Targeting FER Kinase Inhibits Melanoma Growth and Metastasis
Abstract
:1. Introduction
2. Results
2.1. Analysis of FER Kinase in Human Melanoma Tumors
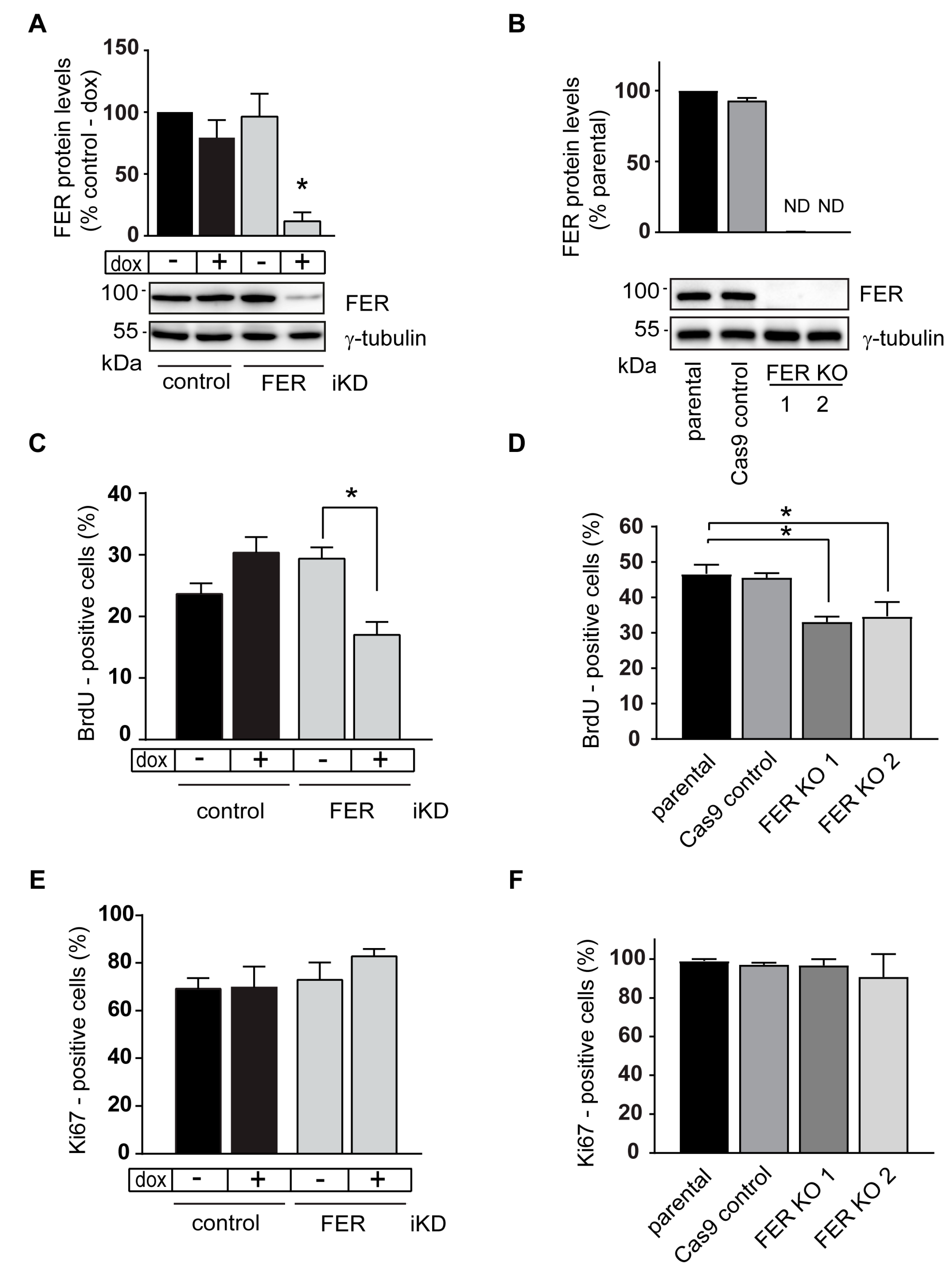
2.2. S-Phase Abnormalities in FER-Deficient Melanoma Cells
2.3. FER Regulates Melanoma Cell Motility
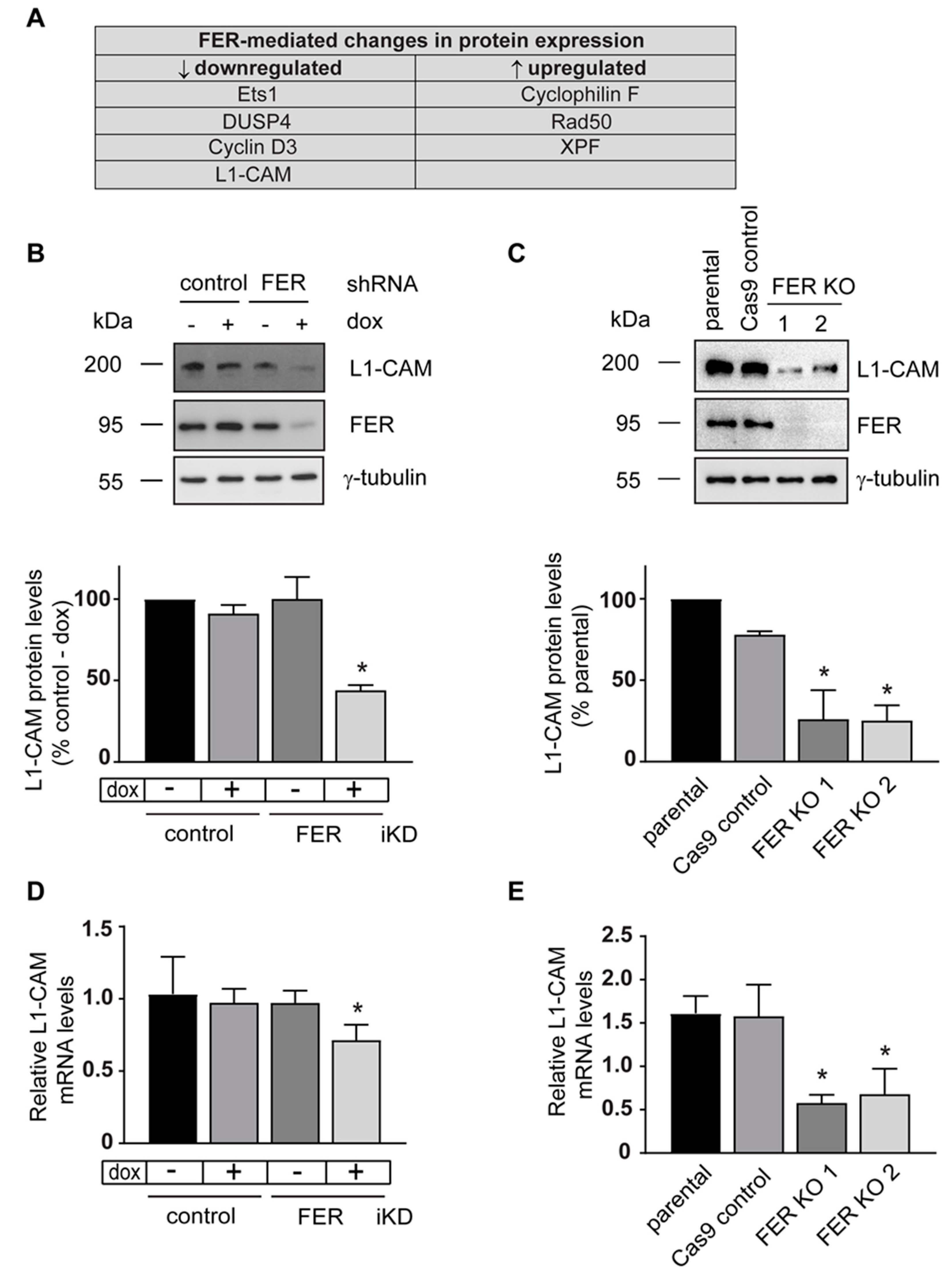
2.4. Discovery of Altered Cellular Signaling Networks in FER-Deficient Cells
2.5. FER Requirement for β-Catenin/T Cell Factor-Lymphoid Enhancer Factor (TCF-LEF) Transcriptional Activity
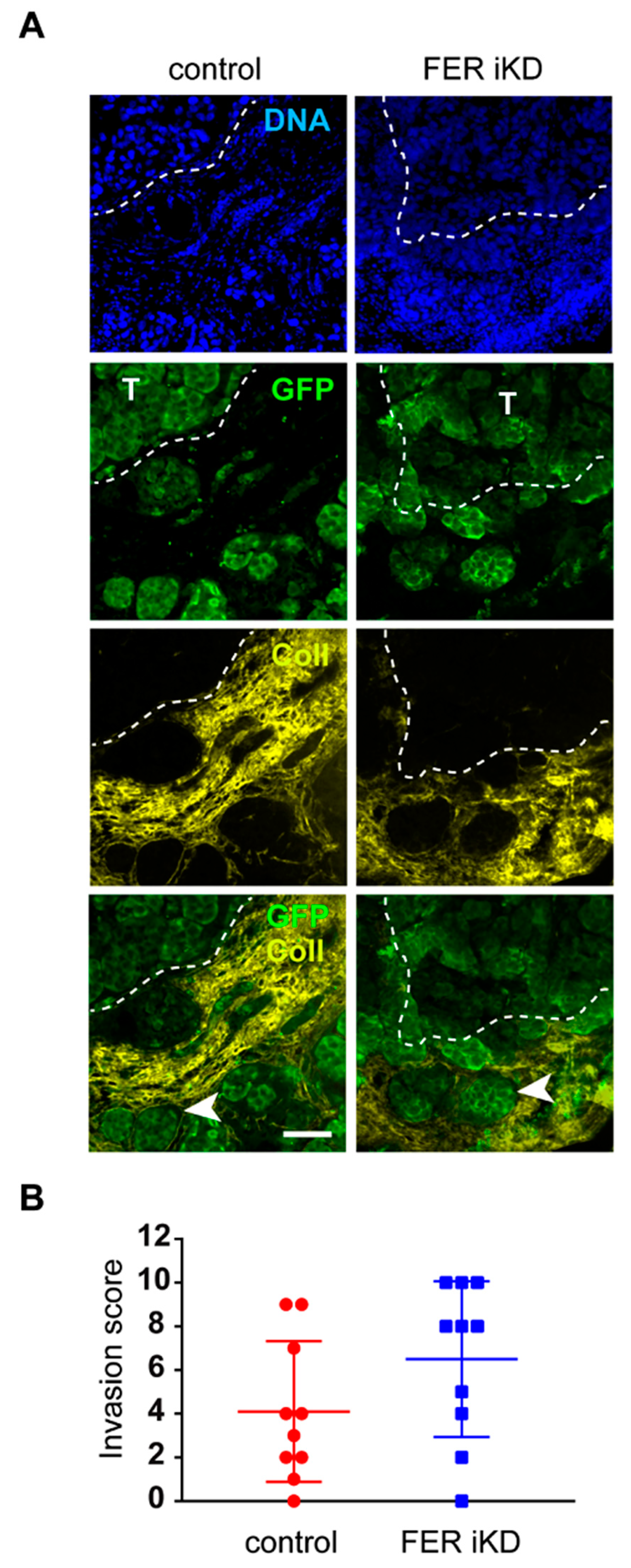
2.6. Role of FER in Melanoma Cell Invasion
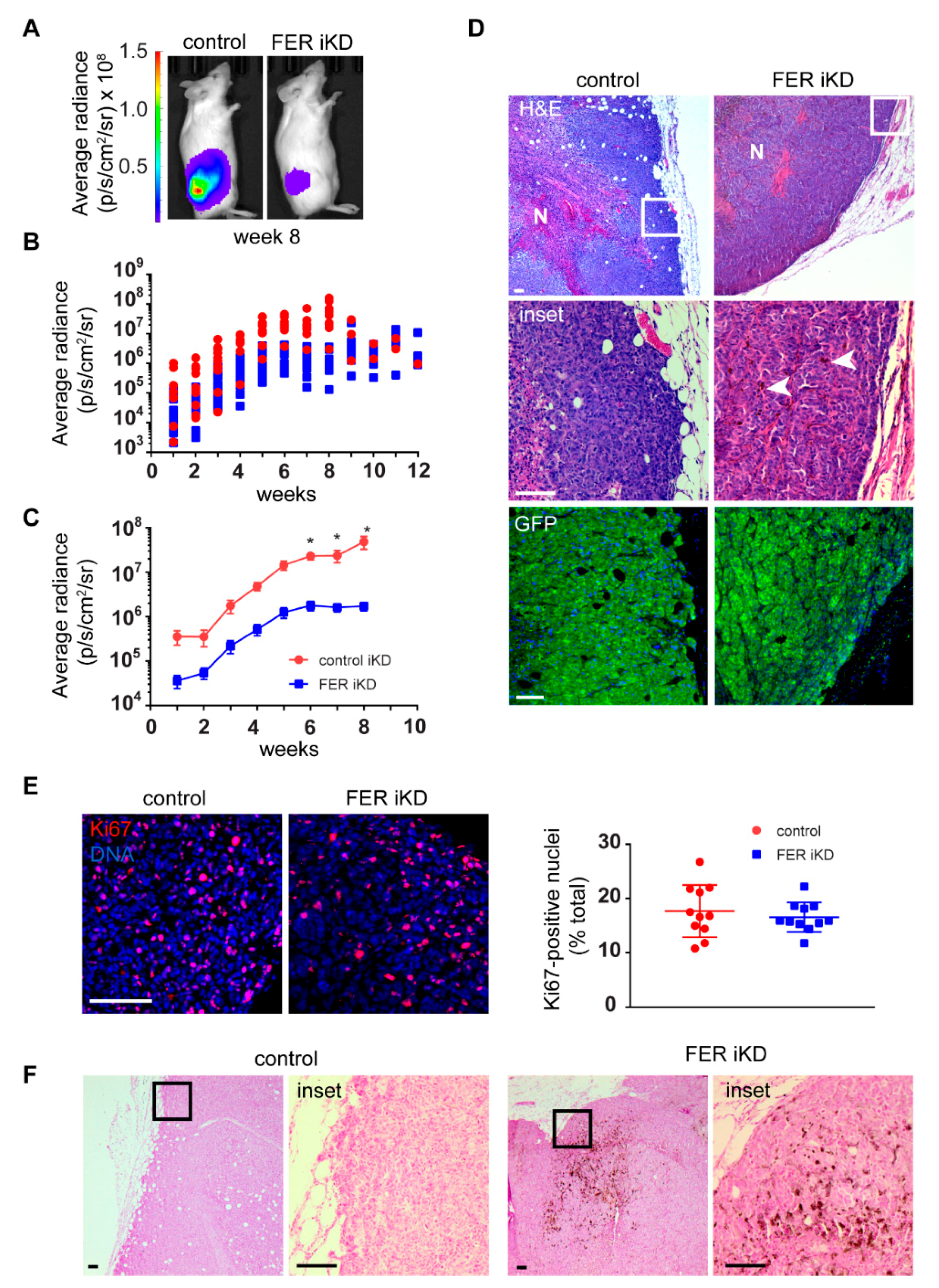
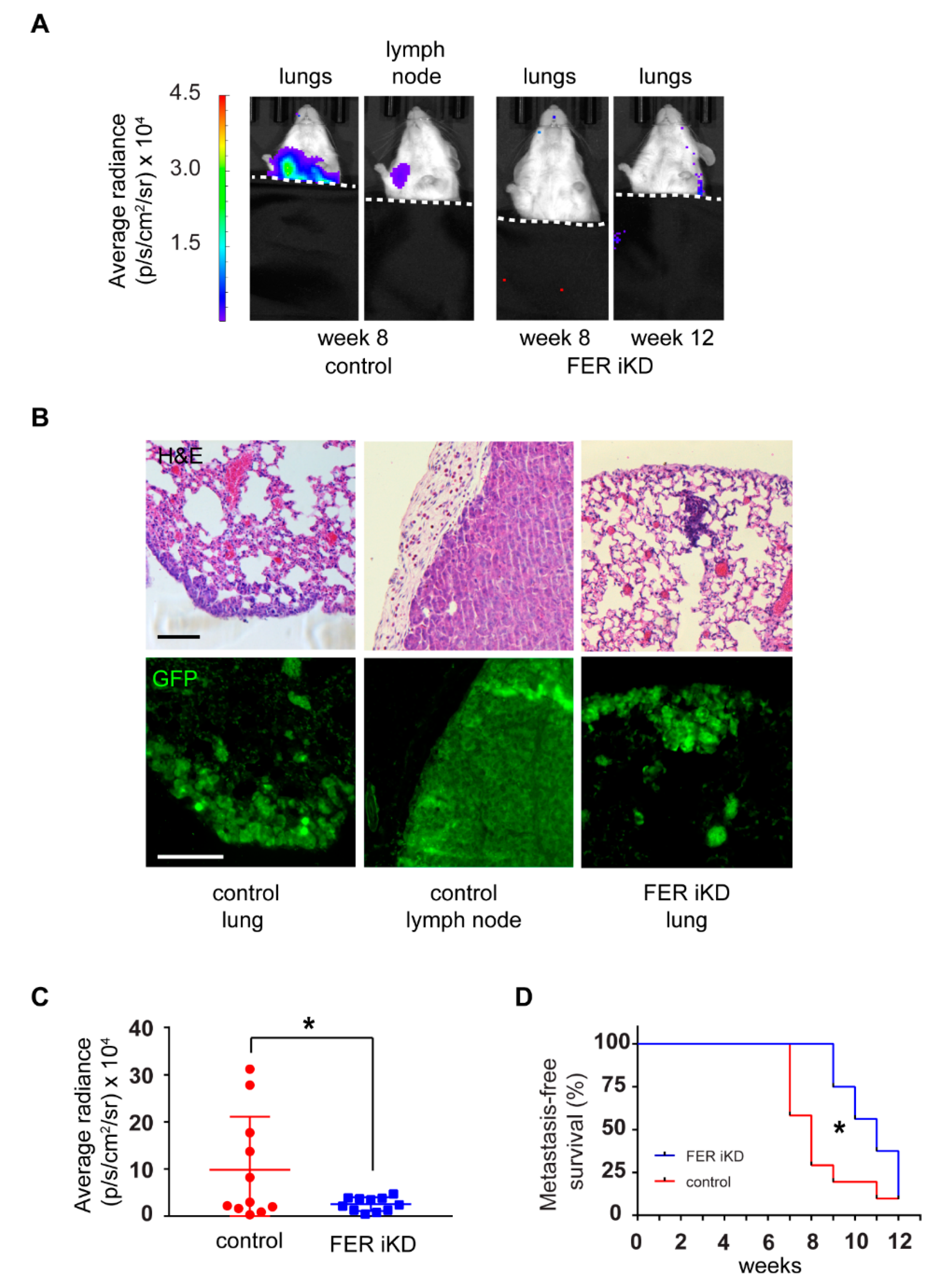
2.7. Reduced Tumor Growth and Formation of Distant Metastases in FER-Deficient Melanoma
3. Discussion
4. Materials and Methods
4.1. Plasmids and Lentivirus
4.2. Cell Culture, Lentiviral Transduction and FER Gene Editing
- FER exon 1 coding 5′-CACCGAGACTGGGAATTACGGTTAC-3′
- FER exon 1 non-coding 5′-AAACGTAACCGTAATTCCCAGTCTC-3′
- FER exon 3 coding 5′-CACCGGCTTTGTCGTATCGTTCCT-3′
- FER exon 3 non-coding 5′- AAACAGGAACGATACGACAAAGCC-3′
- FER exon 1 forward 5′-GGCTAAGACCTCTGGAGTAATGTT-3′
- FER exon 1 reverse 5′-TCTCCATGAGATTTTCATACTCTGTA-3′
- FER exon 3 forward 5′-AGTATCCTACATGCCTCCTTGGCAA-3′
- FER exon 3 reverse 5′-CAACTCTCTGGAGGCAGATATTCAC-3′
4.3. Immunoblot Analysis
4.4. Immunofluorescence Microscopy
4.5. Cell Motility Assays
4.6. Cell Adhesion Assays
4.7. Reverse Phase Protein Array (RPPA) Analysis
4.8. Gene Expression Analysis
4.9. Measurement of TCF/LEF Activity
4.10. Ex Ovo CAM Assays
4.11. In Vivo Xenograft Tumorigenesis Studies
4.12. Immunohistochemistry (IHC)
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2018; Canadian Cancer Statistics: Toronto, ON, Canada, 2018. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Guy, G.P., Jr. Stemming the Rising Incidence of Melanoma: Calling Prevention to Action. J. Natl. Cancer Inst. 2016, 108, djv381. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [Green Version]
