Exosomal miRNAs as Novel Pharmacodynamic Biomarkers for Cancer Chemopreventive Agent Early Stage Treatments in Chemically Induced Mouse Model of Lung Squamous Cell Carcinoma
Abstract
1. Introduction
2. Results
2.1. General Characteristics of Exosomal miRNA-seq Data
2.2. Differentially Expressed (DE) Exosomal miRNAs by CPA Treatments
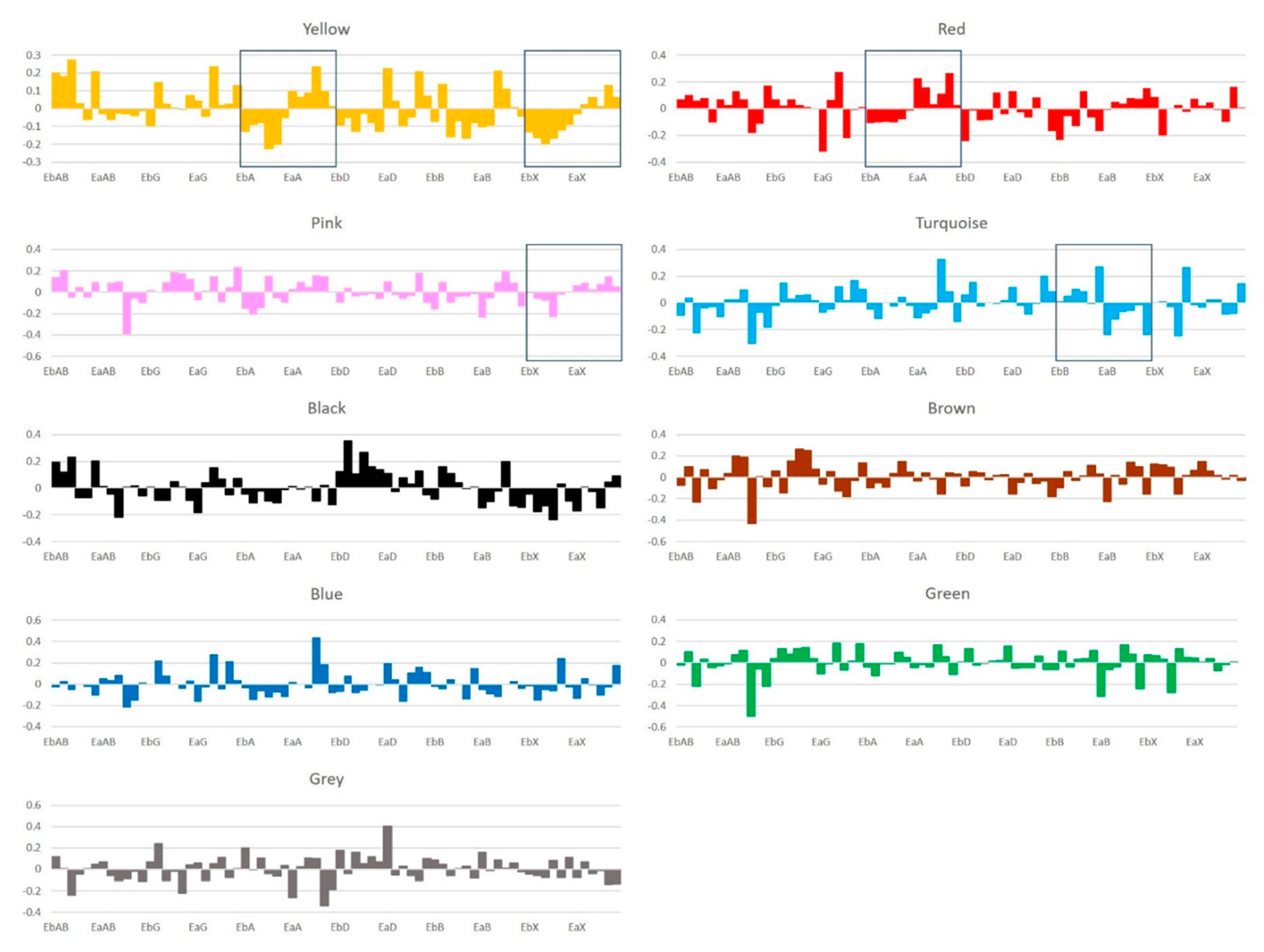
2.3. Exosomal miRNAs Co-expression Network Modules
2.4. Signatures of Exosomal miRNA Expression Change after CPA Treatments
2.5. Pathway Enrichment Analysis
2.6. Conservation Score Analysis
3. Discussion
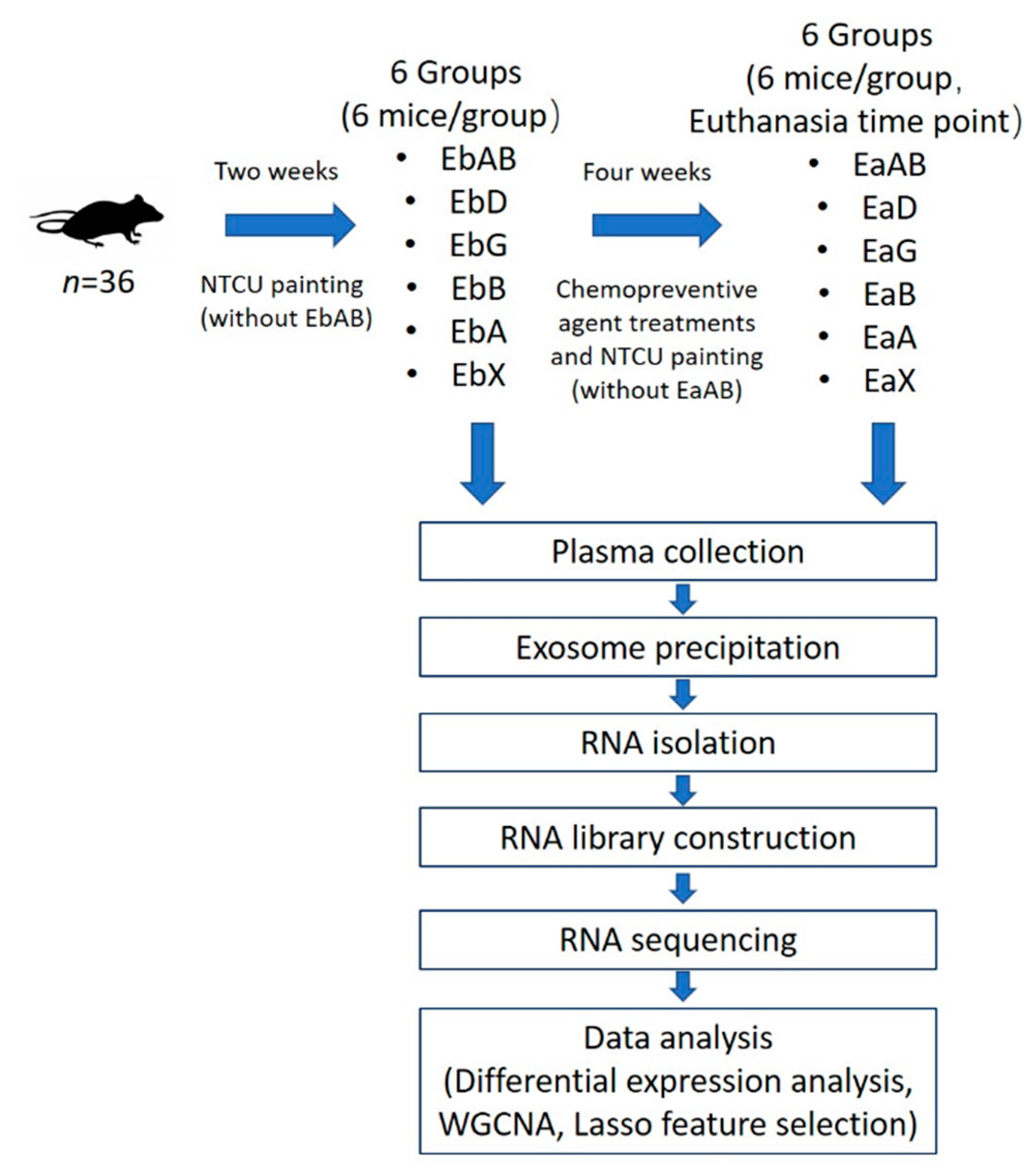
4. Materials and Methods
4.1. Reagents and Animals
4.2. Exosome Precipitation and RNA Isolation
4.3. RNA Library Preparation and Sequencing
4.4. Sequencing Data Analysis
4.4.1. Differential Expression Analysis
4.4.2. Weighted Gene Co-expression Network Analysis
4.4.3. Least Absolute Shrinkage and Selection Operator
4.4.4. Molecular Pathway Analysis
4.4.5. Conservation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations and Definitions
| CPA | Chemopreventive agent |
| DE | Differential expression |
| EaA | Early treatment after AZD6244 |
| EaAB | Early treatment after absolute control |
| EaB | Early treatment after budesonide |
| EaD | Early treatment after diet control |
| EaG | Early treatment after early gavage control |
| EaX | Early treatment after XL-147 |
| EbA | Early treatment before AZD6244 |
| EbAB | Early treatment before absolute control |
| EbB | Early treatment before budesonide |
| EbD | Early treatment before diet control |
| EbG | Early treatment before early gavage control |
| EbX | Early treatment before XL-147 |
| FDR | False discovery rate |
| Lasso | Least absolute shrinkage and selection operator |
| MEK | Mitogen-activated protein kinase kinase |
| miRNA | MicroRNA |
| mRNA | Messenger RNA |
| NTCU | N-nitroso-tris-chloroethylurea |
| PCA | Principal component analysis |
| PI3K | Phosphoinositide 3-kinase |
| Read Count | A term to represent the number of the RNA molecules in the RNA-sequencing libraries. |
| SCC | Squamous cell carcinoma |
| WGCNA | Weighted Gene Co-expression Network Analysis |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Johnson, C.D.; Esquela-Kerscher, A.; Stefani, G.; Byrom, M.; Kelnar, K.; Ovcharenko, D.; Wilson, M.; Wang, X.; Shelton, J.; Shingara, J.; et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007, 67, 7713–7722. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Govindan, R.; Wang, L.; Liu, P.Y.; Goodgame, B.; Wen, W.; Sezhiyan, A.; Pfeifer, J.; Li, Y.F.; Hua, X.; et al. MicroRNA profiling and prediction of recurrence/relapse-free survival in stage I lung cancer. Carcinogenesis 2012, 33, 1046–1054. [Google Scholar] [CrossRef]
