Retinoic Acid Receptors in Acute Myeloid Leukemia Therapy
Abstract
:1. Introduction
2. Retinoid Acid Receptors: Structure and Mechanism of Action
2.1. Retinoid Acid Signaling Pathway
2.1.1. Vitamin A Deficient (VAD) Mice
2.1.2. Rars and Rxrs Knockout Mice
2.2. Retinoid Acid Signaling Pathway in Cancer and Leukemia
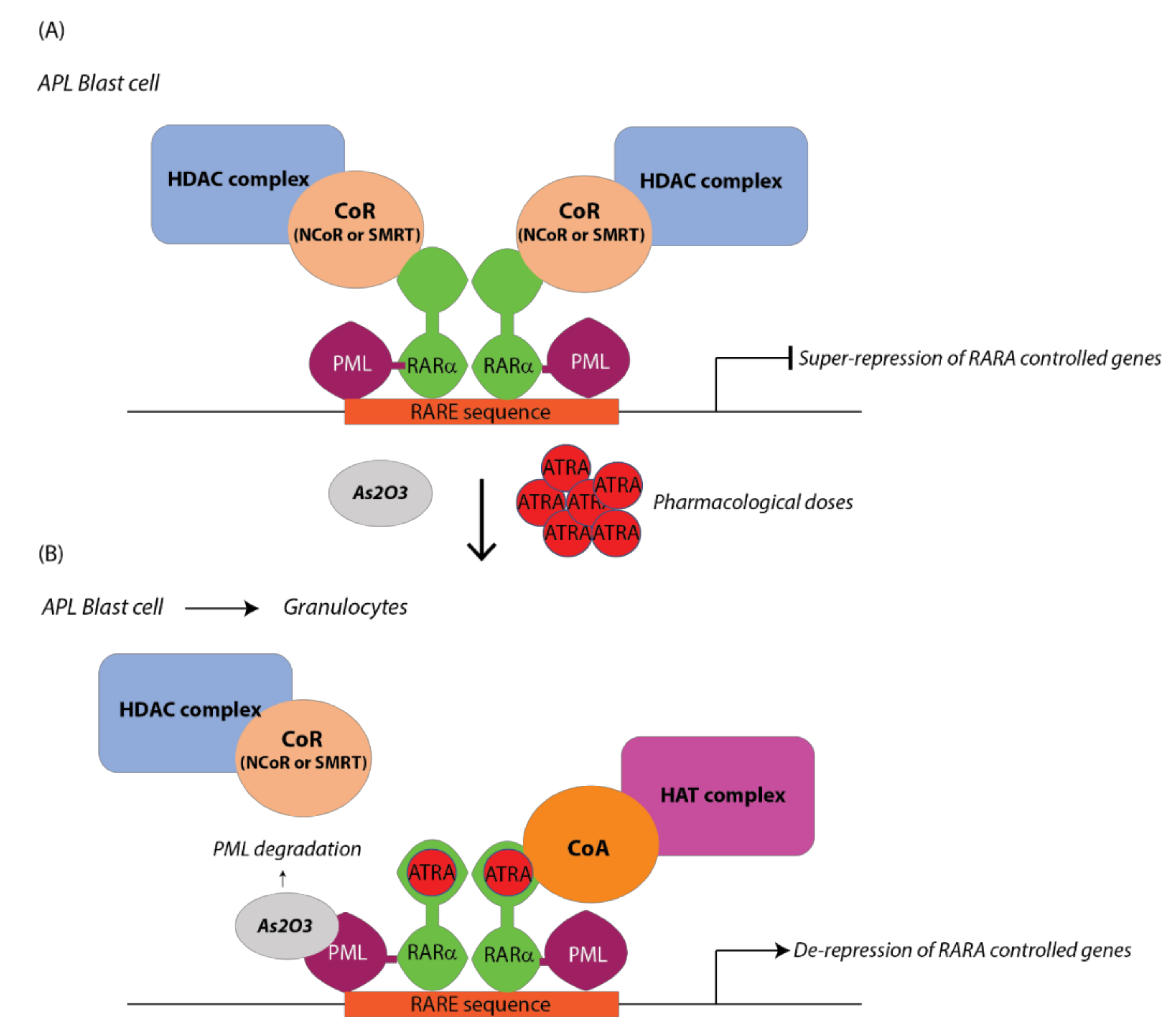
2.2.1. Retinoid Therapy in Acute Promyelocytic Leukemia
2.2.2. Resistance to Differentiation Therapy in APL Patients
2.2.3. Retinoid Acid Signaling Pathway as a Potential Target for Acute Myeloid Leukemia Therapy
2.2.4. Role of Autophagy in Acute Myeloid Leukemia Retinoid Therapy
2.2.5. Rexinoids in Acute Myeloid Leukemia Therapy
3. Conclusions
Funding
Conflicts of Interest
References
- Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors, RXR, and the Big Bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef]
- Baniahmad, A.; Thormeyer, D.; Renkawitz, R. tau4/tau c/AF-2 of the thyroid hormone receptor relieves silencing of the retinoic acid receptor silencer core independent of both tau4 activation function and full dissociation of corepressors. Mol. Cell. Biol. 1997, 17, 4259–4271. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Ma, X.; Bernard, D. Regulation of cellular senescence by retinoid X receptors and their partners. Mech. Ageing Dev. 2019, 183, 111131. [Google Scholar] [CrossRef] [PubMed]
- Bushue, N.; Wan, Y.J. Retinoid pathway and cancer therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Oren, T.; Sher, J.A.; Evans, T. Hematopoiesis and retinoids: Development and disease. Leuk. Lymphoma 2003, 44, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Kastner, P.; Chan, S. Function of RARalpha during the maturation of neutrophils. Oncogene 2001, 20, 7178–7185. [Google Scholar] [CrossRef]
- Purton, L.E.; Dworkin, S.; Olsen, G.H.; Walkley, C.R.; Fabb, S.A.; Collins, S.J.; Chambon, P. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J. Exp. Med. 2006, 203, 1283–1293. [Google Scholar] [CrossRef]
- Lefebvre, P.; Benomar, Y.; Staels, B. Retinoid X receptors: Common heterodimerization partners with distinct functions. Trends Endocrinol. Metab. 2010, 21, 676–683. [Google Scholar] [CrossRef]
- O’Byrne, S.M.; Blaner, W.S. Retinol and retinyl esters: Biochemistry and physiology. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef]
- Guo, X.; Ruiz, A.; Rando, R.R.; Bok, D.; Gudas, L.J. Esterification of all-trans-retinol in normal human epithelial cell strains and carcinoma lines from oral cavity, skin and breast: Reduced expression of lecithin:retinol acyltransferase in carcinoma lines. Carcinogenesis 2000, 21, 1925–1933. [Google Scholar] [CrossRef]
- Marchetti, M.; Vignoli, A.; Bani, M.R.; Balducci, D.; Barbui, T.; Falanga, A. All-Trans retinoic acid modulates microvascular endothelial cell hemostatic properties. Haematologica 2003, 88, 895–905. [Google Scholar] [PubMed]
- Ross, A.C.; Zolfaghari, R. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu. Rev. Nutr. 2011, 31, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Giguere, V.; Ong, E.S.; Segui, P.; Evans, R.M. Identification of a receptor for the morphogen retinoic acid. Nature 1987, 330, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Ablain, J.; de The, H. Retinoic acid signaling in cancer: The parable of acute promyelocytic leukemia. Int. J. Cancer 2014, 135, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M.; Guo, M.; Moreno, V.; Peinado, M.A.; Capella, G.; Galm, O.; Baylin, S.B.; Herman, J.G. Hypermethylation-Associated Inactivation of the Cellular Retinol-Binding-Protein 1 Gene in Human Cancer. Cancer Res. 2002, 62, 5902–5905. [Google Scholar] [PubMed]
