Abstract
Colorectal cancer (CRC) has been ranked as the third most prevalent cancer worldwide. Indeed, it represents 10.2% of all cancer cases. It is also the second most common cause of cancer mortality, and accounted for about 9.2% of all cancer deaths in 2018. Early detection together with a correct diagnosis and staging remains the most effective clinical strategy in terms of disease recovery. Thanks to advances in diagnostic techniques, and improvements of surgical adjuvant and palliative therapies, the mortality rate of CRC has decreased by more than 20% in the last decade. Cancer biomarkers for the early detection of CRC, its management, treatment and follow-up have contributed to the decrease in CRC mortality. Herein, we provide an overview of molecular biomarkers from tumor tissues and liquid biopsies that are approved for use in the CRC clinical setting for early detection, follow-up, and precision therapy, and of biomarkers that have not yet been officially validated and are, nowadays, under investigation.
1. Introduction
Colorectal cancer (CRC) is the second most common cause of cancer death, with 881,000 new deaths in 2018, and is the third most prevalent cancer worldwide, with about 1.8 million new cases in 2018. According to the GLOBOCAN 2018 database, the incidence rate of colon cancer is high in parts of Europe, while it tends to be low in most regions of Africa and Southern Asia. Notably, the incidence of CRC varies greatly between countries depending on their economic development. Arnold et al. [1] defined CRC a “socioeconomic development marker”. In fact, they divided countries into three groups based on CRC incidence and mortality: group 1, constituted by countries with a high CRC incidence and mortality, namely populations in Eastern Europe, Latin America, and Asia; group 2 constituted by countries with a high CRC incidence and a low mortality, namely European countries, Canada, and Singapore; and group 3, constituted by countries with a low CRC incidence and mortality, namely Australia, Iceland, New Zealand, and Japan, all of which have a high Human Development Index [2]. Overall, despite the increase in the incidence of CRC over the last 20 years, the incidence of CRC mortality has decreased in many countries probably due to prevention strategies, early detection and improvements in treatment [1].
Colorectal carcinogenesis is characterized by genetic and epigenetic alterations that transform normal cells into cancer cells. A characteristic of CRC is high inter- and intra-tumor heterogeneity at both clinical and molecular level. Intra-tumor variability refers to changes in distinct regions of a tumor, while temporal heterogeneity refers to changes observed overtime between the primary tumor and its matched metastases. Chromosomal instability, microsatellite instability, aberrant DNA methylation and DNA repair defects are all mechanisms that generate tumor genetic variability during colorectal epithelial cell transformation that, in turns, are responsible for patient prognosis and response to specific therapy. In the era of biological personalized care, precise molecular characterization of the tumor is crucial in defining the therapeutic plan. Consequently, the identification and standardization of cancer prognostic and predictive molecular biomarkers is becoming increasingly more relevant [3,4]. Herein we provide an overview on the approved and promising molecular biomarkers currently available for CRC. We also try to shed light on the molecular basis of CRC onset and progression, its epidemiology and principal approved therapeutic regimens in the attempt to understand the role of molecular biomarkers in the management of CRC.
In this review, we summarize bibliographic sources to analyze, interpret and critically evaluate the data available. We searched the literature related to our topic using PubMed and the PubMed Central database of the MEDLINE database, and the U.S. National Library of Medicine® (NLM, Rockville Pike, Bethesda, Maryland) database. The key-words used were: “Colorectal cancer (and CRC) onset”, “Colorectal cancer (and CRC) progression”, “Colorectal Cancer (and CRC) Molecular Biomarkers”, “Colorectal cancer (and CRC) diagnostic Biomarkers”, ”Colorectal cancer (and CRC) prognostic biomarkers”, “Colorectal cancer (and CRC) predictive biomarkers”, and “Colorectal cancer (and CRC) biomarkers and therapy”. We first scrutinized the most relevant papers by the abstract, journal ranking and years of publication. We also checked the reference lists of the selected papers to identify relevant publications not found using our key-words, while also taking care to avoid duplicate citations.
2. Colorectal Cancer: An Overview
About 75% of CRC cases are sporadic, while only about 10% are hereditary; the remaining 10–20% are familial cases, defined as a familial cluster of CRC patients in which the genetic mechanism of onset remains unclear [5]. Usually, this group of patients, in which the disease is probably associated with low-penetrance DNA variants, does not show Mendelian inheritance, but rather high-phenotypic heterogeneity. Notably, CRC onset and progression are characterized by the well-known adenoma-carcinoma sequence.
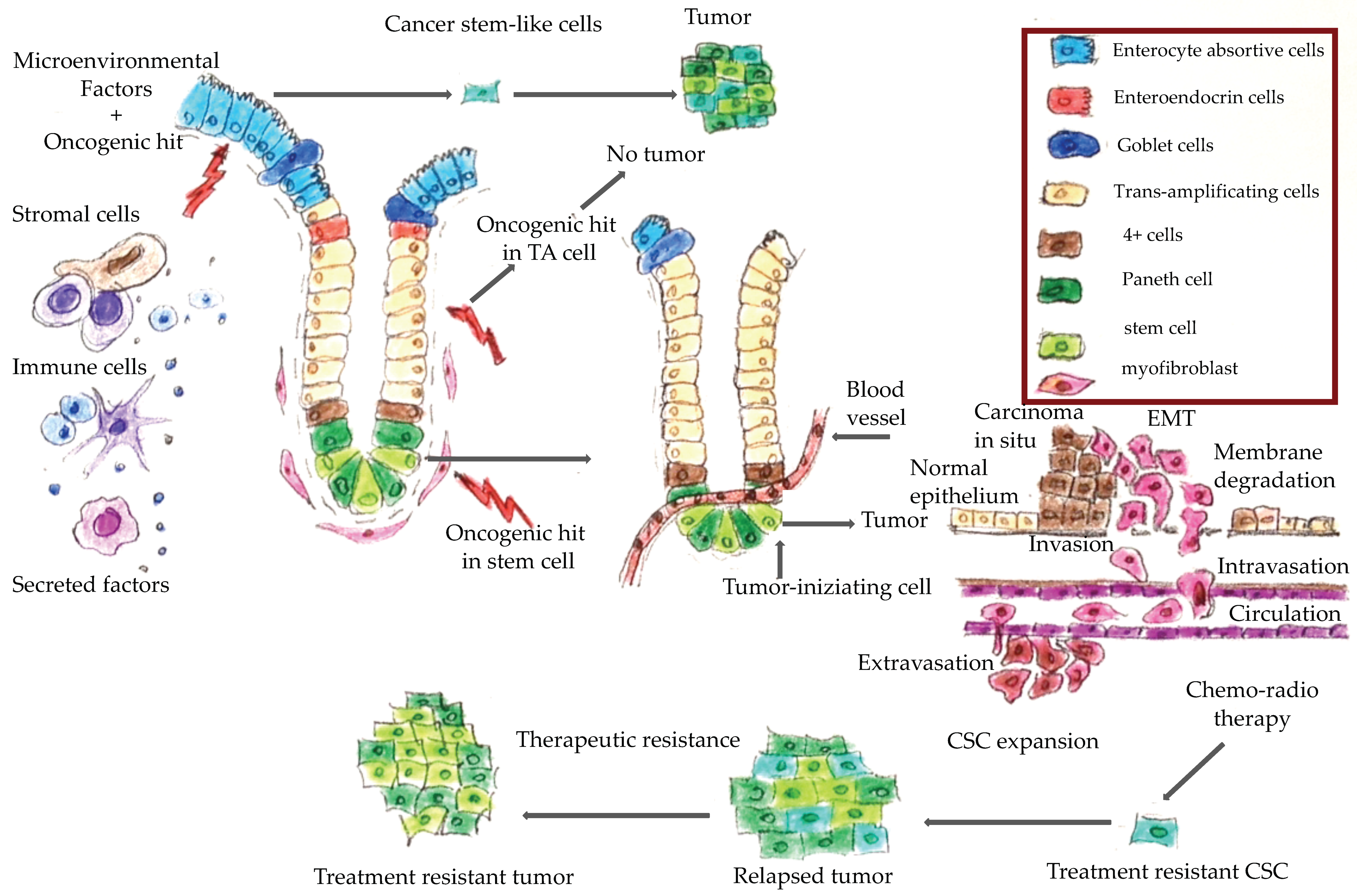
Figure 1 shows a schematic representation of CRC onset and progression. It has been hypothesized that only cancer stem cells are able to trigger neoplastic transformation and promote tumor progression [6,7]. Epithelial cells of colorectal mucosa are organized along the crypt villus axis. At the base of the crypt are the colon stem cells, which are the more undifferentiated cells that have self-renewal and pluripotency capacity. An oncogenic hit in these cells generates a cancer stem cell, which can give rise to a cancer. An oncogenic hit in a trans-amplifying differentiated cell does not produce a cancer. However, several factors, including microenvironment factors, could induce cell de-differentiation thereby generating stem-like cells from trans-amplifying cells. An oncogenic hit in these stem-like cells could also give rise to neoplastic transformation [8].
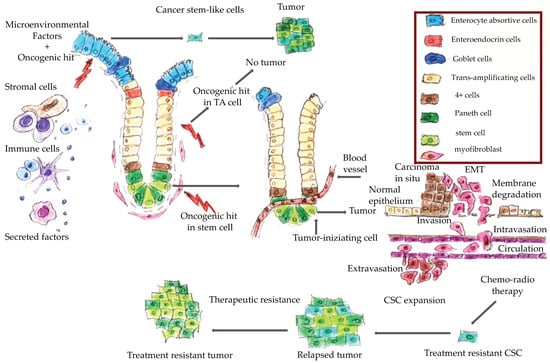
Figure 1.