- Thery, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A common pathway for a specialized function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef]
- Mears, R.; Craven, R.A.; Hanrahan, S.; Totty, N.; Upton, C.; Young, S.L.; Patel, P.; Selby, P.J.; Banks, R.E. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics 2004, 4, 4019–4031. [Google Scholar] [CrossRef]
- Skog, J.; Wurdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Ohshima, K.; Inoue, K.; Fujiwara, A.; Hatakeyama, K.; Kanto, K.; Watanabe, Y.; Muramatsu, K.; Fukuda, Y.; Ogura, S.; Yamaguchi, K.; et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS ONE 2010, 5, e13247. [Google Scholar] [CrossRef]
- Peinado, H.; Aleckovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Rabinowits, G.; Gercel-Taylor, C.; Day, J.M.; Taylor, D.D.; Kloecker, G.H. Exosomal microRNA: A diagnostic marker for lung cancer. Clin. Lung Cancer 2009, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Rupp, A.K.; Rupp, C.; Keller, S.; Brase, J.C.; Ehehalt, R.; Fogel, M.; Moldenhauer, G.; Marme, F.; Sultmann, H.; Altevogt, P. Loss of EpCAM expression in breast cancer derived serum exosomes: Role of proteolytic cleavage. Gynecol. Oncol. 2011, 122, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Yan, Y.; Lemon, W.J.; LaRegina, M.; Morrison, C.; Lubet, R.; You, M. A chemically induced model for squamous cell carcinoma of the lung in mice: Histopathology and strain susceptibility. Cancer Res. 2004, 64, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.W.; Garon, E.B. Targeting MEK for the treatment of non-small-cell lung cancer. J. Thorac. Oncol. 2012, 7, S377–S378. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.; Yamaguchi, K.; Hsu, P.P.; Qian, F.; Du, X.; Wu, J.; Won, K.A.; Yu, P.; Jaeger, C.T.; Zhang, W.; et al. The Selective PI3K Inhibitor XL147 (SAR245408) Inhibits Tumor Growth and Survival and Potentiates the Activity of Chemotherapeutic Agents in Preclinical Tumor Models. Mol. Cancer Ther. 2015, 14, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Kastens, E.; Lubet, R.A.; You, M. Mice with alterations in both p53 and Ink4a/Arf display a striking increase in lung tumor multiplicity and progression: Differential chemopreventive effect of budesonide in wild-type and mutant A/J mice. Cancer Res. 2003, 63, 4389–4395. [Google Scholar] [PubMed]
- Loeb, G.B.; Khan, A.A.; Canner, D.; Hiatt, J.B.; Shendure, J.; Darnell, R.B.; Leslie, C.S.; Rudensky, A.Y. Transcriptome-wide miR-155 binding map reveals widespread noncanonical microRNA targeting. Mol. Cell 2012, 48, 760–770. [Google Scholar] [CrossRef]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef]
