Progressive Oncological Surgery Is Associated with Increased Curative Resection Rates and Improved Survival in Metastatic Colorectal Cancer
Abstract
1. Introduction
2. Results
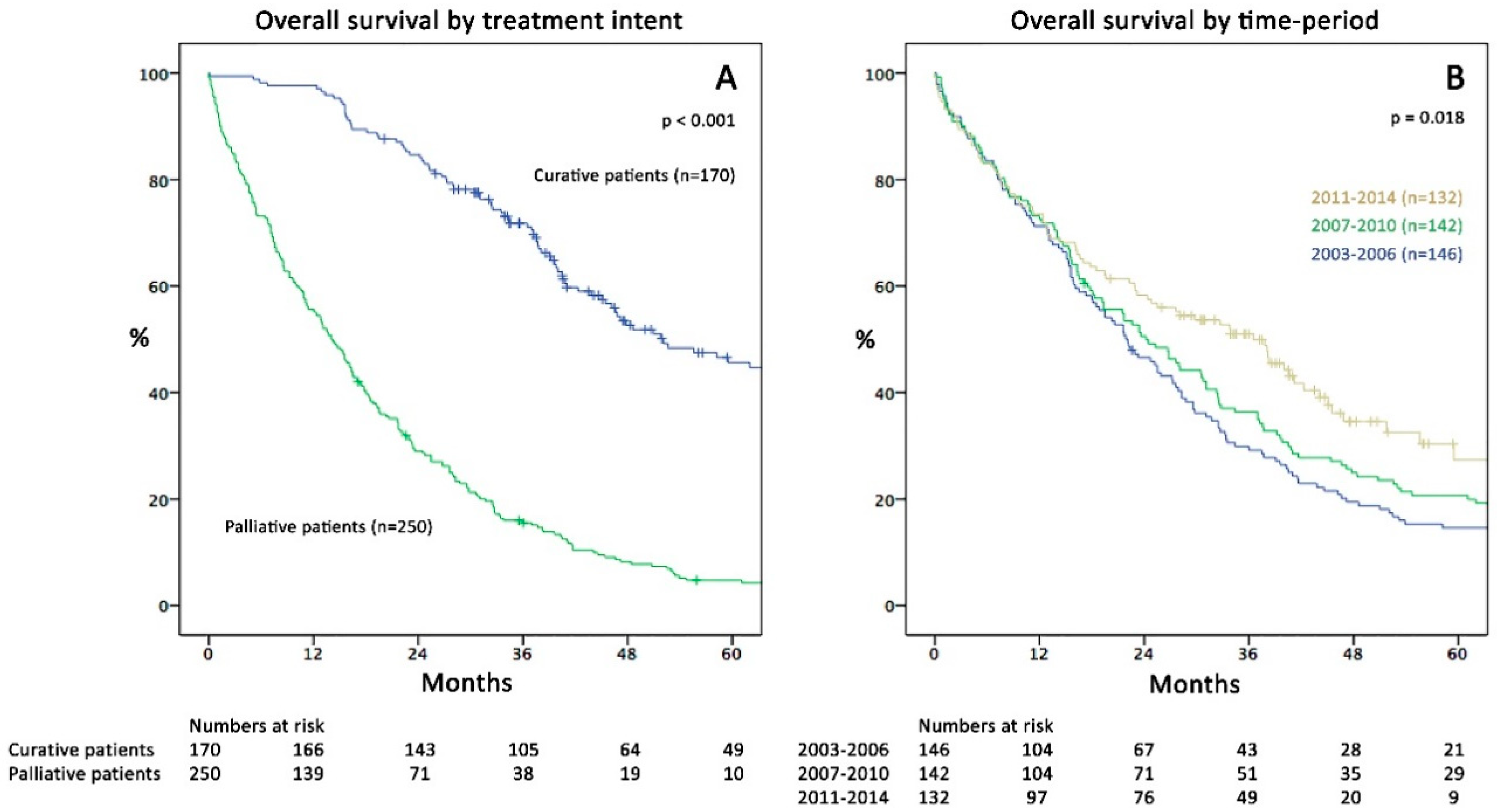
2.1. Patient Characteristics and Overall Survival
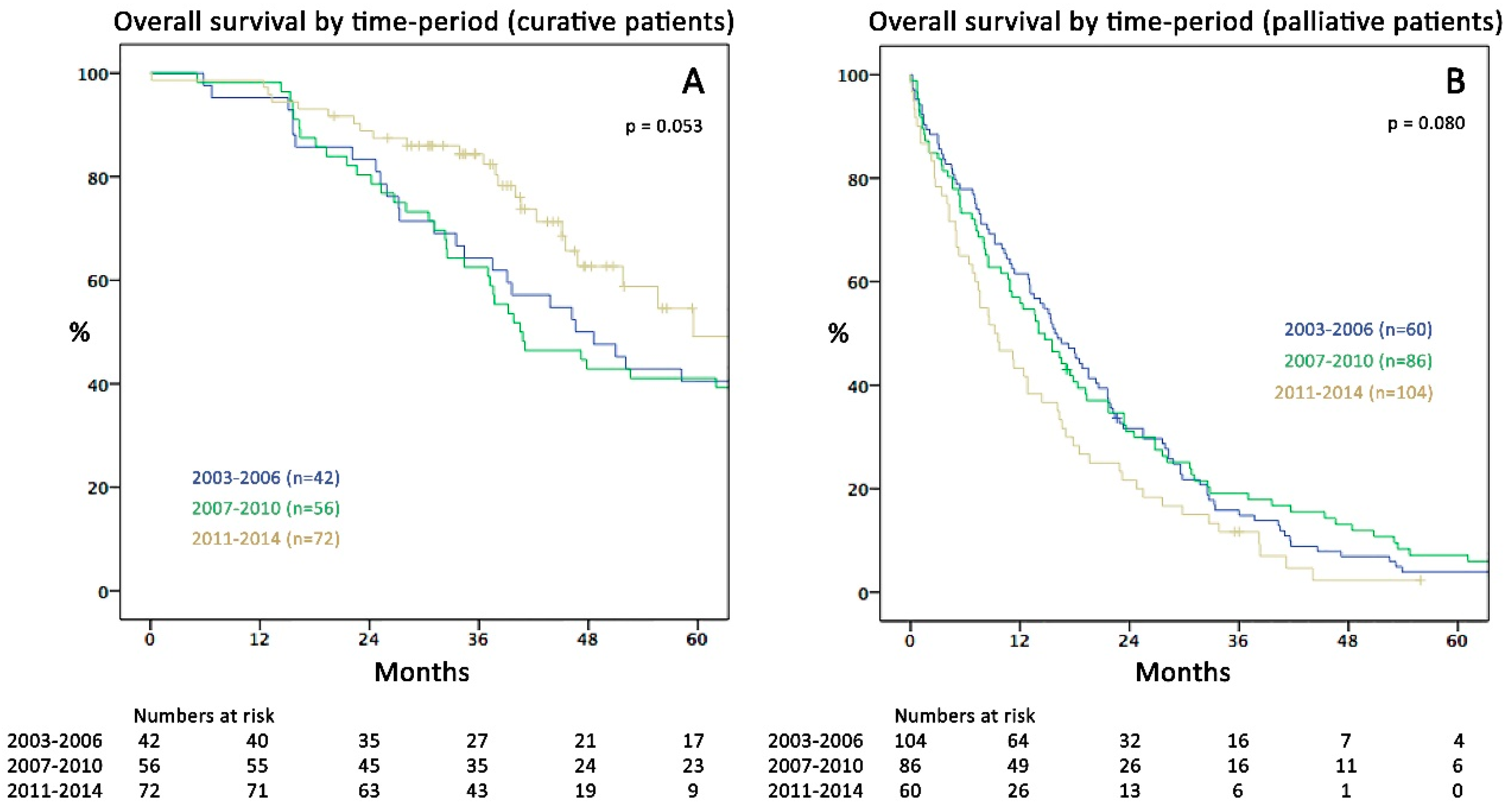
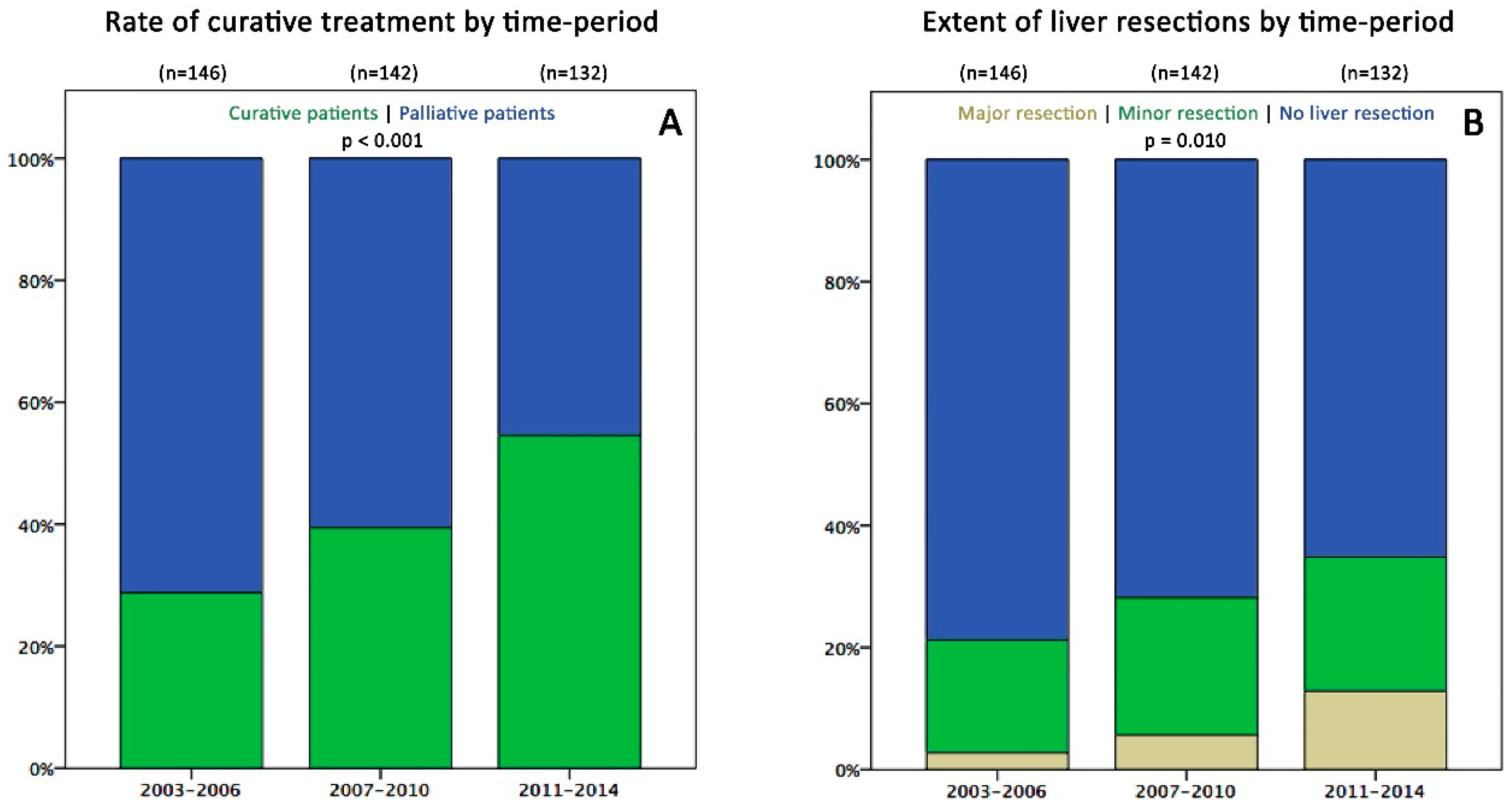
2.2. Survival and Surgical Data in the Three Time Periods
2.3. Disease Recurrence
2.4. Chemotherapeutic Regimens
2.5. Factors Associated with Survival and Treatment Strategy:
3. Discussion
4. Materials and Methods
4.1. Follow-Up
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Capocaccia, R.; Sant, M.; Bell, C.M.; Coebergh, J.W.; Damhuis, R.A.; Faivre, J.; Martinez-Garcia, C.; Pawlega, J.; Ponz de Leon, M.; et al. Understanding variations in survival for colorectal cancer in europe: A eurocare high resolution study. Gut 2000, 47, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Mantke, R.; Schmidt, U.; Wolff, S.; Kube, R.; Lippert, H. Incidence of synchronous liver metastases in patients with colorectal cancer in relationship to clinico-pathologic characteristics. Results of a german prospective multicentre observational study. Eur. J. Surg. Oncol. 2012, 38, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Mitry, E.; Guiu, B.; Cosconea, S.; Jooste, V.; Faivre, J.; Bouvier, A.M. Epidemiology, management and prognosis of colorectal cancer with lung metastases: A 30-year population-based study. Gut 2010, 59, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, V.E.; Klaver, Y.L.; Verwaal, V.J.; Rutten, H.J.; Coebergh, J.W.; de Hingh, I.H. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: A population-based study. Int. J. Cancer 2011, 128, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Meulenbeld, H.J.; van Steenbergen, L.N.; Janssen-Heijnen, M.L.; Lemmens, V.E.; Creemers, G.J. Significant improvement in survival of patients presenting with metastatic colon cancer in the south of the netherlands from 1990 to 2004. Ann. Oncol. 2008, 19, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Sugrue, M.M.; Purdie, D.M.; Dong, W.; Sargent, D.; Hedrick, E.; Kozloff, M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: Results from a large observational cohort study (brite). J. Clin. Oncol. 2008, 26, 5326–5334. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Chang, G.J.; Overman, M.J.; Eng, C.; Sargent, D.J.; Larson, D.W.; Grothey, A.; Vauthey, J.N.; Nagorney, D.M.; McWilliams, R.R. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J. Clin. Oncol. 2009, 27, 3677–3683. [Google Scholar] [CrossRef]
