1. Introduction
Hepatocellular carcinoma (HCC), which accounts for 90% of primary liver cancer cases, is the fourth leading cause of cancer-related deaths worldwide [
1]. East Asia and sub-Saharan Africa have higher incidence rates; however, the incidence rate is increasing in the USA [
2,
3]. Risk factors for HCC include hepatitis B and C virus infection, alcoholic and non-alcoholic liver diseases, iron storage diseases and aflatoxin. The risk factors affecting the HCC incidence rate have regional differences [
4]. The pathogenesis of HCC is a multistep progression involving chronic inflammation, steatosis, fibrosis, cirrhosis, hyperplasia, dysplasia, and the development of a malignant tumor [
5].
Preventions of HCC can be classified as primary, secondary, and tertiary. Primary prevention includes the reduction of exposure to risk factors for liver cancer and vaccine injection against hepatitis B, especially in developing countries. Secondary prevention includes curative treatments and palliative treatments. Tertiary preventions inhibit the recurrence of liver cancer [
6]. Several treatment methods are available for the different stages of HCC. The curative treatment for HCC is resection in the very early stage (single), liver transplantation in the early stage (three nodules <3 cm, but no disease), and radiofrequency ablation (RFA) in the early stage (three nodules <3 cm, with disease), but most patients are in the advanced stage and cannot undergo surgery. Other palliative treatments include transarterial chemoembolization (TACE) in the intermediate stage (multi-nodular), chemotherapy, and radiotherapy. The systemic drug Sorafenib is the first line treatment in the advanced stage (portal invasion) [
5,
7]. Sorafenib combined with radiotherapy can increase survival time and may be used for the prevention of recurrence after resection or ablation [
8]. Regorafenib and lenvatinib showed positive results in Phase III studies [
9].
The safety and efficacy of Bevacizumab plus TACE were evaluated in carefully selected patients and demonstrated antitumor activity in patients with un-resectable HCC [
10,
11]. Drugs selectively block the specific target pathways of HCC progression, such as angiogenesis, signal transduction, and the epigenetic dysregulation of tumors [
5]. Vitamin K1 plus Sorafenib synergistically inhibited HCC cell growth [
12]. HCC is a highly vascularized solid tumor, so angiogenesis can be an important target for therapy [
13]. The role of angiogenesis in HCC has provided systematic therapeutic means for HCC [
14]. Anti-angiogenesis therapies mainly involve the blocking of vascular endothelial growth factor (Cediranib), and are sometimes combined with other angiogenic receptors, such as platelet-derived growth factor receptor (Sorafenib, Sunitinib, Linifanib, and Pazopanib) or the fibroblast growth factor receptor (Brivanib). Anti-vascular endothelial growth factor (VEGF) antibody (Bevacizumab) can block VEGF-mediated angiogenic signals [
8].
Zebrafish (
Danio rerio) is a tropical freshwater fish, and has become a popular vertebrate model in biological research, as it plays a bridging role between in vitro cell-based models and in vivo mammalian models. Zebrafish are small in size, have a large number of progeny, have ex utero development, the optically transparent embryo allows direct morphological observation, and the genomics are highly conserved to humans [
15]. Zebrafish have emerged as a human disease model for toxicology, angiogenesis, DNA damage, cancer, aging, and drug screening [
16,
17,
18,
19].
The zebrafish cancer model can accelerate the discovery of therapeutic means. During zebrafish embryonic development, intersegmental vessel (ISV) formation is an angiogenic process [
20]. ISVs sprout from the dorsal aorta (DA), connecting to the dorsal longitudinal anastomotic vessel (DLAV) between 24 and 48 h post fertilization (hpf) [
21,
22]. In this study, the embryos were immersed with drugs at 24 hpf and we observed the inhibition of ISV angiogenesis at about 50 hpf, in order to identify drugs that inhibit ISV formation for compounds which may target cancer angiogenesis.
Toxicity assays have been developed for zebrafish teratogenicity [
23], cardiotoxicity, and neuro-sensory organ toxicity [
24]. The liver is the organ that metabolizes drugs. Drug hepatoxicity was observed to significantly reduce both liver red fluorescent protein (RFP) intensity and liver size in LiPan
Tg(fabp10a:DsRed;elaA:egfp) transgenic zebrafish [
25]. In this study, we immersed three days post-fertilization (dpf)
Tg(fabp10a:EGFP-mCherry) embryos with drugs for two days, and followed the above study to detect two measurable points as hepatotoxicity indicators: RFP intensity and liver size.
Zebrafish are an excellent animal model for studying liver cancer. Neoplasia can be induced by carcinogens [
26,
27,
28]. Stable overexpression of
krasV12 was generated in transgenic zebrafish-induced liver tumorigenesis [
29]. Pathways and genes responsible for liver development (hepatogenesis) and liver cancer formation (hepatocarcinogenesis) are largely conserved between human and zebrafish [
30,
31]. Zebrafish liver tumors are highly analogous to human tumors in terms of comparative analysis of microarray data and ultrasound biomicroscopy [
27,
28]. Therefore, using the transgenic zebrafish liver cancer model is a useful tool for HCC research and identifying new therapeutic drugs [
32].
