transl. Med. 2018, 10, eaan4488. [Google Scholar] [CrossRef] [PubMed]
- Chester, C.; Sanmamed, M.F.; Wang, J.; Melero, I. Immunotherapy targeting 4-1BB: Mechanistic rationale, clinical results, and future strategies. Blood 2018, 131, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, A.; Dutt, S.; Chester, C.; Kim, J.; Kohrt, H.E. Boosting Cancer Immunotherapy with Anti-CD137 Antibody Therapy. Clin. Cancer Res. 2015, 21, 3113–3120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Ruiz, E.; Etxeberria, I.; Rodriguez-Ruiz, M.E.; Melero, I. Anti-CD137 and PD-1/PD-L1 Antibodies En Route toward Clinical Synergy. Clin. Cancer Res. 2017, 23, 5326–5328. [Google Scholar] [CrossRef]
- Chin, S.M.; Kimberlin, C.R.; Roe-Zurz, Z.; Zhang, P.; Xu, A.; Liao-Chan, S.; Sen, D.; Nager, A.R.; Oakdale, N.S.; Brown, C.; et al. Structure of the 4-1BB/4-1BBL complex and distinct binding and functional properties of utomilumab and urelumab. Nat. Commun. 2018, 9, 4679. [Google Scholar] [CrossRef]
- Tran, B.; Carvajal, R.D.; Marabelle, A.; Patel, S.P.; LoRusso, P.M.; Rasmussen, E.; Juan, G.; Upreti, V.V.; Beers, C.; Ngarmchamnanrith, G.; et al. Dose escalation results from a first-in-human, phase 1 study of glucocorticoid-induced TNF receptor-related protein agonist AMG 228 in patients with advanced solid tumors. J. Immunother. Cancer 2018, 6, 93. [Google Scholar] [CrossRef]
- Zappasodi, R.; Sirard, C.; Li, Y.; Budhu, S.; Abu-Akeel, M.; Liu, C.; Yang, X.; Zhong, H.; Newman, W.; Qi, J.; et al. Rational design of anti-GITR-based combination immunotherapy. Nat. Med. 2019, 25, 759–766. [Google Scholar] [CrossRef]
- Dong, C.; Juedes, A.E.; Temann, U.A.; Shresta, S.; Allison, J.P.; Ruddle, N.H.; Flavell, R.A. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 2001, 409, 97–101. [Google Scholar] [CrossRef]
- Amatore, F.; Gorvel, L.; Olive, D. Inducible Co-Stimulator (ICOS) as a potential therapeutic target for anti-cancer therapy. Expert Opin. Ther. Targets 2018, 22, 343–351. [Google Scholar] [CrossRef]
- Busse, M.; Krech, M.; Meyer-Bahlburg, A.; Hennig, C.; Hansen, G. ICOS mediates the generation and function of CD4+CD25+Foxp3+ regulatory T cells conveying respiratory tolerance. J. Immunol. 2012, 189, 1975–1982. [Google Scholar] [CrossRef]
- Israel, B.F.; Gulley, M.; Elmore, S.; Ferrini, S.; Feng, W.H.; Kenney, S.C. Anti-CD70 antibodies: A potential treatment for EBV+ CD70-expressing lymphomas. Mol. Cancer 2005, 4, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.A.; Ciavattone, N.G.; Atkinson, R.; Woldergerima, N.; Wolf, J.; Clements, V.K.; Sinha, P.; Poudel, M.; Ostrand-Rosenberg, S. CD3xPDL1 bi-specific T cell engager (BiTE) simultaneously activates T cells and NKT cells, kills PDL1(+) tumor cells, and extends the survival of tumor-bearing humanized mice. Oncotarget 2017, 8, 57964–57980. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, M.P.; Coleman, I.M.; Brown, L.G.; True, L.D.; Kollath, L.; Lakely, B.; Nguyen, H.M.; Yang, Y.C.; da Costa, R.M.G.; Kaipainen, A.; et al. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J. Clin. Investig. 2019, 130, 4492–4505. [Google Scholar] [CrossRef]
- Djureinovic, D.; Hallstrom, B.M.; Horie, M.; Mattsson, J.S.M.; La Fleur, L.; Fagerberg, L.; Brunnstrom, H.; Lindskog, C.; Madjar, K.; Rahnenfuhrer, J.; et al. Profiling cancer testis antigens in non-small-cell lung cancer. JCI Insight 2016, 1, e86837. [Google Scholar] [CrossRef]