Colorectal cancer tumorigenesis. During tumor progression, epithelial cancer cells undergo the epithelial-to mesenchymal-transition (EMT) program that is characterized by acquisition of mesenchymal and stem-like cell properties consequent to which the cancer cells can invade the extracellular matrix and migrate into the surrounding tissues. They then join the endothelial cells from vessels and arrive in the lumen in a process known as ‘intravasation’. These cells can survive in the vessel lumen, then exit the vases (i.e., ‘extravasation’), disseminate into the adjacent organs, and colonize them to generate micrometastases. Chemo-radiotherapy often kills differentiated cancer cells, while the mesenchymal, stem-like cells are treatment-resistant and can give rise to a treatment-resistant tumor.
The epithelial-to mesenchymal-transition (EMT) is a physiological process typical of epithelial cells by which the latter lose their epithelial features and acquire mesenchymal characteristics, namely motility, resistance to programmed cell death, self-renewal capability and all the features of stem cells. Mesenchymal cancer cells can alter the basement membrane components and the extracellular matrix and so trigger a metastatic process. These findings suggested that the EMT is a mechanism that generates a pool of stem-like cells that enable cancer progression, and represents a common biological mechanism that could be a target for therapeutic intervention. Ultimately, it is the combined effect of the EMT and the mesenchymal–epithelial transition (MET), that enables the metastatic progression of CRC: the EMT enables primary tumor escape and spread by way of mesenchymal intermediates, and the MET restores the CRC highly-proliferative epithelial stem cell phenotype [8]. It would be interesting to evaluate whether genes involved in the EMT and cell plasticity could serve as molecular markers in CRC follow-up and for the early detection of metastatic disease.
The main mechanisms involved in the accumulation of tumor alterations underlying cancer progression are chromosomal instability (CIN), microsatellite instability (MSI), aberrant DNA methylation (i.e., the CpG island methylator phenotype [CIMP]), and DNA repair defects [9,10,11,12]. The CIN phenotype, which is characterized by chromosome alterations, represents about 60% of all CRCs [10]. MSI, namely, the variations in the numbers of repetitive units in each microsatellite sequence, results from inactivation of the mismatch repair (MMR) system caused by mutations hitting one of the DNA MMR genes (i.e., MLH1, MSH2, MSH6, PMS1, PMS2) [13,14,15,16]. MSI accounts for only about 15–22% of sporadic CRCs, while it is a characteristic of the tumor in patients with Lynch syndrome. MSI phenotypes are distinguished based on the degree of instability: MSI-high (MSI-H) and MSI-low (MSI-L); or microsatellite stable (MSS) [9,17,18]. When the percentage of altered mono- and di-nucleotide microsatellite markers exceeds 20%, the instability is defined “High” (MSI-High). MSI is usually associated to alterations in MMR function mainly in the MSH2 or MLH1 proteins. Microsatellite alterations below 20%, which are usually found only in dinucleotide markers are referred to as “MSI-Low” [11,19,20]. Alterations of tri- and tetra-nucleotides have been associated with MSH3 dysfunction [19,20]. This kind of alteration is called “elevated microsatellite alterations at selected tetra-nucleotide repeats” (EMAST) [19,21,22,23,24]. Genes that are more often altered consequent to MMR inactivation, are the TGF-β tumor suppressor gene [25], the TGF-β type II receptor (TGF-βR2), BAX, caspase 5 apoptotic regulator [26,27], and the tumor suppressor gene TCF4 (which has been implicated in deregulation of the Wnt/β-catenin/TCF signaling pathway) [28]. Sporadic and hereditary CRCs have different mechanisms of MMR inactivation, that mostly consist in point mutations of the hMLH1 or hMSH2 genes in the case of hereditary CRC, and in promoter hyper-methylation of the hMLH1 gene in sporadic CRC [29]. Sporadic MSI CRCs are a consequent of epigenetic silencing induced by the BRAF V600E mutation [30]. Therefore, the latter is a diagnostic marker with which to distinguish sporadic from hereditary MSI CRC [31]. Finally, a tumor is defined “CIMP” if it shows methylation of at least three of the following markers: CACNA1G, IGF2, NEUROG1, RUNX3 and SOCS1 [32].
The microenvironment, including the immune system and the extracellular matrix, also affects tumor heterogeneity and determines different behavior of apparently similar tumors [33,34]. Dendritic cells, tumor-associated macrophages (TAMs) and tumor infiltrating lymphocytes (TILs) are the main immunological cells involved in the host immune response to cancer cells and they have recently been identified as prognostic markers and potential targets for adjuvant therapy [35,36]. Dendritic cells are antigen-presenting cells that generate the adaptive immune response. TAMs produce components of the immunosuppressive tumor microenvironment, namely cytokines, chemokines, growth factors, and trigger T-cell activity by releasing inhibitory immune checkpoint proteins. They affect tumor progression and response to chemo-radiotherapy by acting on tumor microenvironment features [37]. Finally, TILs kill tumor cells and have thus been associated with disease outcomes [38].
Various factors contribute to the incidence of CRC. Sporadic CRCs arise consequent to somatic mutations while germline-inactivating mutations in oncogenes or tumor suppressor genes cause hereditary CRC. First-degree relatives of CRC patients have a threefold greater risk of developing CRC than individuals without familial predisposition. Patients with inflammatory bowel diseases are also at an increased risk of CRC [39]. The prognosis of CRC depends largely on the cancer stage at the time of diagnosis. The five-year survival of patients with stage I CRC is about 90% versus 10% in patients at stage IV [40].
Surgery plays a pivotal role in the treatment of patients diagnosed at an early stage of cancer. However, many patients are diagnosed at an advanced stage of disease, and sometimes have distant metastases. Adjuvant therapy may be effective in such cases, although drug resistance may affect response and concur to recurrent disease [41]. The chemotherapy approved for CRC is a combination of 5-fluorouracil and leucovorin (e.g., oxaliplatin–FOLFOX, irinotecan–FOLFIRI). In addition, two monoclonal antibodies against the epidermal growth factor receptor (cetuximab and panitumumab) are used in combination with well-established treatment regimens [42,43,44]. Biological chemotherapy also includes the vascular endothelial growth factor (VEGF)-A-targeted antibodies bevacizumab and aflibercept, which are recombinant proteins that target VEGF-A, VEGF-B and placental growth factor (PlGF) [3]. Immunotherapy results in a good response in several types of solid tumors, including CRCs. The monoclonal antibodies pembrolizumab and nivolumab, which block programmed cell death 1 (PD1), are approved by the USA Food and Drug Administration for the treatment of mismatch-repair-deficient (dMMR) and microsatellite instability-high (dMMR–MSI-H) mCRC. However, mismatch-repair-proficient (pMMR) and microsatellite instability-low (pMMR–MSI-L) CRC do not response to the immune checkpoint target therapy.
3. Role of Molecular Biomarkers in CRC Management
Biomarkers are defined as a multitude of biological features, such as imaging or radiomic alterations, and biological molecules found in blood and in other body fluids and tissues that are a sign of a normal or disease condition. DNA, RNA, microRNA, antibodies, and epigenetic changes are examples of biomarkers. Biomarkers play an important role in the management of CRC, indeed, they can reveal predisposition for the disease and detect the disease at an early stage. They are also useful for monitoring the efficacy of treatment, neo-adjuvant therapy, follow-up, and disease recurrence. They can also help to select the most appropriate chemotherapeutic drug across a broad spectrum of patients [41].
As we discussed previously [3], the tumor-node-metastases staging, tumor budding, and immunoscore are the best means with which to classify colon cancer and they are a guide in CRC follow-up and in therapy decision-making. With the advent of immunotherapy, CRCs are also classified based on mismatch-repair-deficiency or proficiency and the level of microsatellite instability, (dMMR–MSI-H; pMMR–MSI-L). It is now known that dMMR–MSI-H CRC is associated with a high tumor mutation burden and immune cell infiltration [45,46].
Although surgery is the gold standard treatment for early CRC, in which it can be curative, most CRC patients are diagnosed at an advanced stage [47]. The five-year survival rate after surgery of early stage (I/II) CRC patients exceeds 90% [48]. However, stage III and stage IV CRCs are characterized by local lymph node invasion and distance metastases and a very low overall survival, respectively [49,50]. This is probably because early stages of the disease are often asymptomatic and most people refuse colonoscopy and the fecal occult blood test [51]. In this scenario, it is important to identify new diagnostic and prognostic molecular biomarkers to detect the disease at an early stage to predict therapeutic response.
4. Molecular Features of Hereditary Colorectal Cancer
Hereditary CRC syndromes are rare diseases usually caused by germline mutations in oncogenes or in tumor suppressor genes that are crucial in such processes and events, namely colorectal mucosa turnover, cell division, cell cycle, and programmed cell death. The incidence of hereditary CRC syndromes now account for about 10% of all CRCs [52]. Notably, the prevalence of germline mutations is highest (about 16–33%) in CRC patients diagnosed before the age of 50 [53,54,55]. People with hereditary CRC syndromes show higher risk of CRC than unaffected people. They also show symptoms in other organs that are typical of each specific syndrome, often including an increased risk of extra intestinal cancer during their lifetime. The molecular diagnosis, which currently consists in the identification of pathogenic genetic variants in genes associated with both a high- and low-penetrance cancer risk, is an essential tool for cancer prevention, follow-up, counseling and survival and represents the gold standard approach in the management of these syndromes.
