Cancer Stem Cells in Head and Neck Squamous Cell Carcinoma: Identification, Characterization and Clinical Implications
Abstract
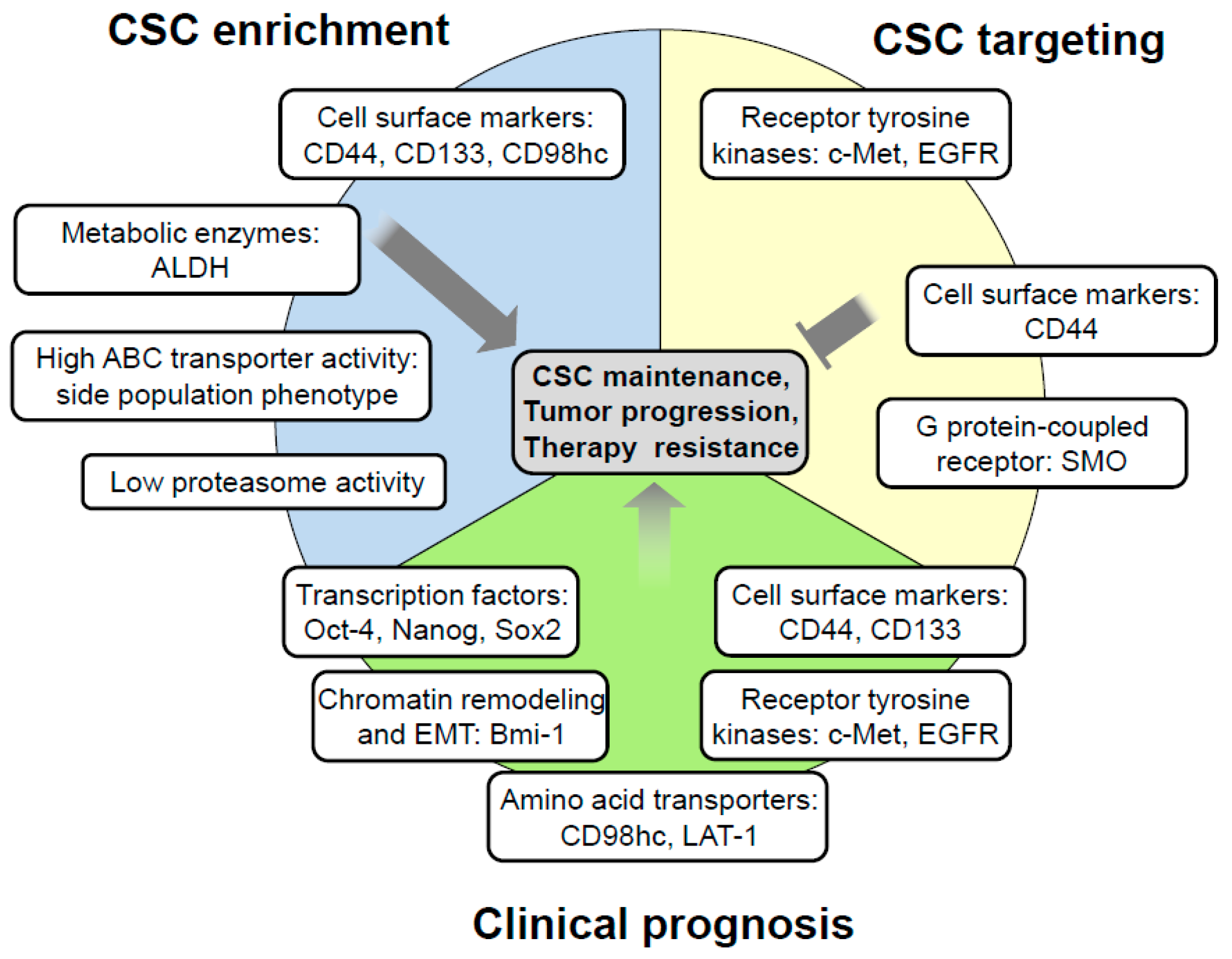
:1. Introduction
2. Current Methods for HNSCC Stem Cell Enrichment and Analysis
2.1. Marker Based CSC Enrichment
2.2. Marker-Independent CSC Enrichment
2.3. CSC Analysis
3. HNSCC Hierarchy, Heterogeneity and Plasticity
3.1. Clonal Evolution and HNSCC Heterogeneity
3.2. Intrinsic and Induced CSC Plasticity
4. Clinical Implication of HNSCC Stem Cells
4.1. The Role of CSCs for Tumor Growth and Metastatic Spread
4.2. Prognostic Role of CSC-Related Biomarkers
4.3. CSC-Targeted Therapies
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550. [Google Scholar] [CrossRef]
- Castellsague, X.; Alemany, L.; Quer, M.; Halec, G.; Quiros, B.; Tous, S.; Clavero, O.; Alos, L.; Biegner, T.; Szafarowski, T.; et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J. Natl. Cancer Inst. 2016, 108, e403. [Google Scholar] [CrossRef] [PubMed]
- Lohaus, F.; Linge, A.; Tinhofer, I.; Budach, V.; Gkika, E.; Stuschke, M.; Balermpas, P.; Rodel, C.; Avlar, M.; Grosu, A.L.; et al. HPV16 DNA status is a strong prognosticator of loco-regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: Results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother. Oncol. 2014, 113, 317–323. [Google Scholar] [CrossRef]
- Bhave, S.L.; Teknos, T.N.; Pan, Q. Molecular parameters of head and neck cancer metastasis. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Arantes, L.; De Carvalho, A.C.; Melendez, M.E.; Lopes Carvalho, A. Serum, plasma and saliva biomarkers for head and neck cancer. Expert. Rev. Mol. Diagn. 2018, 18, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kiess, A.; Chung, C.H. Emerging biomarkers in head and neck cancer in the era of genomics. Nat. Rev. Clin. Oncol. 2015, 12, 11–26. [Google Scholar] [CrossRef]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef]
- Jun, H.J.; Ahn, M.J.; Kim, H.S.; Yi, S.Y.; Han, J.; Lee, S.K.; Ahn, Y.C.; Jeong, H.S.; Son, Y.I.; Baek, J.H.; et al. ERCC1 expression as a predictive marker of squamous cell carcinoma of the head and neck treated with cisplatin-based concurrent chemoradiation. Br. J. Cancer 2008, 99, 167–172. [Google Scholar] [CrossRef]
- Cullen, K.J.; Schumaker, L.; Nikitakis, N.; Goloubeva, O.; Tan, M.; Sarlis, N.J.; Haddad, R.I.; Posner, M.R. beta-Tubulin-II expression strongly predicts outcome in patients receiving induction chemotherapy for locally advanced squamous carcinoma of the head and neck: A companion analysis of the TAX 324 trial. J. Clin. Oncol. 2009, 27, 6222–6228. [Google Scholar] [CrossRef]
- Lassen, P.; Lacas, B.; Pignon, J.P.; Trotti, A.; Zackrisson, B.; Zhang, Q.; Overgaard, J.; Blanchard, P.; Group, M.C. Prognostic impact of HPV-associated p16-expression and smoking status on outcomes following radiotherapy for oropharyngeal cancer: The MARCH-HPV project. Radiother. Oncol. 2018, 126, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Sturgis, E.M.; Burtness, B.; Ridge, J.A.; Ringash, J.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Licitra, L.F.; Burtness, B.; Fayette, J.; Gauler, T.; Clement, P.M.; Grau, J.J.; Del Campo, J.M.; Mailliez, A.; Haddad, R.I.; et al. Biomarkers predict enhanced clinical outcomes with afatinib versus methotrexate in patients with second-line recurrent and/or metastatic head and neck cancer. Ann. Oncol. 2017, 28, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Peitzsch, C.; Kurth, I.; Ebert, N.; Dubrovska, A.; Baumann, M. Cancer stem cells in radiation response: Current views and future perspectives in radiation oncology. Int. J. Radiat. Biol. 2019, 2019, 1–42. [Google Scholar] [CrossRef]
- Krause, M.; Dubrovska, A.; Linge, A.; Baumann, M. Cancer stem cells: Radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv. Drug Deliv. Rev. 2017, 109, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.C. Embryology of testicular teratomas in strain 129 mice. J. Natl. Cancer Inst. 1959, 23, 1249–1295. [Google Scholar] [PubMed]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells: Current status and evolving complexities. Cell Stem Cell 2012, 10, 717–728. [Google Scholar] [CrossRef]
- Kreso, A.; Dick, J.E. Evolution of the cancer stem cell model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef]
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978. [Google Scholar] [CrossRef]
- Wei, X.D.; Zhou, L.; Cheng, L.; Tian, J.; Jiang, J.J.; Maccallum, J. In vivo investigation of CD133 as a putative marker of cancer stem cells in Hep-2 cell line. Head Neck 2009, 31, 94–101. [Google Scholar] [CrossRef]
- Martens-de Kemp, S.R.; Brink, A.; Stigter-van Walsum, M.; Damen, J.M.; Rustenburg, F.; Wu, T.; van Wieringen, W.N.; Schuurhuis, G.J.; Braakhuis, B.J.; Slijper, M.; et al. CD98 marks a subpopulation of head and neck squamous cell carcinoma cells with stem cell properties. Stem Cell Res. 2013, 10, 477–488. [Google Scholar] [CrossRef]
- Fukusumi, T.; Ishii, H.; Konno, M.; Yasui, T.; Nakahara, S.; Takenaka, Y.; Yamamoto, Y.; Nishikawa, S.; Kano, Y.; Ogawa, H.; et al. CD10 as a novel marker of therapeutic resistance and cancer stem cells in head and neck squamous cell carcinoma. Br. J. Cancer 2014, 111, 506–514. [Google Scholar] [CrossRef]
- Song, J.; Chang, I.; Chen, Z.; Kang, M.; Wang, C.Y. Characterization of side populations in HNSCC: Highly invasive, chemoresistant and abnormal Wnt signaling. PLoS ONE 2010, 5, e11456. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, Y.W.; Hsu, H.S.; Tseng, L.M.; Huang, P.I.; Lu, K.H.; Chen, D.T.; Tai, L.K.; Yung, M.C.; Chang, S.C.; et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem. Biophys. Res. Commun. 2009, 385, 307–313. [Google Scholar] [CrossRef]
- Lagadec, C.; Vlashi, E.; Bhuta, S.; Lai, C.; Mischel, P.; Werner, M.; Henke, M.; Pajonk, F. Tumor cells with low proteasome subunit expression predict overall survival in head and neck cancer patients. BMC Cancer 2014, 14, e152. [Google Scholar] [CrossRef]
- Linge, A.; Lock, S.; Gudziol, V.; Nowak, A.; Lohaus, F.; von Neubeck, C.; Jutz, M.; Abdollahi, A.; Debus, J.; Tinhofer, I.; et al. Low Cancer Stem Cell Marker Expression and Low Hypoxia Identify Good Prognosis Subgroups in HPV(-) HNSCC after Postoperative Radiochemotherapy: A Multicenter Study of the DKTK-ROG. Clin. Cancer Res. 2016, 22, 2639–2649. [Google Scholar] [CrossRef]