- Lee, J.K.; Bangayan, N.J.; Chai, T.; Smith, B.A.; Pariva, T.E.; Yun, S.; Vashisht, A.; Zhang, Q.; Park, J.W.; Corey, E.; et al. Systemic surfaceome profiling identifies target antigens for immune-based therapy in subtypes of advanced prostate cancer. Proc. Natl. Acad. Sci. USA 2018, 115, E4473–E4482. [Google Scholar] [CrossRef] [Green Version]
- Young, A.R.; Duarte, J.D.G.; Coulson, R.; O’Brien, M.; Deb, S.; Lopata, A.; Behren, A.; Mathivanan, S.; Lim, E.; Meeusen, E. Immunoprofiling of Breast Cancer Antigens Using Antibodies Derived from Local Lymph Nodes. Cancers 2019, 11, 682. [Google Scholar] [CrossRef]

| Ligand | Cellular Distribution of the Ligand Expression | Immune Checkpoint Receptor | Cellular Expression of the Receptor Expression |
|---|---|---|---|
| Suppressive (negative) immune checkpoints | |||
| CD80 or CD86 | Antigen-presenting cells | CTLA4 | Activated T cells, Tregs |
| PD-L1 (CD274) or PD-L2 (CD273) | DCs, macrophages, peripheral non-lymphoid tissues | PD-1 | Activated B and T cells, APCs, NK cells |
| MHC class II/Lectins | Antigen-presenting cells | LAG3 | Activated T cells, Tregs, NK cells, B cells, DCs |
| CD155/CD112 | Normal epithelial, endothelial, neuronal, and fibroblastic cells | TIGIT | Activated T cells, Tregs, NK cells |
| Galectin 9/ PtdSer /HMGB1 | Multiple tissues | TIM3 | Activated T cells |
| VSIG-3 | Neurons and glial cells | VISTA | Naïve and activated T cells |
| CEACAM1 | T and NK cells | CEACAM1 | Activated T and NK cells |
| Stimulatory (positive) immune checkpoints | |||
| B7 molecules: CD80 or CD86 | Antigen-presenting cells | CD28 | T cells |
| OX40L | DCs, macrophages, B cells, endothelial cells, smooth muscle cells | OX40 | Activated T cells, Tregs, NK cells, neutrophils |
| CD137L | Antigen-presenting cells | CD137 (4-1BB) | Activated Tcells, NK cells, B cells, DCs, endothelial cells |
| GITRL | Antigen-presenting cells and endothelium | GITR | T and NK cells, Tregs |
| ICOSLG | APCs, B cells, DCs and macrophages | ICOS | Naïve and activated T cells |
| CD70 | Activated lymphocytes | CD27 | Activated T and NK cells |
| Checkpoint Inhibitor | Antibody Format | Examples of Types of Cancers with FDA-Approved Use | Year of First Approval |
|---|---|---|---|
| Ipilimumab | Human anti-CTLA4 IgG1 | Melanoma, renal cell carcinoma, metastatic colorectal cancer | 2011 |
| Pembrolizumab | Humanized anti-PD-1 IgG4 | Melanoma, non-small-cell lung cancer, renal cell carcinoma, urothelial bladder cancer, Hodgkin lymphoma, head and neck cancer, Merkel cell carcinoma, microsatellite instability-high cancer, gastric cancer, hepatocellular carcinoma, cervical cancer, primary mediastinal large B-cell lymphoma | 2014 |
| Nivolumab | Human anti-PD-1 IgG4 | Melanoma, non-small-cell lung cancer, renal cell carcinoma, urothelial bladder cancer, Hodgkin lymphoma, head and neck cancer, colorectal cancer, hepatocellular carcinoma, small cell lung cancer | 2014 |
| Atezolizumab | Humanized anti-PD-L1 IgG1 | Non-small-cell lung cancer, urothelial bladder cancer, small cell lung cancer, breast cancer | 2016 |
| Avelumab | Human anti-PD-L1 IgG1 | Merkel cell carcinoma, urothelial bladder cancer | 2017 |