We showed that hepatitis B virus X antigen (HBx) plays an important role in hepatocarcinogenesis, causing genomic instability, activating signal pathways, and affecting the epigenomic status [
31]. Using the HBx-induced HCC mouse model, we identified five common regulator genes:
Edn1,
Bmp7,
Bmp4,
Spib, and
Src that were up-regulated in the pre-cancer stage [
33]. Using transgenic zebrafish, we found that HBx induced steatosis, inflammation, and hyperplasia upon aflatoxin treatment [
34]. Overexpression of
HBx in
p53 mutant (
p53−, tumor suppressor gene) zebrafish can cause HCC formation at 11-months-old. Overexpression of
src also induced HCC at 11 months, but together with the
p53 mutation can produce earlier HCC formation at seven-months-old [
34]. Our HBx-induced HCC zebrafish model is more similar to human HCC, as the animal progresses from steatosis to fibrosis, hyperplasia and dysplasia, prior to developing HCC. Our zebrafish model also shares similar molecular mechanisms with human hepatocarcinogenesis in terms of the activation of
src and its downstream signaling pathways. This phenomenon resembles human HCC formation and provides a potential platform for in vivo drug screening for therapies for human liver cancer platforms [
34]. In this work, we used
Tg(fabp10a:HBx,p53−) and
Tg(fabp10a:src,p53−) transgenic fish at 11- and 9-months-old injected with novel small molecules and observed the therapeutic effects in comparison to Sorafenib. We also used
Tg(fabp10a:HBx,src,
p53−/+) transgenic fish overfeeding to accelerate HCC formation at five months of age, then orally fed drugs for one month, and then observed the therapeutic effect.
Due to heterogeneity of cancers, one-treatment-fits-all medicine approaches have been replaced by a precision approach and personalized medicine [
35,
36]. In the genomic era, next-generation sequencing has gradually improved personalized medicine by identifying the cancer-causing variants of target genes; however, predicting the outcome simply replying on genomic data is difficult [
37]. Patient-derived xenotransplantation (PDX) provides rapid screening for selecting drugs that can inhibit tumor cell proliferation and migration. Injecting human cancer cells into zebrafish embryos at 2 dpf when the immune response of zebrafish is not yet established can avoid rejection [
17], and does not require prior immunosuppressive treatment [
38,
39]. Integrating the genomic approach, the PDX model, and a high-throughput drug screening platform will help the clinical identification of effective personalized medicine [
36]. In this study, we used tumor cells labeled with CM-DiI from patients to microinject 2 dpf embryos, let the tumor grow for one day, and then treated with drugs for two days, and then observed the anti-proliferation effects of the drugs.
3. Discussion
In response to the lack of effective anti-HCC drugs, we established a drug screening platform in zebrafish to quickly find novel anti-HCC drugs for targeted therapy. Our first strategy involved finding anti-angiogenic small molecules for anti-HCC treatment. Malignant tumors are always accompanied by angiogenesis to supply oxygen and nutrients for tumor growth. In particular, HCC is one of the most vascularized solid tumors; thus, anti-angiogenesis provides a potential therapeutic target. By observing inter-segmental vessels (ISVs) of the trunk part of the