- Fahy, B.N.; D’Angelica, M.; DeMatteo, R.P.; Blumgart, L.H.; Weiser, M.R.; Ostrovnaya, I.; Gonen, M.; Jarnagin, W.R. Synchronous hepatic metastases from colon cancer: Changing treatment strategies and results of surgical intervention. Ann. Surg. Oncol. 2009, 16, 361–370. [Google Scholar] [CrossRef]
- Choti, M.A.; Sitzmann, J.V.; Tiburi, M.F.; Sumetchotimetha, W.; Rangsin, R.; Schulick, R.D.; Lillemoe, K.D.; Yeo, C.J.; Cameron, J.L. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann. Surg. 2002, 235, 759–766. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kotake, K.; Sugihara, K. Prognostic scoring system for stage iv colorectal cancer: Is the ajcc sub-classification of stage iv colorectal cancer appropriate? Int. J. Clin. Oncol. 2013, 18, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Paty, P.; Fong, Y.; Grace, A.; Cohen, A.; DeMatteo, R.; Jarnagin, W.; Blumgart, L. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J. Am. Coll. Surg. 2003, 197, 233–241, discussion 241–232. [Google Scholar] [CrossRef]
- Jones, R.P.; Vauthey, J.N.; Adam, R.; Rees, M.; Berry, D.; Jackson, R.; Grimes, N.; Fenwick, S.W.; Poston, G.J.; Malik, H.Z. Effect of specialist decision-making on treatment strategies for colorectal liver metastases. Br. J. Surg. 2012, 99, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Wicherts, D.A.; de Haas, R.J.; Adam, R. Bringing unresectable liver disease to resection with curative intent. Eur. J. Surg. Oncol. 2007, 33 (Suppl. 2), S42–S51. [Google Scholar] [CrossRef]
- Chun, Y.S.; Vauthey, J.N. Extending the frontiers of resectability in advanced colorectal cancer. Eur. J. Surg. Oncol. 2007, 33 (Suppl. 2), S52–S58. [Google Scholar] [CrossRef]
- Folprecht, G.; Gruenberger, T.; Bechstein, W.O.; Raab, H.R.; Lordick, F.; Hartmann, J.T.; Lang, H.; Frilling, A.; Stoehlmacher, J.; Weitz, J.; et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The celim randomised phase 2 trial. Lancet Oncol. 2010, 11, 38–47. [Google Scholar] [CrossRef]