The hereditary syndromes predisposing to CRC are listed in Table 1. Specific genetic variants are associated with each syndrome, each with its typical onset age and responsiveness to drugs. The advent of genetic predisposition markers open the way to the prevention of cancer onset in at risk subjects, and to early cancer detection as well as to precision therapy. Interestingly, effects of NSAIDS and aspirin have long been studied to treat familial adenomatous polyposis (FAP) patients. A recent placebo-controlled randomized trial showed that treatment with a combination of sulindac and erlotinib resulted in a significant decrease of colorectal polyp onset in FAP patients after six months of treatment versus placebo [56]. The molecular identification of specific pathogenic APC gene variants in FAP patients revealed people with inherited disease in at-risk families, who must undergo follow-up. On the other hand, the absence of a disease-causing variant reduces the risk of CRC to that of the general population, and endoscopic surveillance could become less burdensome. Endoscopic surveillance is advisable in FAP patients without a pathogenic mutation in the APC gene or in one of the other genes responsible for CRC. Children carriers of an APC pathogenic variant should also undergo ultrasonography and alpha-fetoprotein screening protocols each 5–10 years, beginning at birth, because of the risk of hepatoblastoma is approximately 800-fold that of the general population [57].

Table 1.
Features of hereditary colorectal cancers.
5. Role of Molecular Biomarkers in the Surgical Approach to Hereditary Colorectal Cancers
Surgical options for patients with hereditary non polyposis colorectal cancer (HNPCC) range from segmental colectomy to total abdominal colectomy with ileorectal anastomosis to restorative proctocolectomy as well as to all the possible procedures for rectal cancer [68]. Identification of the specific pathogenic variants in the MMR genes that confirm the clinical diagnosis of Lynch syndrome could help to guide surgical decision-making. Total abdominal colectomy is considered because of the elevated risk of metachronous lesions. Patients, especially postmenopausal women, should be offered the option of prophylactically extended surgery (hysterectomy and oophorectomy) [69]. Patients with polyposis syndromes are usually offered prophylactic colectomy to prevent cancer [70]. A crucial issue is rectal sparing procedures in patients with limited rectal polyposis. Polypectomy or limited resection can be considered for patients with amartomatous polyposis syndromes. In general, surgical decision-making is based on risk factors, age of the patient, and acceptation of an intensive follow-up policy.
To our knowledge, there is no consensus as to whether genetics and molecular biomarkers could improve surgical options offered to patients with hereditary forms of CRC. The relationship between APC mutations, genotype and the severity of polyposis along the colon and in the rectum of FAP patients, has led to the hypothesis of a schematic surgical strategy, especially in terms of rectum saving procedures. Nieuwenhuis et al. showed that, despite no difference in cancer risk, the risk of deferred proctectomy after ileorectal anastomosis is increasingly higher in patients with severe polyposis [71]. However, according to Dodaro et al. [72], the decision regarding type, extension and timing of surgery should take into consideration the patient’s genotype together with her/his clinical data. The use of minimally invasive surgery has dramatically improved perioperative and long-term results [73].
6. Predictive Biomarkers in CRC Therapy and Prognosis
The first target of 5-FU is the thymidylate synthase (TS) protein, which is encoded by the TYMS gene. As expected, the response to 5-FU depends on the expression of the TS protein and of the TYMS gene that therefore have significant prognostic value in overall survival prediction after chemotherapy [74,75]. Moreover, the expression of molecules involved in the metabolism of 5-FU, namely thymidine phosphorylase (TP), uridine phosphorylase (UP), orotate phosphoribosyl transferase and dihydropyrimidine dehydrogenase (DPD), have been associated with the response to drugs [41]. Capecitabine is an oral drug that is converted to 5-FU consequent to the activity of the TP enzyme. Therefore, TP has prognostic value in predicting the response to capecitabine. Patients with high TP expression have a better response than patients with low TP expression, and loss of TP function causes capecitabine-resistance [76,77]. Similarly, metabolic intermediates involved in the uptake and metabolism of irinotecan, such as carboxylesterases, uridine diphosphate glucuronosyltransferase, the hepatic cytochrome P-450 enzymes CYP3A, β-glucuronidase, and the ATP-binding cassette transporter protein, are prognostic markers of response to this drug. Resistance to oxaliplatin is correlated to the expression of the nucleotide excision repair pathway [78,79].
As discussed above, the EMT and stemness confer resistance to programmed cell death to CRC cells, thereby giving rise to tumors resistant to chemoradiotherapy (Figure 1). Accordingly, stemness surface markers, such as CD133, EphB2high, EpCAMhigh, and CD44+ have been suggested as markers of colon cancer aggressiveness and resistance to therapy [80,81]. The anti-EGFR antibodies, cetuximab and panitumumab, inhibit the EGF signaling pathways thereby regulating cell proliferation. The CRYSTAL trial demonstrated the efficacy of cetuximab, in combination with a FOLFOX or FOLFIRI regimen, only in patients with CRC negative for KRAS or NRAS mutations [44,80,82] The RAS mutation is also a negative predictive marker for panitimumab biological therapy [83], except in the case of the G13D KRAS mutation, which has been associated with a positive response to the anti-EGFR antibody, comparable to that of patients with a KRAS wild-type tumor [84,85]. However, the prospective ICECREAM (Irinotecan Cetuximab Evaluation and Cetuximab Response Evaluation Among Patients with a G13D Mutation) study demonstrated that in patients with KRAS G13D-mutated chemotherapy-refractory mCRC, neithercetuximab monotherapy nor cetuximab plus irinotecan resulted in a statistically significant improvement in terms of two-year overall survival [86]. Similarly, in a meta-analysis, Rowland et al. [87] did not find any significant difference between KRAS G13D and other KRAS mutated tumors in mCRC patients treated with anti-EGFR mAbs biological therapy. Several studies have investigated the role of mutations in other genes of the EGFR pathway, namely, PI3K, BRAF and the quantitative expression of the PTEN protein. However, due to insufficient and/or discordant findings, those mutations are not recommended as predictive therapeutic biomarkers in clinical practice [69]. Monoclonal antibodies against vascular endothelial growth factor (VEGF) are also approved for mCRC therapy; however, their survival benefit is limited to a few months due to acquired resistance [88]. Although there are no validated predictive biomarkers relating to the use of anti-angiogenic drugs, VEGF itself has prognostic value. Indeed, high VEGF expression is associated to a poor prognosis for CRC patients, a low response to preoperative radiotherapy, and relapses. Furthermore, VEGF-C could be a prognostic biomarker in rectal cancer [89].
With regard to immunotherapy that targets the immune checkpoint, dMMR–MSI-H status is the only CRC that responds to this treatment. In this context, MMR and MSI are important predictive biomarkers for therapeutic decision-making in case of CRC, and have entered into clinical practice [90]. Interestingly, TAM infiltration is associated with a better prognosis in CRC than in other solid tumors, in which, on the contrary, TAMs have been associated with a poor prognosis [91,92,93]. Furthermore, Malesci et al. [94] observed that TAMs are positive prognostic factors for 5-FU response in stage III CRC patients. Indeed, TAM infiltration has a clear beneficial effect in patients treated with 5-FU, which has not observed in untreated patients [95,96,97,98,99,100]. Tumor infiltrating lymphocytes, and specifically the density of memory T cells (CD45RO+) in tumors, has been associated with improved survival [101]. Furthermore, in accordance with previous data showing a relationship between MSI and TILs, a high-frequency of MSI correlated with higher CD45RO+ cell density [102,103,104]. It has been suggested that MSI causes immunogenicity of tumor cells by improving the synthesis of truncated peptides [102] thereby stimulating the adaptive immune responses of mCRC. In a phase II clinical trial, the observation that the number of TILs was higher in MSI tumors than in microsatellite stable (MSS) tumors is in accordance with this hypothesis [99,105,106]. As expected, MSI-H is an important predictive biomarker with which to select patients who may benefit from immunotherapy. Indeed, treatment with pembrolizumab (anti-PD-1) and nivolumab (anti-PD-L1) resulted in a better objective response, stable disease, and progression-free survival in MSI-H patients, but not in MSS mCRC patients. In addition, the levels of PD-1 and PD-L1 were significantly higher in dMMR tumors than in proficient MMR (pMMR) tumors. These observations led to the approval of immunotherapy for these MSI patients [107]. Furthermore, measurement of the tumor mutation burden in the primary tumor and/or in blood samples from melanoma or lung cancer patients has been suggested as a biomarker of therapeutic efficacy of the immune checkpoint inhibitors [108,109]. The presence of a high number of tumor-associated neoantigens could improve the identification of cancer cells by the immune system. In this respect, MSI-H CRCs are correlated with increased infiltration of TILs, such as the CD8+ cytotoxic lymphocytes, which are Th1-activated cells that produces IFNγ, and CD45 RO+ T memory cells, which, in turn, are also correlated with a better survival versus MSS CRC [110,111,112,113].
7. Future Perspectives in the Field of Cancer Biomarkers
7.1. Molecular Subtypes
Next-generation sequencing spurred a broad spectrum of data regarding the molecular characterization of solid tumors, including CRC. The CRC Subtyping Consortium classified CRC into subgroups based on a common molecular “core signature” [114]. They identified four consensus molecular subtypes (CMS) and defined the biological features of each subtype. The features of CMS1 are hypermutated phenotype, MSI and CIMP phenotype with BRAF mutations, immune infiltration, and shorter post-relapse survival. CMS1 has been defined “MSI-immune” and accounts for about 14% of CRCs. Conversely, CMS2, CMS3, and CMS4 show high CIN phenotype. CMS2, which is the canonical subtype, represents about 37% of all CRC cases and is characterized by high somatic copy number alterations (SCNAs) and by activation of the WNT and p53 pathways. CMS3, the metabolic subtype, accounts for about of 13% of all CRCs and is characterized by metabolic deregulations, KRAS mutations, a mixed MSI status, SCNA and CIMP low. Finally, CMS4, which is the mesenchymal subtype, represents about 13% of all CRCs and is characterized by TGF-beta activation, angiogenesis, stromal infiltration, high SCNA, and worse relapse-free and overall survival [114].