- McArthur, G.A.; Chapman, P.B.; Robert, C.; Larkin, J.; Haanen, J.B.; Dummer, R.; Ribas, A.; Hogg, D.; Hamid, O.; Ascierto, P.A.; et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): Extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014, 15, 323–332. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Tentori, L.; Lacal, P.M.; Graziani, G. Challenging resistance mechanisms to therapies for metastatic melanoma. Trends Pharmacol. Sci. 2013, 34, 656–666. [Google Scholar] [CrossRef]
- Greer, P. Closing in on the biological functions of Fps/Fes and Fer. Nat. Rev. Mol. Cell Biol. 2002, 3, 278–289. [Google Scholar] [CrossRef]
- Kim, L.; Wong, T.W. Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase FER. J. Biol. Chem. 1998, 273, 23542–23548. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Wong, T.W. The cytoplasmic tyrosine kinase FER is associated with the catenin-like substrate pp120 and is activated by growth factors. Mol. Cell. Biol. 1995, 15, 4553–4561. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.W.; Greer, P.A. Fer kinase is required for sustained p38 kinase activation and maximal chemotaxis of activated mast cells. Mol. Cell. Biol. 2002, 22, 6363–6374. [Google Scholar] [CrossRef]
- Itoh, T.; Hasegawa, J.; Tsujita, K.; Kanaho, Y.; Takenawa, T. The tyrosine kinase Fer is a downstream target of the PLD-PA pathway that regulates cell migration. Sci. Signal. 2009, 2, ra52. [Google Scholar] [CrossRef] [PubMed]
- Sangrar, W.; Gao, Y.; Scott, M.; Truesdell, P.; Greer, P.A. Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion. Mol. Cell. Biol. 2007, 27, 6140–6152. [Google Scholar] [CrossRef] [PubMed]