- Folprecht, G.; Gruenberger, T.; Bechstein, W.; Raab, H.R.; Weitz, J.; Lordick, F.; Hartmann, J.T.; Stoehlmacher-Williams, J.; Lang, H.; Trarbach, T.; et al. Survival of patients with initially unresectable colorectal liver metastases treated with folfox/cetuximab or folfiri/cetuximab in a multidisciplinary concept (celim study). Ann. Oncol. 2014, 25, 1018–1025. [Google Scholar] [CrossRef]
- Vallance, A.E.; vanderMeulen, J.; Kuryba, A.; Botterill, I.D.; Hill, J.; Jayne, D.G.; Walker, K. Impact of hepatobiliary service centralization on treatment and outcomes in patients with colorectal cancer and liver metastases. Br. J. Surg. 2017, 104, 918–925. [Google Scholar] [CrossRef]
- Statistik-Austria. Jahrbuch der Gesundheitsstatistik Österreich. Available online: https://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/gesundheit/krebserkrankungen/dickdarm_enddarm/index.html (accessed on 1 August 2018).
- Haidinger, G.; Waldhoer, T.; Hackl, M.; Vutuc, C. Survival of patients with colorectal cancer in austria by sex, age, and stage. Wien. Med. Wochenschr. 2006, 156, 549–551. [Google Scholar] [CrossRef]
- Dunne, D.F.; Yip, V.S.; Jones, R.P.; McChesney, E.A.; Lythgoe, D.T.; Psarelli, E.E.; Jones, L.; Lacasia-Purroy, C.; Malik, H.Z.; Poston, G.J.; et al. Enhanced recovery in the resection of colorectal liver metastases. J. Surg. Oncol. 2014, 110, 197–202. [Google Scholar] [CrossRef]
- Gasteiger, L.; Eschertzhuber, S.; Tiefenthaler, W. Perioperative management of liver surgery-review on pathophysiology of liver disease and liver failure. Eur. Surg. 2018, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Mattar, R.E.; Al-Alem, F.; Simoneau, E.; Hassanain, M. Preoperative selection of patients with colorectal cancer liver metastasis for hepatic resection. World J. Gastroenterol. 2016, 22, 567–581. [Google Scholar] [CrossRef]
- Cancer-Research-UK. Bowel Cancer Survival Statistics, Former Anglia Cancer Network, 2002–2006. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/survival#heading-Three (accessed on 1 August 2018).
- Sorbye, H.; Cvancarova, M.; Qvortrup, C.; Pfeiffer, P.; Glimelius, B. Age-dependent improvement in median and long-term survival in unselected population-based nordic registries of patients with synchronous metastatic colorectal cancer. Ann. Oncol. 2013, 24, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. Seer Cancer Statistics Review, 1975–2013. National Cancer Institute: Bethesda, MD, USA, April 2016. Available online: http://seer.cancer.gov/csr/1975_2013/ (accessed on 1 August 2018).