embryo’s body in Tg(fli1:EGFP) embryos with green fluorescent protein expressed in the vessels, we tested compounds for anti-angiogenic activity. Although embryonic angiogenesis and tumor-induced angiogenesis use similar pathways and molecules, zebrafish angiogenesis is not exactly the same as tumor-induced angiogenesis. In a future study, we will apply the patient-derived xenograft model and tumor-induced angiogenesis will be monitored under time-lapse microscopy, and we will test the drugs for anti-tumor induced angiogenesis directly.
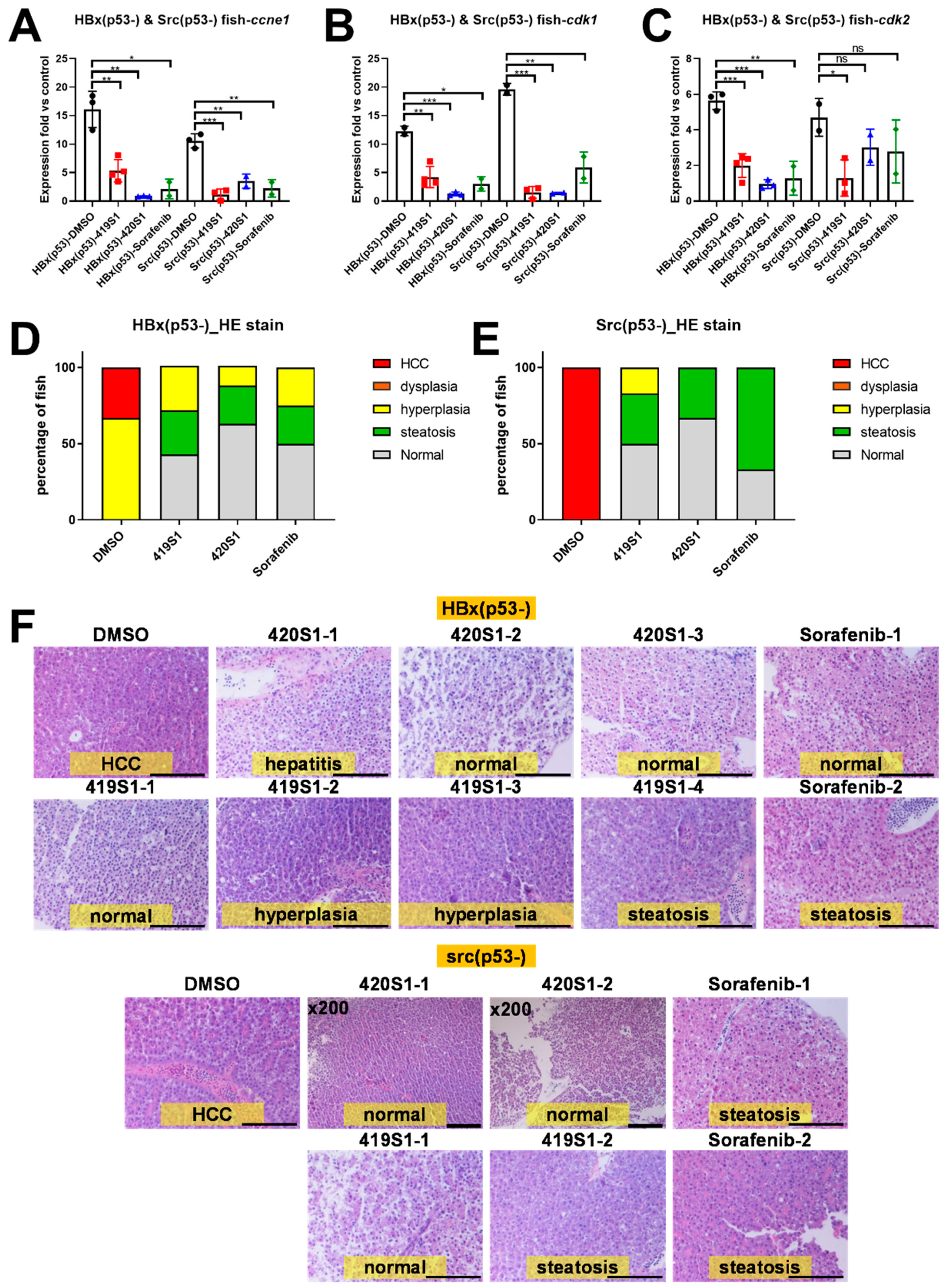
Further candidate drugs were tested in adult transgenic zebrafish with HCC formation to see if they can prevent HCC formation. We established many transgenic zebrafish that developed HCC at 11 or 9 months; we established HBx, Src, and p53 triple mutant transgenic fish with over-feeding-induced obesity, hoping to create an earlier onset and increase HCC incidence. Using both transgenic fish, we proved the effectiveness of 419S1 and 420S1 in preventing HCC formation.
We also established a xenotransplantation assay to test if the candidate drugs have anti-proliferative abilities in a high throughput manner. Using high-content imaging platforms, we enhanced the ability to screen for the anti-proliferative and anti-migratory effects of small molecules in zebrafish embryos. The culture temperature for zebrafish is 28 °C, and for mammalian cells is 37 °C. We adjusted the temperature by gradually increasing the temperature from 28 °C to 37 °C in two days, so the embryos carrying the cancer cells can survive in 37 °C for several days.
Primary tumors directly transplanted from patients into an immuno-deficient mouse model (also known as patient-derived-xenograft, PDX) has become the emerging personalized medicine model [
50,
51]. Previous studies revealed that tumor cell behavior is similar in zebrafish embryos compared to within the human body after xenotransplanting into zebrafish embryos, i.e., if these cells are metastatic tumor cells, they will migrate to other parts of the body of the zebrafish one to three days post-injection. Therefore, we performed a xenotransplantation experiment using patient-derived tumor cells transplanted into zebrafish embryos, and tested whether 419S1 and 420S1 inhibited tumor cell growth and metastasis, with cancer chemotherapy patients as a reference. There are some limitations in using zebrafish as a PDX model: there is little knowledge about their niche structures and micro-environmental cues, differences in size (small zebrafish vessels versus large human cells), and no adult immune-permissive zebrafish lines are available yet.
We were surprised to find both 419S1 and 420S1 are better than Sorafenib. These compounds would be useful in clinical trials for future development. We found 419S1 has the most potential as an anti-HCC drug, because 419S1 had a lower IC50 compared to 420S1, exhibits anti-proliferative and anti-migratory activities in the xenotransplantation assay, and most importantly, 419S1 may have less hepatotoxicity. We also observed 420S1 treatment had the highest cardiac edema rate compared to 419S1 and Sorafenib treatments. 419S1 has less cardiac edema compared to Sorafenib at 6 dpf.
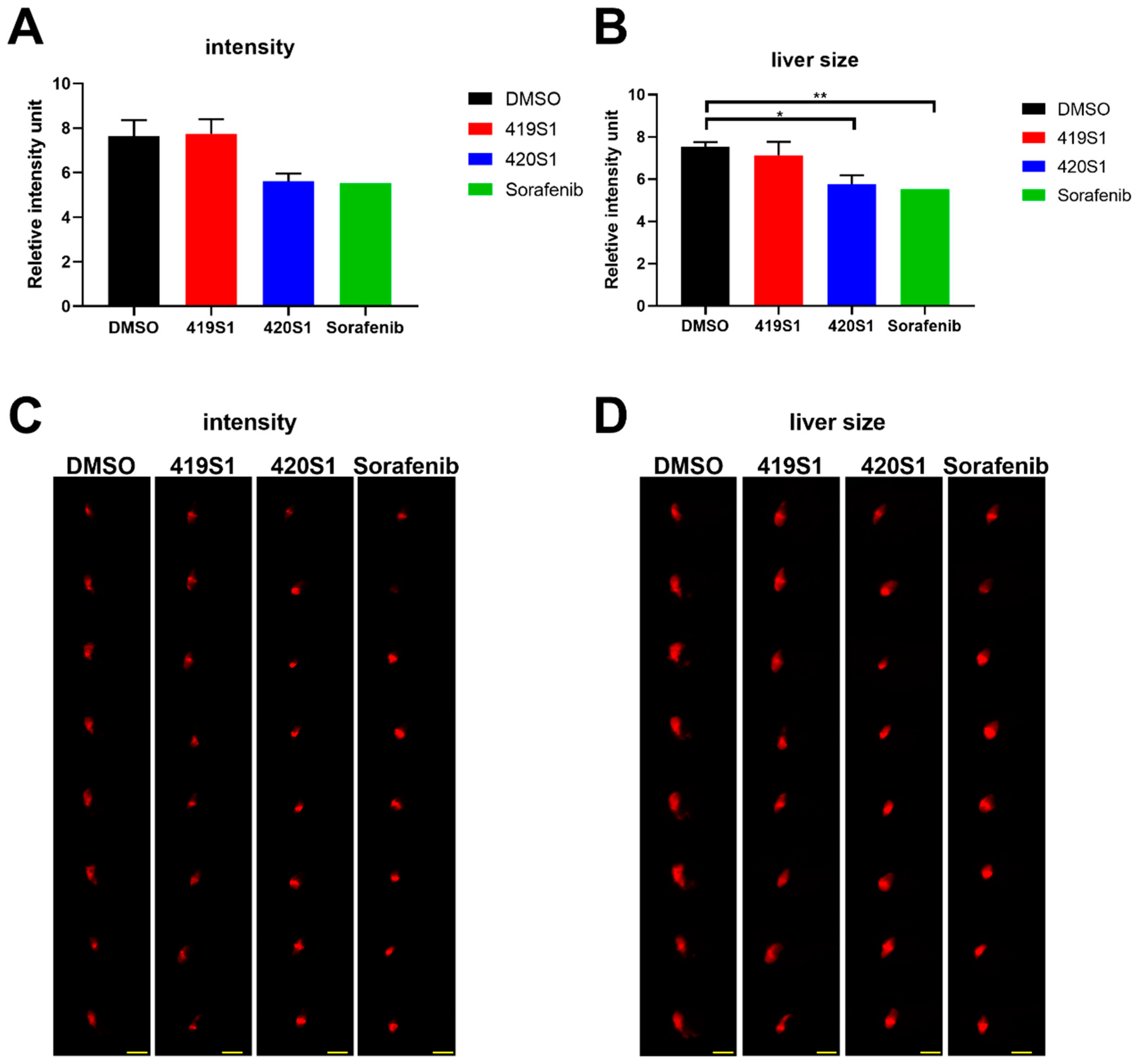