- de Jong, M.C.; Pramana, J.; van der Wal, J.E.; Lacko, M.; Peutz-Kootstra, C.J.; de Jong, J.M.; Takes, R.P.; Kaanders, J.H.; van der Laan, B.F.; Wachters, J.; et al. CD44 expression predicts local recurrence after radiotherapy in larynx cancer. Clin. Cancer Res. 2010, 16, 5329–5338. [Google Scholar] [CrossRef]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef]
- Schober, M.; Fuchs, E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-βand integrin/focal adhesion kinase (FAK) signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 10544–10549. [Google Scholar] [CrossRef]
- Desai, A.; Yan, Y.; Gerson, S.L. Concise Reviews: Cancer Stem Cell Targeted Therapies: Toward Clinical Success. Stem Cells Transl. Med. 2019, 8, 75–81. [Google Scholar] [CrossRef]
- Kaseb, H.O.; Fohrer-Ting, H.; Lewis, D.W.; Lagasse, E.; Gollin, S.M. Identification, expansion and characterization of cancer cells with stem cell properties from head and neck squamous cell carcinomas. Exp. Cell Res. 2016, 348, 75–86. [Google Scholar] [CrossRef]
- Okamoto, A.; Chikamatsu, K.; Sakakura, K.; Hatsushika, K.; Takahashi, G.; Masuyama, K. Expansion and characterization of cancer stem-like cells in squamous cell carcinoma of the head and neck. Oral Oncol. 2009, 45, 633–639. [Google Scholar] [CrossRef]
- Wei, X.; Wang, J.; He, J.; Ma, B.; Chen, J. Biological characteristics of CD133(+) cancer stem cells derived from human laryngeal carcinoma cell line. Int. J. Clin. Exp. Med. 2014, 7, 2453–2462. [Google Scholar]
- Yan, M.; Yang, X.; Wang, L.; Clark, D.; Zuo, H.; Ye, D.; Chen, W.; Zhang, P. Plasma membrane proteomics of tumor spheres identify CD166 as a novel marker for cancer stem-like cells in head and neck squamous cell carcinoma. Mol. Cell Proteomics. 2013, 12, 3271–3284. [Google Scholar] [CrossRef]
- Imai, T.; Tamai, K.; Oizumi, S.; Oyama, K.; Yamaguchi, K.; Sato, I.; Satoh, K.; Matsuura, K.; Saijo, S.; Sugamura, K.; et al. CD271 defines a stem cell-like population in hypopharyngeal cancer. PLoS ONE 2013, 8, e62002. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Tsakmaki, V.; Danielidis, V.; Sivridis, E. Cancer stem cell phenotype relates to radio-chemotherapy outcome in locally advanced squamous cell head-neck cancer. Br. J. Cancer 2012, 106, 846–853. [Google Scholar] [CrossRef]
- Lim, Y.C.; Kang, H.J.; Moon, J.H. C-Met pathway promotes self-renewal and tumorigenecity of head and neck squamous cell carcinoma stem-like cell. Oral Oncol. 2014, 50, 633–639. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Z. Head neck squamous cell carcinoma c-Met(+) cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int. J. Cancer 2011, 129, 2337–2348. [Google Scholar] [CrossRef]
- Moreb, J.S.; Ucar-Bilyeu, D.A.; Khan, A. Use of retinoic acid/aldehyde dehydrogenase pathway as potential targeted therapy against cancer stem cells. Cancer Chemother. Pharmacol. 2017, 79, 295–301. [Google Scholar] [CrossRef]
- Kurth, I.; Hein, L.; Mabert, K.; Peitzsch, C.; Koi, L.; Cojoc, M.; Kunz-Schughart, L.; Baumann, M.; Dubrovska, A. Cancer stem cell related markers of radioresistance in head and neck squamous cell carcinoma. Oncotarget 2015, 6, 34494–34509. [Google Scholar] [CrossRef] [PubMed]
- Keysar, S.B.; Le, P.N.; Miller, B.; Jackson, B.C.; Eagles, J.R.; Nieto, C.; Kim, J.; Tang, B.; Glogowska, M.J.; Morton, J.J.; et al. Regulation of Head and Neck Squamous Cancer Stem Cells by PI3K and SOX2. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Koo, B.S.; Lee, S.H.; Kim, J.M.; Huang, S.; Kim, S.H.; Rho, Y.S.; Bae, W.J.; Kang, H.J.; Kim, Y.S.; Moon, J.H.; et al. Oct4 is a critical regulator of stemness in head and neck squamous carcinoma cells. Oncogene 2015, 34, 2317–2324. [Google Scholar] [CrossRef]
- Chiou, S.H.; Yu, C.C.; Huang, C.Y.; Lin, S.C.; Liu, C.J.; Tsai, T.H.; Chou, S.H.; Chien, C.S.; Ku, H.H.; Lo, J.F. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.T.; Ryu, C.J. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017, 50, 285–298. [Google Scholar] [CrossRef]
- Mack, B.; Gires, O. CD44s and CD44v6 expression in head and neck epithelia. PLoS ONE 2008, 3, e3360. [Google Scholar] [CrossRef]
- Quintana, E.; Shackleton, M.; Foster, H.R.; Fullen, D.R.; Sabel, M.S.; Johnson, T.M.; Morrison, S.J. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell 2010, 18, 510–523. [Google Scholar] [CrossRef]
- Dittfeld, C.; Dietrich, A.; Peickert, S.; Hering, S.; Baumann, M.; Grade, M.; Ried, T.; Kunz-Schughart, L.A. CD133 expression is not selective for tumor-initiating or radioresistant cell populations in the CRC cell line HCT-116. Radiother. Oncol. 2010, 94, 375–383. [Google Scholar] [CrossRef]
- Prasmickaite, L.; Engesaeter, B.O.; Skrbo, N.; Hellenes, T.; Kristian, A.; Oliver, N.K.; Suo, Z.; Maelandsmo, G.M. Aldehyde dehydrogenase (ALDH) activity does not select for cells with enhanced aggressive properties in malignant melanoma. PLoS ONE 2010, 5, e10731. [Google Scholar] [CrossRef] [PubMed]
- Nor, C.; Zhang, Z.; Warner, K.A.; Bernardi, L.; Visioli, F.; Helman, J.I.; Roesler, R.; Nor, J.E. Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia 2014, 16, 137–146. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.W.; Earle, C.; Shiina, M. Activation of Matrix Hyaluronan-Mediated CD44 Signaling, Epigenetic Regulation and Chemoresistance in Head and Neck Cancer Stem Cells. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Gao, W.; Li, F.; Bo, Y.; Zhu, M.; Fu, R.; Liu, Q.; Wen, S.; Wang, B. Identification and characterization of CD133(+)CD44(+) cancer stem cells from human laryngeal squamous cell carcinoma cell lines. J. Cancer 2017, 8, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Fujisawa, T.; Husain, S.R.; Puri, R.K. Identification and characterization of cancer stem cells in human head and neck squamous cell carcinoma. BMC Cancer 2014, 14, e173. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, G.; Maalouf, M.; Boivin, A.; Battiston-Montagne, P.; Beuve, M.; Levy, A.; Jalade, P.; Fournier, C.; Ardail, D.; Magne, N.; et al. Targeting head and neck cancer stem cells to overcome resistance to photon and carbon ion radiation. Stem Cell Rev. 2014, 10, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, V.; Sartini, D.; Rocchetti, R.; Santarelli, A.; Rubini, C.; Morganti, S.; Giuliante, R.; Calabrese, S.; Di Ruscio, G.; Orlando, F.; et al. Identification and characterization of cancer stem cells from head and neck squamous cell carcinoma cell lines. Cell Physiol. Biochem. 2015, 36, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Vlashi, E.; Chen, A.M.; Boyrie, S.; Yu, G.; Nguyen, A.; Brower, P.A.; Hess, C.B.; Pajonk, F. Radiation-Induced Dedifferentiation of Head and Neck Cancer Cells Into Cancer Stem Cells Depends on Human Papillomavirus Status. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.P.; Zhou, L.; Xie, M.; Du, H.D.; Tian, J.; Sun, S.; Li, J.Y. Identification of cancer stem-like side population cells in purified primary cultured human laryngeal squamous cell carcinoma epithelia. PLoS ONE 2013, 8, e65750. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.; Nel, I.; Hassenkamp, P.; Gauler, T.; Schlueter, A.; Lang, S.; Dountsop, P.; Hoffmann, A.C.; Lehnerdt, G. Detection of circulating tumor cell subpopulations in patients with head and neck squamous cell carcinoma (HNSCC). PLoS ONE 2014, 9, e113706. [Google Scholar] [CrossRef]