- Turke, A.B.; Song, Y.; Costa, C.; Cook, R.; Arteaga, C.L.; Asara, J.M.; Engelman, J.A. MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer Res. 2012, 72, 3228–3237. [Google Scholar] [CrossRef]
- Kassel, O.; Sancono, A.; Kratzschmar, J.; Kreft, B.; Stassen, M.; Cato, A.C. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001, 20, 7108–7116. [Google Scholar] [CrossRef] [PubMed]
- Ayroldi, E.; Cannarile, L.; Migliorati, G.; Nocentini, G.; Delfino, D.V.; Riccardi, C. Mechanisms of the anti-inflammatory effects of glucocorticoids: Genomic and nongenomic interference with MAPK signaling pathways. FASEB J. 2012, 26, 4805–4820. [Google Scholar] [CrossRef]
- Leis, H.; Page, A.; Ramirez, A.; Bravo, A.; Segrelles, C.; Paramio, J.; Barettino, D.; Jorcano, J.L.; Perez, P. Glucocorticoid Receptor Counteracts Tumorigenic Activity of Akt in Skin through Interference with the Phosphatidylinositol 3-Kinase Signaling Pathway. Mol. Endocrinol. 2004, 18, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, J.; Price, S.R. Inhibition of PI3-kinase signaling by glucocorticoids results in increased branched-chain amino acid degradation in renal epithelial cells. Am. J. Physiol. Cell Physiol. 2007, 292, C1874–C1879. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, S.; Benitez, D.; Nunez, L.E.; Jewell, C.M.; Langjahr, P.; Candia, E.; Zapata-Torres, G.; Cidlowski, J.A.; Gonzalez, M.J.; Hermoso, M.A. Phosphatidylinositol 3-kinase interacts with the glucocorticoid receptor upon TLR2 activation. J. Cell. Mol. Med. 2011, 15, 339–349. [Google Scholar] [CrossRef]
- Hou, Y.; Zhen, J.; Xu, X.; Zhen, K.; Zhu, B.; Pan, R.; Zhao, C. miR-215 functions as a tumor suppressor and directly targets ZEB2 in human non-small cell lung cancer. Oncol. Lett. 2015, 10, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Sinh, N.D.; Endo, K.; Miyazawa, K.; Saitoh, M. Ets1 and ESE1 reciprocally regulate expression of ZEB1/ZEB2, dependent on ERK1/2 activity, in breast cancer cells. Cancer Sci. 2017, 108, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.H.; Wen, D.Y.; Cai, X.Y.; Liang, L.; Wu, P.R.; Qin, H.; Yang, H.; He, Y.; Chen, G. The protective value of miR-204-5p for prognosis and its potential gene network in various malignancies: A comprehensive exploration based on RNA-seq high-throughput data and bioinformatics. Oncotarget 2017, 8, 104960–104980. [Google Scholar] [CrossRef] [PubMed]