We used Tg(fabp10a:EGFP-mCherry) embryos expressing red florescent protein in liver as a hepatotoxicity model, and observed liver RFP intensity and liver size to examine the hepatotoxicity of the new drugs. Hepatotoxicity is one of the main causes of drug attrition in the pharmaceutical industry. Zebrafish phenotypic assays showed considerable advantages in this assay. We would like to find new drugs that not only have strong anti-cancer effects but also have little effect on the liver and other normal cells of the organism. We can achieve this goal by using the zebrafish animal model.
Drug metabolism occurs primarily in the liver. The expression of
fabp10a, which encodes for a liver fatty acid binding protein, is possibly an appropriate marker of chemical-induced hepatotoxicity. If lipid metabolism changes, hepatotoxicity is reflected in RFP intensity. When compounds produce toxicity via a non-lipid mechanism, RFP in the liver also promotes the observation and measurement of the liver size. Changes in liver size due to toxicity has severe effects on the liver, inducing hypertrophy or atrophy [
25]. Molecular- or cellular-level analysis should also be conducted to determine the real cause for the hepatotoxicity of 420S1.
4. Materials and Methods
4.1. Ethics Statement
The HCC tissues were obtained from Taipei Municipal Wanfang Hospital (Taipei, Taiwan); procedures were undertaken in accordance with the Taipei Medical University Joint Institutional Review Board (TMU-JIRB, 201404018) and National Health Research Institutes Institutional Review Board (NHRI-IRB, EC1030206). All adult participants provided written informed consent and there were no child participants.
All zebrafish experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the NHRI and were in accordance with the International Association for the Study of Pain guidelines (protocol number: NHRI-IACUC-103021-A). Taiwan Zebrafish Core Facility (TZCF) at NHRI or TZeNH is a government-funded core facility, and since 2015, the TZeNH has been accredited by Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
4.2. Transgenic Zebrafish Lines
Four transgenic zebrafish lines
Tg(fli1:EGFP);
Tg(fabp10a:EGFP-mCherry),
tp53zdf1/zdf1;
Tg(fabp10a:HBV-HBx-mCherry,myl7:EGFP) i.e.,
Tg(fabp10a:HBx,,p53−),
tp53zdf1/zdf1;
Tg(fabp10a:src,myl7:EGFP) i.e.,
Tg(fabp10a:Src,,p53−), and the cross of those two fish generated
Tg(fabp10a:HBx,src,p53−) were used in this study. The
Tg(fli1:EGFP) containing
fli1 (friend leukemia integration 1 transcription factor, 15 kb) promoter, driving the expression of enhanced green fluorescent protein (EGFP) in all blood vessels throughout embryogenesis [
52], enables anti-angiogenesis readout for drug treatment.
Tg(fabp10a:EGFP-mCherry) contains liver-specific
fabp10a (fatty acid binding protein 10a) promoter driving the expression of EGFP and mCherry fusion proteins [
53], facilitating observation of hepatotoxicity.
Tg(fabp10a:HBx,p53−),
Tg(fabp10a:Src,p53−) were established by Dr. Lu in our laboratory—the fish developing HCC at 11 and 9 months, respectively [
43].
4.3. Zebrafish Maintenance
Zebrafish (Danio rerio) were maintained in the Zebrafish Core Facility at NTHU-NHRI (ZeTH). The zebrafish were incubated at 28 °C under continuous flow of air in the zebrafish core facility and with automatic control of a 14 h light/10 h dark cycle. All zebrafish experiments were conducted under the approval of the Institutional Animal Care and Use Committee (IACUC) at NHRI (NHRI-IACUC-103021-A).