- Pasder, O.; Shpungin, S.; Salem, Y.; Makovsky, A.; Vilchick, S.; Michaeli, S.; Malovani, H.; Nir, U. Downregulation of Fer induces PP1 activation and cell-cycle arrest in malignant cells. Oncogene 2006, 25, 4194–4206. [Google Scholar] [CrossRef] [Green Version]
- Voisset, E.; Lopez, S.; Chaix, A.; Georges, C.; Hanssens, K.; Prebet, T.; Dubreuil, P.; De Sepulveda, P. FES kinases are required for oncogenic FLT3 signaling. Leukemia 2010, 24, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Zoubeidi, A.; Rocha, J.; Zouanat, F.Z.; Hamel, L.; Scarlata, E.; Aprikian, A.G.; Chevalier, S. The Fer tyrosine kinase cooperates with interleukin-6 to activate signal transducer and activator of transcription 3 and promote human prostate cancer cell growth. Mol. Cancer Res. 2009, 7, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, Z.; Kang, X.; Zhang, L.; Li, X.; Wang, Y.; Xue, T.; Shen, Y.; Liu, Y. Identification of tyrosine-phosphorylated proteins associated with metastasis and functional analysis of FER in human hepatocellular carcinoma cells. BMC Cancer 2009, 9, 366. [Google Scholar] [CrossRef]
- Ivanova, I.A.; Vermeulen, J.F.; Ercan, C.; Houthuijzen, J.M.; Saig, F.A.; Vlug, E.J.; van der Wall, E.; van Diest, P.J.; Vooijs, M.; Derksen, P.W. FER kinase promotes breast cancer metastasis by regulating alpha- and beta-integrin-dependent cell adhesion and anoikis resistance. Oncogene 2013, 32, 5582–5592. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Truesdell, P.; Meens, J.; Kadish, C.; Yang, X.; Boag, A.H.; Craig, A.W. Fer protein-tyrosine kinase promotes lung adenocarcinoma cell invasion and tumor metastasis. Mol. Cancer Res. 2013, 11, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Zhang, S.; Gao, Y.; Greer, P.A.; Tonks, N.K. HGF-independent regulation of MET and GAB1 by nonreceptor tyrosine kinase FER potentiates metastasis in ovarian cancer. Genes Dev. 2016, 30, 1542–1557. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Munoz, W.; Man, S.; Xu, P.; Kerbel, R.S. Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res. 2008, 68, 4500–4505. [Google Scholar] [CrossRef]