Notably, the CMS classification has been proposed as a predictive factor for chemotherapy response in mCRC. Indeed, in a retrospective study, both progression-free and overall survival were better in CMS4 patients treated with an irinotecan regimen in first-line therapy than in those treated with oxaliplatin chemotherapy. On the other hand, in CRC patients undergoing EGFR treatment, the worse progression-free and overall survival occurred in CMS1 and the best in CMS2 patients [115]. Similarly, in an in vitro study, 5-FU induced high apoptosis in cancer cell lines belonging to CMS subtypes 1 to 3, and low or no apoptosis in CMS4 cells [116]. Furthermore, the response to oxaliplatin was poor or absent in CMS4 cells, and the best in CMS2 cells [117].
Another CRC classification is based on the CRC intrinsic subtype (CRIS) that consists in the features own of the patient’s colon cancer cells, not affected by their non-neoplastic tissue components, primarily cancer-associated fibroblasts (CAFs), that are a strong indicator of tumor aggressiveness [118,119].
The authors defined its role as a prognostic and predictive biomarker. In accordance with this classification, the CRIS-A subtype is constituted by BRAF-mutated-MSI and KRAS-mutated-MSS tumors. Although these tumors are unresponsiveness to the therapy now available, it is conceivable that they could respond to anti-metabolic therapies that are now under investigation because they have strong glycolytic/hypoxic features [120]. CRIS-B tumors are characterized by activation of TGF-beta signaling and EMT program and by high invasiveness and a poor prognosis. However, they are unrelated to the CMS4 mesenchymal subtype, which has the same features, but is of stromal origin. The CRIS-C subtype is constituted by tumors with elevated EGFR signaling and sensitivity to EGFR inhibitors, independently of all known gene mutations. The CRIS-D subtype is constituted by tumors that activate the WNT pathway and in which IGF2 is overexpressed, which probably induces resistance to biological therapy with EGFR antibody [121]. Finally, CRIS-E is constituted by tumors with high WNT pathway activation, a Paneth cell-like phenotype and KRAS mutations, and are thus resistant to anti-EGFR antibody treatment. The CRIS components -C, -D, and -E are characterized by high WNT pathway activity, which suggests they could benefit from drugs targeting this pathway [122,123].
However, the CRIS tumor categorization classifies the tumor taking into account only the specific features of cancer cells, whereas the relevance of the stromal compartment is well-known in cancer aggressiveness, progression, and response to therapy. Thus, the integration of stromal signatures, mainly CAF infiltration and CRIS traits results in a tumor classification more powerful in terms of prognosis and prediction than CRIS traits or CAFs alone. For example, patients with low CAF infiltration and non-CRIS-B subtype have a good prognosis and do not require adjuvant chemotherapy, while, patients with low CAF infiltration and CRIS-B subtype have a poor prognosis and are predicted to be unresponsive to traditional chemotherapy. However, it is conceivable that patients in this group could benefit from drugs that target the TGF-β pathway and that are now under investigation [124].
In our opinion, the classification of CRC molecular subtypes can easily be applied in clinical practice. Moreover, confirmation and validation of these concepts and findings will lead to a more precise understanding of the role and power of each molecular subtype as a prognostic and predictive tool for the management of CRC.
7.2. Circulating Biomarkers
The term “liquid biopsy”, initially referred to the detection of circulating tumor cells (CTCs) [125], whereas it now refers to the detection of many tumor traits in the peripheral blood of patients [126,127,128]. Circulating tumor DNA (ctDNA), CTCs, exosomes, and microRNAs present in the bloodstream of patients are considered promising biomarkers for the management of CRC. Circulating cell-free DNA (cfDNA) present in blood and other body fluids are produced from cellular apoptosis, necrosis, phagocytosis, and active secretion [129]. ctDNA is the fraction of cfDNA that originates from tumor cells; it can easily be quantified by digital PCR on small volumes of plasma, and can rapidly identify somatic tumor mutations [130,131,132]. It has been suggested that the increased levels of ctDNA in the blood of advanced and metastatic cancer patients versus the ctDNA level observed in early stage cancer patients [133], may account for the tumor burden [134,135]. The presence of ctDNA in the peripheral blood of patients can be used to determine genotypic changes that occur during systemic treatment, and that can render such therapy ineffective [136]. In this context, serial ctDNA measurements can reveal the response of mCRC patients to treatment, which suggests that ctDNA could be an early predictor of treatment response, to complement the standard Response Evaluation Criteria In Solid Tumors-based disease assessment [137], and a guide for anti-EGFR therapy [138]. Moreover, the presence of ctDNA in the peripheral blood of CRC patients, negatively impacts on their survival and it has been considered a prognostic factor in clinical studies [139,140].
CTCs are cells that, after undergoing the EMT, have detached from the primary tumor and are shed daily into bloodstream at a rate of approximately 10 million cells per tumor gram [141,142]. However, due to platelet cloaks or coagulation factors that surround CTCs, a fraction of cells elude detection and are found in a low concentration in peripheral blood [143]. Although many CTC detection methods have been described, only the Cell Search System (Veridex LLC, Raritan, NJ) has been approved by the US Food and Drug Administration for CRC and for breast and prostate cancer [144]. As described for ctDNA, peripheral blood CTCs were reported to be of considerable importance in early stage and metastatic cancer. CTC evaluation is a non-invasive procedure with which to diagnose cancer at an early stage [145] and a useful prognostic factor for cancer progression and survival [126]. Notably, CTCs proved to be a prognostic marker in cases of mCRC, in which levels of CEA and other markers were not measurable [141]. Moreover, elevated CTC levels were associated with worse clinical outcome parameters, overall survival and progression-free survival in CRC patients [141,146,147].
Exosomes are small cellular vesicles, spontaneously released by many cell types. They contain protein and nucleic acid and are involved in both physiological and pathological processes. Exosomes derived from CRCs have been implicated in such tumor processes as EMT [148], migration [149], and metastasis [150]. In recent years, many attempts have been made to identify diagnostic, prognostic, and treatment response biomarkers in CRC exosomes. Some studies focused on isolating miRNAs from tumor exosomes as potential biomarkers for the detection of CRC disease [151]. Other studies have been conducted on serum miRNAs, which however seem to be less stable than the exosomal miRNA [152]. Ogata-Kawata and colleagues [153] showed that serum levels of seven miRNAs (let-7a, miR-1229, miR-1246, miR150, miR-21, miR-223, and miR-23a) were significantly higher in CRC patients than in healthy controls, which indicates that these miRNAs may detect CRCs. In addition, the sensitivity of miR23a and miR-1246 was much higher than that of the CA19-9 and CEA markers for stage I CRC, which again suggests that these miRNAs are potential biomarkers for the detection of early stage CRC [153].
8. Conclusions
Colorectal cancer is a heterogeneous disease, characterized by inter- and intra-tumor variability. Molecular alterations accumulate in the colorectal mucosa via various mechanisms, i.e., MMR gene alterations, chromosomal instability, and CpG island methylation alterations, which, in turn, lead to cancer onset and progression. All these mechanisms confer specific features to each tumor in terms of malignancy, aggressiveness, invasion and response to therapy. Notwithstanding the increase in CRC, its mortality has decreased probably due to prevention approaches, early detection and improvements in therapeutic strategies. Precision therapy, based on the tumor’s molecular features, is often combined with chemoradiotherapy. In this context, molecular biomarkers, defined as biological molecules that are a sign of a normal or tumor condition, and also of tumor predisposition, play a crucial role.
An overview of diagnostic, prognostic, and predictive biomarkers that could be used in the management of CRC is provided in Table 2.

Table 2.
Diagnostic, prognostic and predictive biomarkers in CRC management.
Several molecular biomarkers have been approved for use in clinical practice and are essential tools that support therapeutic decisions. This is the case of KRAS mutations, BRAF mutations and MSI/MSS status. On the other hand, germline genetic variants in specific disease-causing genes are associated to hereditary CRC syndrome, which strongly suggests tumor predisposition. High throughput screening technology has produced a large quantity of data. The classification of these data is shedding light on the nature of tumors, and could, in the near future, upturn the clinical and therapeutic approaches to CRC. This applies also to the classification of molecular subtypes. A better classification and validation of molecular subtypes could help to improve the outcome of precision therapy by providing information about the cancer that single molecular biomarkers alone could not provide.
Finally, molecular circulating biomarkers are promising tools in the management of CRC. Liquid biopsies can be used in cancer screening, and to determine the tumor burden and residual disease. They are also prognostic and predictive biomarkers, not all of which have been approved for clinical practice. Consequently, given the large body of evidence of the efficacy of these biomarkers, a concerted effort should be made to validate them for the benefit of patients.