4.4. Embryos Collection
One day prior to fertilization, male and female adult zebrafish were placed individually into mating tanks with inner mesh. Male and female fish were separated by a separator and left in mating cages overnight. The next morning after the removal of the separator, the couple zebrafish stimulated by the light started to chase each other and lay eggs and sperm. After 1 h, the embryos were collected and transferred to a 100 mm dish with E3 solution (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl
2, and 0.33 mM MgSO
4, pH 7.0) [
54] and incubated at 28 °C for 6 h. The unfertilized and dead embryos were removed, and the remaining live embryos were replenished with fresh E3 solution and kept for incubation.
4.5. Angiogenesis Inhibition Drug Screening Platform
At about 24 h post-fertilization (hpf), the chorion was removed with the protease (1.5 mg/mL for 5 min) from Streptomyces griseus (Sigma-Aldrich Inc., St. Louis, MO, USA). The de-chorion embryos were distributed into 24-well culture plates with eight embryos per well containing E3/PTU (1-phenyl-2-thiourea, 0.003%) buffer. The Tg(fli1:EGFP) embryos were treated with drugs at various concentrations and resuspended in 1% DMSO. The embryos were anesthetized with tricaine (ethyl 3-aminobenzoate methanesulfonate, MS-222 (Sigma-Aldrich In., St. Louis, MO, USA) final concentration 0.016%) for about 50 hpf to prevent movement and their images were captured. The images were analyzed by measurement of the length of inter-segmental vessels to determine the anti-angiogenesis effect of individual compounds.
4.6. Sources of Compounds
BPR1J419S1 and BPR1J420S1 are multiple kinase inhibitors involving Src (one of Src family kinase, a family of non-receptor tyrosine kinases) and Flt-3 (Fms-related tyrosine kinase-3 is a receptor tyrosine kinase), and both can inhibit VEGFR. Sorafenib, a Src inhibitor, was used as a positive control. Sorafenib was the first FDA-approved multi-kinase inhibitor for the treatment of advanced HCC in 2007 [
5]. It inhibits tumor cell proliferation and tumor angiogenesis by targeting different signaling pathways, cell proliferation (via the serine/threonine RAF kinases), and angiogenesis (via VEGFR and platelet-derived growth factor receptor (PDGFR)), significantly increasing the survival of patients with advanced HCC [
55]. The compounds were provided by Dr. Tsu-An Hsu and Dr. Weir-Torn Jiaang of the Institute of Biotechnology and Pharmaceutical Research, National Health Research Institutes (Zhunan Town, Miaoli County, Taiwan). Vatalanib 2HCl (PTK787), used as a positive control for anti-angiogenesis, was purchased from Selleckchem Inc. (Houston, TX, USA).
4.7. Retro Orbital Injection (RO Injection)
The Tg(fabp10a:HBx,p53−) adult fish at 11.3 months old and Tg(fabp10a:src,p53−) adult fish at 8.7 months old were used to inject the drugs and observe the histopathological changes of the hepatocytes. DMSO was used as the negative control; the experimental drugs (419S1 and 420S1) and the positive control (Sorafenib) were injected with final concentrations of 15 μg, 30 μg, and 30 μg per gram of body weight, respectively. Phenol Red was added to the injection solution as a color indicator. The adult fish were injected with the same volume (1.9 μL) per fish, which weighed 0.1 g. The adult fish were anesthetized with 0.016% tricaine to prevent their movement and placed with dorsal side up and faced right on damp sponge. The injection needle was positioned with the bevel facing up such that if the fish’s eye were a clock, the needle was pointed at the seven o’clock position and at a 45-degree angle to the fish. The injection duration was twice a week for one month for a total of eight times.
4.8. Procedure of Diet-Induced Obesity (DIO)
DMSO, 419S1, and 420S1 treatment were applied to diet-induced obesity (DIO) Tg(fabp10a:HBx,src,p53−) fish. Three-month-old Tg(fabp10a:HBx,src,p53−) fish were divided into three groups containing 10 or 12 fish in 2 L tanks, and fed three times per day with four times over the regular amount of hatched Artemia salina (about 83 mg cyst/fish/day) to induce obesity. This procedure was sustained for eight weeks.
4.9. Oral Gavage
Tg(fabp10a:HBx,src,p53−) DIO zebrafish were oral gavaged with drugs to test the anti-tumorigenesis effect. After the diet adjustment for two months, fish were orally fed with 5 μL drug solution twice per week for a month, and then were sacrificed and livers were collected for further molecular and pathological analyses. For oral feeding, we anaesthetized the fish and placed them on a wet sponge, then fed with 5 μL drug solution using microliter syringes (Hamilton, MA, USA) and FTP-22-25 plastic feeding tubes (Instech Laboratories, Plymouth Meeting, Inc. PA, USA). The fish were put back into fresh water immediately after oral gavage to recover from anaesthetization. The dosages of 419S1, 420S1, and Sorafenib were 15, 30, and 30 μg per gram of body weight, respectively, and the average weight of fish was 425 ng. All the fish were weighed and length measured before and after the whole process of oral gavage.
4.10. Liver Tissue Collection and Paraffin Section
After one month of RO injection or oral gavage, the fish were sacrificed, and the livers were removed and divided into two parts for RNA isolation and paraffin section. The liver tissues were frozen in liquid nitrogen immediately after sectioning and stored at −80 °C for later RNA isolation. For histochemistry analysis, liver tissues were fixed in a 10% formalin solution (Sigma-Aldrich Inc., St. Louis, MO, USA). The fixed tissue was embedded in paraffin, and sectioned into 5-μm thicknesses mounted on poly-L-lysine coated slides, and the sections were stained with hematoxylin and eosin (H&E) stain, which was performed at the Pathology core facility.