- Rhinn, M.; Schuhbaur, B.; Niederreither, K.; Dolle, P. Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc. Natl. Acad. Sci. USA 2011, 108, 16687–16692. [Google Scholar] [CrossRef]
- Rhinn, M.; Dolle, P. Retinoic acid signalling during development. Development 2012, 139, 843–858. [Google Scholar] [CrossRef]
- Cabezas-Wallscheid, N.; Buettner, F.; Sommerkamp, P.; Klimmeck, D.; Ladel, L.; Thalheimer, F.B.; Pastor-Flores, D.; Roma, L.P.; Renders, S.; Zeisberger, P.; et al. Vitamin A-Retinoic Acid Signaling Regulates Hematopoietic Stem Cell Dormancy. Cell 2017, 169, 807–823.e19. [Google Scholar] [CrossRef]
- Perissi, V.; Rosenfeld, M.G. Controlling nuclear receptors: The circular logic of cofactor cycles. Nat. Rev. Mol. Cell Biol. 2005, 6, 542–554. [Google Scholar] [CrossRef]
- Cordeiro, T.N.; Sibille, N.; Germain, P.; Barthe, P.; Boulahtouf, A.; Allemand, F.; Bailly, R.; Vivat, V.; Ebel, C.; Barducci, A.; et al. Interplay of Protein Disorder in Retinoic Acid Receptor Heterodimer and Its Corepressor Regulates Gene Expression. Structure 2019, 27, 1270–1285. [Google Scholar] [CrossRef]
- Efficace, F.; Mandelli, F.; Platzbecker, U.; Cottone, F.; Lo Coco, F. Time to improve health-related quality of life outcomes in patients with acute promyelocytic leukemia. Blood 2015, 126, 2523–2524. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.B.; Zhang, J.; Wang, Z.Y.; Chen, S.J.; Chen, Z. Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: A paradigm of synergistic molecular targeting therapy. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Noguera, N.I.; Piredda, M.L.; Taulli, R.; Catalano, G.; Angelini, G.; Gaur, G.; Nervi, C.; Voso, M.T.; Lunardi, A.; Pandolfi, P.P.; et al. PML/RARa inhibits PTEN expression in hematopoietic cells by competing with PU.1 transcriptional activity. Oncotarget 2016, 7, 66386–66397. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.S.; Klco, J.M.; Varghese, N.; Nagarajan, R.; Ley, T.J. Rara haploinsufficiency modestly influences the phenotype of acute promyelocytic leukemia in mice. Blood 2011, 117, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Lo-Coco, F.; Avvisati, G.; Vignetti, M.; Thiede, C.; Orlando, S.M.; Iacobelli, S.; Ferrara, F.; Fazi, P.; Cicconi, L.; Di Bona, E.; et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N. Engl. J. Med. 2013, 369, 111–121. [Google Scholar] [CrossRef]
- Brown, G.; Hughes, P. Retinoid differentiation therapy for common types of acute myeloid leukemia. Leuk. Res. Treat. 2012, 2012, 939021. [Google Scholar] [CrossRef]
- Martelli, M.P.; Gionfriddo, I.; Mezzasoma, F.; Milano, F.; Pierangeli, S.; Mulas, F.; Pacini, R.; Tabarrini, A.; Pettirossi, V.; Rossi, R.; et al. Arsenic trioxide and all-trans retinoic acid target NPM1 mutant oncoprotein levels and induce apoptosis in NPM1-mutated AML cells. Blood 2015, 125, 3455–3465. [Google Scholar] [CrossRef]
- Falini, B.; Nicoletti, I.; Martelli, M.F.; Mecucci, C. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): Biologic and clinical features. Blood 2007, 109, 874–885. [Google Scholar] [CrossRef]
- Verhagen, H.J.; Smit, M.A.; Rutten, A.; Denkers, F.; Poddighe, P.J.; Merle, P.A.; Ossenkoppele, G.J.; Smit, L. Primary acute myeloid leukemia cells with overexpression of EVI-1 are sensitive to all-trans retinoic acid. Blood 2016, 127, 458–463. [Google Scholar] [CrossRef]
- Mugoni, V.; Panella, R.; Cheloni, G.; Chen, M.; Pozdnyakova, O.; Stroopinsky, D.; Guarnerio, J.; Monteleone, E.; Lee, J.D.; Mendez, L.; et al. Vulnerabilities in mIDH2 AML confer sensitivity to APL-like targeted combination therapy. Cell Res. 2019, 29, 446–459. [Google Scholar] [CrossRef]