Author Contributions
M.D.R. and M.T. participated in conceptualization; all authors: discussed the findings; P.D. and D.R. contributed to description of clinical and surgical approaches; P.I. and F.D. contributed to description of hereditary colorectal cancers; A.P. and F.C. contributed to introduction and references sections; M.D.R. contributed to description of molecular biomarkers; M.T. contributed to description of circulating biomarkers; M.D.R. wrote the final manuscript; M.T. critically revised the manuscript. All authors edited and approved the final version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thanks to Jaen Ann Gilder for the text editing, Scientific Communication srl., 80131, Naples, Italy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Rega, D.; Costabile, V.; Duraturo, F.; Niglio, A.; Izzo, P.; Pace, U.; Delrio, P. The biological complexity of colorectal cancer: Insights into biomarkers for early detection and personalized care. Ther. Adv. Gastroenterol. 2016, 9, 861–886. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Pace, U.; Rega, D.; Costabile, V.; Duraturo, F.; Izzo, P.; Delrio, P. Genetics, diagnosis and management of colorectal cancer. Oncol. Rep. 2015, 34, 1087–1096. [Google Scholar] [CrossRef]
- Armelao, F.; de Pretis, G. Familial colorectal cancer: A review. World J. Gastroenterol. 2014, 20, 9292–9298. [Google Scholar] [CrossRef]
- Fanali, C.; Lucchetti, D.; Farina, M.; Corbi, M.; Cufino, V.; Cittadini, A.; Sgambato, A. Cancer stem cells in colorectal cancer from pathogenesis to therapy: Controversies and perspectives. World J. Gastroenterol. 2014, 20, 923–942. [Google Scholar] [CrossRef]
- Zhou, Y.; Xia, L.; Wang, H.; Oyang, L.; Su, M.; Liu, Q.; Lin, J.; Tan, S.; Tian, Y.; Liao, Q.; et al. Cancer stem cells in progression of colorectal cancer. Oncotarget 2017, 9, 33403–33415. [Google Scholar] [CrossRef]
- Ong, B.A.; Vega, K.J.; Houchen, C.W. Intestinal stem cells and the colorectal cancer microenvironment. World J. Gastroenterol. 2014, 20, 1898–1909. [Google Scholar]
- Sideris, M.; Papagrigoriadis, S. Molecular biomarkers and classification models in the evaluation of the prognosis of colorectal cancer. Anticancer Res. 2014, 34, 2061–2068. [Google Scholar]
- Pino, M.S.; Chung, D.C. The chromosomal instability pathway in colon cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Colussi, D.; Brandi, G.; Bazzoli, F.; Ricciardiello, L. Molecular pathways involved in colorectal cancer: Implications for disease behavior and prevention. Int. J. Mol. Sci. 2013, 14, 16365–16385. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, L.A.; Peltomäki, P.; Leach, F.S.; Sistonen, P.; Pylkkänen, L.; Mecklin, J.P.; Järvinen, H.; Powell, S.M.; Jen, J.; Hamilton, S.R.; et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993, 260, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Ionov, Y.; Peinado, M.A.; Malkhosyan, S.; Shibata, D.; Perucho, M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, S.N.; French, A.J.; Roche, P.C.; Cunningham, J.M.; Tester, D.J.; Lindor, N.M.; Moslein, G.; Baker, S.M.; Liskay, R.M.; Burgart, L.J.; et al. Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res. 1996, 56, 4836–4840. [Google Scholar]
- Vasen, H.F.; Watson, P.; Mecklin, J.P.; Lynch, H.T. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999, 116, 1453–1456. [Google Scholar] [CrossRef]
- Jass, J.R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007, 50, 113–130. [Google Scholar] [CrossRef]
- Duraturo, F.; Liccardo, R.; Cavallo, A.; De Rosa, M.; Rossi, G.B.; Izzo, P. Multivariate analysis as a method for evaluating the pathogenicity of novel genetic MLH1 variants in patients with colorectal cancer and microsatellite instability. Int. J. Mol. Med. 2015, 36, 511–517. [Google Scholar] [CrossRef]
- Carethers, J.M.; Koi, M.; Tseng-Rogenski, S.S. EMAST is a form of microsatellite instability that is initiated by inflammation and modulates colorectal cancer progression. Genes 2015, 6, 185–205. [Google Scholar] [CrossRef]
- Huang, S.C.; Lee, J.K.; Smith, E.J.; Doctolero, R.T.; Tajima, A.; Beck, S.E.; Weidner, N.; Carethers, J.M. Evidence for an hMSH3 defect in familial hamartomatous polyps. Cancer 2011, 117, 492–500. [Google Scholar] [CrossRef]
- Carethers, J.M.; Jung, B.H. Genetics and Genetic Biomarkers in Sporadic Colorectal Cancer. Gastroenterology 2015, 149, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Choi, C.; Kim, H.R.; Daoud, Y.; Toiyama, Y.; Takahashi, M.; Goel, A.; Boland, C.R.; Koi, M. Association between recurrent metastasis from stage II and III primary colorectal tumors and moderate microsatellite instability. Gastroenterology 2012, 143, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Tseng-Rogenski, S.S.; Chung, H.; Wilk, M.B.; Zhang, S.; Iwaizumi, M.; Carethers, J.M. Oxidative stress induces nuclear-to-cytosol shift of hMSH3, a potential mechanism for EMAST in colorectal cancer cells. PLoS ONE 2012, 7, e50616. [Google Scholar] [CrossRef] [PubMed]
- Campregher, C.; Schmid, G.; Ferk, F.; Knasmüller, S.; Khare, V.; Kortüm, B.; Dammann, K.; Lang, M.; Scharl, T.; Spittler, A.; et al. MSH3-deficiency initiates EMAST without oncogenic transformation of human colon epithelial cells. PLoS ONE 2012, 7, e50541. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.; Wang, J.; Myeroff, L.; Parsons, R.; Sun, L.; Lutterbaugh, J.; Fan, R.S.; Zborowska, E.; Kinzler, K.W.; Vogelstein, B.; et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 1995, 268, 1336–1338. [Google Scholar] [CrossRef]
- Duval, A.; Hamelin, R. Genetic instability in human mismatch repair deficient cancers. Ann. Genet. 2002, 45, 71–75. [Google Scholar] [CrossRef]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef]
- Cuilliere-Dartigues, P.; El-Bchiri, J.; Krimi, A.; Buhard, O.; Fontanges, P.; Fléjou, J.F.; Hamelin, R.; Duval, A. TCF-4 isoforms absent in TCF-4 mutated MSI-H colorectal cancer cells colocalize with nuclear CtBP and repress TCF-4-mediated transcription. Oncogene 2006, 25, 4441–4448. [Google Scholar] [CrossRef]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular origins of cancer: Molecular basis of colorectal cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef]
- Fang, M.; Hutchinson, L.; Deng, A.; Green, M.R. Common BRAF(V600E)-directed pathway mediates widespread epigenetic silencing in colorectal cancer and melanoma. Proc. Natl. Acad. Sci. USA 2016, 113, 1250–1255. [Google Scholar] [CrossRef]
- Capper, D.; Voigt, A.; Bozukova, G.; Ahadova, A.; Kickingereder, P.; von Deimling, A.; von Knebel Doeberitz, M.; Kloor, M. BRAF V600E-specific immunohistochemistry for the exclusion of Lynch syndrome in MSI-H colorectal cancer. Int. J. Cancer 2013, 133, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.; Wu, C.; Yu, M.; Gourgioti, G.; Wirtz, R.; Raptou, G.; Gkakou, C.; Kotoula, V.; Pentheroudakis, G.; Papaxoinis, G. Evaluation of CpG Island Methylator Phenotype as a Biomarker in Colorectal Cancer Treated With Adjuvant Oxaliplatin. Clin. Colorectal Cancer 2016, 15, 164–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vermeulen, L.; De Sousa E Melo, F.; van der Heijden, M.; Cameron, K.; de Jong, J.H.; Borovski, T.; Tuynman, J.B.; Todaro, M.; Merz, C.; Rodermond, H.; et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010, 12, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Espejo-Herrera, N.; Gràcia-Lavedan, E.; Boldo, E.; Aragonés, N.; Pérez-Gómez, B.; Pollán, M.; Molina, A.J.; Fernández, T.; Martín, V.; La Vecchia, C.; et al. Colorectal cancer risk and nitrate exposure through drinking water and diet. Int. J. Cancer 2016, 139, 334–346. [Google Scholar] [CrossRef]
- Tang, X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett. 2013, 332, 3–10. [Google Scholar] [CrossRef]
- Xuan, Q.J.; Wang, J.X.; Nanding, A.; Wang, Z.P.; Liu, H.; Lian, X.; Zhang, Q.Y. Tumor-associated macrophages are correlated with tamoxifen resistance in the postmenopausal breast cancer patients. Pathol. Oncol. Res. 2014, 20, 619–624. [Google Scholar] [CrossRef]
- Yuxin, L.; Jianxin, X.; Huiyin, L. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar]
- Yoon, N.; Han, K.M.; Cho, S.Y.; Kim, S.W.; Lee, J.E.; Nam, S.J.; Cho, E.Y. Tumor-associated macrophages (TAMs) and tumor-infiltrating lymphocytes (TILs) in pretherapeutic breast cancer core biopsies: Anti-tumoral effect of immune cells associated with neoadjuvant chemotherapy. Int. J. Clin. Exp. Pathol. 2017, 10, 1738–1746. [Google Scholar]
- Clarke, W.T.; Feuerstein, J.D. Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. World J. Gastroenterol. 2019, 25, 4148–4157. [Google Scholar] [CrossRef]