- Toll, A.; Salgado, R.; Espinet, B.; Diaz-Lagares, A.; Hernandez-Ruiz, E.; Andrades, E.; Sandoval, J.; Esteller, M.; Pujol, R.M.; Hernandez-Munoz, I. MiR-204 silencing in intraepithelial to invasive cutaneous squamous cell carcinoma progression. Mol. Cancer 2016, 15, 53. [Google Scholar] [CrossRef]
- Lee, J.W.; Guan, W.; Han, S.; Hong, D.K.; Kim, L.S.; Kim, H. MicroRNA-708-3p mediates metastasis and chemoresistance through inhibition of epithelial-to-mesenchymal transition in breast cancer. Cancer Sci. 2018, 109, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.Y.; Hsin, I.L.; Yang, T.Y.; Sung, W.W.; Chi, J.Y.; Chang, J.T.; Ko, J.L.; Sheu, G.T. The ERK-ZEB1 pathway mediates epithelial-mesenchymal transition in pemetrexed resistant lung cancer cells with suppression by vinca alkaloids. Oncogene 2017, 36, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dang, J.; Chang, K.Y.; Yau, E.; Aza-Blanc, P.; Moscat, J.; Rana, T.M. miR-1298 Inhibits Mutant KRAS-Driven Tumor Growth by Repressing FAK and LAMB3. Cancer Res. 2016, 76, 5777–5787. [Google Scholar] [CrossRef]
- Cai, B.; Ma, M.; Chen, B.; Li, Z.; Abdalla, B.A.; Nie, Q.; Zhang, X. MiR-16-5p targets SESN1 to regulate the p53 signaling pathway, affecting myoblast proliferation and apoptosis, and is involved in myoblast differentiation. Cell Death Dis. 2018, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, Q.; Chang, J.; Wang, E.; Qiu, X. MicroRNA HSA-miR-125a-5p induces apoptosis by activating p53 in lung cancer cells. Exp. Lung Res. 2011, 37, 387–398. [Google Scholar] [CrossRef]
- Aziz, M.H.; Shen, H.; Maki, C.G. Glucocorticoid receptor activation inhibits p53-induced apoptosis of MCF10Amyc cells via induction of protein kinase Cepsilon. J. Biol. Chem. 2012, 287, 29825–29836. [Google Scholar] [CrossRef]
- Sengupta, S.; Vonesch, J.L.; Waltzinger, C.; Zheng, H.; Wasylyk, B. Negative cross-talk between p53 and the glucocorticoid receptor and its role in neuroblastoma cells. EMBO J. 2000, 19, 6051–6064. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.Q.; Liu, L.; Yu, Z. Clinical value of microRNA-23a upregulation in non-small cell lung cancer. Int. J. Clin. Exp. Med. 2015, 8, 13598–13603. [Google Scholar]
- Hu, X.; Wang, Y.; Liang, H.; Fan, Q.; Zhu, R.; Cui, J.; Zhang, W.; Zen, K.; Zhang, C.Y.; Hou, D.; et al. miR-23a/b promote tumor growth and suppress apoptosis by targeting PDCD4 in gastric cancer. Cell Death Dis. 2017, 8, e3059. [Google Scholar] [CrossRef]
- Ribeiro, J.R.; Freiman, R.N. Estrogen signaling crosstalk: Implications for endocrine resistance in ovarian cancer. J. Steroid Biochem. Mol. Biol. 2014, 143, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Hew, K.E.; Miller, P.; El-Ashry, D.; Wei, Z.; Sun, J.; Zhang, G.; Guo, W.; Brafford, P.; Mills, G.; Slingerland, J.; et al. The effects of combined MEK inhibition and antiestrogen therapy in the treatment of ovarian cancer. Gynecol. Oncol. 2015, 137, 182. [Google Scholar] [CrossRef]