- Kizaki, M.; Dawson, M.I.; Heyman, R.; Elster, E.; Morosetti, R.; Pakkala, S.; Chen, D.L.; Ueno, H.; Chao, W.; Morikawa, M.; et al. Effects of novel retinoid X receptor-selective ligands on myeloid leukemia differentiation and proliferation in vitro. Blood 1996, 87, 1977–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altucci, L.; Rossin, A.; Hirsch, O.; Nebbioso, A.; Vitoux, D.; Wilhelm, E.; Guidez, F.; De Simone, M.; Schiavone, E.M.; Grimwade, D.; et al. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase A-mediated desubordination of retinoid X receptor. Cancer Res. 2005, 65, 8754–8765. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, P.V.; Glantz, S.T.; Scotland, S.; Kasner, M.T.; Carroll, M. Induced differentiation of acute myeloid leukemia cells by activation of retinoid X and liver X receptors. Leukemia 2014, 28, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Germain, P.; Chambon, P.; Eichele, G.; Evans, R.M.; Lazar, M.A.; Leid, M.; De Lera, A.R.; Lotan, R.; Mangelsdorf, D.J.; Gronemeyer, H. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol. Rev. 2006, 58, 760–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, S.P.; Cidlowski, J.A.; Kelly, E.; Marrion, N.V.; Peters, J.A.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Nuclear hormone receptors. Br. J. Pharmacol. 2017, 174, S208–S224. [Google Scholar] [CrossRef] [Green Version]
- Gronemeyer, H.; Gustafsson, J.A.; Laudet, V. Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 2004, 3, 950–964. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Wang, X.; Wang, Z.; Zhang, J.; Wu, J.; Xu, W. Design, synthesis, and biological characterization of tamibarotene analogs as anticancer agents. Chem. Biol. Drug Des. 2016, 88, 542–555. [Google Scholar] [CrossRef]
- Das, B.C.; Thapa, P.; Karki, R.; Das, S.; Mahapatra, S.; Liu, T.C.; Torregroza, I.; Wallace, D.P.; Kambhampati, S.; Van Veldhuizen, P.; et al. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2014, 22, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Roszer, T.; Menendez-Gutierrez, M.P.; Cedenilla, M.; Ricote, M. Retinoid X receptors in macrophage biology. Trends Endocrinol. Metab. 2013, 24, 460–468. [Google Scholar] [CrossRef]
- Altucci, L.; Gronemeyer, H. The promise of retinoids to fight against cancer. Nat. Rev. Cancer 2001, 1, 181–193. [Google Scholar] [CrossRef]
- Horlein, A.J.; Naar, A.M.; Heinzel, T.; Torchia, J.; Gloss, B.; Kurokawa, R.; Ryan, A.; Kamei, Y.; Soderstrom, M.; Glass, C.K.; et al. Ligand-Independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 1995, 377, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Evans, R.M. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 1995, 377, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, T.; Lavinsky, R.M.; Mullen, T.M.; Soderstrom, M.; Laherty, C.D.; Torchia, J.; Yang, W.M.; Brard, G.; Ngo, S.D.; Davie, J.R.; et al. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 1997, 387, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Kao, H.Y.; Love, J.D.; Li, C.; Banayo, E.; Gooch, J.T.; Krishna, V.; Chatterjee, K.; Evans, R.M.; Schwabe, J.W. Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 1999, 13, 3209–3216. [Google Scholar] [CrossRef] [Green Version]
- Bourguet, W.; Vivat, V.; Wurtz, J.M.; Chambon, P.; Gronemeyer, H.; Moras, D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol. Cell 2000, 5, 289–298. [Google Scholar] [CrossRef]
- Glass, C.K.; Rosenfeld, M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000, 14, 121–141. [Google Scholar]
- Lonard, D.M.; O’Malley, B.W. Nuclear receptor coregulators: Judges, juries, and executioners of cellular regulation. Mol. Cell 2007, 27, 691–700. [Google Scholar] [CrossRef]
- Perez, E.; Bourguet, W.; Gronemeyer, H.; de Lera, A.R. Modulation of RXR function through ligand design. Biochim. Biophys. Acta 2012, 1821, 57–69. [Google Scholar] [CrossRef]
