Is there an Exposure–Response Relationship for Nivolumab in Real-World NSCLC Patients?
Abstract
:1. Introduction
2. Results
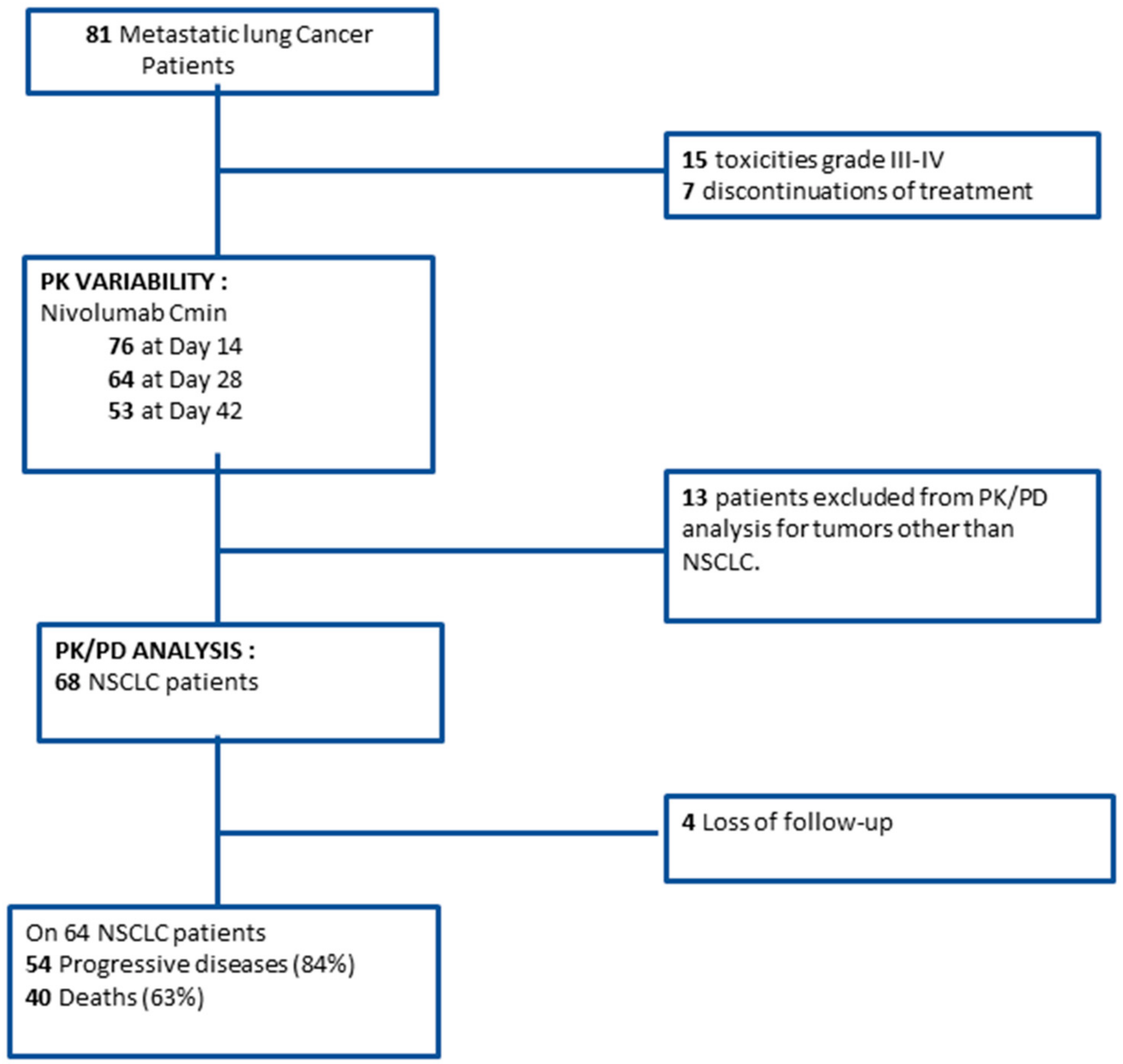
2.1. Study Population
2.2. Pharmacokinetic Variability
2.3. Exposure-Survival Relationship
2.4. Exposure-Toxicity Relationship
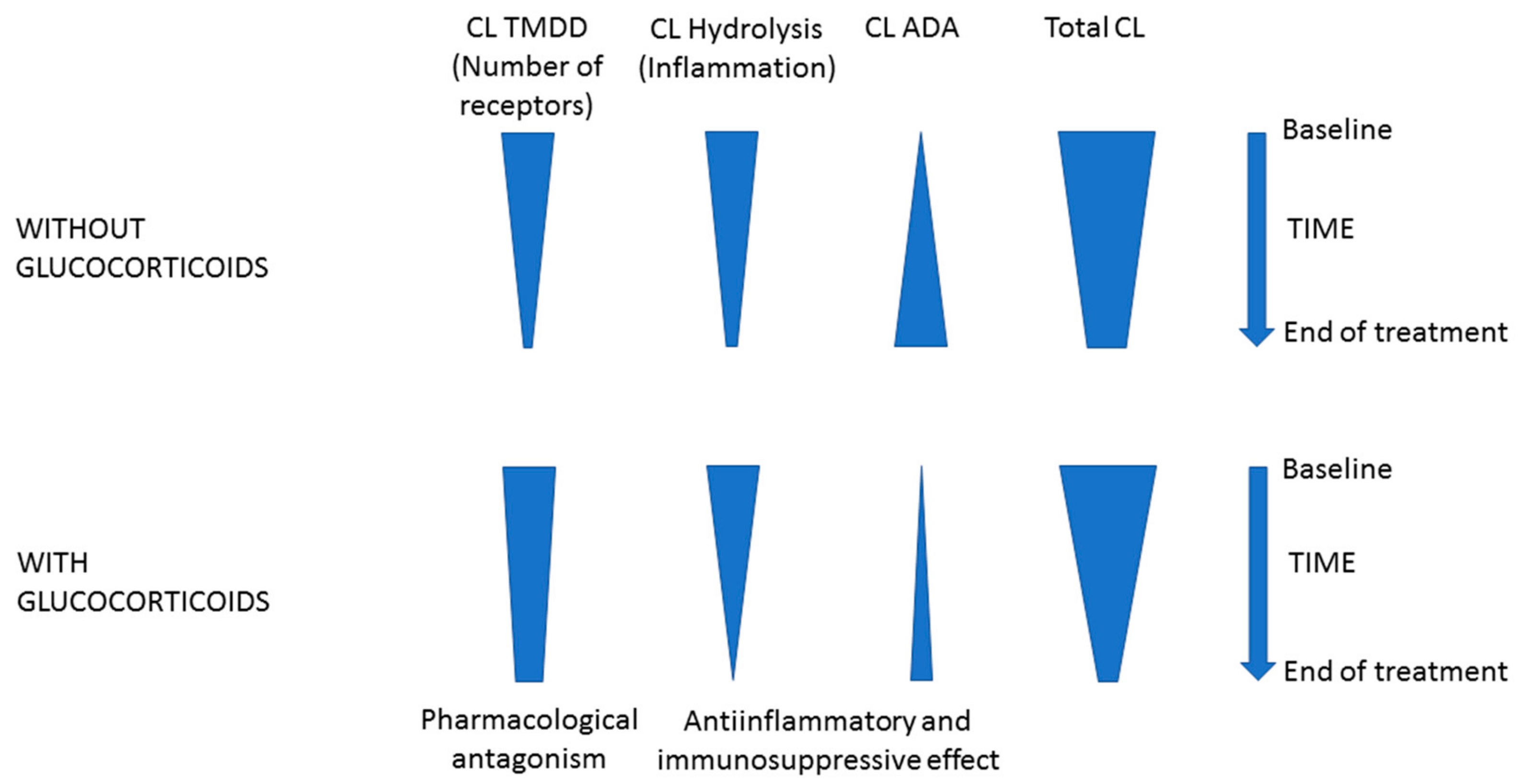
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Treatment
4.3. Pharmacokinetic Measurements
4.4. PD-L1 Expression Analysis
4.5. Study Endpoints
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Sheng, J.; Srivastava, S.; Sanghavi, K.; Lu, Z.; Schmidt, B.J.; Bello, A.; Gupta, M. Clinical Pharmacology Considerations for the Development of Immune Checkpoint Inhibitors. J. Clin. Pharmacol. 2017, 57, S26–S42. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Bassanelli, M.; Sioletic, S.; Martini, M.; Giacinti, S.; Viterbo, A.; Staddon, A.; Liberati, F.; Ceribelli, A. Heterogeneity of PD-L1 Expression and Relationship with Biology of NSCLC. Anticancer Res. 2018, 38, 3789–3796. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Bajaj, G.; Agrawal, S.; Bello, A.; Lestini, B.; Finckenstein, F.G.; Park, J.-S.; Roy, A. Nivolumab Exposure-Response Analyses of Efficacy and Safety in Previously Treated Squamous or Nonsquamous Non-Small Cell Lung Cancer. Clin. Cancer Res. 2017, 23, 5394–5405. [Google Scholar] [CrossRef]
- Zhao, X.; Suryawanshi, S.; Hruska, M.; Feng, Y.; Wang, X.; Shen, J.; Vezina, H.E.; McHenry, M.B.; Waxman, I.M.; Achanta, A.; et al. Assessment of nivolumab benefit-risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann. Oncol. 2017, 28, 2002–2008. [Google Scholar] [CrossRef]
- Long, G.V.; Tykodi, S.S.; Schneider, J.G.; Garbe, C.; Gravis, G.; Rashford, M.; Agrawal, S.; Grigoryeva, E.; Bello, A.; Roy, A.; et al. Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat-dosing schedule in patients with cancer. Ann. Oncol. 2018, 29, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, G.; Gupta, M.; Feng, Y.; Statkevich, P.; Roy, A. Exposure-Response Analysis of Nivolumab in Patients with Previously Treated or Untreated Advanced Melanoma. J. Clin. Pharmacol. 2017, 57, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.; Bajaj, G.; Gupta, M.; Agrawal, S.; Yang, A.; Park, J.