- Hew, K.E.; Miller, P.C.; El-Ashry, D.; Sun, J.; Besser, A.H.; Ince, T.A.; Gu, M.; Wei, Z.; Zhang, G.; Brafford, P.; et al. MAPK Activation Predicts Poor Outcome and the MEK Inhibitor, Selumetinib, Reverses Antiestrogen Resistance in ER-Positive High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2016, 22, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lee, J.; Seo, H.H.; Shin, S.; Kim, S.W.; Lee, S.; Lim, S.; Hwang, K.C. TAK733 attenuates adrenergic receptor-mediated cardiomyocyte hypertrophy via inhibiting ErkThr188 phosphorylation. Clin. Hemorheol. Microcirc. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.; Musi, E.; Ho, A.L.; de Stanchina, E.; Schwartz, G.K. Inhibition of mutant GNAQ signaling in uveal melanoma induces AMPK-dependent autophagic cell death. Mol. Cancer Ther. 2013, 12, 768–776. [Google Scholar] [CrossRef]
- Martinez-Anton, A.; de Bolos, C.; Alobid, I.; Benitez, P.; Roca-Ferrer, J.; Picado, C.; Mullol, J. Corticosteroid therapy increases membrane-tethered while decreases secreted mucin expression in nasal polyps. Allergy 2008, 63, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, H.; Carraway, K.L., 3rd. MUC1 and MUC4: Switching the emphasis from large to small. Cancer Biother. Radiopharm. 2011, 26, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Devinoy, E.; Houdebine, L.M. Effects of glucocorticoids on casein gene expression in the rabbit. Eur. J. Biochem. 1977, 75, 411–416. [Google Scholar] [CrossRef]
- Huangfu, D.; Maehr, R.; Guo, W.; Eijkelenboom, A.; Snitow, M.; Chen, A.E.; Melton, D.A. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 2008, 26, 795–797. [Google Scholar] [CrossRef]
- Kiezun, A.; Artzi, S.; Modai, S.; Volk, N.; Isakov, O.; Shomron, N. miRviewer: A multispecies microRNA homologous viewer. BMC Res. Notes 2012, 5, 92. [Google Scholar] [CrossRef]
- Poon, E.; Mullins, S.; Watkins, A.; Williams, G.S.; Koopmann, J.O.; Di Genova, G.; Cumberbatch, M.; Veldman-Jones, M.; Grosskurth, S.E.; Sah, V.; et al. The MEK inhibitor selumetinib complements CTLA-4 blockade by reprogramming the tumor immune microenvironment. J. Immunother. Cancer 2017, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.; Soo, K.C.; Chow, P.K.; Tran, E. Targeted inhibition of the extracellular signal-regulated kinase kinase pathway with AZD6244 (ARRY-142886) in the treatment of hepatocellular carcinoma. Mol. Cancer Ther. 2007, 6, 138–146. [Google Scholar] [CrossRef]