- Niu, H.; Fujiwara, H.; di Martino, O.; Hadwiger, G.; Frederick, T.E.; Menendez-Gutierrez, M.P.; Ricote, M.; Bowman, G.R.; Welch, J.S. Endogenous retinoid X receptor ligands in mouse hematopoietic cells. Sci. Signal 2017, 10, eaan1011. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, B.P.; Stunnenberg, H.G. 9-Cis retinoic acid signaling: Changing partners causes some excitement. Genes Dev. 1995, 9, 1811–1816. [Google Scholar] [CrossRef] [Green Version]
- Di Masi, A.; Leboffe, L.; De Marinis, E.; Pagano, F.; Cicconi, L.; Rochette-Egly, C.; Lo-Coco, F.; Ascenzi, P.; Nervi, C. Retinoic acid receptors: From molecular mechanisms to cancer therapy. Mol. Aspects Med. 2015, 41, 1–115. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Chacko, J.; Hadwiger, G.; Welch, J.S. Absence of natural intracellular retinoids in mouse bone marrow cells and implications for PML-RARA transformation. Blood Cancer J. 2015, 5, e284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muenzner, M.; Tuvia, N.; Deutschmann, C.; Witte, N.; Tolkachov, A.; Valai, A.; Henze, A.; Sander, L.E.; Raila, J.; Schupp, M. Retinol-Binding protein 4 and its membrane receptor STRA6 control adipogenesis by regulating cellular retinoid homeostasis and retinoic acid receptor alpha activity. Mol. Cell. Biol. 2013, 33, 4068–4082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Crofton, E.J.; Smith, T.E.S.; Koshy, S.; Li, D.; Green, T.A. Manipulation of retinoic acid signaling in the nucleus accumbens shell alters rat emotional behavior. Behav. Brain Res. 2019, 376, 112177. [Google Scholar] [CrossRef]
- Altucci, L.; Leibowitz, M.D.; Ogilvie, K.M.; de Lera, A.R.; Gronemeyer, H. RAR and RXR modulation in cancer and metabolic disease. Nat. Rev. Drug Discov. 2007, 6, 793–810. [Google Scholar] [CrossRef]
- Idres, N.; Marill, J.; Flexor, M.A.; Chabot, G.G. Activation of retinoic acid receptor-dependent transcription by all-trans-retinoic acid metabolites and isomers. J. Biol. Chem. 2002, 277, 31491–31498. [Google Scholar] [CrossRef] [Green Version]
- De Urquiza, A.M.; Liu, S.; Sjoberg, M.; Zetterstrom, R.H.; Griffiths, W.; Sjovall, J.; Perlmann, T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 2000, 290, 2140–2144. [Google Scholar] [CrossRef]
- Lengqvist, J.; Mata De Urquiza, A.; Bergman, A.C.; Willson, T.M.; Sjovall, J.; Perlmann, T.; Griffiths, W.J. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Mol. Cell. Proteom. 2004, 3, 692–703. [Google Scholar] [CrossRef] [Green Version]
- Carman, J.A.; Pond, L.; Nashold, F.; Wassom, D.L.; Hayes, C.E. Immunity to Trichinella spiralis infection in vitamin A-deficient mice. J. Exp. Med. 1992, 175, 111–120. [Google Scholar] [CrossRef]
- Van Bennekum, A.M.; Wong Yen Kong, L.R.; Gijbels, M.J.; Tielen, F.J.; Roholl, P.J.; Brouwer, A.; Hendriks, H.F. Mitogen response of B cells, but not T cells, is impaired in adult vitamin A-deficient rats. J. Nutr. 1991, 121, 1960–1968. [Google Scholar] [CrossRef]
- Canete, A.; Cano, E.; Munoz-Chapuli, R.; Carmona, R. Role of Vitamin A/Retinoic Acid in Regulation of Embryonic and Adult Hematopoiesis. Nutrients 2017, 9, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, A.C.; Chen, Q.; Ma, Y. Vitamin A and retinoic acid in the regulation of B-cell development and antibody production. Vitam. Horm. 2011, 86, 103–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ertesvag, A.; Naderi, S.; Blomhoff, H.K. Regulation of B cell proliferation and differentiation by retinoic acid. Semin. Immunol. 2009, 21, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Dolle, P. Developmental expression of retinoic acid receptors (RARs). Nucl. Recept. Signal. 2009, 7, e006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duong, V.; Rochette-Egly, C. The molecular physiology of nuclear retinoic acid receptors. From health to disease. Biochim. Biophys. Acta 2011, 1812, 1023–1031. [Google Scholar] [CrossRef] [Green Version]