- Kim, H.S.; Pearson, A.T.; Nor, J.E. Isolation and Characterization of Cancer Stem Cells from Primary Head and Neck Squamous Cell Carcinoma Tumors. Methods Mol. Biol. 2016, 1395, 241–249. [Google Scholar] [CrossRef]
- Yuan, X.; Curtin, J.; Xiong, Y.; Liu, G.; Waschsmann-Hogiu, S.; Farkas, D.L.; Black, K.L.; Yu, J.S. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene 2004, 23, 9392–9400. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Nor, J.E. Orosphere assay: A method for propagation of head and neck cancer stem cells. Head Neck 2013, 35, 1015–1021. [Google Scholar] [CrossRef]
- Dontu, G.; Abdallah, W.M.; Foley, J.M.; Jackson, K.W.; Clarke, M.F.; Kawamura, M.J.; Wicha, M.S. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003, 17, 1253–1270. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Cheaito, K.; Chalhoub, R.M.; Hadadeh, O.; Monzer, A.; Ballout, F.; El-Hajj, A.; Mukherji, D.; Liu, Y.N.; Daoud, G.; et al. Sphere-Formation Assay: Three-Dimensional in vitro Culturing of Prostate Cancer Stem/Progenitor Sphere-Forming Cells. Front Oncol. 2018, 8, e347. [Google Scholar] [CrossRef]
- Lim, Y.C.; Oh, S.Y.; Cha, Y.Y.; Kim, S.H.; Jin, X.; Kim, H. Cancer stem cell traits in squamospheres derived from primary head and neck squamous cell carcinomas. Oral Oncol. 2011, 47, 83–91. [Google Scholar] [CrossRef]
- Hirschmann-Jax, C.; Foster, A.E.; Wulf, G.G.; Nuchtern, J.G.; Jax, T.W.; Gobel, U.; Goodell, M.A.; Brenner, M.K. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl. Acad. Sci. USA 2004, 101, 14228–14233. [Google Scholar] [CrossRef]
- Goodell, M.A.; Brose, K.; Paradis, G.; Conner, A.S.; Mulligan, R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996, 183, 1797–1806. [Google Scholar] [CrossRef]
- Tabor, M.H.; Clay, M.R.; Owen, J.H.; Bradford, C.R.; Carey, T.E.; Wolf, G.T.; Prince, M.E. Head and neck cancer stem cells: The side population. Laryngoscope 2011, 121, 527–533. [Google Scholar] [CrossRef]
- Loebinger, M.R.; Giangreco, A.; Groot, K.R.; Prichard, L.; Allen, K.; Simpson, C.; Bazley, L.; Navani, N.; Tibrewal, S.; Davies, D.; et al. Squamous cell cancers contain a side population of stem-like cells that are made chemosensitive by ABC transporter blockade. Br. J. Cancer 2008, 98, 380–387. [Google Scholar] [CrossRef]
- Vlashi, E.; Kim, K.; Lagadec, C.; Donna, L.D.; McDonald, J.T.; Eghbali, M.; Sayre, J.W.; Stefani, E.; McBride, W.; Pajonk, F. In vivo imaging, tracking, and targeting of cancer stem cells. J. Natl. Cancer Inst. 2009, 101, 350–359. [Google Scholar] [CrossRef]
- Wilson, G.D.; Marples, B.; Galoforo, S.; Geddes, T.J.; Thibodeau, B.J.; Grenman, R.; Akervall, J. Isolation and genomic characterization of stem cells in head and neck cancer. Head Neck 2013, 35, 1573–1582. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Dong, Z.; Vodopyanov, D.; Imai, A.; Helman, J.I.; Prince, M.E.; Wicha, M.S.; Nor, J.E. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010, 70, 9969–9978. [Google Scholar] [CrossRef]
- Yaromina, A.; Krause, M.; Thames, H.; Rosner, A.; Krause, M.; Hessel, F.; Grenman, R.; Zips, D.; Baumann, M. Pre-treatment number of clonogenic cells and their radiosensitivity are major determinants of local tumour control after fractionated irradiation. Radiother. Oncol. 2007, 83, 304–310. [Google Scholar] [CrossRef]
- Alvarez-Teijeiro, S.; Garcia-Inclan, C.; Villaronga, M.A.; Casado, P.; Hermida-Prado, F.; Granda-Diaz, R.; Rodrigo, J.P.; Calvo, F.; Del-Rio-Ibisate, N.; Gandarillas, A.; et al. Factors Secreted by Cancer-Associated Fibroblasts that Sustain Cancer Stem Properties in Head and Neck Squamous Carcinoma Cells as Potential Therapeutic Targets. Cancers 2018, 10. [Google Scholar] [CrossRef]
- Le, P.N.; Keysar, S.B.; Miller, B.; Eagles, J.R.; Chimed, T.S.; Reisinger, J.; Gomez, K.E.; Nieto, C.; Jackson, B.C.; Somerset, H.L.; et al. Wnt signaling dynamics in head and neck squamous cell cancer tumor-stroma interactions. Mol. Carcinog 2019, 58, 398–410. [Google Scholar] [CrossRef]
- Chinn, S.B.; Darr, O.A.; Owen, J.H.; Bellile, E.; McHugh, J.B.; Spector, M.E.; Papagerakis, S.M.; Chepeha, D.B.; Bradford, C.R.; Carey, T.E.; et al. Cancer stem cells: Mediators of tumorigenesis and metastasis in head and neck squamous cell carcinoma. Head Neck 2015, 37, 317–326. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Yu, T.S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef]
- Driessens, G. Deciphering tumor growth by clonal analysis. Crit. Rev. Oncog. 2014, 19, 317–325. [Google Scholar] [CrossRef]
- Driessens, G.; Beck, B.; Caauwe, A.; Simons, B.D.; Blanpain, C. Defining the mode of tumour growth by clonal analysis. Nature 2012, 488, 527–530. [Google Scholar] [CrossRef]
- Chen, D.; Wu, M.; Li, Y.; Chang, I.; Yuan, Q.; Ekimyan-Salvo, M.; Deng, P.; Yu, B.; Yu, Y.; Dong, J.; et al. Targeting BMI1(+) Cancer Stem Cells Overcomes Chemoresistance and Inhibits Metastases in Squamous Cell Carcinoma. Cell Stem Cell 2017, 20, 621–634.e626. [Google Scholar] [CrossRef]
- Zscheppang, K.; Kurth, I.; Wachtel, N.; Dubrovska, A.; Kunz-Schughart, L.A.; Cordes, N. Efficacy of Beta1 Integrin and EGFR Targeting in Sphere-Forming Human Head and Neck Cancer Cells. J. Cancer 2016, 7, 736–745. [Google Scholar] [CrossRef]
- Warrier, S.; Bhuvanalakshmi, G.; Arfuso, F.; Rajan, G.; Millward, M.; Dharmarajan, A. Cancer stem-like cells from head and neck cancers are chemosensitized by the Wnt antagonist, sFRP4, by inducing apoptosis, decreasing stemness, drug resistance and epithelial to mesenchymal transition. Cancer Gene Ther. 2014, 21, 381–388. [Google Scholar] [CrossRef]