4.11. Total RNA Isolation
Total RNA was isolated by NucleoSpin® RNA kit (MACHEREY-NAGEL INC., Bethlehem, PA, USA). About 30 mg of tissue were collected and placed in 350 μL RA1 buffer and 3.5 μL β-mercaptoethanol (MilliporeSigma, St. Louis, MO, USA) mixture, and stored at −80 °C at this step. Upon RNA isolation, the samples were thawed slowly at room temperature and then disrupted by pestles to lyse tissue. The lysate was filtrated with NucleoSpin® Filter (violet ring) by centrifuging at 11,000× g for 1 min to reduce viscosity and clear the lysate. After centrifugation, 350 μL of 70% ethanol prepared by DEPC water (diethyl pyrocabonate water) was added to the filtrate and mixed well by pipetting up and down. The lysate was loaded into a NucleoSpin® RNA column (light blue ring) and centrifuged at 11,000× g for 30 s. Following, 350 μL of membrane desalting buffer was added to the column and centrifuged at 11,000× g for 1 min.
To each column, we added 95 μL DNAse reaction mixture containing 10% RNase-free DNase and 90% reaction buffer for DNase, and placed at room temperature for 30 min to digest the genomic DNA. After DNase digestion, 200 μL RAW2 buffer was added to the column to inactivate the DNase and centrifuged at 11,000× g for 30 s. Then, the columns containing RNA were transferred to new 2-mL collection tubes, and 600 μL and 250 μL RA3 buffer were added sequentially followed by centrifuged at 11,000× g for 30 s and 2 min to clean up the RNA samples twice. Finally, the columns were transferred into new 1.5-mL tubes that were RNase free. We eluted the RNA samples in 40-μL RNase-free H2O and then centrifuged at 11,000× g for 1 min. All RNA samples were stored at −80 °C.
4.12. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Complementary DNA (cDNA) was synthesized using a High Capacity RNA-to-cDNA Kit (Thermo Fisher Scientific, Waltham, MA, USA). The reverse transcription (RT) reaction mixture contained: 2 × RT Buffer (10 μL), 20 × enzyme mix (1 μL), RNA sample (1 μg), and RNase-free H2O for a total volume 20 μL.
The reaction mixtures were mixed and spun down in the PCR tubes to collect all the samples in the bottom of the tubes. The reverse transcription in thermal cycling program in the PCR machine was set as: 37 °C for 60 min to start RT reaction, 95 °C for 5 min to inactive enzyme activity, then 4 °C for preservation. For long-term storage, we put the samples in a −20 °C freezer.
4.13. Quantitative PCR(Q-PCR)
After the RT was finished, we diluted the cDNA to 100 × with RNase-free water. For each sample, the following reaction mixture was added to one well of a 384-well Q-PCR plate: quantitative real-time PCR reaction mixture contains: cDNA (diluted with RNase-free water, 3.8 μL), 2.5 μM primer mix (forward and reverse, 1.2 μL), and 2 × SybrGreen Mix (5.0 μL), for a total volume of 10.0 μL.
The 2 × SybrGreen was added last because it is photosensitive. When the whole plate was ready, we covered it with an optical adhesive cover and smoothed out the bubbles with a sealing comb. The Q-PCR program was set as follows in an ABI HT-7900 (Thermo Fisher Scientific, Waltham, MA, USA) machine:
Stage I: 50 °C—2 min; 95 °C—5 min; 4 °C
Stage II: 95 °C—10 min
Stage III (40 cycles): 95 °C—15 s; 60 °C—1 min
Stage IV: 95 °C—15 s; 60 °C—15 s; 95 °C—15 s
Dissociation Protocol: Start Temp: 60 °C
The resulting first-strand cDNA was used as a template for qualitative PCR performed in triplicate using the SYBR Green Q-PCR Master Mix Kit (Thermo Fisher Scientific, Waltham, MA, USA) using an ABI PRISM 7900 System (Thermo Fisher Scientific, Waltham, MA, USA). After normalization to internally controlled actin, the expression ratio between the experimental and control groups was calculated using the comparative Ct method. The relative expression ratio (fold change) was calculated based on △△Ct, △△Ct = (Ct target – Ct actin)treatment – (Ct target – Ct actin)control, and fold change = 2−△△Ct. All experiments were performed in triplicate, and the mean values of three values are presented. At least three independent samples were used for Q-PCR, and the standard error was calculated and incorporated into the presented data as medians ± standard error. Differences among variables were assessed using a two-tailed Student’s t-test. A p < 0.05 was considered statistically significant and is shown as: *: 0.01 < p ≤ 0.05; **: 0.001 < p ≤ 0.01; and ***: p ≤ 0.001.