-S.; Lestini, B.; Roy, A. Quantitative Characterization of the Exposure-Response Relationship for Cancer Immunotherapy: A Case Study of Nivolumab in Patients with Advanced Melanoma. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Basak, E.A.; Koolen, S.L.W.; Hurkmans, D.P.; Schreurs, M.W.J.; Bins, S.; Oomen-de Hoop, E.; Wijkhuijs, A.J.M.; den Besten, I.; Sleijfer, S.; Debets, R.; et al. Correlation between nivolumab exposure and treatment outcomes in non-small-cell lung cancer. Eur. J. Cancer 2019, 109, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, G.; Wang, X.; Agrawal, S.; Gupta, M.; Roy, A.; Feng, Y. Model-Based Population Pharmacokinetic Analysis of Nivolumab in Patients with Solid Tumors. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Horn, L.; Jackman, D.; Spigel, D.; Antonia, S.; Hellmann, M.; Powderly, J.; Heist, R.; Sequist, L.V.; Smith, D.C.; et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results from the CA209-003 Study. J. Clin. Oncol. 2018, 36, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Brody, R.; Zhang, Y.; Ballas, M.; Siddiqui, M.K.; Gupta, P.; Barker, C.; Midha, A.; Walker, J. PD-L1 expression in advanced NSCLC: Insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer 2017, 112, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.C.; Kondic, A.G.; Anderson, K.M.; Robinson, A.G.; Garon, E.B.; Riess, J.W.; Jain, L.; Mayawala, K.; Kang, J.; Ebbinghaus, S.W.; et al. Pembrolizumab Exposure-Response Assessments Challenged by Association of Cancer Cachexia and Catabolic Clearance. Clin. Cancer Res. 2018, 24, 5841–5849. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, J.; Li, H.; Liu, J.; Xu, Y.; Song, P.; Liu, Q.; Zhao, H.; Xu, J.; Maher, V.E.; et al. Association of time-varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin. Pharmacol. Ther. 2017, 101, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Ogungbenro, K.; Patel, A.; Duncombe, R.; Nuttall, R.; Clark, J.; Lorigan, P. Dose Rationalization of Pembrolizumab and Nivolumab Using Pharmacokinetic Modeling and Simulation and Cost Analysis. Clin. Pharmacol. Ther. 2018, 103, 582–590. [Google Scholar] [CrossRef]