| Durvalumab | Human anti-PD-L1 IgG1 | Non-small-cell lung cancer, urothelial bladder cancer | 2017 |
| Cemiplimab | Human anti-PD-1 IgG4 | Cutaneous squamous-cell carcinoma | 2018 |
| Receptor | Antagonistic Compounds | Example Clinical Trials (Phase) | Comments |
|---|---|---|---|
| Inhibitory immune checkpoint molecules | |||
| LAG-3 | MGD013 (Anti-PD-1, anti-LAG-3 dual checkpoint inhibitor) | NCT04082364 (Phase 2/3) | HER2-positive gastric cancer or gastroesophageal junction cancer to determine the efficacy of margetuximab combined with anti-HER2 monoclonal antibody and margetuximab combined with anti-HER2 monoclonal antibody or MGD013 and chemotherapy compared to trastuzumab combined with chemotherapy (Cohort B) |
| Relatlimab (BMS-986016) | NCT01968109 (Phase 1) | Administered alone and in combination with nivolumab in patients with solid tumors: non-small cell lung cancer, gastric cancer, hepatocellular carcinoma, renal cell carcinoma, bladder cancer, squamous cell carcinoma of the head and neck, and melanoma. | |
| TIGIT | BGB-A1217 | NCT04047862 (Phase 2) | Evaluation of anti-tumor effect of BGB-A1217 in combination with tislelizumab in patients with advanced solid tumors. |
| BMS-986207 | NCT02913313 (Phase 1/2a) | Advanced or spread solid cancers. Administered alone and in combination with nivolumab | |
| TIM-3 (HAVcr2) | Sym023 | NCT03489343 (Phase 1) | As a monotherapy in patients with locally advanced/unresectable or metastatic solid tumor malignancies or lymphomas |
| TSR-022 | NCT02817633 (Phase 1) | As a monotherapy and in combination with an anti-PD-1 antibody and/or an anti-LAG-3 antibody, in patients with advanced solid tumors | |
| MBG453 | NCT03961971 (Phase 1) | MBG453 with stereotactic radiosurgery and spartalizumab in treating patients with recurrent glioblastoma multiforme | |
| NCT03066648 (Phase 1) | As a monotherapy and in combination with an anti-PD-1 antibody (PDR001) and/or Decitabine in acute myeloid leukemia and high risk myelodysplastic syndromes patients | ||
| VISTA | JNJ-61610588 | NCT02671955 (Phase 1) | Evaluation the safety and tolerability of JNJ-61610588 in participants with advanced cancer—study terminated. |
| CEACAM1 | CM-24 (MK-6018) | NCT02346955 | Advanced or recurrent malignancies, administered as monotherapy or in combination with pembrolizumab—study terminated. |
| Stimulatory immune checkpoint molecules | |||
| CD28 | Theralizumab (TAB08) | NCT03006029 (Phase 1) | Metastatic or unresectable advanced solid malignancies |
| OX40 (CD134) | BMS 986178 | NCT03831295 (Phase 1) | Advanced solid malignancies, combination with TLR9 agonist SD-101 |
| MEDI6469 | NCT02274155 (Phase 1) | Head and neck squamous cell carcinoma | |
| PF-04518600 | NCT03971409 (Phase 2) | Triple negative breast cancer, combination with nivolumab | |
| GSK3174998 | NCT02528357 (Phase 1) | Advanced solid tumors, combination with pembrolizumab | |
| MOXR0916 | NCT02219724 (Phase 1) | Locally advanced or metastatic solid tumors | |
| 4-1BB (CD137) | Utomilumab (PF-05082566) | NCT03364348 (Phase 1) | Advanced HER2-positive breast cancer, combination with trastuzumab |
| NCT02179918 (Phase 1) | Advanced solid tumors, combination with PD-1 inhibitor MK-3475 | ||