- Harper, L.J.; Piper, K.; Common, J.; Fortune, F.; Mackenzie, I.C. Stem cell patterns in cell lines derived from head and neck squamous cell carcinoma. J. Oral Pathol. Med. 2007, 36, 594–603. [Google Scholar] [CrossRef]
- Kuch, V.; Schreiber, C.; Thiele, W.; Umansky, V.; Sleeman, J.P. Tumor-initiating properties of breast cancer and melanoma cells in vivo are not invariably reflected by spheroid formation in vitro, but can be increased by long-term culturing as adherent monolayers. Int. J. Cancer 2013, 132, 94–105. [Google Scholar] [CrossRef]
- Bartfeld, S.; Clevers, H. Stem cell-derived organoids and their application for medical research and patient treatment. J. Mol. Med. 2017, 95, 729–738. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, C.; Hu, Y.; Mu, L.; Huang, K.; Li, Q.; Li, X.; Tao, D.; Qin, J. Sphereforming assay vs. organoid culture: Determining longterm stemness and the chemoresistant capacity of primary colorectal cancer cells. Int. J. Oncol. 2019, 54, 893–904. [Google Scholar] [CrossRef]
- Tanaka, N.; Osman, A.A.; Takahashi, Y.; Lindemann, A.; Patel, A.A.; Zhao, M.; Takahashi, H.; Myers, J.N. Head and neck cancer organoids established by modification of the CTOS method can be used to predict in vivo drug sensitivity. Oral Oncol. 2018, 87, 49–57. [Google Scholar] [CrossRef]
- Hubert, C.G.; Rivera, M.; Spangler, L.C.; Wu, Q.; Mack, S.C.; Prager, B.C.; Couce, M.; McLendon, R.E.; Sloan, A.E.; Rich, J.N. A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas Recapitulates the Hypoxic Gradients and Cancer Stem Cell Heterogeneity of Tumors Found In Vivo. Cancer Res. 2016, 76, 2465–2477. [Google Scholar] [CrossRef]
- Odoux, C.; Fohrer, H.; Hoppo, T.; Guzik, L.; Stolz, D.B.; Lewis, D.W.; Gollin, S.M.; Gamblin, T.C.; Geller, D.A.; Lagasse, E. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 2008, 68, 6932–6941. [Google Scholar] [CrossRef]
- Grenman, R.; Burk, D.; Virolainen, E.; Buick, R.N.; Church, J.; Schwartz, D.R.; Carey, T.E. Clonogenic cell assay for anchorage-dependent squamous carcinoma cell lines using limiting dilution. Int. J. Cancer 1989, 44, 131–136. [Google Scholar] [CrossRef]
- Fedr, R.; Pernicova, Z.; Slabakova, E.; Strakova, N.; Bouchal, J.; Grepl, M.; Kozubik, A.; Soucek, K. Automatic cell cloning assay for determining the clonogenic capacity of cancer and cancer stem-like cells. Cytometry 2013, 83, 472–482. [Google Scholar] [CrossRef]
- Zhang, M.; Kumar, B.; Piao, L.; Xie, X.; Schmitt, A.; Arradaza, N.; Cippola, M.; Old, M.; Agrawal, A.; Ozer, E.; et al. Elevated intrinsic cancer stem cell population in human papillomavirus-associated head and neck squamous cell carcinoma. Cancer 2014, 120, 992–1001. [Google Scholar] [CrossRef]
- Tang, B.; Raviv, A.; Esposito, D.; Flanders, K.C.; Daniel, C.; Nghiem, B.T.; Garfield, S.; Lim, L.; Mannan, P.; Robles, A.I.; et al. A flexible reporter system for direct observation and isolation of cancer stem cells. Stem Cell Rep. 2015, 4, 155–169. [Google Scholar] [CrossRef]
- Jin, C.; Jin, Y.; Wennerberg, J.; Akervall, J.; Dictor, M.; Mertens, F. Karyotypic heterogeneity and clonal evolution in squamous cell carcinomas of the head and neck. Cancer Genet. Cytogenet. 2002, 132, 85–96. [Google Scholar] [CrossRef]
- Jin, Y.; Jin, C.; Lv, M.; Tsao, S.W.; Zhu, J.; Wennerberg, J.; Mertens, F.; Kwong, Y.L. Karyotypic evolution and tumor progression in head and neck squamous cell carcinomas. Cancer Genet. Cytogenet. 2005, 156, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.C.; Xu, C.; Mitchell, R.M.; Zhang, B.; Zhao, D.; Li, Y.; Huang, X.; Fan, W.; Wang, H.; Lerma, L.A.; et al. Tumor evolution and intratumor heterogeneity of an oropharyngeal squamous cell carcinoma revealed by whole-genome sequencing. Neoplasia 2013, 15, 1371–1378. [Google Scholar] [CrossRef]
- Mroz, E.A.; Tward, A.D.; Hammon, R.J.; Ren, Y.; Rocco, J.W. Intra-tumor genetic heterogeneity and mortality in head and neck cancer: Analysis of data from the Cancer Genome Atlas. PLoS Med. 2015, 12, e1001786. [Google Scholar] [CrossRef]
- Roh, V.; Abramowski, P.; Hiou-Feige, A.; Cornils, K.; Rivals, J.P.; Zougman, A.; Aranyossy, T.; Thielecke, L.; Truan, Z.; Mermod, M.; et al. Cellular Barcoding Identifies Clonal Substitution as a Hallmark of Local Recurrence in a Surgical Model of Head and Neck Squamous Cell Carcinoma. Cell Rep. 2018, 25, 2208–2222.e2207. [Google Scholar] [CrossRef]
- Geissler, C.; Hambek, M.; Leinung, M.; Diensthuber, M.; Gassner, D.; Stover, T.; Wagenblast, J. The challenge of tumor heterogeneity--different phenotypes of cancer stem cells in a head and neck squamous cell carcinoma xenograft mouse model. In Vivo 2012, 26, 593–598. [Google Scholar]
- Spencer, S.L.; Berryman, M.J.; Garcia, J.A.; Abbott, D. An ordinary differential equation model for the multistep transformation to cancer. J. Theor. Biol. 2004, 231, 515–524. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Gentry, S.N.; Jackson, T.L. A mathematical model of cancer stem cell driven tumor initiation: Implications of niche size and loss of homeostatic regulatory mechanisms. PLoS ONE 2013, 8, e71128. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.C.; Agus, D.B. Stochasticity and determinism in cancer creation and progression. Converg. Sci. Phys. Oncol. 2015, 1. [Google Scholar] [CrossRef]
- Poleszczuk, J.; Hahnfeldt, P.; Enderling, H. Evolution and phenotypic selection of cancer stem cells. PLoS Comput. Biol. 2015, 11, e1004025. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.V.; Nor, J.E.; Jackson, T.L. Modeling the VEGF-Bcl-2-CXCL8 pathway in intratumoral agiogenesis. Bull. Math. Biol. 2008, 70, 89–117. [Google Scholar] [CrossRef]
- Jain, H.V.; Nor, J.E.; Jackson, T.L. Quantification of endothelial cell-targeted anti-Bcl-2 therapy and its suppression of tumor growth and vascularization. Mol. Cancer Ther. 2009, 8, 2926–2936. [Google Scholar] [CrossRef]
- Dong, Z.; Zeitlin, B.D.; Song, W.; Sun, Q.; Karl, E.; Spencer, D.M.; Jain, H.V.; Jackson, T.; Nunez, G.; Nor, J.E. Level of endothelial cell apoptosis required for a significant decrease in microvessel density. Exp. Cell Res. 2007, 313, 3645–3657. [Google Scholar] [CrossRef]