- Xu, L.; Shen, S.S.; Hoshida, Y.; Subramanian, A.; Ross, K.; Brunet, J.P.; Wagner, S.N.; Ramaswamy, S.; Mesirov, J.P.; Hynes, R.O. Gene expression changes in an animal melanoma model correlate with aggressiveness of human melanoma metastases. Mol. Cancer Res. 2008, 6, 760–769. [Google Scholar] [CrossRef]
- Gupta, P.B.; Kuperwasser, C.; Brunet, J.P.; Ramaswamy, S.; Kuo, W.L.; Gray, J.W.; Naber, S.P.; Weinberg, R.A. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat. Genet. 2005, 37, 1047–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, M.; Leclerc, V.; Dagnino, L. A reporter mouse model for in vivo tracing and in vitro molecular studies of melanocytic lineage cells and their diseases. Biol. Open 2017, 6, 1219–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moullan, N.; Mouchiroud, L.; Wang, X.; Ryu, D.; Williams, E.G.; Mottis, A.; Jovaisaite, V.; Frochaux, M.V.; Quiros, P.M.; Deplancke, B.; et al. Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research. Cell Rep. 2015. [Google Scholar] [CrossRef]
- Colombo, F.; Meldolesi, J. L1-CAM and N-CAM: From Adhesion Proteins to Pharmacological Targets. Trends Pharmacol. Sci. 2015, 36, 769–781. [Google Scholar] [CrossRef]
- Fogel, M.; Mechtersheimer, S.; Huszar, M.; Smirnov, A.; Abu-Dahi, A.; Tilgen, W.; Reichrath, J.; Georg, T.; Altevogt, P.; Gutwein, P. L1 adhesion molecule (CD 171) in development and progression of human malignant melanoma. Cancer Lett. 2003, 189, 237–247. [Google Scholar] [CrossRef]
- Thies, A.; Schachner, M.; Moll, I.; Berger, J.; Schulze, H.J.; Brunner, G.; Schumacher, U. Overexpression of the cell adhesion molecule L1 is associated with metastasis in cutaneous malignant melanoma. Eur. J. Cancer 2002, 38, 1708–1716. [Google Scholar] [CrossRef]