4.14. Tissue Preparation for Transplantation into Zebrafish Embryos
Human material from surgical resection specimens was obtained at the Wan-Fang Hospital (Taipei, Taiwan) according to the ethical guidelines reviewed by the Taipei Medical University Joint Institutional Review Board and after obtaining informed patient consent. Tissue samples from patients were cut into small pieces using scissors. Tissue pieces were then transferred to 15 mL containers with 3 mL isolation media (180 mL Dulbecco’s Modified Eagle Medium (DMEM) high glucose, 20 mL 100 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 46 mL 5% BSA (bovine serum albumin) and collagenase (Thermo Fisher Scientific, Waltham, MA, USA) (50 μL of a 6 μg/μL stock solution (HBSS as solvent) for each 12 mL of isolation media), and then incubated the tissue in a water bath for 15 min at 37 °C. The supernatant was filtered through cell strainers (70 μm) and tissue pieces were cut further into smaller pieces using scissors. Tissue pieces were again incubated 15 min at 37 °C in 3 mL isolation media with collagenase. The cell suspension was centrifuged 5 min at 1500 rpm. The supernatant was discarded and cells re-suspended in isolation media. The described procedure was then repeated once. For injections, cells were stained with DiI or CellTrace™ CFSE/PBS (Thermo Fisher Scientific, Waltham, MA, USA) working solution (25 μM) for 15 min at 37 °C. The cells were centrifuged at 1500 rpm for 5 min, and washed with PBS. The supernatant was discarded, and the cells were resuspended in PBS and diluted at 4.35 × 104 cells/μL.
4.15. Xenotransplantation and Imaging for Monitoring Drug Response
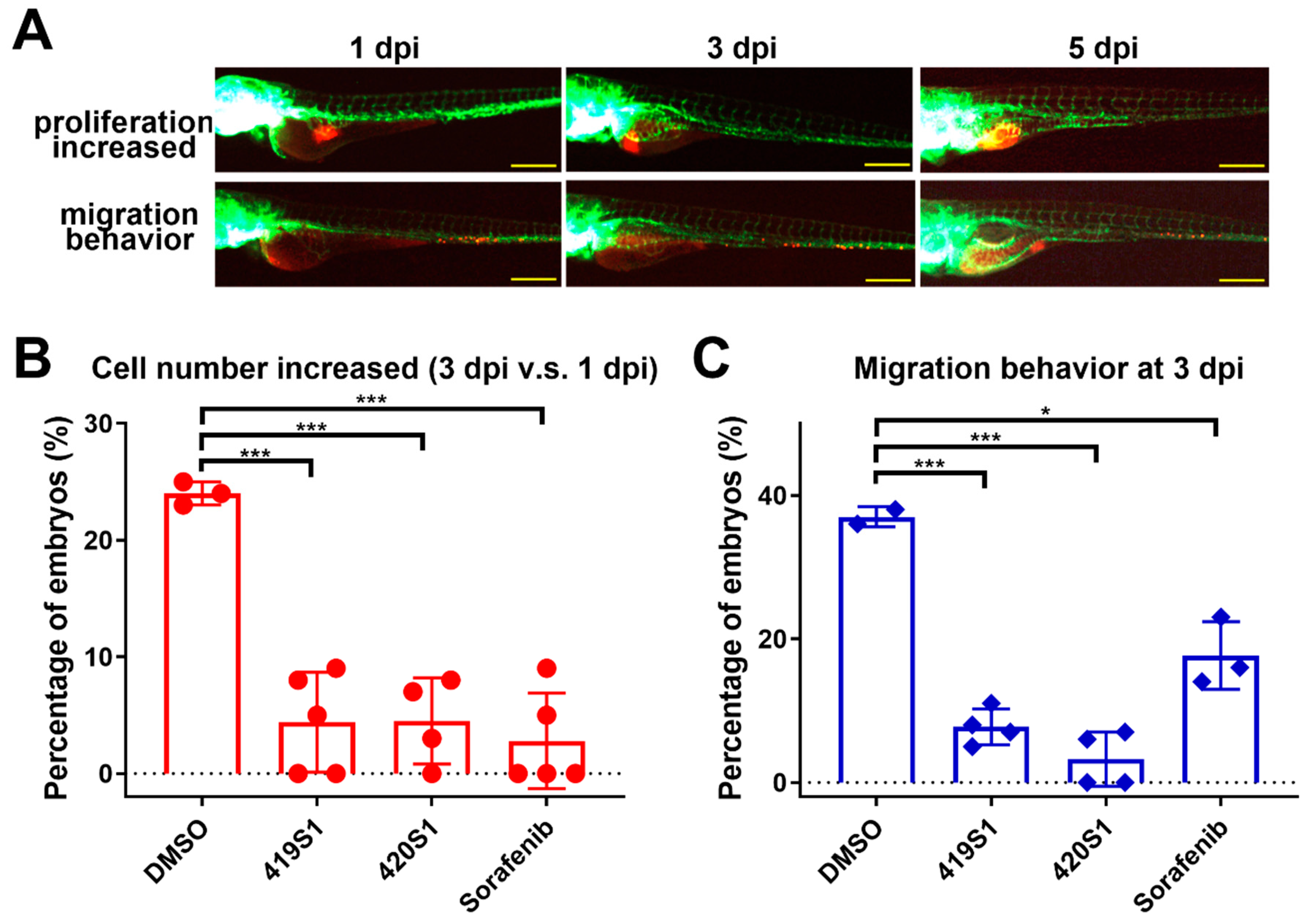
The fertilized eggs of Tg(fli1:EGFP) zebrafish were incubated at 28 °C in E3/PTU solution and raised under standard zebrafish laboratory conditions. Zebrafish embryos two days post-fertilization were dechorionated and anesthetized with 0.016% tricaine methanesulfonate (MS-222) (MilliporeSigma, St. Louis, MO, USA) before microinjection. The 293T/EDN1 cells collected in PBS were labeled in vitro with CM-Dil (red fluorescence) (Vybrant; Invitrogen, Carlsbad, CA, USA). Each injection volume of 4.6 nL contained about 200 cells and implanted into each yolk of 2 dpf zebrafish embryo via a glass capillary using a Nanoject IITM nanoliter injector (Drummond Scientific, Broomall, PA, USA). After injection, zebrafish embryos were washed once with E3/PTU solution, and incubated for 1 h at 28 °C and checked for the presence of fluorescent cells at 2 h post-transplantation. After 24 h post-transplantation, the zebrafish were treated with BPR1J419S1 and BPR1J420S1 separately, and we observed the cell proliferation and migration abilities in the following 3–5 days using a LEICA DM IRB fluorescent microscope (Leica Microsystems Inc., Buffalo Grove, IL, USA)
Xenotransplantation was performed in 2-dpf embryos. For microinjection of human tumor cells to the fish embryos, we followed these protocols: fish embryos were treated with mild protease to remove the protective chorions at 1 dpf. Approximately 200 cells per 4.6 nL were injected into 2 dpf embryos. The injection site was the yolk. The Nanoject II system (Drummond Scientific Company, Broomall, PA, USA) was used with capillaries of 3.5′ (Drummond #3-000-203-G/X; Drummond Scientific Company, Broomall, PA, USA) pulled by a flaming/brown micropipette puller model P-87 (Sutter Instrument Co., Novato, CA, USA) in a fixed parameter (heat: 607, pull: 180, velocity: 150, delay time: 100 and pressure: 500). The end of the pulled needle was then removed using watchmaker forceps to produce an opening, and ground using a EG-400 Microgrinder (NARISHIGE Tokyo, Japan) to create a 40-degree grinding plane and a 125-μm diameter section opening. Injections were performed under an XL-720 microscope (SAGE VISION, Bala Cynwyd, PA, USA). After injection, the embryos were cultured in E3/PTU buffer (E3: 5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4) in petri dishes in temperature-controlled incubators (28 °C). The embryos with leaking injected cells were removed at 2 h post-injection (hpi) and placed in the temperature gradient incubator that automatically increased 1 °C every six hours, and reached 37 °C in two days.