- Peer, C.J.; Goldstein, D.A.; Ratain, M.J.; Figg, W.D. A modeling and simulation study of less frequent dosing of nivolumab 480 mg. J. Clin. Oncol. 2019. [Google Scholar] [CrossRef]
- Agrawal, S.; Feng, Y.; Roy, A.; Kollia, G.; Lestini, B. Nivolumab dose selection: Challenges, opportunities, and lessons learned for cancer immunotherapy. J. Immunother. Cancer 2016, 4, 72. [Google Scholar] [CrossRef]
- Wang, X.; Ludwig, E.A.; Passarell, J.; Bello, A.; Roy, A.; Hruska, M.W. Population Pharmacokinetics and Exposure—Safety Analyses of Nivolumab in Patients with Relapsed or Refractory Classical Hodgkin Lymphoma. J. Clin. Pharmacol. 2019, 59, 364–373. [Google Scholar] [CrossRef]
- Brezski, R.J.; Jordan, R.E. Cleavage of IgGs by proteases associated with invasive diseases: An evasion tactic against host immunity? MAbs 2010, 2, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Statkevich, P.; Bajaj, G.; Feng, Y.; Saeger, S.; Desai, D.D.; Park, J.-S.; Waxman, I.M.; Roy, A.; Gupta, M. Evaluation of Immunogenicity of Nivolumab Monotherapy and Its Clinical Relevance in Patients with Metastatic Solid Tumors. J. Clin. Pharmacol. 2017, 57, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Ryman, J.T.; Meibohm, B. Pharmacokinetics of Monoclonal Antibodies. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Hurkmans, D.P.; Basak, E.A.; van Dijk, T.; Mercieca, D.; Schreurs, M.W.J.; Wijkhuijs, A.J.M.; Bins, S.; Hoop, E.O.; Debets, R.; Joerger, M.; et al. A prospective cohort study on the pharmacokinetics of nivolumab in metastatic non-small cell lung cancer, melanoma, and renal cell cancer patients. J. Immunother. Cancer 2019, 7, 192. [Google Scholar] [CrossRef]
- Almawi, W.Y.; Hess, D.A.; Rieder, M.J. Multiplicity of glucocorticoid action in inhibiting allograft rejection. Cell Transpl. 1998, 7, 511–523. [Google Scholar] [CrossRef]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients with Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.C.; Pennell, N.A. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 1771–1775. [Google Scholar] [CrossRef]
- Larimer, B.M.; Bloch, E.; Nesti, S.; Austin, E.E.; Wehrenberg-Klee, E.; Boland, G.; Mahmood, U. The Effectiveness of Checkpoint Inhibitor Combinations and Administration Timing Can Be Measured by Granzyme B PET Imaging. Clin. Cancer Res. 2019, 25, 1196–1205. [Google Scholar] [CrossRef]