- Sing Vink, G.; Jafri, M.; Mehdi, S.; Ashley, C. Staging and survival of colorectal cancer (CRC) in octogenarians: Nationwide Study of US Veterans. J. Gastrointest. Oncol. 2019, 10, 12–18. [Google Scholar] [CrossRef]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef] [PubMed]
- Tveit, K.M.; Guren, T.; Glimelius, B.; Pfeiffer, P.; Sorbye, H.; Pyrhonen, S.; Sigurdsson, F.; Kure, E.; Ikdahl, T.; Skovlund, E. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: The NORDIC-VII study. J. Clin. Oncol. 2012, 30, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; André, T.; Chan, E.; Lordick, F.; Punt, C.J.; et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J. Clin. Oncol. 2010, 28, 4706–4713. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Köhne, C.H.; Hitre, E.; Zaluski, J.; Chang Chien, C.R.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Duraturo, F.; Liccardo, R.; De Rosa, M.; Izzo, P. Genetics, diagnosis and treatment of Lynch syndrome: Old lessons and current challenges. Oncol. Lett. 2019, 17, 3048–3054. [Google Scholar] [CrossRef]
- Sargent, D.; Sobrero, A.; Grothey, A.; O’Connell, M.J.; Buyse, M.; Andre, T.; Zheng, Y.; Green, E.; Labianca, R.; O’Callaghan, C.; et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J. Clin. Oncol. 2009, 27, 872–877. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Siegel, R.; Desantis, C.; Jemal, A. Colorectal cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef]
- Edwards, B.K.; Ward, E.; Kohler, B.A.; Eheman, C.; Zauber, A.G.; Anderson, R.N.; Jemal, A.; Schymura, M.J.; Lansdorp-Vogelaar, I.; Seeff, L.C.; et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010, 116, 544–573. [Google Scholar] [CrossRef]
- Adler, A.; Geiger, S.; Keil, A.; Bias, H.; Schatz, P.; deVos, T.; Dhein, J.; Zimmermann, M.; Tauber, R.; Wiedenmann, B. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol. 2014, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.; Vilar, E.; Tavtigianand, S.V.; Stoffel, E.M. Genetic predisposition to colorectal cancer: Syndromes, genes, classification of genetic variants and implications for precision medicine. J. Pathol. 2019, 247, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, R.; Frankel, W.L.; Swanson, B.; Zhao, W.; Yilmaz, A.; Miller, K.; Bacher, J.; Bigley, C.; Nelsen, L.; Goodfellow, P.J.; et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol. 2017, 3, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Koeppe, E.; Everett, J.; Ulintz, P.; Kiel, M.; Osborne, J.; Williams, L.; Hanson, K.; Gruber, S.B.; Rozek, L.S. Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology 2018, 154, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Mork, M.E.; You, Y.N.; Ying, J.; Bannon, S.A.; Lynch, P.M.; Rodriguez-Bigas, M.A.; Vilar, E. High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer. J. Clin. Oncol. 2015, 33, 3544–3549. [Google Scholar] [CrossRef]
- Samadder, N.J.; Kuwada, S.K.; Boucher, K.M.; Byrne, K.; Kanth, P.; Samowitz, W.; Jones, D.; Tavtigian, S.V.; Westover, M.; Berry, T.; et al. Association of Sulindac and Erlotinib vs Placebo With Colorectal Neoplasia in Familial Adenomatous Polyposis Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 671–677. [Google Scholar] [CrossRef]
- Barnard, J. Screening and Surveillance Recommendations for Pediatric Gastrointestinal Polyposis Syndromes. J. Pediatr. Gastroenterol. Nutr. 2009, 48 (Suppl. 2), S75–S78. [Google Scholar] [CrossRef]
- Talseth-Palmer, B. The genetic basis of colonic adenomatous polyposis syndromes. Hered. Cancer Clin. Pract. 2017, 15, 5. [Google Scholar] [CrossRef]
- Esteban-Jurado, C.; Giménez-Zaragoza, D.; Muñoz, J.; Franch-Expósito, S.; Álvarez-Barona, M.; Ocaña, T.; Cuatrecasas, M.; Carballal, S.; López-Cerón, M.; Marti-Solano, M. POLE and POLD1 screening in 155 patients with multiple polyps and early-onset colorectal cancer. Oncotarget 2017, 8, 26732–26743. [Google Scholar] [CrossRef]
- Lubbe, S.J.; Di Bernardo, M.C.; Chandler, I.P.; Houlston, R.S. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J. Clin. Oncol. 2009, 27, 3975–3980. [Google Scholar] [CrossRef]
- Boland, P.M.; Yurgelun, M.B.; Boland, R.C. Recent Progress in Lynch Syndrome and Other Familial Colorectal Cancer Syndromes. CA Cancer J. Clin. 2018, 68, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Carethers, J.M. Microsatellite Instability Pathway and EMAST in Colorectal Cancer. Curr. Colorectal Cancer Rep. 2017, 13, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Brosens, L.A.; van Hattem, A.; Hylind, L.M.; Iacobuzio-Donahue, C.; Romans, K.E.; Axilbund, J.; Cruz-Correa, M.; Tersmette, A.C.; Offerhaus, G.J.; Giardiello, F.M. Risk of colorectal cancer in juvenile polyposis. Gut 2007, 56, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.N.; Kopetz, E.S. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: Clinical characteristics, clinical behavior, and response to targeted therapies. J. Gastrointest. Oncol. 2015, 6, 660–667. [Google Scholar] [CrossRef]
- Rosty, C.; Buchanan, D.D.; Walsh, M.D.; Pearson, S.A.; Pavluk, E.; Walters, R.J.; Clendenning, M.; Spring, K.J.; Jenkins, M.A.; Win, A.K. Phenotype and polyp landscape in serrated polyposis syndrome: A series of 100 patients from genetics clinics. Am. J. Surg. Pathol. 2012, 36, 876. [Google Scholar] [CrossRef]
- Syngal, S.; Brand, R.E.; Church, J.M.; Giardiello, F.M.; Hampel, H.L.; Burt, R.W. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am. J. Gastroenterol. 2015, 110, 223–263. [Google Scholar] [CrossRef]
- Nieminen, T.T.; O’Donohue, M.F.; Wu, Y.; Lohi, H.; Scherer, S.W.; Paterson, A.D.; Ellonen, P.; Abdel-Rahman, W.M.; Valo, S.; Mecklin, J.P.; et al. Germline mutation of RPS20, encoding a ribosomal protein, causes predisposition to hereditary nonpolyposis colorectal carcinoma without DNA mismatch repair deficiency. Gastroenterology 2014, 147, 595–598. [Google Scholar] [CrossRef]
- Herzig, D.O.; Buie, W.D.; Weiser, M.R.; You, Y.N.; Rafferty, J.F.; Feingold, D.; Steele, S.R. Clinical Practice Guidelines for the Surgical Treatment of Patients With Lynch Syndrome. Dis. Colon Rectum 2017, 60, 137–143. [Google Scholar] [CrossRef]
- Sepulveda, A.R.; Hamilton, S.R.; Allegra, C.J.; Grody, W.; Cushman-Vokoun, A.M.; Funkhouser, W.K.; Kopetz, S.E.; Lieu, C.; Lindor, N.M.; Minsky, B.D.; et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. J. Mol. Diagn. 2017, 19, 187–225. [Google Scholar] [CrossRef]
- Herzig, D.; Hardiman, K.; Weiser, M.; You, N.; Paquette, I.; Feingold, D.L.; Steele, S.R. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Inherited Polyposis Syndromes. Dis. Colon Rectum 2017, 60, 881–894. [Google Scholar] [CrossRef]
- Nieuwenhuis, M.H.; Douma, K.F.; Bleiker, E.M.; Bemelman, W.A.; Aaronson, N.K.; Vasen, H.F. Female fertility after colorectal surgery for familial adenomatous polyposis: A nationwide cross-sectional study. Ann. Surg. 2010, 252, 341–344. [Google Scholar] [CrossRef]
- Dodaro, C.; Grifasi, C.; Florio, J.; Santangelo, M.L.; Duraturo, F.; De Rosa, M.; Izzo, P.; Renda, A. The role of mutation analysis of the APC gene in the management of FAP patients. A controversial issue. Ann. Ital. Chir. 2016, 87, 321–325. [Google Scholar] [PubMed]
- Dicks, E.; Pullman, D.; Kao, K.; MacMillan, A.; Simmonds, C.; Etchegary, H. Universal tumor screening for Lynch syndrome: Perspectives of Canadian pathologists and genetic counselors. Commun. Genet. 2019, 10, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.X.; Tang, Q.Y.; Bai, J.L.; Qian, X.P.; Li, R.T.; Liu, B.R.; Zheng, M.H. Predictive value of thymidylate synthase expression in advanced colorectal cancer patients receiving fluoropyrimidine-based chemotherapy: Evidence from 24 studies. Int. J. Cancer 2008, 123, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.A.; Fanelli, M.F.; Buim, M.E.; Machado Netto, M.C.; Gasparini Junior, J.L.; Souza ESilva, V.; Dettino, A.L.; Mingues, N.B.; Romero, J.V.; Ocea, L.M.; et al. Thymidylate synthase expression in circulating tumor cells: A new tool to predict 5-fluorouracil resistance in metastatic colorectal cancer patients. Int. J. Cancer 2015, 137, 1397–1405. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Shi, R.; Yu, H.L.; Zeng, Y.; Zheng, W.L.; Ma, W.L. The association between two polymorphisms in the TS gene and risk of cancer: A systematic review and pooled analysis. Int. J. Cancer 2012, 131, 2103–2116. [Google Scholar] [CrossRef]
- Stark, M.; Bram, E.E.; Akerman, M.; Mandel-Gutfreund, Y.; Assaraf, Y.G. Heterogeneous nuclear ribonucleoprotein H1/H2-dependent unsplicing of thymidine phosphorylase results in anticancer drug resistance. J. Biol. Chem. 2011, 286, 3741–3754. [Google Scholar] [CrossRef]