- Morris, M.; Iacopetta, B.; Platell, C. Comparing survival outcomes for patients with colorectal cancer treated in public and private hospitals. Med. J. Aust. 2007, 186, 296–300. [Google Scholar] [PubMed]
- Evrard, S.; Poston, G.; Kissmeyer-Nielsen, P.; Diallo, A.; Desolneux, G.; Brouste, V.; Lalet, C.; Mortensen, F.; Stattner, S.; Fenwick, S.; et al. Combined ablation and resection (care) as an effective parenchymal sparing treatment for extensive colorectal liver metastases. PLoS ONE 2014, 9, e114404. [Google Scholar] [CrossRef]
- Stattner, S.; Primavesi, F.; Yip, V.S.; Jones, R.P.; Ofner, D.; Malik, H.Z.; Fenwick, S.W.; Poston, G.J. Evolution of surgical microwave ablation for the treatment of colorectal cancer liver metastasis: Review of the literature and a single centre experience. Surg. Today 2015, 45, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Zampino, M.G.; Maisonneuve, P.; Ravenda, P.S.; Magni, E.; Casiraghi, M.; Solli, P.; Petrella, F.; Gasparri, R.; Galetta, D.; Borri, A.; et al. Lung metastases from colorectal cancer: Analysis of prognostic factors in a single institution study. Ann. Thorac. Surg. 2014, 98, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Bredt, L.C.; Rachid, A.F. Predictors of recurrence after a first hepatectomy for colorectal cancer liver metastases: A retrospective analysis. World J. Surg. Oncol. 2014, 12, 391. [Google Scholar] [CrossRef]
- Vigano, L.; Capussotti, L.; Majno, P.; Toso, C.; Ferrero, A.; De Rosa, G.; Rubbia-Brandt, L.; Mentha, G. Liver resection in patients with eight or more colorectal liver metastases. Br. J. Surg. 2015, 102, 92–101. [Google Scholar] [CrossRef]
- Hunt, S.L.; McKay, A.; Kelly, L.M.; Kirk, A.J. A case series of pulmonary resection for metastatic colorectal cancer in a uk regional thoracic center. Future Oncol. 2015, 11, 35–36. [Google Scholar] [CrossRef]
- Eckardt, J.; Licht, P.B. Thoracoscopic versus open pulmonary metastasectomy: A prospective, sequentially controlled study. Chest 2012, 142, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kiyoshima, M.; Kitahara, M.; Asato, Y.; Amemiya, R. Long-term outcomes after surgical resection of pulmonary metastases from colorectal cancer. Ann. Thorac. Surg. 2015, 99, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Mirnezami, R.; Moran, B.J.; Harvey, K.; Cecil, T.; Chandrakumaran, K.; Carr, N.; Mohamed, F.; Mirnezami, A.H. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal metastases. World J. Gastroenterol. 2014, 20, 14018–14032. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Sa Cunha, A.; Adam, R.; Goere, D.; Bachellier, P.; Azoulay, D.; Ayav, A.; Gregoire, E.; Navarro, F.; Pessaux, P.; et al. Factors influencing recurrence following initial hepatectomy for colorectal liver metastases. Br. J. Surg. 2016, 103, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Kulaylat, A.N.; Bhayani, N.H.; Stokes, A.L.; Schubart, J.R.; Wong, J.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Kaifi, J.T.; Gusani, N.J. Determinants of repeat curative intent surgery in colorectal liver metastasis. J. Gastrointest. Surg. 2014, 18, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Sponholz, S.; Schirren, M.; Baldes, N.; Oguzhan, S.; Schirren, J. Repeat resection for recurrent pulmonary metastasis of colorectal cancer. Langenbecks Arch. Surg. 2017, 402, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Alberts, S.; Poston, G. Oncosurge: A strategy for long-term survival in metastatic colorectal cancer. Colorectal Dis. 2003, 5 (Suppl. 3), 20–28. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Rivera, F.; Karthaus, M.; Fasola, G.; Canon, J.L.; Hecht, J.R.; Yu, H.; Oliner, K.S.; Go, W.Y. Peak: A randomized, multicenter phase ii study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mfolfox6) or bevacizumab plus mfolfox6 in patients with previously untreated, unresectable, wild-type kras exon 2 metastatic colorectal cancer. J. Clin. Oncol. 2014, 32, 2240–2247. [Google Scholar]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmuller, C.; Kahl, C.; Seipelt, G.; et al. Folfiri plus cetuximab versus folfiri plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (fire-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Andres, A.; Mentha, G.; Adam, R.; Gerstel, E.; Skipenko, O.G.; Barroso, E.; Lopez-Ben, S.; Hubert, C.; Majno, P.E.; Toso, C. Surgical management of patients with colorectal cancer and simultaneous liver and lung metastases. Br. J. Surg. 2015, 102, 691–699. [Google Scholar] [CrossRef]