- Larimer, B.M.; Wehrenberg-Klee, E.; Dubois, F.; Mehta, A.; Kalomeris, T.; Flaherty, K.; Boland, G.; Mahmood, U. Granzyme B PET Imaging as a Predictive Biomarker of Immunotherapy Response. Cancer Res. 2017, 77, 2318–2327. [Google Scholar] [CrossRef]
- Hruba, P.; Tycova, I.; Krepsova, E.; Girmanova, E.; Sekerkova, A.; Slatinska, J.; Striz, I.; Honsova, E.; Viklicky, O. Steroid free immunosuppression is associated with enhanced Th1 transcripts in kidney transplantation. Transpl. Immunol. 2017, 42, 18–23. [Google Scholar] [CrossRef]
- Costantini, A.; Julie, C.; Dumenil, C.; Hélias-Rodzewicz, Z.; Tisserand, J.; Dumoulin, J.; Giraud, V.; Labrune, S.; Chinet, T.; Emile, J.-F.; et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. Oncoimmunology 2018, 7, e1452581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohira, M.; Nishida, S.; Tryphonopoulos, P.; Ruiz, P.; Ohdan, H.; Tzakis, A.G. Impact of Steroids on Natural Killer Cells Against Cytotoxicity and Hepatitis C Virus Replication. Transpl. Proc. 2017, 49, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Mazzaschi, G.; Facchinetti, F.; Missale, G.; Canetti, D.; Madeddu, D.; Zecca, A.; Veneziani, M.; Gelsomino, F.; Goldoni, M.; Buti, S.; et al. The circulating pool of functionally competent NK and CD8+ cells predicts the outcome of anti-PD1 treatment in advanced NSCLC. Lung Cancer 2019, 127, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Puszkiel, A.; Noé, G.; Boudou-Rouquette, P.; Cossec, C.L.; Arrondeau, J.; Giraud, J.-S.; Thomas-Schoemann, A.; Alexandre, J.; Vidal, M.; Goldwasser, F.; et al. Development and validation of an ELISA method for the quantification of nivolumab in plasma from non-small-cell lung cancer patients. J Pharm Biomed. Anal. 2017, 139, 30–36. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Janmahasatian, S.; Duffull, S.B.; Ash, S.; Ward, L.C.; Byrne, N.M.; Green, B. Quantification of lean bodyweight. Clin. Pharmacokinet. 2005, 44, 1051–1065. [Google Scholar] [CrossRef]