- Meier, F.; Busch, S.; Gast, D.; Goppert, A.; Altevogt, P.; Maczey, E.; Riedle, S.; Garbe, C.; Schittek, B. The adhesion molecule L1 (CD171) promotes melanoma progression. Int. J. Cancer 2006, 119, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Li, F.Z.; Dhillon, A.S.; Anderson, R.L.; McArthur, G.; Ferrao, P.T. Phenotype switching in melanoma: Implications for progression and therapy. Front. Oncol. 2015, 5, 31. [Google Scholar] [CrossRef]
- Lokman, N.A.; Elder, A.S.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef]
- Redelsperger, I.M.; Taldone, T.; Riedel, E.R.; Lepherd, M.L.; Lipman, N.S.; Wolf, F.R. Stability of Doxycycline in Feed and Water and Minimal Effective Doses in Tetracycline-Inducible Systems. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 467–474. [Google Scholar] [PubMed]
- Millet, A.; Martin, A.R.; Ronco, C.; Rocchi, S.; Benhida, R. Metastatic Melanoma: Insights Into the Evolution of the Treatments and Future Challenges. Med. Res. Rev. 2017, 37, 98–148. [Google Scholar] [CrossRef] [PubMed]
- Spofford, L.S.; Abel, E.V.; Boisvert-Adamo, K.; Aplin, A.E. Cyclin D3 expression in melanoma cells is regulated by adhesion-dependent phosphatidylinositol 3-kinase signaling and contributes to G1-S progression. J. Biol. Chem. 2006, 281, 25644–25651. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Kawada, Y.; Kai-Murozono, M.; Komatsu, S.; Han, S.A.; Takeuchi, K.; Mizushima, H.; Miyazaki, K.; Irimura, T. Regulation of melanoma cell migration and invasion by laminin-5 and alpha3beta1 integrin (VLA-3). Clin. Exp. Metastasis 2002, 19, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, T.; Angata, K.; Bao, X.; Courtneidge, S.; Chanda, S.K.; Fukuda, M. Fer kinase regulates cell migration through alpha-dystroglycan glycosylation. Mol. Biol. Cell 2012, 23, 771–780. [Google Scholar] [CrossRef]