- Menendez-Gutierrez, M.P.; Roszer, T.; Fuentes, L.; Nunez, V.; Escolano, A.; Redondo, J.M.; De Clerck, N.; Metzger, D.; Valledor, A.F.; Ricote, M. Retinoid X receptors orchestrate osteoclast differentiation and postnatal bone remodeling. J. Clin. Investig. 2015, 125, 809–823. [Google Scholar] [CrossRef]
- Ricote, M.; Snyder, C.S.; Leung, H.Y.; Chen, J.; Chien, K.R.; Glass, C.K. Normal hematopoiesis after conditional targeting of RXRalpha in murine hematopoietic stem/progenitor cells. J. Leukoc. Biol. 2006, 80, 850–861. [Google Scholar] [CrossRef] [Green Version]
- Szanto, A.; Narkar, V.; Shen, Q.; Uray, I.P.; Davies, P.J.; Nagy, L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004, 11 Suppl. 2, S126–S143. [Google Scholar] [CrossRef] [Green Version]
- Kuppumbatti, Y.S.; Bleiweiss, I.J.; Mandeli, J.P.; Waxman, S.; Mira, Y.L.R. Cellular retinol-binding protein expression and breast cancer. J. Natl. Cancer Inst. 2000, 92, 475–480. [Google Scholar] [CrossRef]
- Osanai, M.; Lee, G.H. Enhanced expression of retinoic acid-metabolizing enzyme CYP26A1 in sunlight-damaged human skin. Med. Mol. Morphol. 2011, 44, 200–206. [Google Scholar] [CrossRef]
- Osanai, M.; Sawada, N.; Lee, G.H. Oncogenic and cell survival properties of the retinoic acid metabolizing enzyme, CYP26A1. Oncogene 2010, 29, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Qiu, Q.; Khanna, A.; Todd, N.W.; Deepak, J.; Xing, L.; Wang, H.; Liu, Z.; Su, Y.; Stass, S.A.; et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol. Cancer Res. 2009, 7, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Lapointe, J.; Kaygusuz, G.; Ong, D.E.; Li, C.; van de Rijn, M.; Brooks, J.D.; Pollack, J.R. The retinoic acid synthesis gene ALDH1a2 is a candidate tumor suppressor in prostate cancer. Cancer Res. 2005, 65, 8118–8124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirchia, S.M.; Ferguson, A.T.; Sironi, E.; Subramanyan, S.; Orlandi, R.; Sukumar, S.; Sacchi, N. Evidence of epigenetic changes affecting the chromatin state of the retinoic acid receptor beta2 promoter in breast cancer cells. Oncogene 2000, 19, 1556–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, M.J.; Park, S.; Uskokovic, M.R.; Dawson, M.I.; Koeffler, H.P. Expression of retinoic acid receptor-beta sensitizes prostate cancer cells to growth inhibition mediated by combinations of retinoids and a 19-nor hexafluoride vitamin D3 analog. Endocrinology 1998, 139, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Nugoli, M.; Laferriere, J.; Saleh, S.M.; Rodrigue-Gervais, I.G.; Saleh, M.; Park, M.; Hallett, M.T.; Muller, W.J.; Giguere, V. Stromal retinoic acid receptor beta promotes mammary gland tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 774–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farinello, D.; Wozinska, M.; Lenti, E.; Genovese, L.; Bianchessi, S.; Migliori, E.; Sacchetti, N.; di Lillo, A.; Bertilaccio, M.T.S.; de Lalla, C.; et al. A retinoic acid-dependent stroma-leukemia crosstalk promotes chronic lymphocytic leukemia progression. Nat. Commun. 2018, 9, 1787. [Google Scholar] [CrossRef]
- Guo, G.; Sun, X.; Chen, C.; Wu, S.; Huang, P.; Li, Z.; Dean, M.; Huang, Y.; Jia, W.; Zhou, Q.; et al. Whole-Genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat. Genet. 2013, 45, 1459–1463. [Google Scholar] [CrossRef]