- Lin, S.; Lai, H.; Qin, Y.; Chen, J.; Lin, Y. Thymidine phosphorylase and hypoxia-inducible factor 1-α expression in clinical stage II/III rectal cancer: Association with response to neoadjuvant chemoradiation therapy and prognosis. Int. J. Clin. Exp. Pathol. 2015, 8, 10680–10688. [Google Scholar]
- Gnoni, A.; Russo, A.; Silvestris, N.; Maiello, E.; Vacca, A.; Marech, I.; Numico, G.; Paradiso, A.; Lorusso, V.; Azzariti, A. Pharmacokinetic and metabolism determinants of fluoropyrimidines and oxaliplatin activity in treatment of colorectal patients. Curr. Drug Metab. 2011, 12, 918–931. [Google Scholar] [CrossRef]
- Zeuner, A.; Todaro, M.; Stassi, G.; De Maria, R. Colorectal cancer stem cells: From the crypt to the clinic. Cell Stem Cell 2014, 15, 692–705. [Google Scholar] [CrossRef]
- Turano, M.; Costabile, V.; Cerasuolo, A.; Duraturo, F.; Liccardo, R.; Delrio, P.; Pace, U.; Rega, D.; Dodaro, C.A.; Milone, M.; et al. Characterisation of mesenchymal colon tumour-derived cells in tumourspheres as a model for colorectal cancer progression. Int. J. Oncol. 2018, 53, 2379–2396. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Köhne, C.H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- De Roock, W.; Jonker, D.J.; Di Nicolantonio, F.; Sartore-Bianchi, A.; Tu, D.; Siena, S.; Lamba, S.; Arena, S.; Frattini, M.; Piessevaux, H.; et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA 2010, 304, 1812–1820. [Google Scholar] [CrossRef] [PubMed]
- Osumi, H.; Shinozaki, E.; Osako, M.; Kawazoe, Y.; Oba, M.; Misaka, T.; Goto, T.; Kamo, H.; Suenaga, M.; Kumekawa, Y.; et al. Cetuximab treatment for metastatic colorectal cancer with KRAS p.G13D mutations improves progression-free survival. Mol. Clin. Oncol. 2015, 3, 1053–1057. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tejpar, S.; Celik, I.; Schlichting, M.; Sartorius, U.; Bokemeyer, C.; van Cutsem, E. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J. Clin. Oncol. 2012, 30, 3570–3577. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef]
- Rowland, A.; Dias, M.M.; Wiese, M.D.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S.; Sorich, M.J. Meta-analysis comparing the efficacy of anti-EGFR monoclonal antibody therapy between KRAS G13D and other KRAS mutant metastatic colorectal cancer tumours. Eur. J. Cancer 2016, 55, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, L.; Zhou, Q. Overall and KRAS- specific results of combined cetuximab treatment and chemotherapy for metastatic colorectal cancer: A meta-analysis. Int. J. Colorectal Dis. 2011, 26, 1025–1033. [Google Scholar] [CrossRef]
- Yin, W.H.; Fan, H.Z.; Sheng, J.W.; Xia, H.M.; Wu, Y.W.; Xie, P. Effect of vascular endothelial growth factor C and collagen triple helix repeat containing 1 expression on prognosis of rectal carcinoma patients. Chin. J. Gastrointest. Surg. 2013, 16, 673–675. [Google Scholar]
- Ciardiello, D.; Vitiello, P.P.; Cardone, C.; Martini, G.; Troiani, T.; Martinelli, E.; Ciardiello, F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy T. Cancer Treat. Rev. 2019, 76, 22–23. [Google Scholar] [CrossRef]
- Cavnar, M.J.; Turcotte, S.; Katz, S.C.; Kuk, D.; Goönen, M.; Shia, J.; Allen, P.J.; Balachandran, V.P.; D’Angelica, M.I.; Kingham, T.P.; et al. Tumor-Associated Macrophage Infiltration in Colorectal Cancer Liver Metastases is Associated With Better Outcome. Ann. Surg. Oncol. 2017, 24, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Koelzer, V.H.; Canonica, K.; Dawson, H.; Sokol, L.; Karamitopoulou-Diamantis, E.; Lugli, A.; Zlobec, I. Phenotyping of tumor- associated macrophages in colorectal cancer: Impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunol 2015, 5, e1106677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Liu, L.; Gong, C.Y.; Shi, H.S.; Zeng, Y.H.; Wang, X.Z.; Zhao, Y.W.; Wei, Y.Q. Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature. PLoS ONE 2012, 7, e50946. [Google Scholar] [CrossRef] [PubMed]
- Malesci, A.; Bianchi, P.; Celesti, G.; Basso, G.; Marchesi, F.; Grizzi, F.; Di Caro, G.; Cavalleri, T.; Rimassa, L.; Palmqvist, R.; et al. Tumor- associated macrophages and response to 5-fluorouracil adjuvant therapy in stage III colorectal cancer. Oncoimmunol 2017, 6, e1342918. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Hurwitz, H. Combinations of bevacizumab with cancer immunotherapy. Cancer J. 2018, 24, 193–204. [Google Scholar] [CrossRef]
- Hegde, P.S.; Wallin, J.J.; Mancao, C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin. Cancer Biol. 2018, 52, 117–124. [Google Scholar] [CrossRef]
- Villadangos, J.A.; Schnorrer, P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat. Rev. Immunol. 2007, 7, 543–555. [Google Scholar] [CrossRef]
- Oyama, T.; Ran, S.; Ishida, T.; Nadaf, S.; Kerr, L.; Carbone, D.P.; Gabrilovich, D.I. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-κB activation in hemopoietic progenitor cells. J. Immunol. 1998, 160, 1224. [Google Scholar]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in anti-angiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Ohm, J.E. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 2003, 101, 487886. [Google Scholar] [CrossRef] [PubMed]
- Smyrk, T.C.; Watson, P.; Kaul, K.; Lynch, H.T. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 2001, 91, 2417–2422. [Google Scholar] [CrossRef]
- Michael-Robinson, J.M.; Biemer-Hüttmann, A.; Purdie, D.M.; Walsh, M.D.; Simms, L.A.; Biden, K.G.; Young, J.P.; Leggett, B.A.; Jass, J.R.; Radford-Smith, G.L. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut 2001, 48, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, A.C.; Sørensen, F.B.; Lindebjerg, J.; Hager, H.; Christensen, R.; Frifeldt, S.K.; Hansen, T.F. The Prognostic Value of Tumor-Infiltrating lymphocytes in Stage II Colon Cancer. A Nationwide Population-Based Study. Transl. Oncol. 2018, 11, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Vreeland, T.; Trappey, A.; Hale, D.; Peace, K.; Tyler, J.; Walker, A.; Brown, R.; Herbert, G.; Yi, F.; et al. Cancer vaccines in colon and rectal cancer over the last decade: Lessons learned and future directions. Exp. Rev. Clin. Immunol. 2017, 13, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Banños, M.; Benitez-Ribas, D.; Tabera, J.; Varea, S.; Vilana, R.; Bianchi, L.; Ayuso, J.R.; Pagés, M.; Carrera, G.; Cuatrecasas, M.; et al. Phase II randomised trial of autologous tumour lysate dendritic cell plus best supportive care compared with best supportive care in pre-treated advanced col- orectal cancer patients. Eur. J. Cancer 2016, 64, 167–174. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Claessen, A.M.; van Tinteren, H.; Gall, H.E.; Ezinga, R.; Meijer, S.; Scheper, R.J.; Meijer, C.J.L.M.; Bloemena, E.; Ransom, J.H.; et al. Active specific immunotherapy for stage II and stage III human colon cancer: A randomised trial. Lancet 1999, 353, 345–350. [Google Scholar] [CrossRef]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199. [Google Scholar] [CrossRef]
- Neal, R.; Hellmann, M.D.; Awad, M.M.; Otterson, G.A.; Gutierrez, M.; Gainor, J.F.; Borghaei, H.; Jolivet, J.; Horn, L.; Mates, M.; et al. Line nivolumab plus ipilimumab in advanced non–small-cell lung cancer (CheckMate 568): Outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. JCO 2019, 37, 992–1000. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune land- scape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pageès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960. [Google Scholar] [CrossRef]
- Watanabe, T.; Wu, T.T.; Catalano, P.J.; Ueki, T.; Satriano, R.; Haller, D.G.; Benson, A.B.; Hamilton, S.R. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2001, 344, 1196–1206. [Google Scholar] [CrossRef]
- Lee, L.H.; Cavalcanti, M.S.; Segal, N.H.; Hechtman, J.F.; Weiser, M.R.; Smith, J.J.; Garcia, A.J.; Sadot, E.; Ntiamoah, P.; Markowitz, A.J.; et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod. Pathol. 2016, 29, 1433–1442. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Okita, A.; Takahashi, S.; Ouchi, K.; Inoue, M.; Watanabe, M.; Endo, M.; Honda, H.; Yamada, Y.; Ishioka, C. Consensus molecular subtypes classification of colorectal cancer as a predictive factor for chemotherapeutic efficacy against metastatic colorectal cancer. Oncotarget 2018, 9, 18698–18711. [Google Scholar] [CrossRef]
- Johnson, K.R.; Wang, L.; Miller, M.C.; Willingham, M.C.; Fan, W. Fluorouracil interferes with paclitaxel cytotoxicity against human solid tumor cells. Clin. Cancer Res. 1997, 3, 1739–1745. [Google Scholar]