- Dave, R.V.; Pathak, S.; White, A.D.; Hidalgo, E.; Prasad, K.R.; Lodge, J.P.; Milton, R.; Toogood, G.J. Outcome after liver resection in patients presenting with simultaneous hepatopulmonary colorectal metastases. Br. J. Surg. 2015, 102, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Q.; Min, Y.; Wang, S.Y.; Yang, X.J.; Liu, Y.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: A systematic review and meta-analysis of current evidence. Oncotarget 2017, 8, 55657–55683. [Google Scholar] [CrossRef]
- Bjornsson, B.; Sparrelid, E.; Rosok, B.; Pomianowska, E.; Hasselgren, K.; Gasslander, T.; Bjornbeth, B.A.; Isaksson, B.; Sandstrom, P. Associating liver partition and portal vein ligation for staged hepatectomy in patients with colorectal liver metastases--intermediate oncological results. Eur. J. Surg. Oncol. 2016, 42, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Bale, R.; Widmann, G.; Schullian, P.; Haidu, M.; Pall, G.; Klaus, A.; Weiss, H.; Biebl, M.; Margreiter, R. Percutaneous stereotactic radiofrequency ablation of colorectal liver metastases. Eur. Radiol. 2012, 22, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Putzer, D.; Schullian, P.; Braunwarth, E.; Fodor, M.; Primavesi, F.; Cardini, B.; Resch, T.; Oberhuber, R.; Maglione, M.; Margreiter, C.; et al. Integrating interventional oncology in the treatment of liver tumors. Eur. Surg. 2018, 50, 117–124. [Google Scholar] [CrossRef]
- Kemeny, N.E.; Chou, J.F.; Boucher, T.M.; Capanu, M.; DeMatteo, R.P.; Jarnagin, W.R.; Allen, P.J.; Fong, Y.C.; Cercek, A.; D’Angelica, M.I. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. J. Surg. Oncol. 2016, 113, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Townsend, A.R.; Chong, L.C.; Karapetis, C.; Price, T.J. Selective internal radiation therapy for liver metastases from colorectal cancer. Cancer Treat. Rev. 2016, 50, 148–154. [Google Scholar] [CrossRef]
- Jones, R.P.; Stattner, S.; Dunne, D.F.; O’Grady, E.; Smethurst, A.; Terlizzo, M.; Malik, H.Z.; Fenwick, S.W.; Poston, G.J. Radiological assessment of response to neoadjuvant transcatheter hepatic therapy with irinotecan-eluting beads (debiri((r))) for colorectal liver metastases does not predict tumour destruction or long-term outcome. Eur. J. Surg. Oncol. 2013, 39, 1122–1128. [Google Scholar] [CrossRef]
- Tufo, A.; Dunne, D.F.J.; Manu, N.; Joshi, H.; Lacasia, C.; Jones, L.; Malik, H.Z.; Poston, G.J.; Fenwick, S.W. Hepatectomy for octogenarians with colorectal liver metastasis in the era of enhanced recovery. Eur. J. Surg. Oncol. 2018, 44, 1040–1047. [Google Scholar] [CrossRef]
- Leal, J.N.; Sadot, E.; Gonen, M.; Lichtman, S.; Kingham, T.P.; Allen, P.J.; DeMatteo, R.P.; Jarnagin, W.R.; D’Angelica, M.I. Operative morbidity and survival following hepatectomy for colorectal liver metastasis in octogenarians: A contemporary case matched series. HPB 2017, 19, 162–169. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The strengthening the reporting of observational studies in epidemiology (strobe) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- De Meijer, V.E.; Kalish, B.T.; Puder, M.; Ijzermans, J.N. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br. J. Surg. 2010, 97, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Barbas, A.S.; Turley, R.S.; Steel, J.L.; Tsung, A.; Marsh, J.W.; Geller, D.A.; Clary, B.M. A standard definition of major hepatectomy: Resection of four or more liver segments. HPB 2011, 13, 494–502. [Google Scholar] [CrossRef] [PubMed]
- OEGHO. Onkopedia-Leitlinien der Österreichischen Gesellschaft für Hämatologie & Medizinische Onkologie (Kolonkarzinom). Available online: http://www.oegho.at/onkopedia-leitlinien/solide-tumore/kolonkarzinom.html (accessed on 1 September 2018).
- Punt, C.J.; Buyse, M.; Kohne, C.H.; Hohenberger, P.; Labianca, R.; Schmoll, H.J.; Pahlman, L.; Sobrero, A.; Douillard, J.Y. Endpoints in adjuvant treatment trials: A systematic review of the literature in colon cancer and proposed definitions for future trials. J. Natl. Cancer Inst. 2007, 99, 998–1003. [Google Scholar] [CrossRef] [PubMed]
| Variable | Curative Group (CIS) | Palliative Group (PAT) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2003–2006 n = 42 | 2007–2010 n = 56 | 2011–2014 n = 72 | p * | 2003–2006 n = 104 | 2007–2010 n = 86 | 2011–2014 n = 60 | p * | |
| Age at mCRC dx: Mean (SD) | 66.0 (9.8) | 65.0 (10.6) | 63.7 (10.7) | 0.507 † | 67.0 (11.5) | 70.2 (11.6) | 74.0 (11.2) | 0.001 † |
| BMI: Mean (SD) | 25.9 (4.1) | 24.9 (3.5) | 25.2 (4.5) | 0.487 † | 25.3 (4.2) | 25.9 (4.7) | 24.7 (4.7) | 0.276 † |
| Sex: Male | 32 (76%) | 32 (57%) | 44 (61%) | 0.130 | 68 (65%) | 52 (61%) | 34 (57%) | 0.524 |
| ASA | 0.519 | 0.144 | ||||||
| I | 3 (7%) | 7 (13%) | 5 (7%) | 10 (10%) | 8 (9%) | 1 (2%) | ||
| II | 25 (60%) | 34 (61%) | 47 (65%) | 46 (45%) | 31 (36%) | 23 (38%) | ||
| III | 14 (33%) | 13 (23%) | 20 (28%) | 38 (37%) | 42 (49%) | 27 (45%) | ||
| IV/V | 0 (0%) | 2 (4%) | 0 (0%) | 9 (9%) | 5 (6%) | 9 (15%) | ||
| Primary TU Location | 0.821 | 0.085 | ||||||