- Halstead, A.M.; Kapadia, C.D.; Bolzenius, J.; Chu, C.E.; Schriefer, A.; Wartman, L.D.; Bowman, G.R.; Arora, V.K. Bladder-Cancer-Associated mutations in RXRA activate peroxisome proliferator-activated receptors to drive urothelial proliferation. Elife 2017, 6. [Google Scholar] [CrossRef]
- Wong, M.M.; Guo, C.; Zhang, J. Nuclear receptor corepressor complexes in cancer: Mechanism, function and regulation. Am. J. Clin. Exp. Urol. 2014, 2, 169–187. [Google Scholar]
- Zhang, J.; Hug, B.A.; Huang, E.Y.; Chen, C.W.; Gelmetti, V.; Maccarana, M.; Minucci, S.; Pelicci, P.G.; Lazar, M.A. Oligomerization of ETO is obligatory for corepressor interaction. Mol. Cell. Biol. 2001, 21, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelmetti, V.; Zhang, J.; Fanelli, M.; Minucci, S.; Pelicci, P.G.; Lazar, M.A. Aberrant recruitment of the nuclear receptor corepressor-histone deacetylase complex by the acute myeloid leukemia fusion partner ETO. Mol. Cell. Biol. 1998, 18, 7185–7191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsogiannouli, E.A.; Wagner, N.; Hader, C.; Pinkerneil, M.; Hoffmann, M.J.; Schulz, W.A. Differential Effects of Histone Acetyltransferase GCN5 or PCAF Knockdown on Urothelial Carcinoma Cells. Int. J. Mol. Sci. 2017, 18, 1449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhong, J.F.; Stucky, A.; Chen, X.L.; Press, M.F.; Zhang, X. Histone acetylation: Novel target for the treatment of acute lymphoblastic leukemia. Clin. Epigenet. 2015, 7, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubbert, M.; Suciu, S.; Baila, L.; Ruter, B.H.; Platzbecker, U.; Giagounidis, A.; Selleslag, D.; Labar, B.; Germing, U.; Salih, H.R.; et al. Low-Dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: Final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J. Clin. Oncol. 2011, 29, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Blagitko-Dorfs, N.; Schlosser, P.; Greve, G.; Pfeifer, D.; Meier, R.; Baude, A.; Brocks, D.; Plass, C.; Lubbert, M. Combination treatment of acute myeloid leukemia cells with DNMT and HDAC inhibitors: Predominant synergistic gene downregulation associated with gene body demethylation. Leukemia 2019, 33, 945–956. [Google Scholar] [CrossRef]
- Uy, G.L.; Lane, A.A.; Welch, J.S.; Grieselhuber, N.R.; Payton, J.E.; Ley, T.J. A protease-resistant PML-RAR{alpha} has increased leukemogenic potential in a murine model of acute promyelocytic leukemia. Blood 2010, 116, 3604–3610. [Google Scholar] [CrossRef]
- Merghoub, T.; Gurrieri, C.; Piazza, F.; Pandolfi, P.P. Modeling acute promyelocytic leukemia in the mouse: New insights in the pathogenesis of human leukemias. Blood Cells Mol. Dis. 2001, 27, 231–248. [Google Scholar] [CrossRef]
- Miller, C.A.; Tricarico, C.; Skidmore, Z.L.; Uy, G.L.; Lee, Y.S.; Hassan, A.; O’Laughlin, M.D.; Schmidt, H.; Tian, L.; Duncavage, E.J.; et al. A case of acute myeloid leukemia with promyelocytic features characterized by expression of a novel RARG-CPSF6 fusion. Blood Adv. 2018, 2, 1295–1299. [Google Scholar] [CrossRef]
- Borrow, J.; Goddard, A.D.; Gibbons, B.; Katz, F.; Swirsky, D.; Fioretos, T.; Dube, I.; Winfield, D.A.; Kingston, J.; Hagemeijer, A.; et al. Diagnosis of acute promyelocytic leukaemia by RT-PCR: Detection of PML-RARA and RARA-PML fusion transcripts. Br. J. Haematol. 1992, 82, 529–540. [Google Scholar] [CrossRef]