- Pearson, A.T.; Jackson, T.L.; Nor, J.E. Modeling head and neck cancer stem cell-mediated tumorigenesis. Cell. Mol. Life Sci. 2016, 73, 3279–3289. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.G. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012, 22, 457–472. [Google Scholar] [CrossRef]
- Rycaj, K.; Tang, D.G. Cell-of-Origin of Cancer versus Cancer Stem Cells: Assays and Interpretations. Cancer Res. 2015, 75, 4003–4011. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Marjanovic, N.D.; Lee, T.; Bell, G.; Kleer, C.G.; Reinhardt, F.; D’Alessio, A.C.; Young, R.A.; Weinberg, R.A. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell 2013, 154, 61–74. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Brueckmann, I.; Scheel, C.; Kaestli, A.J.; Wiggins, P.A.; Rodrigues, L.O.; Brooks, M.; Reinhardt, F.; Su, Y.; Polyak, K.; et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc. Natl. Acad. Sci. USA 2011, 108, 7950–7955. [Google Scholar] [CrossRef]
- Tsai, L.L.; Yu, C.C.; Chang, Y.C.; Yu, C.H.; Chou, M.Y. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J. Oral Pathol. Med. 2011, 40, 621–628. [Google Scholar] [CrossRef]
- Keith, B.; Simon, M.C. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007, 129, 465–472. [Google Scholar] [CrossRef]
- Covello, K.L.; Kehler, J.; Yu, H.; Gordan, J.D.; Arsham, A.M.; Hu, C.J.; Labosky, P.A.; Simon, M.C.; Keith, B. HIF-2alpha regulates Oct-4: Effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006, 20, 557–570. [Google Scholar] [CrossRef]
- McMahon, S.; Charbonneau, M.; Grandmont, S.; Richard, D.E.; Dubois, C.M. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J. Biol. Chem. 2006, 281, 24171–24181. [Google Scholar] [CrossRef]
- da Silva-Diz, V.; Lorenzo-Sanz, L.; Bernat-Peguera, A.; Lopez-Cerda, M.; Munoz, P. Cancer cell plasticity: Impact on tumor progression and therapy response. Semin. Cancer Biol. 2018, 53, 48–58. [Google Scholar] [CrossRef]
- Peitzsch, C.; Tyutyunnykova, A.; Pantel, K.; Dubrovska, A. Cancer stem cells: The root of tumor recurrence and metastases. Semin. Cancer Biol. 2017, 44, 10–24. [Google Scholar] [CrossRef]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.C.; Wu, L.; Yu, G.T.; Zhang, W.F.; Huang, C.F.; Sun, Z.J. TRAF6 regulates tumour metastasis through EMT and CSC phenotypes in head and neck squamous cell carcinoma. J. Cell Mol. Med. 2018, 22, 1337–1349. [Google Scholar] [CrossRef]
- Chung, M.K.; Jung, Y.H.; Lee, J.K.; Cho, S.Y.; Murillo-Sauca, O.; Uppaluri, R.; Shin, J.H.; Sunwoo, J.B. CD271 Confers an Invasive and Metastatic Phenotype of Head and Neck Squamous Cell Carcinoma through the Upregulation of Slug. Clin. Cancer Res. 2018, 24, 674–683. [Google Scholar] [CrossRef]
- Steinbichler, T.B.; Alshaimaa, A.; Maria, M.V.; Daniel, D.; Herbert, R.; Jozsef, D.; Ira-Ida, S. Epithelial-mesenchymal crosstalk induces radioresistance in HNSCC cells. Oncotarget 2018, 9, 3641–3652. [Google Scholar] [CrossRef]
- Miyazaki, H.; Takahashi, R.U.; Prieto-Vila, M.; Kawamura, Y.; Kondo, S.; Shirota, T.; Ochiya, T. CD44 exerts a functional role during EMT induction in cisplatin-resistant head and neck cancer cells. Oncotarget 2018, 9, 10029–10041. [Google Scholar] [CrossRef]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef]
- Schliekelman, M.J.; Taguchi, A.; Zhu, J.; Dai, X.; Rodriguez, J.; Celiktas, M.; Zhang, Q.; Chin, A.; Wong, C.H.; Wang, H.; et al. Molecular portraits of epithelial, mesenchymal, and hybrid States in lung adenocarcinoma and their relevance to survival. Cancer Res. 2015, 75, 1789–1800. [Google Scholar] [CrossRef]
- Chinn, S.B.; Darr, O.A.; Peters, R.D.; Prince, M.E. The role of head and neck squamous cell carcinoma cancer stem cells in tumorigenesis, metastasis, and treatment failure. Front. Endocrinol. 2012, 3, e90. [Google Scholar] [CrossRef]
- Biddle, A.; Gammon, L.; Liang, X.; Costea, D.E.; Mackenzie, I.C. Phenotypic Plasticity Determines Cancer Stem Cell Therapeutic Resistance in Oral Squamous Cell Carcinoma. BioMedicine 2016, 4, 138–145. [Google Scholar] [CrossRef]
- Nieto, M.A. Epithelial plasticity: A common theme in embryonic and cancer cells. Science 2013, 342, 1234850. [Google Scholar] [CrossRef]
- Lim, K.P.; Cirillo, N.; Hassona, Y.; Wei, W.; Thurlow, J.K.; Cheong, S.C.; Pitiyage, G.; Parkinson, E.K.; Prime, S.S. Fibroblast gene expression profile reflects the stage of tumour progression in oral squamous cell carcinoma. J. Pathol. 2011, 223, 459–469. [Google Scholar] [CrossRef]
- Eke, I.; Deuse, Y.; Hehlgans, S.; Gurtner, K.; Krause, M.; Baumann, M.; Shevchenko, A.; Sandfort, V.; Cordes, N. beta(1)Integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J. Clin. Investig. 2012, 122, 1529–1540. [Google Scholar] [CrossRef]
- Yang, M.H.; Wu, M.Z.; Chiou, S.H.; Chen, P.M.; Chang, S.Y.; Liu, C.J.; Teng, S.C.; Wu, K.J. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat. Cell Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef]
- Song, L.B.; Li, J.; Liao, W.T.; Feng, Y.; Yu, C.P.; Hu, L.J.; Kong, Q.L.; Xu, L.H.; Zhang, X.; Liu, W.L.; et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J. Clin. Investig. 2009, 119, 3626–3636. [Google Scholar] [CrossRef]
- Jouppila-Matto, A.; Narkio-Makela, M.; Soini, Y.; Pukkila, M.; Sironen, R.; Tuhkanen, H.; Mannermaa, A.; Kosma, V.M. Twist and snai1 expression in pharyngeal squamous cell carcinoma stroma is related to cancer progression. BMC Cancer 2011, 11, 350. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Chen, M.H.; Chang, P.M.; Chen, P.M.; Tzeng, C.H.; Chu, P.Y.; Chang, S.Y.; Yang, M.H. Prolonged facial edema is an indicator of poor prognosis in patients with head and neck squamous cell carcinoma. Support Care Cancer 2010, 18, 1313–1319. [Google Scholar] [CrossRef]
- Lyons, J.G.; Patel, V.; Roue, N.C.; Fok, S.Y.; Soon, L.L.; Halliday, G.M.; Gutkind, J.S. Snail up-regulates proinflammatory mediators and inhibits differentiation in oral keratinocytes. Cancer Res. 2008, 68, 4525–4530. [Google Scholar] [CrossRef]
- Hayry, V.; Makinen, L.K.; Atula, T.; Sariola, H.; Makitie, A.; Leivo, I.; Keski-Santti, H.; Lundin, J.; Haglund, C.; Hagstrom, J. Bmi-1 expression predicts prognosis in squamous cell carcinoma of the tongue. Br. J. Cancer 2010, 102, 892–897. [Google Scholar] [CrossRef]
- Mendelsohn, A.H.; Lai, C.K.; Shintaku, I.P.; Fishbein, M.C.; Brugman, K.; Elashoff, D.A.; Abemayor, E.; Dubinett, S.M.; St John, M.A. Snail as a novel marker for regional metastasis in head and neck squamous cell carcinoma. Am. J. Otolaryngol. 2012, 33, 6–13. [Google Scholar] [CrossRef]