| Urelumab (BMS-663513) ES101 | NCT02534506 (Phase 1) | Advanced malignancies, alone or in combination with nivolumab | |
| NCT04009460 (Phase 1) | Advanced solid tumors, anti-PD-L1/4-1BB bispecific antibody | ||
| GITRL | BMS-986156 | NCT02598960 (Phase 1/2) | Advanced solid tumors, alone or with nivolumab |
| TRX-518 | NCT01239134 (Phase 1) | Solid malignancies | |
| NCT02628574 (Phase 1) | Advanced solid tumors, in combination with gemcitabine, pembrolizumab, or nivolumab | ||
| AMG 228 | NCT02437916 (Phase 1) | Advanced solid tumors | |
| ICOSLG, (CD275) | JTX-2011 | NCT02904226 (Phase 1/2) | Advanced solid malignancies, alone or in combination with nivolumab |
| GSK3359609 | NCT02723955 (Phase 1) | Advanced solid tumors, alone or in combination with pembrolizumab | |
| BMS-986226 | NCT03251924 (Phase 1/2) | Advanced solid tumors, alone or in combination with nivolumab or ipilimumab | |
| MEDI-570 | NCT02520791 (Phase 1) | T-cell lymphomas, antagonistic antibody | |
| CD27 | Varlilumab (CDX-1127) | NCT04081688 (Phase 1) NCT02335918 (Phase 1/2) | Non-small cell lung carcinoma, combination with atezolizumab and radiation therapy Five types of solid tumors, combination with nivolumab |
| Antigens | Name | Cancer Type | Reference or Clinical Trial No. |
|---|---|---|---|
| Redirectors of cytotoxic effector cells | |||
| Anti-PD-L1/CD3 | PD-L1-positive human cancers | Preclinical [104] | |
| Dual immunomodulators | |||
| Anti-PD-1/TIM3 | LY3415244 | Advanced solid tumors | NCT03752177 (Phase 1) |
| Anti-PD-1/TIM3 | RO7121661 | Metastatic Melanoma Non-small Cell Lung Cancer (NSCLC) Small Cell Lung Cancer (SCLC) | NCT03708328 (Phase 1) |
| Anti-PD-1/PD-L1 | LY3434172 | Advanced solid tumors | NCT0393695 9(Phase 1) |
| Anti-PD-1/CTLA-4 | AK104 | Gastric Adenocarcinoma Advanced Solid Tumors Gastroesophageal Junction Adenocarcinoma | NCT03852251 (Phase 1/2) |
| Advanced Cancer | NCT03261011 (Phase 1) | ||
| Anti-CTLA-4/OX40 | ATOR-1015 | Advanced and/or Refractory Solid Malignancies | NCT03782467 (Phase 1) |
| Anti-LAG-3/PD-L1 | FS118 | Advanced Cancer Metastatic Cancer | NCT03440437 (Phase 1) |
| Tumor-associated antigen-targeted immunomodulators | |||
| Anti-Her2/4-1BB | PRS343 | HER2-positive Solid Tumors | NCT03330561 (Phase 1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marhelava, K.; Pilch, Z.; Bajor, M.; Graczyk-Jarzynka, A.; Zagozdzon, R. Targeting Negative and Positive Immune Checkpoints with Monoclonal Antibodies in Therapy of Cancer. Cancers 2019, 11, 1756. https://doi.org/10.3390/cancers11111756
Marhelava K, Pilch Z, Bajor M, Graczyk-Jarzynka A, Zagozdzon R. Targeting Negative and Positive Immune Checkpoints with Monoclonal Antibodies in Therapy of Cancer. Cancers. 2019; 11(11):1756. https://doi.org/10.3390/cancers11111756
Chicago/Turabian StyleMarhelava, Katsiaryna, Zofia Pilch, Malgorzata Bajor, Agnieszka Graczyk-Jarzynka, and Radoslaw Zagozdzon. 2019. "Targeting Negative and Positive Immune Checkpoints with Monoclonal Antibodies in Therapy of Cancer" Cancers 11, no. 11: 1756. https://doi.org/10.3390/cancers11111756
APA StyleMarhelava, K., Pilch, Z., Bajor, M., Graczyk-Jarzynka, A., & Zagozdzon, R. (2019). Targeting Negative and Positive Immune Checkpoints with Monoclonal Antibodies in Therapy of Cancer. Cancers, 11(11), 1756. https://doi.org/10.3390/cancers11111756