- Agrawal, P.; Fontanals-Cirera, B.; Sokolova, E.; Jacob, S.; Vaiana, C.A.; Argibay, D.; Davalos, V.; McDermott, M.; Nayak, S.; Darvishian, F.; et al. A Systems Biology Approach Identifies FUT8 as a Driver of Melanoma Metastasis. Cancer Cell 2017, 31, 804–819. [Google Scholar] [CrossRef]
- Piedra, J.; Miravet, S.; Castano, J.; Palmer, H.G.; Heisterkamp, N.; Garcia de Herreros, A.; Dunach, M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol. Cell. Biol. 2003, 23, 2287–2297. [Google Scholar] [CrossRef]
- Xu, G.; Craig, A.W.; Greer, P.; Miller, M.; Anastasiadis, P.Z.; Lilien, J.; Balsamo, J. Continuous association of cadherin with beta-catenin requires the non-receptor tyrosine-kinase Fer. J. Cell Sci. 2004, 117, 3207–3219. [Google Scholar] [CrossRef]
- Sinnberg, T.; Menzel, M.; Ewerth, D.; Sauer, B.; Schwarz, M.; Schaller, M.; Garbe, C.; Schittek, B. beta-Catenin signaling increases during melanoma progression and promotes tumor cell survival and chemoresistance. PLoS ONE 2011, 6, e23429. [Google Scholar] [CrossRef]
- Gallagher, S.J.; Rambow, F.; Kumasaka, M.; Champeval, D.; Bellacosa, A.; Delmas, V.; Larue, L. Beta-catenin inhibits melanocyte migration but induces melanoma metastasis. Oncogene 2013, 32, 2230–2238. [Google Scholar] [CrossRef] [PubMed]
- Sinnberg, T.; Levesque, M.P.; Krochmann, J.; Cheng, P.F.; Ikenberg, K.; Meraz-Torres, F.; Niessner, H.; Garbe, C.; Busch, C. Wnt-signaling enhances neural crest migration of melanoma cells and induces an invasive phenotype. Mol. Cancer 2018, 17, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Su, Y.; Wesslowski, J.; Hagemann, A.I.; Ramialison, M.; Wittbrodt, J.; Scholpp, S.; Davidson, G. Tyrosine phosphorylation of LRP6 by Src and Fer inhibits Wnt/beta-catenin signalling. EMBO Rep. 2014, 15, 1254–1267. [Google Scholar] [CrossRef]