- Yang, M.H.; Hsu, D.S.; Wang, H.W.; Wang, H.J.; Lan, H.Y.; Yang, W.H.; Huang, C.H.; Kao, S.Y.; Tzeng, C.H.; Tai, S.K.; et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat. Cell Biol. 2010, 12, 982–992. [Google Scholar] [CrossRef]
- Chaffer, C.L.; San Juan, B.P.; Lim, E.; Weinberg, R.A. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016, 35, 645–654. [Google Scholar] [CrossRef]
- Jolly, M.K.; Ware, K.E.; Gilja, S.; Somarelli, J.A.; Levine, H. EMT and MET: Necessary or permissive for metastasis? Mol. Oncol. 2017, 11, 755–769. [Google Scholar] [CrossRef]
- Tam, W.L.; Weinberg, R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013, 19, 1438–1449. [Google Scholar] [CrossRef]
- Shi, H.; Chen, X.; Lu, C.; Gu, C.; Jiang, H.; Meng, R.; Niu, X.; Huang, Y.; Lu, M. Association between P16INK4a promoter methylation and HNSCC: A meta-analysis of 21 published studies. PLoS ONE 2015, 10, e0122302. [Google Scholar] [CrossRef]
- Almeida, L.O.; Abrahao, A.C.; Rosselli-Murai, L.K.; Giudice, F.S.; Zagni, C.; Leopoldino, A.M.; Squarize, C.H.; Castilho, R.M. NFkappaB mediates cisplatin resistance through histone modifications in head and neck squamous cell carcinoma (HNSCC). FEBS Open Biol. 2014, 4, 96–104. [Google Scholar] [CrossRef]
- Le, J.M.; Squarize, C.H.; Castilho, R.M. Histone modifications: Targeting head and neck cancer stem cells. World J. Stem Cells 2014, 6, 511–525. [Google Scholar] [CrossRef]
- Ferlito, A.; Shaha, A.R.; Silver, C.E.; Rinaldo, A.; Mondin, V. Incidence and sites of distant metastases from head and neck cancer. ORL J. Otorhinolaryngol. Relat Spec. 2001, 63, 202–207. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Perry, C.; Jovanovic, L.; Nelson, C.; Punyadeera, C. Circulating tumour cells in metastatic head and neck cancers. Int. J. Cancer 2015, 136, 2515–2523. [Google Scholar] [CrossRef]
- Cho, J.K.; Lee, G.J.; Kim, H.D.; Moon, U.Y.; Kim, M.J.; Kim, S.; Baek, K.H.; Jeong, H.S. Differential impact of circulating tumor cells on disease recurrence and survivals in patients with head and neck squamous cell carcinomas: An updated meta-analysis. PLoS ONE 2018, 13, e0203758. [Google Scholar] [CrossRef]
- Wu, X.L.; Tu, Q.; Faure, G.; Gallet, P.; Kohler, C.; Bittencourt Mde, C. Diagnostic and Prognostic Value of Circulating Tumor Cells in Head and Neck Squamous Cell Carcinoma: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 20210. [Google Scholar] [CrossRef]
- Kulasinghe, A.; Perry, C.; Kenny, L.; Warkiani, M.E.; Nelson, C.; Punyadeera, C. PD-L1 expressing circulating tumour cells in head and neck cancers. BMC Cancer 2017, 17, e333. [Google Scholar] [CrossRef]
- Mazel, M.; Jacot, W.; Pantel, K.; Bartkowiak, K.; Topart, D.; Cayrefourcq, L.; Rossille, D.; Maudelonde, T.; Fest, T.; Alix-Panabieres, C. Frequent expression of PD-L1 on circulating breast cancer cells. Mol. Oncol. 2015, 9, 1773–1782. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Wang, S.J.; Bourguignon, L.Y. Role of hyaluronan-mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am. J. Pathol. 2011, 178, 956–963. [Google Scholar] [CrossRef]
- Liang, G.; Li, S.; Du, W.; Ke, Q.; Cai, J.; Yang, J. Hypoxia regulates CD44 expression via hypoxia-inducible factor-1alpha in human gastric cancer cells. Oncol. Lett. 2017, 13, 967–972. [Google Scholar] [CrossRef]
- Wang, S.J.; Wong, G.; de Heer, A.M.; Xia, W.; Bourguignon, L.Y. CD44 variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope 2009, 119, 1518–1530. [Google Scholar] [CrossRef]
- Linge, A.; Lohaus, F.; Lock, S.; Nowak, A.; Gudziol, V.; Valentini, C.; von Neubeck, C.; Jutz, M.; Tinhofer, I.; Budach, V.; et al. HPV status, cancer stem cell marker expression, hypoxia gene signatures and tumour volume identify good prognosis subgroups in patients with HNSCC after primary radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother. Oncol. 2016, 121, 364–373. [Google Scholar] [CrossRef]
- Kim, H.S.; Chen, Y.C.; Nor, F.; Warner, K.A.; Andrews, A.; Wagner, V.P.; Zhang, Z.; Zhang, Z.; Martins, M.D.; Pearson, A.T.; et al. Endothelial-derived interleukin-6 induces cancer stem cell motility by generating a chemotactic gradient towards blood vessels. Oncotarget 2017, 8, 100339–100352. [Google Scholar] [CrossRef]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef]
- Tinhofer, I.; Budach, V.; Saki, M.; Konschak, R.; Niehr, F.; Johrens, K.; Weichert, W.; Linge, A.; Lohaus, F.; Krause, M.; et al. Targeted next-generation sequencing of locally advanced squamous cell carcinomas of the head and neck reveals druggable targets for improving adjuvant chemoradiation. Euro. J. Cancer 2016, 57, 78–86. [Google Scholar] [CrossRef]
- Balermpas, P.; Rodel, F.; Rodel, C.; Krause, M.; Linge, A.; Lohaus, F.; Baumann, M.; Tinhofer, I.; Budach, V.; Gkika, E.; et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int. J. Cancer 2016, 138, 171–181. [Google Scholar] [CrossRef]
- Kimple, R.J.; Harari, P.M. The prognostic value of HPV in head and neck cancer patients undergoing postoperative chemoradiotherapy. Ann. Transl. Med. 2015, 3, e14. [Google Scholar] [CrossRef]
- Lohaus, F.; Linge, A.; Baumann, M. HPV and beyond-looking out for biomarkers for distinguishing the good prognosis from the bad prognosis group in locally advanced and clinically high risk HNSCC. Ann. Transl. Med. 2015, 3, e255. [Google Scholar] [CrossRef]
- Brandwein-Gensler, M.; Smith, R.V. Prognostic indicators in head and neck oncology including the new 7th edition of the AJCC staging system. Head Neck Pathol. 2010, 4, 53–61. [Google Scholar] [CrossRef]
- Worsham, M.J. Identifying the risk factors for late-stage head and neck cancer. Expert Rev. Anticancer Ther. 2011, 11, 1321–1325. [Google Scholar] [CrossRef]
- Digomann, D.; Kurth, I.; Tyutyunnykova, A.; Chen, O.; Lock, S.; Gorodetska, I.; Peitzsch, C.; Skvortsova, I.; Negro, G.; Aschenbrenner, B.; et al. The CD98 heavy chain is a marker and regulator of head and neck squamous cell carcinoma radiosensitivity. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef]
- Kokko, L.L.; Hurme, S.; Maula, S.M.; Alanen, K.; Grenman, R.; Kinnunen, I.; Ventela, S. Significance of site-specific prognosis of cancer stem cell marker CD44 in head and neck squamous-cell carcinoma. Oral Oncol. 2011, 47, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Faber, A.; Barth, C.; Hormann, K.; Kassner, S.; Schultz, J.D.; Sommer, U.; Stern-Straeter, J.; Thorn, C.; Goessler, U.R. CD44 as a stem cell marker in head and neck squamous cell carcinoma. Oncol. Rep. 2011, 26, 321–326. [Google Scholar] [CrossRef]