- Bosbach, B.; Rossi, F.; Yozgat, Y.; Loo, J.; Zhang, J.Q.; Berrozpe, G.; Warpinski, K.; Ehlers, I.; Veach, D.; Kwok, A.; et al. Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor. Proc. Natl. Acad. Sci. USA 2017, 114, E8448–E8457. [Google Scholar] [CrossRef] [PubMed]
- Clissold, S.P.; Heel, R.C. Budesonide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy in asthma and rhinitis. Drugs 1984, 28, 485–518. [Google Scholar] [CrossRef] [PubMed]
- Heijer, A.; Hesser, G.; Holm, P.; Salde, L. Comparison between two non-halogenated glucocorticoid ointments in psoriasis. J. Int. Med. Res. 1981, 9, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Edsbacker, S.; Andersson, P.; Lindberg, C.; Paulson, J.; Ryrfeldt, A.; Thalen, A. Liver metabolism of budesonide in rat, mouse, and man. Comparative aspects. Drug Metab. Dispos. 1987, 15, 403–411. [Google Scholar] [PubMed]
- Wang, Y.; Zhang, Z.; Garbow, J.R.; Rowland, D.J.; Lubet, R.A.; Sit, D.; Law, F.; You, M. Chemoprevention of lung squamous cell carcinoma in mice by a mixture of Chinese herbs. Cancer Prev. Res. (Phila) 2009, 2, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; James, M.; Wen, W.; Lu, Y.; Szabo, E.; Lubet, R.A.; You, M. Chemopreventive effects of pioglitazone on chemically induced lung carcinogenesis in mice. Mol. Cancer Ther. 2010, 9, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhang, Q.; Li, K.; Liu, Q.; Wang, Y.; You, M. Chemoprevention of lung squamous cell carcinoma by ginseng. Cancer Prev. Res. (Phila) 2013, 6, 530–539. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Q.; Liu, Q.; Komas, S.M.; Kalyanaraman, B.; Lubet, R.A.; Wang, Y.; You, M. Honokiol inhibits lung tumorigenesis through inhibition of mitochondrial function. Cancer Prev. Res. (Phila) 2014, 7, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 319. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011, 39, D152–D157. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. miRBase: Tools for microRNA genomics. Nucleic Acids Res. 2008, 36, D154–D158. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef]
- Griffiths-Jones, S. The microRNA Registry. Nucleic Acids Res. 2004, 32, D109–D111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Horvath, S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 2005, 4, 17. [Google Scholar] [CrossRef]
- Langfelder, P.; Zhang, B.; Horvath, S. Defining clusters from a hierarchical cluster tree: The Dynamic Tree Cut package for R. Bioinformatics 2008, 24, 719–720. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R.; Bien, J.; Friedman, J.; Hastie, T.; Simon, N.; Taylor, J.; Tibshirani, R.J. Strong rules for discarding predictors in lasso-type problems. J. R. Stat. Soc. Ser. B Stat. Methodol. 2012, 74, 245–266. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef] [PubMed]