- Jurcic, J.G.; Soignet, S.L.; Maslak, A.P. Diagnosis and treatment of acute promyelocytic leukemia. Curr. Oncol. Rep. 2007, 9, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Segalla, S.; Rinaldi, L.; Kilstrup-Nielsen, C.; Badaracco, G.; Minucci, S.; Pelicci, P.G.; Landsberger, N. Retinoic acid receptor alpha fusion to PML affects its transcriptional and chromatin-remodeling properties. Mol. Cell. Biol. 2003, 23, 8795–8808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.X.; Shi, Z.Z.; Fang, J.; Gu, B.W.; Li, J.M.; Zhu, Y.M.; Shi, J.Y.; Zheng, P.Z.; Yan, H.; Liu, Y.F.; et al. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 2004, 101, 5328–5335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grignani, F.; De Matteis, S.; Nervi, C.; Tomassoni, L.; Gelmetti, V.; Cioce, M.; Fanelli, M.; Ruthardt, M.; Ferrara, F.F.; Zamir, I.; et al. Fusion proteins of the retinoic acid receptor-alpha recruit histone deacetylase in promyelocytic leukaemia. Nature 1998, 391, 815–818. [Google Scholar] [CrossRef]
- Lallemand-Breitenbach, V.; Zhu, J.; Puvion, F.; Koken, M.; Honore, N.; Doubeikovsky, A.; Duprez, E.; Pandolfi, P.P.; Puvion, E.; Freemont, P.; et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation. J. Exp. Med. 2001, 193, 1361–1371. [Google Scholar] [CrossRef]
- Ding, W.; Li, Y.P.; Nobile, L.M.; Grills, G.; Carrera, I.; Paietta, E.; Tallman, M.S.; Wiernik, P.H.; Gallagher, R.E. Leukemic cellular retinoic acid resistance and missense mutations in the PML-RARalpha fusion gene after relapse of acute promyelocytic leukemia from treatment with all-trans retinoic acid and intensive chemotherapy. Blood 1998, 92, 1172–1183. [Google Scholar] [CrossRef]
- Tobita, T.; Takeshita, A.; Kitamura, K.; Ohnishi, K.; Yanagi, M.; Hiraoka, A.; Karasuno, T.; Takeuchi, M.; Miyawaki, S.; Ueda, R.; et al. Treatment with a new synthetic retinoid, Am80, of acute promyelocytic leukemia relapsed from complete remission induced by all-trans retinoic acid. Blood 1997, 90, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Tomita, A.; Kiyoi, H.; Naoe, T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O 3) in acute promyelocytic leukemia. Int. J. Hematol. 2013, 97, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, J.; Zhang, L. Characterization of atypical acute promyelocytic leukaemia: Three cases report and literature review. Medicine 2019, 98, e15537. [Google Scholar] [CrossRef]
- Kitamura, K.; Kiyoi, H.; Yoshida, H.; Tobita, T.; Takeshita, A.; Ohno, R.; Naoe, T. New retinoids and arsenic compounds for the treatment of refractory acute promyelocytic leukemia: Clinical and basic studies for the next generation. Cancer Chemother. Pharmacol. 1997, 40, S36–S41. [Google Scholar] [CrossRef]
- Takeshita, A.; Asou, N.; Atsuta, Y.; Sakura, T.; Ueda, Y.; Sawa, M.; Dobashi, N.; Taniguchi, Y.; Suzuki, R.; Nakagawa, M.; et al. Tamibarotene maintenance improved relapse-free survival of acute promyelocytic leukemia: A final result of prospective, randomized, JALSG-APL204 study. Leukemia 2019, 33, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Glasow, A.; Barrett, A.; Petrie, K.; Gupta, R.; Boix-Chornet, M.; Zhou, D.C.; Grimwade, D.; Gallagher, R.; von Lindern, M.; Waxman, S.; et al. DNA methylation-independent loss of RARA gene expression in acute myeloid leukemia. Blood 2008, 111, 2374–2377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Lan, F.; Matson, C.; Mulligan, P.; Whetstine, J.R.; Cole, P.A.; Casero, R.A.; Shi, Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004, 119, 941–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenk, T.; Chen, W.C.; Gollner, S.; Howell, L.; Jin, L.; Hebestreit, K.; Klein, H.U.; Popescu, A.C.; Burnett, A.; Mills, K.; et al. Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat. Med. 2012, 18, 605–611. [Google Scholar] [CrossRef] [PubMed]