- Zhang, G.; Ai, D.; Yang, X.; Ji, S.; Wang, Z.; Feng, S. MicroRNA-610 inhibits tumor growth of melanoma by targeting LRP6. Oncotarget 2017, 8, 97361–97370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolligs, F.T.; Hu, G.; Dang, C.V.; Fearon, E.R. Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol. Cell. Biol. 1999, 19, 5696–5706. [Google Scholar] [CrossRef]
- Hlavin, M.L.; Lemmon, V. Molecular structure and functional testing of human L1CAM: An interspecies comparison. Genomics 1991, 11, 416–423. [Google Scholar] [CrossRef]
- Veeman, M.T.; Slusarski, D.C.; Kaykas, A.; Louie, S.H.; Moon, R.T. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 2003, 13, 680–685. [Google Scholar] [CrossRef]
- Herold, M.J.; van den Brandt, J.; Seibler, J.; Reichardt, H.M. Inducible and reversible gene silencing by stable integration of an shRNA-encoding lentivirus in transgenic rats. Proc. Natl. Acad. Sci. USA 2008, 105, 18507–18512. [Google Scholar] [CrossRef] [Green Version]
- Schackmann, R.C.; van Amersfoort, M.; Haarhuis, J.H.; Vlug, E.J.; Halim, V.A.; Roodhart, J.M.; Vermaat, J.S.; Voest, E.E.; van der Groep, P.; van Diest, P.J.; et al. Cytosolic p120-catenin regulates growth of metastatic lobular carcinoma through Rock1-mediated anoikis resistance. J. Clin. Investig. 2011, 121, 3176–3188. [Google Scholar] [CrossRef] [Green Version]
- Freeman, T.J.; Sayedyahossein, S.; Johnston, D.; Sanchez-Pupo, R.E.; O’Donnell, B.; Huang, K.; Lakhani, Z.; Nouri-Nejad, D.; Barr, K.J.; Harland, L.; et al. Inhibition of Pannexin 1 Reduces the Tumorigenic Properties of Human Melanoma Cells. Cancers 2019, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Wang, T.; Li, Z.; Guan, G.; Hong, Y. Efficient detection, quantification and enrichment of subtle allelic alterations. DNA Res. 2012, 19, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.A.; D’Souza, S.J.; Dagnino, L. E2F1 stability is regulated by a novel-PKC/p38beta MAP kinase signaling pathway during keratinocyte differentiation. Oncogene 2006, 25, 430–437. [Google Scholar] [CrossRef]