- Facchinetti, F.; Veneziani, M.; Buti, S.; Gelsomino, F.; Squadrilli, A.; Bordi, P.; Bersanelli, M.; Cosenza, A.; Ferri, L.; Rapacchi, E.; et al. Clinical and hematologic parameters address the outcomes of non-small-cell lung cancer patients treated with nivolumab. Immunotherapy 2018, 10, 681–694. [Google Scholar] [CrossRef]
- Shah, S.; Wood, K.; Labadie, B.; Won, B.; Brisson, R.; Karrison, T.; Hensing, T.; Kozloff, M.; Bao, R.; Patel, J.D.; et al. Clinical and molecular features of innate and acquired resistance to anti-PD-1/PD-L1 therapy in lung cancer. Oncotarget 2018, 9, 4375–4384. [Google Scholar] [CrossRef] [Green Version]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Früh, M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef]
- Bagley, S.J.; Kothari, S.; Aggarwal, C.; Bauml, J.M.; Alley, E.W.; Evans, T.L.; Kosteva, J.A.; Ciunci, C.A.; Gabriel, P.E.; Thompson, J.C.; et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017, 106, 1–7. [Google Scholar] [CrossRef]
- El-Osta, H.; Jafri, S. Predictors for clinical benefit of immune checkpoint inhibitors in advanced non-small-cell lung cancer: A meta-analysis. Immunotherapy 2019, 11, 189–199. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | n = 81 |
| Demographic data | |
| Sex, n (%) | |
| Male | 49 (60) |
| Female | 32 (40) |
| Age (years) | 65.0 (57.0–69.0) |
| Body weight (kg) | 69.0 (62.0–78.0) |
| BMI (kg/m2) | 23.7 (21.7–26.5) |
| Lean body mass (kg) | 51.7 (41.3–57.2) |
| ECOG performance status, n (%) | |
| 0–1 | 45 (56) |
| 2 | 36 (44) |
| Corticosteroids therapy at nivolumab initiation, n (%) | 14 (17) |
| Corticosteroids daily dose (mg) | 20 (10–25) |
| Disease characteristics | |
| Histological tumor type, n (%) | |
| Adenocarcinoma | 52 (64) |
| Squamous cell carcinoma | 16 (20) |
| Other | 13 (16) |
| Number of previous treatment line, n (%) | |
| 1 | 58 (72) |
| 2 | 13 (16) |
| ≥3 | 10 (12) |
| Metastasis, n (%) | |
| Synchronous | 55 (68) |
| Metachronous | 26 (32) |
| Number of extrathoracic metastatic sites, n (%) | |
| 0 | 20 (25) |
| 1 | 29 (36) |
| 2 | 17 (21) |
| ≥3 | 15 (18) |
| Cerebral metastasis, n (%) | |
| Yes | 20 (25) |
| No | 61 (75) |
| Tumor cells PD-L1 expression (%) | 5 (0–28) |
| Baseline Biological data | |
| Hemoglobin (g/dL) (n = 80) | 12.2 (11.2–13.3) |