- Maragkakis, M.; Reczko, M.; Simossis, V.A.; Alexiou, P.; Papadopoulos, G.L.; Dalamagas, T.; Giannopoulos, G.; Goumas, G.; Koukis, E.; Kourtis, K.; et al. DIANA-microT web server: Elucidating microRNA functions through target prediction. Nucleic Acids Res. 2009, 37, W273–W276. [Google Scholar] [CrossRef]
- Reczko, M.; Maragkakis, M.; Alexiou, P.; Grosse, I.; Hatzigeorgiou, A.G. Functional microRNA targets in protein coding sequences. Bioinformatics 2012, 28, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Kanellos, I.; Vergoulis, T.; Sacharidis, D.; Dalamagas, T.; Hatzigeorgiou, A.; Sartzetakis, S.; Sellis, T. MR-microT: A MapReduce-based MicroRNA target prediction method. In Proceedings of the 26th International Conference on Scientific and Statistical Database Management, Aalborg, Denmark, 30 June–2 July 2014; pp. 1–4. [Google Scholar]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]


| Mouse | NTCU | Number of Mice/Group | Treated Groups | Group Symbol (Before Treatment) | Group Symbol (After Treatment) |
|---|---|---|---|---|---|
| NIH Swiss | − | 6 | AIN 76A diet | EbAB | EaAB |
| NIH Swiss | + | 6 | AIN 76A diet | EbD | EaD |
| NIH Swiss | + | 6 | AIN 76A diet-gavage control | EbG | EaG |
| NIH Swiss | + | 6 | Budesonide (1.5 mg/kg diet) | EbB | EaB |
| NIH Swiss | + | 6 | AZD6244 (40 mg/kg body weight-gavage) | EbA | EaA |
| NIH Swiss | + | 6 | XL-147 (100 mg/kg body weight-gavage) | EbX | EaX |
| Differentially Expressed Exosomal miRNAs Affected by AZD6244 Treatment | ||||||
| Rank | miRNA ID | Mean EbA (log2) | Mean EaA (log2) | p-Value | Fold Change (log2) | False Discovery Rate (B&H) |
| 1 | mmu-miR-149-5p | 8.586 | 6.707 | 0 | −1.879 | 0.012 |
| 2 | mmu-miR-24-2-5p | 5.737 | 7.2 | 0 | 1.463 | 0.012 |
| 3 | mmu-miR-27a-3p | 9.493 | 10.636 | 0 | 1.143 | 0.012 |
| 4 | mmu-miR-215-5p | 9.917 | 11.846 | 0 | 1.929 | 0.023 |
| 5 | mmu-miR-543-3p | 12.329 | 11.283 | 0.001 | −1.046 | 0.035 |
| 6 | mmu-miR-92b-3p | 11.735 | 11.56 | 0.001 | −0.175 | 0.041 |
| 7 | mmu-miR-192-5p | 10.437 | 11.537 | 0.002 | 1.1 | 0.041 |
| 8 | mmu-miR-744-5p | 13.204 | 12.14 | 0.002 | −1.064 | 0.041 |
| Differentially expressed exosomal miRNAs affected by XL-147 treatment | ||||||
| Rank | miRNA ID | Mean EbX (log2) | Mean EaX (log2) | p-Value | Fold Change (log2) | False Discovery Rate (B&H) |
| 1 | mmu-miR-224-5p | 4.676 | 6.227 | 0 | 1.551 | 0.023 |