| Colon | 22 (52%) | 30 (54%) | 37 (51%) | 64 (62%) | 59 (69%) | 47 (78%) | ||
| Rectum | 19 (45%) | 26 (46%) | 33 (49%) | 40 (39%) | 25 (29%) | 12 (20%) | ||
| Both | 1 (2%) | 0 (0%) | 2 (3%) | 0 (0%) | 2 (2%) | 1 (0%) | ||
| Primary TU UICC Stage | 0.116 | 0.885 | ||||||
| I | 2 (5%) | 3 (5%) | 4 (6%) | 4 (4%) | 2 (2%) | 2 (3%) | ||
| II | 5 (12%) | 8 (14%) | 4 (6%) | 9 (9%) | 5 (6%) | 5 (8%) | ||
| III | 19 (45%) | 12 (21%) | 20 (28%) | 23 (22%) | 17 (20%) | 9 (15%) | ||
| IV | 16 (38%) | 33 (59%) | 44 (61%) | 68 (65%) | 62 (72%) | 44 (73%) | ||
| Timing of mCRC: synchronous | 18 (43%) | 35 (63%) | 45 (63%) | 0.082 | 75 (72%) | 61 (71%) | 46 (77%) | 0.730 |
| CEA at stage IV: Mean—ng/mL (SD) | 128.4 (680) | 116.3 (398) | 98.0 (331) | 0.946 † | 335.4 (988) | 276.3 (714) | 461.0 (1404) | 0.585 † |
| Initial metastatic site | ||||||||
| Hepatic | 31 (74%) | 40 (71%) | 46 (64%) | 0.477 | 77 (74%) | 64 (74%) | 50 (83%) | 0.348 |
| Pulmonary | 9 (21%) | 20 (36%) | 23 (32%) | 0.299 | 26 (25%) | 27 (31%) | 27 (45%) | 0.030 |
| Hepatic + Pulmonary | 1 (2%) | 4 (7%) | 5 (7%) | 0.650 | 18 (17%) | 18 (21%) | 21 (36%) | 0.025 |
| Peritoneal | 0 (0%) | 2 (4%) | 10 (14%) | 0.009 | 29 (28%) | 26 (30%) | 17 (28%) | 0.935 |
| Distant lymph nodes | 4 (10%) | 2 (4%) | 3 (4%) | 0.461 | 15 (14%) | 10 (12%) | 3 (5%) | 0.173 |
| Others | 1 (2%) | 2 (4%) | 5 (7%) | 0.593 | 13 (13%) | 10 (12%) | 8 (13%) | 0.969 |
| Variable | Curative (CIS; n = 170) | Palliative (PAT; n = 250) | p * |
|---|---|---|---|
| Patient-related factors | |||
| Sex: male | 108 (63%) | 154 (62%) | 0.689 |
| Age at first diagnosis of metastases: mean (SD) | 64.7 (10.5) | 69.8 (11.7) | <0.001 § |
| ≥70 years | 58 (34%) | 131 (52%) | <0.001 |
| ASA Score | <0.001 | ||
| I | 15 (9%) | 19 (8%) | |
| II | 106 (62%) | 100 (40%) | |
| III | 47 (28%) | 108 (43%) | |
| IV/V | 2 (1%) | 23 (9%) | |
| BMI: mean (SD) | 25.3 (4.1) | 25.4 (4.5) | 0.914 § |
| Disease/Tumour-related factors | |||
| Primary tumour | |||
| T stage | <0.001 | ||
| T1 & T2 | 20 (12%) | 18 (7%) | |
| T3 | 113 (67%) | 123 (49%) | |
| T4 & Tx | 37 (22%) | 109 (44%) | |
| Nodal status | <0.001 | ||
| N negative | 46 (27%) | 43 (17%) | |
| N positive | 122 (72%) | 181 (73%) | |
| Nx (unknown) | 2 (1%) | 26 (10%) | |
| R-Status (missing = 8) | <0.001 | ||
| R0 | 152 (91%) | 88 (36%) | |
| R1 | 8 (5%) | 11 (5%) | |
| R2 or no resection | 8 (5%) | 145 (60%) | |
| Tumour differentiation (missing = 12) | 0.090 | ||
| G1 | 2 (1%) | 3 (1%) | |
| G2 | 116 (71%) | 147 (60%) | |
| G3 | 46 (28%) | 91 (37%) | |
| G4 | 0 (0%) | 3 (1%) | |
| Tumour sidedness | 0.002 | ||
| Right Colon | 38 (22%) | 91 (36%) | |
| Left Colon incl. Rectum | 132 (78%) | 159 (64%) | |
| Histology | 0.745 | ||
| Adeno-Carcinoma | 167 (98%) | 244 (98%) | |
| Mucinous or Signet-Cell | 3 (2%) | 6 (2%) | |
| Metastases | |||
| Occurrence of metastases | 0.001 | ||
| Synchronous (≤6 months) | 98 (58%) | 182 (73%) | |
| Metachronous (>6 months) | 72 (42%) | 68 (27%) | |
| CEA at diagnosis of metastases (missing = 39) | <0.001 | ||
| ≤200 ng/mL | 138 (93%) | 174 (75%) | |
| >200 ng/mL | 10 (7%) | 59 (25%) | |
| Metastatic extent | <0.001 | ||
| Liver limited | 98 (58%) | 90 (36%) | |
| Lung limited | 39 (23%) | 18 (7%) | |
| Liver-lung limited | 11 (7%) | 31 (12%) | |
| Other organs involvement | 22 (13%) | 111 (44%) | |
| Treatment-related factors | |||
| CTX received at any time since mets-dx (missing = 3) | 151 (89%) | 190 (77%) | <0.001 |
| Type of CTX (missing = 11) | 0.006 | ||
| Including IRI and/or OX | 139 (84%) | 67 (28%) | |
| 5-FU-based only or no CTX (BSC) | 26 (16%) | 177 (73%) | |
| Received biological anytime since mets-dx (missing = 10) | 105 (63%) | 144 (59%) | 0.389 |
| Time period of diagnosis of metastases | <0.001 | ||
| 2003–2006 | 42 (25%) | 104 (42%) | |
| 2007–2010 | 56 (33%) | 86 (34%) | |
| 2011–2014 | 72 (42%) | 60 (24%) |
| Variable | 2003–2006 | 2007–2010 | 2011–2014 | p * |
|---|---|---|---|---|
| Curative patients (% of total) | 42 (29%) | 56 (39%) | 72 (55%) | <0.001 |
| (A) Surgical data | ||||
| Liver resections (% of total) | 31 (21%) | 40 (28%) | 46 (35%) | 0.041 |
| Liver first concept (% of LR) | 0 (0%) | 5 (13%) | 6 (13%) | 0.122 |
| Major resections (% of LR) | 4 (13%) | 8 (20%) | 17 (37%) | 0.039 |
| Laparoscopic resection (% of LR) | 0 (0%) | 3 (8%) | 6 (13%) | 0.109 |
| Liver metastases | ||||
| Involvement: bilobar (% of LR) | 6 (19%) | 10 (25%) | 24 (52%) | 0.004 |
| Number: mean (range) | 2.2 (1–8) | 2.5 (1–9) | 5.5 (1–40) | 0.005 |
| Diameter of largest lesion (cm): mean (range) | 2.9 (0.9–5.7) | 2.6 (0.4–7.0) | 3.0 (0.4–16.0) | 0.807 † |
| Surgical outcome LR | ||||
| Length of stay (days): mean (range) | 11.2 (3–34) | 14.7 (2–126) | 12.4 (4–50) | 0.514 † |