| Platelets (×109/L) (n = 80) | 244 (199–322) |
| Lymphocytes (×109/L) (n = 74) | 1.18 (0.85–1.72) |
| Neutrophils (×109/L) (n = 80) | 5.31 (3.89–6.84) |
| NLR (n = 74) | 4.31 (2.99–5.67) |
| IgG (UI/mL) (n = 77) | 10.2 (7.6–13.2) |
| PAL (UI/L) (n = 78) | 87 (71–109) |
| AST (UI/L) (n = 78) | 25 (21–31) |
| ALT (UI/L) (n = 78) | 22 (18–35) |
| Total bilirubin (µmol/L) (n = 79) | 5.9 (4.3–7.1) |
| Albumin (g/L) (n = 81) | 38 (34–42) |
| CRP (mg/L) (n = 77) | 11.2 (3.4–31.9) |
| Creatinine (µmol/L) (n = 81) | 78 (64–90) |
| Univariate | Multivariate | ||
|---|---|---|---|
| Linear regression coefficient estimate (95% CI) | p-value | p-value | |
| Age (year) (n = 75) | 0.016 (−0.084; 0.117) | 0.746 | |
| Age > 70 years old (n = 75) | 1.54 (−1.77; 4.85) | 0.357 | |
| Sex (male) (n=75) | −2.48 (−5.25; 0.28) | 0.0777 | 0.36 |
| Total body weight (kg) (n = 75) | 0.146 (0.045; 0.247) | 0.005 | |
| BMI (kg.m-2) (n = 75) | 0.78 (0.48; 1.09) | 0.0000016 | <0.0001 |
| Lean body mass (kg) (n = 75) | 0.012 (−0.13; 0.15) | 0.862 | |
| CRP (mg/L) (n = 72) | −0.054 (−0.094; −0.014) | 0.0089 | 0.69 |
| Albumin (g/L) (n = 75) | 0.42 (0.12; 0.72) | 0.0068 | 0.10 |
| IgG (UI) (n = 71) | −0.277 (−0.661; 0.108) | 0.156 | |
| AST (UI) (n = 72) | −0.043 (−0.159; 0.074) | 0.47 | |
| ALT(UI) (n = 72) | 0.044 (−0.050;0.138) | 0.352 | |
| Creatinine clearance (mL/min) a | 0.019 (−0.025; 0.064) | 0.388 | |
| PD-L1 expression | −0.0163 (−0.0643; 0.0318) | 0.498 | |
| Stage III vs stage IV (reference = stage III) | −1.571 (−4.882; 1.740) | 0.348 | |
| Adenocarcinoma vs SCC | −2.036 (−5.578; 1.506) | 0.255 | |
| Cerebral metastasis (reference = no) | −3.946 (0.873; 7.018) | 0.013 | 0.145 |
| Baseline use of corticosteroids | 2.1 (−1.55; 5.76) | 0.255 | |
| Univariate Model HR (95%CI) | p-Value | Multivariate Model HR (95%CI) (n = 46) | p-value | |
|---|---|---|---|---|
| Risk of death | ||||
| Cmin D14 ≤median (n = 62) | 1.03 (0.55–1.96) | 0.9145 | ||
| Cmin D28 ≤median (n = 53) | 0.51 (0.24–1.09) | 0.0786 | 1.37 (0.44-4.32) | 0.586 |
| ECOG Performance Status (n = 64) | 2.06 (1.28–3.30) | 0.0034 | 1.75 (1.86–3.57) | 0.122 |
| Number of metastasis (n = 64) | 1.04 (0.80–1.35) | 0.7987 | ||