- Vespa, A.; D’Souza, S.J.; Dagnino, L. A novel role for integrin-linked kinase in epithelial sheet morphogenesis. Mol. Biol. Cell 2005, 16, 4084–4095. [Google Scholar] [CrossRef] [PubMed]
- Goldfinger, L.E.; Stack, M.S.; Jones, J.C. Processing of laminin-5 and its functional consequences: Role of plasmin and tissue-type plasminogen activator. J. Cell Biol. 1998, 141, 255–265. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, I.A.; Arulanantham, S.; Barr, K.; Cepeda, M.; Parkins, K.M.; Hamilton, A.M.; Johnston, D.; Penuela, S.; Hess, D.A.; Ronald, J.A.; et al. Targeting FER Kinase Inhibits Melanoma Growth and Metastasis. Cancers 2019, 11, 419. https://doi.org/10.3390/cancers11030419
Ivanova IA, Arulanantham S, Barr K, Cepeda M, Parkins KM, Hamilton AM, Johnston D, Penuela S, Hess DA, Ronald JA, et al. Targeting FER Kinase Inhibits Melanoma Growth and Metastasis. Cancers. 2019; 11(3):419. https://doi.org/10.3390/cancers11030419
Chicago/Turabian StyleIvanova, Iordanka A., Shinthujah Arulanantham, Kevin Barr, Mario Cepeda, Katie M. Parkins, Amanda M. Hamilton, Danielle Johnston, Silvia Penuela, David A. Hess, John A. Ronald, and et al. 2019. "Targeting FER Kinase Inhibits Melanoma Growth and Metastasis" Cancers 11, no. 3: 419. https://doi.org/10.3390/cancers11030419
APA StyleIvanova, I. A., Arulanantham, S., Barr, K., Cepeda, M., Parkins, K. M., Hamilton, A. M., Johnston, D., Penuela, S., Hess, D. A., Ronald, J. A., & Dagnino, L. (2019). Targeting FER Kinase Inhibits Melanoma Growth and Metastasis. Cancers, 11(3), 419. https://doi.org/10.3390/cancers11030419