- Chung, J.H.; Jung, H.R.; Jung, A.R.; Lee, Y.C.; Kong, M.; Lee, J.S.; Eun, Y.G. SOX2 activation predicts prognosis in patients with head and neck squamous cell carcinoma. Sci. Rep. 2018, 8, e1677. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kang, Y.H.; Lee, J.S.; Byun, J.H.; Kim, U.K.; Jang, S.J.; Rho, G.J.; Park, B.W. Positive expression of NANOG, mutant p53, and CD44 is directly associated with clinicopathological features and poor prognosis of oral squamous cell carcinoma. BMC Oral Health 2015, 15, e153. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, G.; Sawant, S.S.; Hague, A.; Kingsley, K.; Devaraj, H. Association of differential beta-catenin expression with Oct-4 and Nanog in oral squamous cell carcinoma and their correlation with clinicopathological factors and prognosis. Head Neck 2015, 37, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, L.; Wu, S.; Gong, X.; Feng, Z.; Ma, L.; Zhu, B.; Yao, N.; Wang, D.; Dong, H. Clinicopathological significance of cancer stem cells marked by CD133 and KAI1/CD82 expression in laryngeal squamous cell carcinoma. World J. Surg. Oncol. 2014, 12, e118. [Google Scholar] [CrossRef]
- Canis, M.; Lechner, A.; Mack, B.; Zengel, P.; Laubender, R.P.; Koehler, U.; Heissmeyer, V.; Gires, O. CD133 is a predictor of poor survival in head and neck squamous cell carcinomas. Cancer Biomark. 2012, 12, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Allegra, E.; Puzzo, L.; Zuccala, V.; Trapasso, S.; Vasquez, E.; Garozzo, A.; Caltabiano, R. Nuclear BMI-1 expression in laryngeal carcinoma correlates with lymph node pathological status. World J. Surg. Oncol. 2012, 10, e206. [Google Scholar] [CrossRef]
- Huber, G.F.; Albinger-Hegyi, A.; Soltermann, A.; Roessle, M.; Graf, N.; Haerle, S.K.; Holzmann, D.; Moch, H.; Hegyi, I. Expression patterns of Bmi-1 and p16 significantly correlate with overall, disease-specific, and recurrence-free survival in oropharyngeal squamous cell carcinoma. Cancer 2011, 117, 4659–4670. [Google Scholar] [CrossRef]
- Rietbergen, M.M.; Martens-de Kemp, S.R.; Bloemena, E.; Witte, B.I.; Brink, A.; Baatenburg de Jong, R.J.; Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. Cancer stem cell enrichment marker CD98: A prognostic factor for survival in patients with human papillomavirus-positive oropharyngeal cancer. Euro. J. Cancer 2014, 50, 765–773. [Google Scholar] [CrossRef]
- Butof, R.; Dubrovska, A.; Baumann, M. Clinical perspectives of cancer stem cell research in radiation oncology. Radiother. Oncol. 2013, 108, 388–396. [Google Scholar] [CrossRef]
- Linge, A.; Löck, S.; Krenn, C.; Appold, S.; Lohaus, F.; Nowak, A.; Gudziol, V.; Baretton, G.B.; Buchholz, F.; Baumann, M.; et al. Independent validation of the prognostic value of cancer stem cell marker expression and hypoxia-induced gene expression for patients with locally advanced HNSCC after postoperative radiotherapy. Clin. Transl. Radiat. Oncol. 2016, 1, 19–26. [Google Scholar] [CrossRef]
- Nasman, A.; Nordfors, C.; Grun, N.; Munck-Wikland, E.; Ramqvist, T.; Marklund, L.; Lindquist, D.; Dalianis, T. Absent/weak CD44 intensity and positive human papillomavirus (HPV) status in oropharyngeal squamous cell carcinoma indicates a very high survival. Cancer Med. 2013, 2, 507–518. [Google Scholar] [CrossRef]
- Motegi, A.; Fujii, S.; Zenda, S.; Arahira, S.; Tahara, M.; Hayashi, R.; Akimoto, T. Impact of Expression of CD44, a Cancer Stem Cell Marker, on the Treatment Outcomes of Intensity Modulated Radiation Therapy in Patients With Oropharyngeal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 461–468. [Google Scholar] [CrossRef]
- Toyoda, M.; Kaira, K.; Shino, M.; Sakakura, K.; Takahashi, K.; Takayasu, Y.; Tominaga, H.; Oriuchi, N.; Nikkuni, O.; Suzuki, M.; et al. CD98 as a novel prognostic indicator for patients with stage III/IV hypopharyngeal squamous cell carcinoma. Head Neck 2015, 37, 1569–1574. [Google Scholar] [CrossRef]
- Vermeulen, L.; de Sousa e Melo, F.; Richel, D.J.; Medema, J.P. The developing cancer stem-cell model: Clinical challenges and opportunities. Lancet Oncol. 2012, 13, 83–89. [Google Scholar] [CrossRef]
- Moreira, J.; Tobias, A.; O’Brien, M.P.; Agulnik, M. Targeted Therapy in Head and Neck Cancer: An Update on Current Clinical Developments in Epidermal Growth Factor Receptor-Targeted Therapy and Immunotherapies. Drugs 2017, 77, 843–857. [Google Scholar] [CrossRef]
- Kerk, S.A.; Finkel, K.A.; Pearson, A.T.; Warner, K.A.; Zhang, Z.; Nor, F.; Wagner, V.P.; Vargas, P.A.; Wicha, M.S.; Hurt, E.M.; et al. 5T4-Targeted Therapy Ablates Cancer Stem Cells and Prevents Recurrence of Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2017, 23, 2516–2527. [Google Scholar] [CrossRef]
- Sun, S.; Liu, S.; Duan, S.Z.; Zhang, L.; Zhou, H.; Hu, Y.; Zhou, X.; Shi, C.; Zhou, R.; Zhang, Z. Targeting the c-Met/FZD8 signaling axis eliminates patient-derived cancer stem-like cells in head and neck squamous carcinomas. Cancer Res. 2014, 74, 7546–7559. [Google Scholar] [CrossRef]
- Kim, J.; Shin, J.H.; Chen, C.H.; Cruz, L.; Farnebo, L.; Yang, J.; Borges, P.; Kang, G.; Mochly-Rosen, D.; Sunwoo, J.B. Targeting aldehyde dehydrogenase activity in head and neck squamous cell carcinoma with a novel small molecule inhibitor. Oncotarget 2017, 8, 52345–52356. [Google Scholar] [CrossRef]
- McDermott, S.C.; Rodriguez-Ramirez, C.; McDermott, S.P.; Wicha, M.S.; Nor, J.E. FGFR signaling regulates resistance of head and neck cancer stem cells to cisplatin. Oncotarget 2018, 9, 25148–25165. [Google Scholar] [CrossRef]
- Liu, J.; Pan, S.; Hsieh, M.H.; Ng, N.; Sun, F.; Wang, T.; Kasibhatla, S.; Schuller, A.G.; Li, A.G.; Cheng, D.; et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. USA 2013, 110, 20224–20229. [Google Scholar] [CrossRef]
- Colnot, D.R.; Quak, J.J.; Roos, J.C.; van Lingen, A.; Wilhelm, A.J.; van Kamp, G.J.; Huijgens, P.C.; Snow, G.B.; van Dongen, G.A. Phase I therapy study of 186Re-labeled chimeric monoclonal antibody U36 in patients with squamous cell carcinoma of the head and neck. J. Nucl. Med. 2000, 41, 1999–2010. [Google Scholar]