- McKeown, M.R.; Corces, M.R.; Eaton, M.L.; Fiore, C.; Lee, E.; Lopez, J.T.; Chen, M.W.; Smith, D.; Chan, S.M.; Koenig, J.L.; et al. Superenhancer Analysis Defines Novel Epigenomic Subtypes of Non-APL AML, Including an RARalpha Dependency Targetable by SY-1425, a Potent and Selective RARalpha Agonist. Cancer Discov. 2017, 7, 1136–1153. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, H.; Dassouki, Z.; Berthier, C.; Raffoux, E.; Ades, L.; Legrand, O.; Hleihel, R.; Sahin, U.; Tawil, N.; Salameh, A.; et al. Retinoic acid and arsenic trioxide trigger degradation of mutated NPM1, resulting in apoptosis of AML cells. Blood 2015, 125, 3447–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugthart, S.; van Drunen, E.; van Norden, Y.; van Hoven, A.; Erpelinck, C.A.; Valk, P.J.; Beverloo, H.B.; Lowenberg, B.; Delwel, R. High EVI1 levels predict adverse outcome in acute myeloid leukemia: Prevalence of EVI1 overexpression and chromosome 3q26 abnormalities underestimated. Blood 2008, 111, 4329–4337. [Google Scholar] [CrossRef] [Green Version]
- Buteyn, N.J.; Fatehchand, K.; Santhanam, R.; Fang, H.; Dettorre, G.M.; Gautam, S.; Harrington, B.K.; Henderson, S.E.; Merchand-Reyes, G.; Mo, X.; et al. Anti-Leukemic effects of all-trans retinoic acid in combination with Daratumumab in acute myeloid leukemia. Int. Immunol. 2018, 30, 375–383. [Google Scholar] [CrossRef]
- White, E. The role for autophagy in cancer. J. Clin. Investig. 2015, 125, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Rothe, K.; Porter, V.; Jiang, X. Current Outlook on Autophagy in Human Leukemia: Foe in Cancer Stem Cells and Drug Resistance, Friend in New Therapeutic Interventions. Int. J. Mol. Sci. 2019, 20, 461. [Google Scholar] [CrossRef] [Green Version]
- Moosavi, M.A.; Djavaheri-Mergny, M. Autophagy: New Insights into Mechanisms of Action and Resistance of Treatment in Acute Promyelocytic leukemia. Int. J. Mol. Sci. 2019, 20, 3559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orfali, N.; O’Donovan, T.R.; Nyhan, M.J.; Britschgi, A.; Tschan, M.P.; Cahill, M.R.; Mongan, N.P.; Gudas, L.J.; McKenna, S.L. Induction of autophagy is a key component of all-trans-retinoic acid-induced differentiation in leukemia cells and a potential target for pharmacologic modulation. Exp. Hematol. 2015, 43, 781–793.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, D.; Yu, X.; Wu, Y.; Chen, Y.; Li, G.; Cheng, F.; Xia, L. Emerging roles of bexarotene in the prevention, treatment and anti-drug resistance of cancers. Expert Rev. Anticancer Ther. 2018, 18, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Desvergne, B. RXR: From partnership to leadership in metabolic regulations. Vitam. Horm. 2007, 75, 1–32. [Google Scholar] [CrossRef]
- Tsai, D.E.; Luger, S.M.; Andreadis, C.; Vogl, D.T.; Kemner, A.; Potuzak, M.; Goradia, A.; Loren, A.W.; Perl, A.E.; Schuster, S.J.; et al. A phase I study of bexarotene, a retinoic X receptor agonist, in non-M3 acute myeloid leukemia. Clin. Cancer Res. 2008, 14, 5619–5625. [Google Scholar] [CrossRef]
- Welch, J.S.; Niu, H.; Uy, G.L.; Westervelt, P.; Abboud, C.N.; Vij, R.; Stockerl-Goldstein, K.E.; Jacoby, M.; Pusic, I.; Schroeder, M.A.; et al. A phase I dose escalation study of oral bexarotene in combination with intravenous decitabine in patients with AML. Am. J. Hematol. 2014, 89, E103–E108. [Google Scholar] [CrossRef] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Martino, O.; Welch, J.S. Retinoic Acid Receptors in Acute Myeloid Leukemia Therapy. Cancers 2019, 11, 1915. https://doi.org/10.3390/cancers11121915
di Martino O, Welch JS. Retinoic Acid Receptors in Acute Myeloid Leukemia Therapy. Cancers. 2019; 11(12):1915. https://doi.org/10.3390/cancers11121915
Chicago/Turabian Styledi Martino, Orsola, and John S. Welch. 2019. "Retinoic Acid Receptors in Acute Myeloid Leukemia Therapy" Cancers 11, no. 12: 1915. https://doi.org/10.3390/cancers11121915
APA Styledi Martino, O., & Welch, J. S. (2019). Retinoic Acid Receptors in Acute Myeloid Leukemia Therapy. Cancers, 11(12), 1915. https://doi.org/10.3390/cancers11121915