| Complications (% of LR) | 9 (29%) | 9 (23%) | 19 (41%) | 0.024 |
| Mild (Clavien-Dindo 1-3a) | 4 (13%) | 4 (10%) | 17 (37%) | |
| Severe (Clavien-Dindo 3b-4b) | 5 (16%) | 5 (13%) | 2 (4%) | |
| Mortality (In-hospital) | 0 (0%) | 0 (0%) | 1 (2%) | 0.459 |
| Lung resections (% of total) | 9 (6%) | 20 (14%) | 23 (17%) | 0.013 |
| Thoracoscopic resection (VATS) (% of lung resections) | 4 (44%) | 4 (20%) | 10 (44%) | 0.215 |
| Lung metastases | ||||
| Involvement: bilateral (% of lung resections) | 0 (0%) | 3 (15%) | 5 (22%) | 0.308 |
| Number: mean (range) | 1.2 (1–2) | 2.5 (1–12) | 2.3 (1–8) | 0.359 † |
| Diameter of largest lesion (cm): mean (range) | 1.9 (0.5–3.1) | 1.8 (0.4–5.0) | 1.4 (0.3–6.0) | 0.496 † |
| Surgical outcome lung resections | ||||
| Length of stay (days): mean (range) | 7.4 (2–18) | 6.1 (1–15) | 6.0 (2–20) | 0.719 |
| Complications (% of lung resections) | 2 (22%) | 3 (15%) | 6 (26%) | 0.216 |
| Mild (Clavien-Dindo 1-3a) | 1 (11%) | 1 (5%) | 6 (26%) | |
| Severe (Clavien-Dindo 3b-4b) | 1 (11%) | 2 (10%) | 0 (0%) | |
| Mortality (In-hospital) | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Peritonectomy (% of total) | 0 (0%) | 2 (1%) | 10 (8%) | 0.009 |
| Including HIPEC (% of Peritonectomy) | 0 (0%) | 0 (0%) | 5 (50%) | - |
| Other curative surgery (LA/abd. organs) (% of total) | 3 (2%) | 3 (2%) | 6 (5%) | 0.372 |
| Further curative treatment after initial metastasectomy | 18 (43%) | 21 (38%) | 28 (39%) | 0.860 |
| Number of further treatments: mean (range) | 1.6 (1–5) | 1.5 (1–4) | 1.6 (1–4) | 0.883 † |
| (B) Chemotherapy in curative patients | ||||
| CTX received since first diagnosis of metastases | 37 (88%) | 52 (93%) | 62 (86%) | 0.460 |
| Number of CTX cycles (months) received: mean (range) | 11.5 (0–31) | 9.3 (0–23) | 8.8 (0–33) | 0.147 † |
| Pseudoneoadjuvant before metastasectomy | 16 (38%) | 21 (38%) | 27 (38%) | 0.998 |
| Adjuvant/palliative after metastasectomy | 33 (79%) | 44 (79%) | 59 (82%) | 0.694 |
| Type of CTX scheme | ||||
| 5-FU-Mono-based | 11 (29%) | 12 (22%) | 21 (29%) | 0.609 |
| Oxaliplatin/Irinotecan-based dual therapy ** | 0.733 | |||
| One agent | 13 (34%) | 23 (41%) | 34 (47%) | |
| Both agents (sequentially) | 18 (47%) | 22 (39%) | 27 (38%) | |
| Oxaliplatin/Irinotecan-based triple (FOLFOXIRI) | 0 (0%) | 2 (4%) | 3 (4%) | 0.456 |
| Biological included *** | 21 (55%) | 38 (68%) | 46 (64%) | 0.457 |
| Other agents **** | 0 (0%) | 2 (4%) | 11 (15%) | 0.007 |
| Variable | No. (%) | Median OS (mo; 95%CI) | Univariable Analysis | p | Multivariable Analysis | p |
|---|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | |||||
| Patient-related factors | ||||||
| Sex | ||||||
| Female | 158 (38%) | 24.4 (16.6–32.2) | ||||
| Male | 262 (62%) | 25.3 (21.2–29.4) | 1.01 (0.81–1.26) | 0.913 | ||
| Age at first mets-dx | ||||||
| <70 | 231 (55%) | 33.4 (27.3–39.5) | ||||
| ≥70 | 189 (45%) | 18.3 (14.0–22.6) | 1.46 (1.18–1.81) | 0.001 | 0.99 (0.78–1.27) | 0.958 |
| ASA Score | ||||||
| I | 34 (8%) | 23.6 (12.9–34.3) | ||||
| II | 206 (49%) | 36.5 (31.0–42.0) | 0.97 (0.62–1.50) | 0.888 | 1.14 (0.72–1.82) | 0.579 |
| III | 155 (37%) | 17.0 (14.4–19.6) | 1.63 (1.05–2.54) | 0.031 | 1.44 (0.89–2.33) | 0.134 |
| IV/V | 25 (6%) | 4.5 (2.1–7.0) | 6.05 (3.40–10.77) | <0.001 | 3.50 (1.85–6.61) | <0.001 |
| BMI (missing = 11) | ||||||
| <25.4 (=Median) | 217 (52%) | 22.6 (17.7–27.5) | ||||
| ≥25.4 | 192 (46%) | 28.1 (23.7–32.5) | 0.90 (0.72–1.12) | 0.342 | ||
| Disease/Tumour-related factors | ||||||
| Primary tumour | ||||||
| T stage | ||||||
| T1 & T2 | 38 (9%) | 30.5 (16.9–44.1) | ||||
| T3 | 236 (56%) | 31.1 (26.8–35.4) | 1.03 (0.70–1.53) | 0.868 | 0.94 (0.61–1.44) | 0.759 |
| T4 & Tx | 146 (35%) | 15.6 (12.6–18.6) | 1.69 (1.13–2.53) | 0.011 | 1.00 (0.64–1.57) | 0.996 |
| Nodal status | ||||||
| N negative | 89 (21%) | 41.0 (27.8–54.3) | ||||
| N positive | 303 (72%) | 23.2 (19.0–27.4) | 1.55 (1.18–2.04) | 0.002 | 1.52 (1.13–2.04) | 0.006 |
| Nx (unknown) | 28 (7%) | 12.8 (3.6–22.0) | 2.91 (1.85–4.58) | <0.001 | 1.36 (0.81–2.26) | 0.245 |
| R-Status | ||||||
| R0 | 240 (58%) | 39.1 (34.5–43.7) | ||||
| R1 | 19 (5%) | 12.9 (9.4–16.42) | 1.94 (1.14–3.30) | 0.014 | 1.13 (0.63–2.03) | 0.686 |
| R2/no resection/Rx | 161 (38%) | 15.1 (12.0–18.2) | 2.84 (2.27–3.58) | <0.001 | 1.05 (0.75–1.46) | 0.782 |
| Tumour differentiation | ||||||
| G1 | 5 (1%) | 20.6 (3.9–37.4) | ||||
| G2 | 263 (63%) | 30.5 (26.6–34.4) | 0.77 (0.32–1.87) | 0.564 | ||
| G3 | 137 (33%) | 16.2 (10.5–21.9) | 1.04 (0.42–2.54) | 0.940 | ||
| G4 | 3 (1%) | 3.7 (10.8–25.2) | 1.43 (0.34–5.99) | 0.626 | ||
| Gx | 12 (3%) | 32.5 (0.0–66.1) | 0.71 (0.24–2.11) | 0.535 | ||
| Tumour location (sidedness) | ||||||
| Left Colon incl. Rectum | 291 (69%) | 29.6 (24.8–34.4) | ||||