| 2 | mmu-miR-184-3p | 9.728 | 13.554 | 0 | 3.826 | 0.025 |
| 3 | mmu-miR-676-3p | 4.068 | 5.608 | 0 | 1.539 | 0.031 |
| 4 | mmu-miR-1198-5p | 7.605 | 8.266 | 0.001 | 0.661 | 0.042 |
| Differentially expressed exosomal miRNAs affected by Budesonide treatment | ||||||
| Rank | miRNA ID | Mean EbB (log2) | Mean EaB (log2) | p-Value | Fold Change (log2) | False Discovery Rate (B&H) |
| 1 | mmu-miR-378c | 7.148 | 7.725 | 0 | 0.576 | 0.013 |
| KEGG Pathway | Adjusted p-Value | #Genes | #miRNAs |
|---|---|---|---|
| Estrogen signaling pathway | 4.30 × 10−5 | 12 | 3 |
| Adrenergic signaling in cardiomyocytes | 0.018869 | 15 | 3 |
| Amphetamine addiction | 0.04725 | 8 | 2 |
| Lysine degradation | 0.04725 | 4 | 3 |
| AMPK signaling pathway | 0.04725 | 12 | 4 |
| KEGG Pathway | Adjusted p-Value | #Genes | #miRNAs |
|---|---|---|---|
| Galactose metabolism | 0.005 | 2 | 1 |
| Mucin type O-Glycan biosynthesis | 0.005 | 3 | 2 |
| Signaling pathways regulating pluripotency of stem cells | 0.019 | 15 | 2 |
| Glycosphingolipid biosynthesis - ganglio series | 0.032 | 3 | 1 |
| Protein processing in endoplasmic reticulum | 0.032 | 16 | 2 |
| Dorso-ventral axis formation | 0.032 | 6 | 2 |
| Other glycan degradation | 0.049 | 1 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, Q.; Du, M.; Xiong, D.; Wang, Y.; Mohammed, A.; Lubet, R.A.; Wang, L.; You, M. Exosomal miRNAs as Novel Pharmacodynamic Biomarkers for Cancer Chemopreventive Agent Early Stage Treatments in Chemically Induced Mouse Model of Lung Squamous Cell Carcinoma. Cancers 2019, 11, 477. https://doi.org/10.3390/cancers11040477
Zhou Y, Zhang Q, Du M, Xiong D, Wang Y, Mohammed A, Lubet RA, Wang L, You M. Exosomal miRNAs as Novel Pharmacodynamic Biomarkers for Cancer Chemopreventive Agent Early Stage Treatments in Chemically Induced Mouse Model of Lung Squamous Cell Carcinoma. Cancers. 2019; 11(4):477. https://doi.org/10.3390/cancers11040477
Chicago/Turabian StyleZhou, Yu, Qi Zhang, Meijun Du, Donghai Xiong, Yian Wang, Altaf Mohammed, Ronald A. Lubet, Liang Wang, and Ming You. 2019. "Exosomal miRNAs as Novel Pharmacodynamic Biomarkers for Cancer Chemopreventive Agent Early Stage Treatments in Chemically Induced Mouse Model of Lung Squamous Cell Carcinoma" Cancers 11, no. 4: 477. https://doi.org/10.3390/cancers11040477
APA StyleZhou, Y., Zhang, Q., Du, M., Xiong, D., Wang, Y., Mohammed, A., Lubet, R. A., Wang, L., & You, M. (2019). Exosomal miRNAs as Novel Pharmacodynamic Biomarkers for Cancer Chemopreventive Agent Early Stage Treatments in Chemically Induced Mouse Model of Lung Squamous Cell Carcinoma. Cancers, 11(4), 477. https://doi.org/10.3390/cancers11040477