- Song, N.; Pogue-Geile, K.L.; Gavin, P.G.; Yothers, G.; Kim, S.R.; Johnson, N.L.; Lipchik, C.; Allegra, C.J.; Petrelli, N.J.; O’Connell, M.J.; et al. Clinical outcome from oxaliplatin treatment in stage II/III colon cancer according to intrinsic subtypes: Secondary analysis of NSABP C-07/NRG oncology randomized clinical trial. JAMA Oncol. 2016, 2, 1162–1169. [Google Scholar] [CrossRef]
- Isella, C.; Terrasi, A.; Bellomo, S.; Petti, C.; Galatola, G.; Muratore, A.; Mellano, A.; Senetta, R.; Cassenti, A.; Sonetto, C.; et al. Stromal contribution to the colorectal cancer transcriptome. Nat. Genet. 2015, 47, 312–319. [Google Scholar] [CrossRef]
- Calon, A.; Lonardo, E.; Berenguer-Llergo, A.; Espinet, E.; Hernando-Momblona, X.; Iglesias, M.; Sevillano, M.; Palomo-Ponce, S.; Tauriello, D.V.; Byrom, D.; et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015, 47, 320–329. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.L.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Zanella, E.R.; Galimi, F.; Sassi, F.; Migliardi, G.; Cottino, F.; Leto, S.M.; Lupo, B.; Erriquez, J.; Isella, C.; Comoglio, P.M.; et al. IGF2 is an actionable target that identifies a distinct subpopulation of colorectal cancer patients with marginal response to anti-EGFR therapies. Sci. Transl. Med. 2015, 7, 272ra12. [Google Scholar] [CrossRef]
- Arqués, O.; Chicote, I.; Puig, I.; Tenbaum, S.P.; Argilés, G.; Dienstmann, R.; Fernández, N.; Caratù, G.; Matito, J.; Silberschmidt, D.; et al. Tankyrase Inhibition Blocks Wnt/β-Catenin Pathway and Reverts Resistance to PI3K and AKT Inhibitors in the Treatment of Colorectal Cancer. Clin. Cancer Res. 2016, 22, 644–656. [Google Scholar] [CrossRef]
- Lau, T.; Chan, E.; Callow, M.; Waaler, J.; Boggs, J.; Blake, R.A.; Magnuson, S.; Sambrone, A.; Schutten, M.; Firestein, R.; et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013, 73, 3132–3144. [Google Scholar] [CrossRef]
- Isella, C.; Brundu, F.; Bellomo, S.E.; Galimi, F.; Zanella, E.; Porporato, R.; Petti, C.; Fiori, A.; Orzan, F.; Senetta, R.; et al. Selective analysis of cancer-cell intrinsic transcriptional traits defines novel clinically relevant subtypes of colorectal cancer. Nat. Comm. 2017, 8, 15107. [Google Scholar] [CrossRef]
- Alix-Panabieres, C.; Pantel, K. Circulating tumor cells: Liquid biopsy ofcancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, R.; Li, Z.; Li, J. Clinical and biological significance of circulating tumor cells, circulating tumor DNA, and exosomes as biomarkers in colorectal cancer. Oncotarget 2017, 8, 55632–55645. [Google Scholar] [CrossRef]
- Toiyama, Y.; Okugawa, Y.; Fleshman, J.; Richard Boland, C.; Goel, A. Micrornas as potential liquid biopsy biomarkers in colorectal cancer: A. systematic review. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 274–282. [Google Scholar] [CrossRef]
- Dominguez-Vigil, I.G.; Moreno-Martinez, A.K.; Wang, J.Y.; Roehrl, M.H.A.; Barrera-Saldana, H.A. The dawn of the liquid biopsy in the fight against cancer. Oncotarget 2018, 9, 2912–2922. [Google Scholar] [CrossRef]
- Thierry, A.R.; El Messaoudi, S.; Gahan, P.B.; Anker, P.; Stroun, M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016, 35, 347–376. [Google Scholar] [CrossRef]
- Vogelstein, B.; Kinzler, K.W. Digital PCR. Proc. Natl. Acad. Sci. USA 1999, 96, 9236–9241. [Google Scholar] [CrossRef]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef]
- Dressman, D.; Yan, H.; Traverso, G.; Kinzler, K.W.; Vogelstein, B. Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc. Natl. Acad. Sci. USA 2003, 100, 8817–8822. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Diaz, L.A.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Earlyand Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Winther-Larsen, A.; Demuth, C.; Fledelius, J.; Madsen, A.T.; Hjorthaug, K.; Meldgaard, P.; Sorensen, B.S. Correlation between circulating mutant DNA and metabolic tumour burden in advanced non–small cell lung cancer patients. Br. J. Cancer 2017, 117, 704–709. [Google Scholar] [CrossRef]
- Thierry, A.R.; Mouliere, F.; Gongora, C.; Ollier, J.; Robert, B.; Ychou, M.; Rio, M.D.; Molina, F. Origin and quantification of circulating DNA in mice with human colorectal cancer xenografts. Nucleic Acids Res. 2010, 38, 6159–6175. [Google Scholar] [CrossRef]
- Diaz, L.A.; Williams, R.T.; Wu, J.; Kinde, I.; Hecht, J.R.; Berlin, J.; Allen, B.; Bozic, I.; Reiter, J.G.; Nowak, M.A.; et al. The molecular evolution of acquired resistance totargeted EGFR blockade in colorectal cancers. Nature 2012, 486, 537–540. [Google Scholar] [CrossRef]
- Tie, J.; Kinde, I.; Wang, Y.; Wong, H.L.; Roebert, J.; Christie, M.; Tacey, M.; Wong, R.; Singh, M.; Karapetis, C.S. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann. Oncol. 2015, 26, 1715–1722. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Bedin, C.; Enzo, M.V.; Del Bianco, P.; Pucciarelli, S.; Nitti, D.; Agostini, M. Diagnostic and prognostic role of cell-free DNA testing for colorectal cancer patients. Int. J. Cancer 2017, 140, 1888–1898. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Tomasetti, C.; Li, L.; Springer, S.; Kinde, I.; Silliman, N.; Tacey, M.; Wong, H.L.; Christie, M.; et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage ii colon cancer. Sci. Transl. Med. 2016, 8. [Google Scholar] [CrossRef]
- Huang, M.Y.; Tsai, H.L.; Huang, J.J.; Wang, J.Y. Clinical Implications and Future Perspectives of Circulating Tumor Cells and Biomarkers in Clinical Outcomes of Colorectal Cancer. Transl. Oncol. 2016, 9, 340–347. [Google Scholar] [CrossRef]
- Burz, C.; Pop, V.V.; Buiga, R.; Daniel, S.; Samasca, G.; Aldea, C.; Lupan, I. Circulating tumor cells in clinical research and monitoring patients with colorectal cancer. Oncotarget 2018, 9, 24561–24571. [Google Scholar] [CrossRef]
- Plaks, V.; Koopman, C.D.; Werb, Z. Cancer. Circulating tumor cells. Science 2013, 341, 1186–1188. [Google Scholar] [CrossRef]
- Bunger, S.; Zimmermann, M.; Habermann, J.K. Diversity of assessing circulating tumor cells (CTCs) emphasizes need for standardization: A CTC Guide to design and report trials. Cancer Metastasis Rev. 2015, 34, 527–545. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, J.Y.; Wu, C.H.; Chen, F.M.; Cheng, T.L.; Lin, S.R. Detection of circulating cancer cells with K-ras oncogene using membrane array. Cancer Lett. 2005, 229, 115–122. [Google Scholar] [CrossRef]
- Steinert, G.; Scholch, S.; Koch, M.; Weitz, J. Biology and significance of circulating and disseminated tumour cells in colorectal cancer. Langenbeck Arch. Surg. 2012, 397, 535–542. [Google Scholar] [CrossRef]
- Hardingham, J.E.; Grover, P.; Winter, M.; Hewett, P.J.; Price, T.J.; Thierry, B. Detection and Clinical Significance of Circulating Tumor Cells in Colorectal Cancer—20 Years of Progress. Mol. Med. 2015, 21, 25–31. [Google Scholar] [CrossRef]
- Greening, D.W.; Gopal, S.K.; Mathias, R.A.; Liu, L.; Sheng, J.; Zhu, H.J.; Simpson, R.J. Emerging roles of exosomes during epithelial-mesenchymal transition and cancer progression. Semin. Cell Dev. Biol. 2015, 40, 60–71. [Google Scholar] [CrossRef]
- Mu, W.; Rana, S.; Zöller, M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia 2013, 15, 875–887. [Google Scholar] [CrossRef]
- Wang, X.; Ding, X.; Nan, L.; Wang, Y.; Wang, J.; Yan, Z.; Zhang, W.; Sun, J.; Zhu, W.; Ni, B.; et al. Investigation of the roles of exosomes in colorectal cancer liver metastasis. Oncol. Rep. 2015, 33, 2445–2453. [Google Scholar] [CrossRef]
- Menéndez, P.; Villarejo, P.; Padilla, D.; Menéndez, J.M.; Montes, J.A.R. Diagnostic and prognostic significance of serum microRNAs in colorectal cancer. J. Surg. Oncol. 2013, 107, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Köberle, V.; Pleli, T.; Schmithals, C.; Alonso, E.A.; Haupenthal, J.; Bönig, H.; Peveling-Oberhag, J.; Biondi, R.M.; Zeuzem, S.; Kronenberger, B.; et al. Differential stability of cell-free circulating microRNAs: Implications for their utilization as biomarkers. PLoS ONE 2013, 8, e75184. [Google Scholar] [CrossRef] [PubMed]
- Ogata-Kawata, H.; Izumiya, M.; Kurioka, D.; Honma, Y.; Yamada, Y.; Furuta, K.; Gunji, T.; Ohta, H.; Okamoto, H.; Sonoda, H.; et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS ONE 2014, 9, e92921. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kobunai, T.; Yamamoto, Y.; Matsuda, K.; Ishihara, S.; Nozawa, K.; Yamada, H.; Hayama, T.; Inoue, E.; Tamura, J.; et al. Chromosomal instability (CIN) phenotype, CIN high or CIN low, predicts survival for colorectal cancer. J. Clin. Oncol. 2012, 30, 2256–2264. [Google Scholar] [CrossRef]
- Mojarad, E.N.; Kuppen, P.J.K.; Aghdaei, H.A.; Reza Zali, M. The CpG island methylator phenotype (CIMP) in colorectal cancer. Gastroenterol. Hepatol. Bed Bench 2013, 6, 120–128. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).