| Right Colon | 129 (31%) | 15.3 (11.8–18.8) | 1.59 (1.26–1.99) | <0.001 | 1.33 (1.04–1.69) | 0.021 |
| Histology primary tumour | ||||||
| Adeno-Carcinoma | 411 (98%) | 25.9 (22.0–29.8) | ||||
| Mucinous or Signet-Cell | 9 (2%) | 12.4 (0.0–27.6) | 2.80 (1.44–5.46) | 0.002 | 3.18 (1.55–6.55) | 0.002 |
| Metastases | ||||||
| Occurrence of metastases | ||||||
| Metachronous (>6 months) | 140 (33%) | 32.5 (24.7–40.3) | ||||
| Synchronous (≤6 months) | 280 (67%) | 21.7 (17.7–25.7) | 1.52 (1.20–1.93) | <0.001 | 1.25 (0.90–1.72) | 0.180 |
| CEA at mets-dx | ||||||
| ≤200 ng/mL | 312 (74%) | 28.3 (23.4–33.2) | ||||
| >200 ng/mL | 69 (16%) | 13.6 (9.0–18.3) | 2.22 (1.68–2.93) | <0.001 | 1.74 (1.27–2.39) | <0.001 |
| Missing | 39 (9%) | 31.7 (16.2–47.2) | 0.83 (0.56–1.24) | 0.833 | 0.94 (0.61–1.45) | 0.779 |
| Metastatic extent | ||||||
| Liver limited | 188 (45%) | 29.8 (24.1–35.6) | ||||
| Lung limited | 57 (13%) | 46.8 (34.7–59.0) | 0.57 (0.40–0.83) | 0.003 | 0.70 (0.47–1.04) | 0.080 |
| Liver-lung limited | 42 (10%) | 25.5 (9.9–41.1) | 1.46 (1.01–2.11) | 0.042 | 0.95 (0.63–1.43) | 0.803 |
| Other organs involvement | 133 (32%) | 15.6 (11.9–19.3) | 1.80 (1.41–2.30) | <0.001 | 1.24 (0.91–1.69) | 0.167 |
| Treatment-related factors | ||||||
| Initial treatment strategy | ||||||
| Curative | 170 (41%) | 52.1 (39.9–54.3) | ||||
| Palliative | 250 (60%) | 14.0 (11.6–16.4) | 4.36 (3.42–5.57) | <0.001 | 3.68 (2.64–5.12) | <0.001 |
| CTX since mets-dx (miss = 3) | ||||||
| Any chemotherapy | 341 (82%) | 29.8 (25.7–40.0) | ||||
| BSC/no chemo | 76 (18%) | 4.0 (2.7–5.3) | 2.42 (1.84–3.18) | <0.001 | ** | |
| Type of CTX | ||||||
| Including IRI and/or OX | 316 (75%) | 30.8 (27.1–34.5) | ||||
| 5-FU-based only/BSC/no CTX | 93 (22%) | 6.7 (3.3–10.1) | 2.00 (1.55–2.59) | <0.001 | 2.57 (1.92–3.45) | <0.001 |
| Unknown | 11 (3%) | 21.6 (17.2–26.0) | 1.17 (0.62–2.20) | 0.627 | 2.05 (1.06–3.99) | 0.034 |
| Received biological | ||||||
| At any time since mets-dx | 249 (59%) | 29.8 (26.4–33.2) | ||||
| Never | 161 (38%) | 14.4 (8.3–20.5) | 1.13 (0.90–1.42) | 0.289 | ||
| Missing | 10 (3%) | 20.6 (16.3–24.9) | 1.04 (0.53–2.03) | 0.905 | ||
| Time period of mets-dx | ||||||
| 2003–2006 | 146 (35%) | 21.9 (17.3–26.5) | ||||
| 2007–2010 | 142 (34%) | 24.2 (17.5–30.9) | 0.91 (0.71–1.16) | 0.428 | ||
| 2011–2014 | 132 (31%) | 36.5 (26.6–46.4) | 0.67 (0.51–0.89) | 0.005 | ** |
| Variable | OR (95% CI) | p |
|---|---|---|
| Patient factors | ||
| Sex: male | 0.95 (0.55–1.65) | 0.859 |
| Age at diagnosis of metastases ≥70 | 2.22 (1.28–3.83) | 0.004 |
| ASA ≥ 3 | 1.39 (0.79–2.42) | 0.250 |
| BMI ≥ 25.4 | 1.30 (0.77–2.22) | 0.326 |
| Tumour factors | ||
| Nodal positive primary tumour | 1.13 (0.61–2.09) | 0.697 |
| Grading ≥ G3 | 0.98 (0.55–1.74) | 0.939 |
| Right-sided primary tumour | 0.59 (0.33–1.07) | 0.083 |
| Synchronous metastases | 2.43 (1.33–4.45) | 0.004 |
| CEA >200 ng/mL | 2.86 (1.26–6.47) | 0.012 |
| Metastatic extent | ||
| Liver limited | 1 (ref) | |
| Lung limited | 1.38 (0.60–3.18) | 0.453 |
| Combined lung & liver or other organ involvement | 6.73 (3.55–12.76) | <0.001 |
| Time period of diagnosis of metastases | ||
| 2003–2006 | 1 (ref) | |
| 2007–2010 | 0.54 (0.29–1.00) | 0.050 |
| 2011–2014 | 0.15 (0.08–0.30) | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primavesi, F.; Stättner, S.; Jäger, T.; Göbel, G.; Presl, J.; Tomanová, K.; Buchner, S.; Maglione, M.; Resch, T.; Hutter, J.; et al. Progressive Oncological Surgery Is Associated with Increased Curative Resection Rates and Improved Survival in Metastatic Colorectal Cancer. Cancers 2019, 11, 218. https://doi.org/10.3390/cancers11020218
Primavesi F, Stättner S, Jäger T, Göbel G, Presl J, Tomanová K, Buchner S, Maglione M, Resch T, Hutter J, et al. Progressive Oncological Surgery Is Associated with Increased Curative Resection Rates and Improved Survival in Metastatic Colorectal Cancer. Cancers. 2019; 11(2):218. https://doi.org/10.3390/cancers11020218
Chicago/Turabian StylePrimavesi, Florian, Stefan Stättner, Tarkan Jäger, Georg Göbel, Jaroslav Presl, Katerina Tomanová, Selina Buchner, Manuel Maglione, Thomas Resch, Jörg Hutter, and et al. 2019. "Progressive Oncological Surgery Is Associated with Increased Curative Resection Rates and Improved Survival in Metastatic Colorectal Cancer" Cancers 11, no. 2: 218. https://doi.org/10.3390/cancers11020218
APA StylePrimavesi, F., Stättner, S., Jäger, T., Göbel, G., Presl, J., Tomanová, K., Buchner, S., Maglione, M., Resch, T., Hutter, J., Öfner, D., & Dinnewitzer, A. (2019). Progressive Oncological Surgery Is Associated with Increased Curative Resection Rates and Improved Survival in Metastatic Colorectal Cancer. Cancers, 11(2), 218. https://doi.org/10.3390/cancers11020218