4.15.1. Manual Imaging
We took zebrafish with 1 × tricaine (0.016 mg/mL) and images were captured using an OLYMPUS SZX10 fluorescence microscope coupled with a DP71 and U-RFL-T camera (OLYMPUS, Tokyo, Japan) at 1 dpi. The embryos were then placed in a 96-well plate, with 280 μL per well containing one zebrafish embryo. The drugs were dissolved in E3/PTU buffer and 1% DMSO as control. At 2 dpi, we changed the media with fresh solution and control. Two days after drug treatment (3 dpi), the images of the same embryo were captured again. The tumor cells in embryos were observed, and the migration and proliferation ability of the injected cells were recorded and analyzed using ImageJ software (National Institutes of Health (NIH), Bethesda, MD, USA).
4.15.2. Automatic Imaging
The 1-dpi embryos were placed in a 96-well half-area plate with 150 μL (0.016 mg/mL) tricaine per well to take images using auto-machine (ImageXpressR Micro, Molecular Devices, Sunnyvale, CA, USA). Then, 180 μL per well containing one zebrafish embryo was selected for drug treatment. The drugs were dissolved in E3/PTU buffer and 1% DMSO as control. At 2 dpi, we changed the media with fresh solution and control. We captured images at 3 dpi with Automated High Throughput Screening System (HTS) (BMG LABTECH, Allmendgrün 8, Ortenberg, Germany). The images were analyzed using MetaXpress MDCStore 2.3 software (Molecular Devices, Sunnyvale, CA, USA).
4.16. Embryonic Toxicity Test
Zebrafish embryos were harvested at 4 hpf and incubated at 28 °C for the duration of the experiments. Forty embryos were placed into each well of 6-well polystyrene tissue culture plates. We added 5 mL of a different concentrations of compounds to each well. The buffer was renewed every day throughout the experiment. The morphology and survival rate were observed at different time points: 12, 24, 36, 48, 72, 96, and 120 h. The images were captured using an Olympus SZX10 stereo fluorescence microscope coupled with a DP71 digital Charge Coupled Device (CCD) camera (OLYMPUS, Tokyo, Japan).
4.17. Hepatotoxicity Test
EGFP-mCherry embryos collection and incubation conditions were previously described in
Section 4.4. At about 3 dpf, 50 embryos were distributed into 10 mL chemical solution/well (chemical/E3 solution) in 6-well plates until 5 dpf and the chemical solution was replaced every day. At 5 dpf, embryos were anesthetized with tricaine (0.016%) and images of 8 to 10 randomly chosen embryos per well were taken with a ZEISS AxioCam MRc (ZEISS, Oberkochen, Germany). We captured three different images per embryo: one with automatic exposure time for the clearest view, one with fixed exposure time to capture RFP (red fluorescent protein) intensity below saturation for intensity measurement and comparison, and the one with sufficient exposure time to show the whole liver region for size measurement. ImageJ software was then used to quantify intensity of RFP and liver size. Average RFP intensity in the liver was calculated and compared within the same group of lateral view fry under the same magnification and fixed exposure time.
4.18. Survival Test
Tg(fli1:EGFP) embryos were used in the survival assay. At 3 dpf, 20 embryos were placed into one well of the 6-well plates with 2 mL E3 medium supplement with drugs. The DMSO control and three different drugs, 419S1, 420S1, and Sorafenib, were serially diluted to determine the survival rate. Two days after exposure, the embryos were counted and the survival curves were measured.
4.19. Statistical Analysis
The statistical analysis of the results was performed using Prism 8 (GraphPad Software, San Diego, CA, USA) and two-tailed Student’s t-test was applied. In all statistical analyses, a p-value < 0.05 was considered to be statistically significant and is shown as: *: 0.01 < p ≤ 0.05; **: 0.001 < p ≤ 0.01; and ***: p ≤ 0.001.