- Odenthal, J.; Rijpkema, M.; Bos, D.; Wagena, E.; Croes, H.; Grenman, R.; Boerman, O.; Takes, R.; Friedl, P. Targeting CD44v6 for fluorescence-guided surgery in head and neck squamous cell carcinoma. Sci. Rep. 2018, 8, e10467. [Google Scholar] [CrossRef]
- Borges, G.A.; Rego, D.F.; Assad, D.X.; Coletta, R.D.; De Luca Canto, G.; Guerra, E.N. In vivo and in vitro effects of curcumin on head and neck carcinoma: A systematic review. J. Oral Pathol. Med. 2017, 46, 3–20. [Google Scholar] [CrossRef]
- Moselhy, J.; Srinivasan, S.; Ankem, M.K.; Damodaran, C. Natural Products That Target Cancer Stem Cells. Anticancer Res. 2015, 35, 5773–5788. [Google Scholar]
- Taylor, W.F.; Jabbarzadeh, E. The use of natural products to target cancer stem cells. Am. J. Cancer Res. 2017, 7, 1588–1605. [Google Scholar]
| Marker | Biological Function | Detection Method | Functional Analysis | Ref. |
|---|---|---|---|---|
| CD44 | Cell surface protein: Receptor for hyaluronic acid, cell-cell and cell-matrix contacts, migration | Flow cytometry, Immunohisto-/Immunocytochemistry | Serial transplantation, xenograft assay | [20] |
| CD133 | Cell surface protein: possible role in membrane organization | Flow cytometry, Immunohisto-/Immnuocytochemistry | Xenograft tumor formation assay | [21] |
| CD98 | Cell surface protein: amino acid transport, integrin signaling | Flow cytometry, Immunohisto-/Immnuocytochemistry | Serial transplanation, xenograft tumor formation assay | [22] |
| Aldehyde dehydrogenase (ALDH) | Intracellular enzyme oxidizing aldehydes: detoxification, retinoic acid production | Aldefluor assay | Xenotranplantation, Sphere formation assay, (Matrigel/Transwell-invasion assay, Colony formation after irradiation) | [25] |
| Side population (SP) | ABC transporter-mediated efflux of endogenous and exogenous substances | DNA dye (Hoechst 33342) exclusion assay | Xenograft assay, Serial sphere formation assay, (Matrigel invasion assay, Cell viability after chemotherapeutic drugs) | [24] |
| ZsGreen-cODC reporter | Low proteasome activity leading to decreased protein degradation | Accumulation of the fluorescent protein ZsGreen fused to the C-terminal degron of murine ornithine decarboxylase (cODC) | Xenograft assay, Sphere formation assay | [26] |
| Enrichment Only from Cell Culture | Enrichment from Surgically Excised or Primary Cultured Patient Material | Enrichment from Blood Samples |
|---|---|---|
| Low proteasome activity (ZsGreen-cODC reporter) [26,56] | Cell surface proteins (antibody-based methods) [20] | Cell surface proteins (antibody-based methods) [58] |
| Aldehyde dehydrogenase (ALDH) activity (Aldefluor assay) [25] | ||
| Stem cell transcription factors (fluorescent reporters) [42] | Sphere formation assay [55] | |
| Side population (Hoechst 33342 exclusion assay) [57] |
| Therapeutic Target | Compound | Model System | Results | Reference |
|---|---|---|---|---|
| 5T4 (oncofetal antigen) | MEDI0641 | tissue microarray, HNSCC cells (UM-SCC-11B, UM-SCC-22B), patient-derived xenograft (PDX) model | Reduction of CSC fraction, tumor regression | [186] |
| Bmi1/AP-1 | PTC-209 | BmiCreER; RosatdTomato mice | cisplatin plus PTC-209 potently eradicates Bmi1+ CSCs and inhibits tumor progression | [80] |
| FGF | BGJ398 | HNSCC cell lines | decreased ALDHhighCD44high, sensitization to cisplatin | [189] |
| Porcupine (PORCN) (Wnt signaling) | LGK974 | HNSCC HN30 cell line | High response in HNSCC with Notch loss of function mutation | [190] |
| ALDH1 | Alda-89, Aldi-6 | HNSCC cells, xenograft model | Combination with cisplatin enhanced tumor cell death and reduced tumor growth | [188] |
| CD44 | Radionuclide 186Re-cmAb (U36) | Patients with Squamous Cell Carcinoma of the Head and Neck | Dose-limiting myelotoxicity, reduction in tumor size | [191] |
| CD44v6 | Anti-CD44v6 antibody BIWA- IRDye800CW and -Indium-111 | Fluorescence-guided surgery, locally invasive xenograft model | Detection of tumor regions and invasive zones | [192] |
| cMET/FZD8 | PF-2341066 | HNSCC patient-derived xenograft (PDX) | Reduced sphere formation, tumor initiation and metastatic spread | [187] |
| CSC Marker | Pathology/Stage | Treatment | Clinical Endpoint | Sample Size | Trial Identifier | Reference |
|---|---|---|---|---|---|---|
| 1. Diagnostic potential | ||||||
| Bmi-1 | HNSCC, OSCC, LSCC, stage I–IV | DSS, DFS, OS | 64–149 | [88,140,176] | ||
| CD133 | HNSCC/LSCC, stage I–IV | OS, DSF | 83–98 | [174,175] | ||
| Oct-4 | HNSCC, stage I–V | OS, DSF | 60 | [173] | ||
| Nanog | OSCC | OS | 57 | [172] | ||
| Sox2 | HNSCC, stage III–IV | Radiation | OS | 725 | [171] | |
| 2. Prognostic potential | ||||||
| CD44 | HNSCC, SCC | RCT | OS, LRC, Distant metastases, Overall survival, Toxicity, Efficiency | 29–221 | NCT03148665 (OncAlert™), NCT02254018 (Bivatuzumab Mertansine) | [27,169,170] |
| cMet | HNSCC | RCT | LRC, Distant metastases, Overall survival | 221 | [27] | |
| CD98 | HNSCC | RCT | LRC, Distant metastases, Overall survival | 197-221 | [27,168] | |
| 3. Therapeutic potential | ||||||
| Hedgehog | Recurrent HNSCC | IPI-926, Cetuximab | Toxicity, Safety, Efficiency | NCT01255800 | ||
| cMet | Tivantinib, INC280, Ficlatuzumab | Toxicity, Safety, Efficiency | NCT01619618 (E1302 Trial) NCT01696955 NCT02205398 NCT03422536 | |||
| CD44 | Bivatuzumab Mertansine | Toxicity, Safety, Efficiency | NCT02254018 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peitzsch, C.; Nathansen, J.; Schniewind, S.I.; Schwarz, F.; Dubrovska, A. Cancer Stem Cells in Head and Neck Squamous Cell Carcinoma: Identification, Characterization and Clinical Implications. Cancers 2019, 11, 616. https://doi.org/10.3390/cancers11050616
Peitzsch C, Nathansen J, Schniewind SI, Schwarz F, Dubrovska A. Cancer Stem Cells in Head and Neck Squamous Cell Carcinoma: Identification, Characterization and Clinical Implications. Cancers. 2019; 11(5):616. https://doi.org/10.3390/cancers11050616
Chicago/Turabian StylePeitzsch, Claudia, Jacqueline Nathansen, Sebastian I. Schniewind, Franziska Schwarz, and Anna Dubrovska. 2019. "Cancer Stem Cells in Head and Neck Squamous Cell Carcinoma: Identification, Characterization and Clinical Implications" Cancers 11, no. 5: 616. https://doi.org/10.3390/cancers11050616
APA StylePeitzsch, C., Nathansen, J., Schniewind, S. I., Schwarz, F., & Dubrovska, A. (2019). Cancer Stem Cells in Head and Neck Squamous Cell Carcinoma: Identification, Characterization and Clinical Implications. Cancers, 11(5), 616. https://doi.org/10.3390/cancers11050616