| NLR > median (n = 58) | 2.38 (1.05–5.55) | 0.0036 | 1.82 (0.74–4.54) | 0.189 |
| PD-L1 > 5% (n = 37) a | 0.43 (0.18–1.02) | 0.055 | ||
| Baseline use of corticosteroids | 2.62 (1.29–5.31) | 0.008 | 6.29 (1.46–27.08) | 0.013 |
| Risk of progression | ||||
| Cmin D14 ≤ median (n = 65) | 1.14 (0.66–1.96) | 0.646 | ||
| Cmin D28 ≤ median (n = 55) | 0.68 (0.37–1.27) | 0.223 | 1.30 (0.49–3.39) | 0.59 |
| ECOG Performance Status (n = 67) | 1.97 (1.28–3.02) | 0.023 | 1.85 (1.02–3.38) | 0.043 |
| Number of metastasis (n = 67) | 1.09 (0.87–1.37) | 0.452 | 1.14 (0.70–1.88) | 0.58 |
| NLR > median (n = 60) | 1.82 (1.02–3.22) | 0.042 | ||
| PD-L1 > 5% (n = 38) a | 0.44 (0.20–0.96) | 0.041 | ||
| Baseline use of corticosteroids | 2.51 (1.30–4.89) | 0.008 | 8.08 [1.78–36.62) | 0.007 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellesoeur, A.; Ollier, E.; Allard, M.; Hirsch, L.; Boudou-Rouquette, P.; Arrondeau, J.; Thomas-Schoemann, A.; Tiako, M.; Khoudour, N.; Chapron, J.; et al. Is there an Exposure–Response Relationship for Nivolumab in Real-World NSCLC Patients? Cancers 2019, 11, 1784. https://doi.org/10.3390/cancers11111784
Bellesoeur A, Ollier E, Allard M, Hirsch L, Boudou-Rouquette P, Arrondeau J, Thomas-Schoemann A, Tiako M, Khoudour N, Chapron J, et al. Is there an Exposure–Response Relationship for Nivolumab in Real-World NSCLC Patients? Cancers. 2019; 11(11):1784. https://doi.org/10.3390/cancers11111784
Chicago/Turabian StyleBellesoeur, Audrey, Edouard Ollier, Marie Allard, Laure Hirsch, Pascaline Boudou-Rouquette, Jennifer Arrondeau, Audrey Thomas-Schoemann, Manuela Tiako, Nihel Khoudour, Jeanne Chapron, and et al. 2019. "Is there an Exposure–Response Relationship for Nivolumab in Real-World NSCLC Patients?" Cancers 11, no. 11: 1784. https://doi.org/10.3390/cancers11111784
APA StyleBellesoeur, A., Ollier, E., Allard, M., Hirsch, L., Boudou-Rouquette, P., Arrondeau, J., Thomas-Schoemann, A., Tiako, M., Khoudour, N., Chapron, J., Giraud, F., Wislez, M., Damotte, D., Lupo, A., Vidal, M., Alexandre, J., Goldwasser, F., Tod, M., & Blanchet, B. (2019). Is there an Exposure–Response Relationship for Nivolumab in Real-World NSCLC Patients? Cancers, 11(11), 1784. https://doi.org/10.3390/cancers11111784






