Non-Invasive Monitoring of Stromal Biophysics with Targeted Depletion of Hyaluronan in Pancreatic Ductal Adenocarcinoma
Abstract
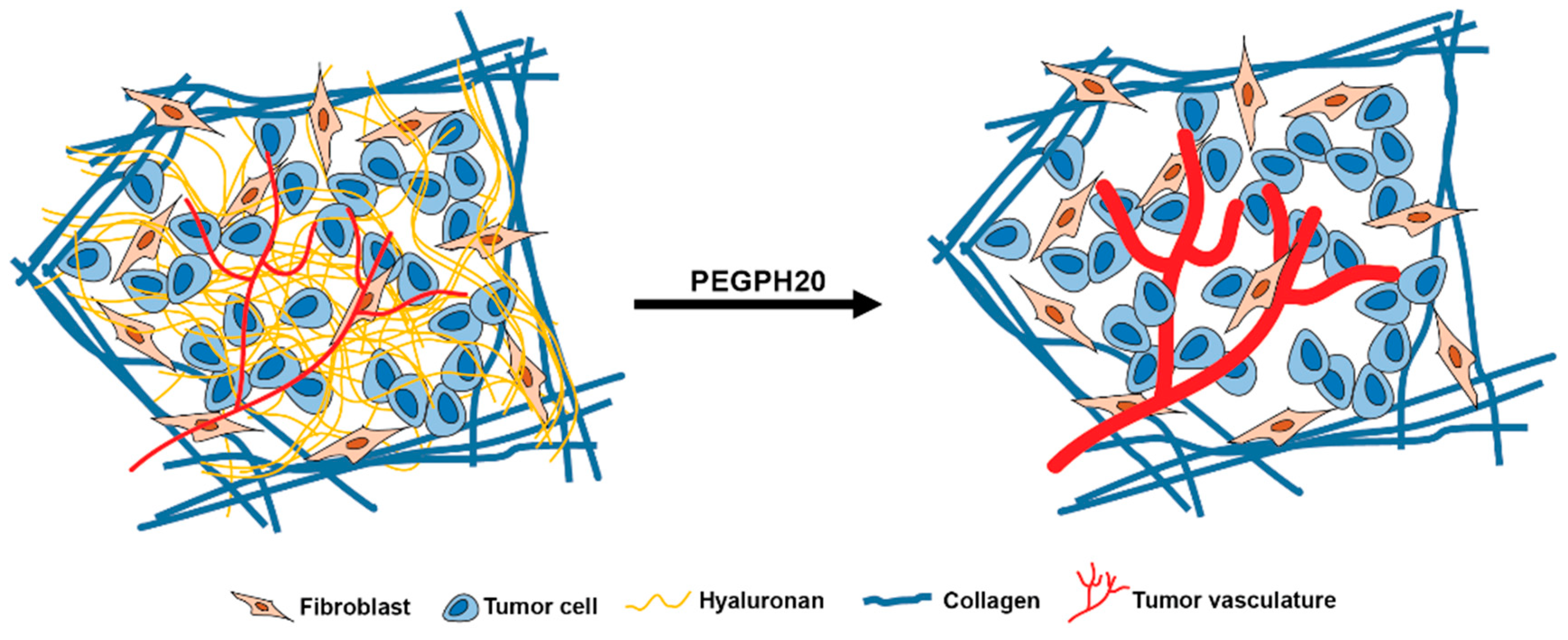
:1. Introduction
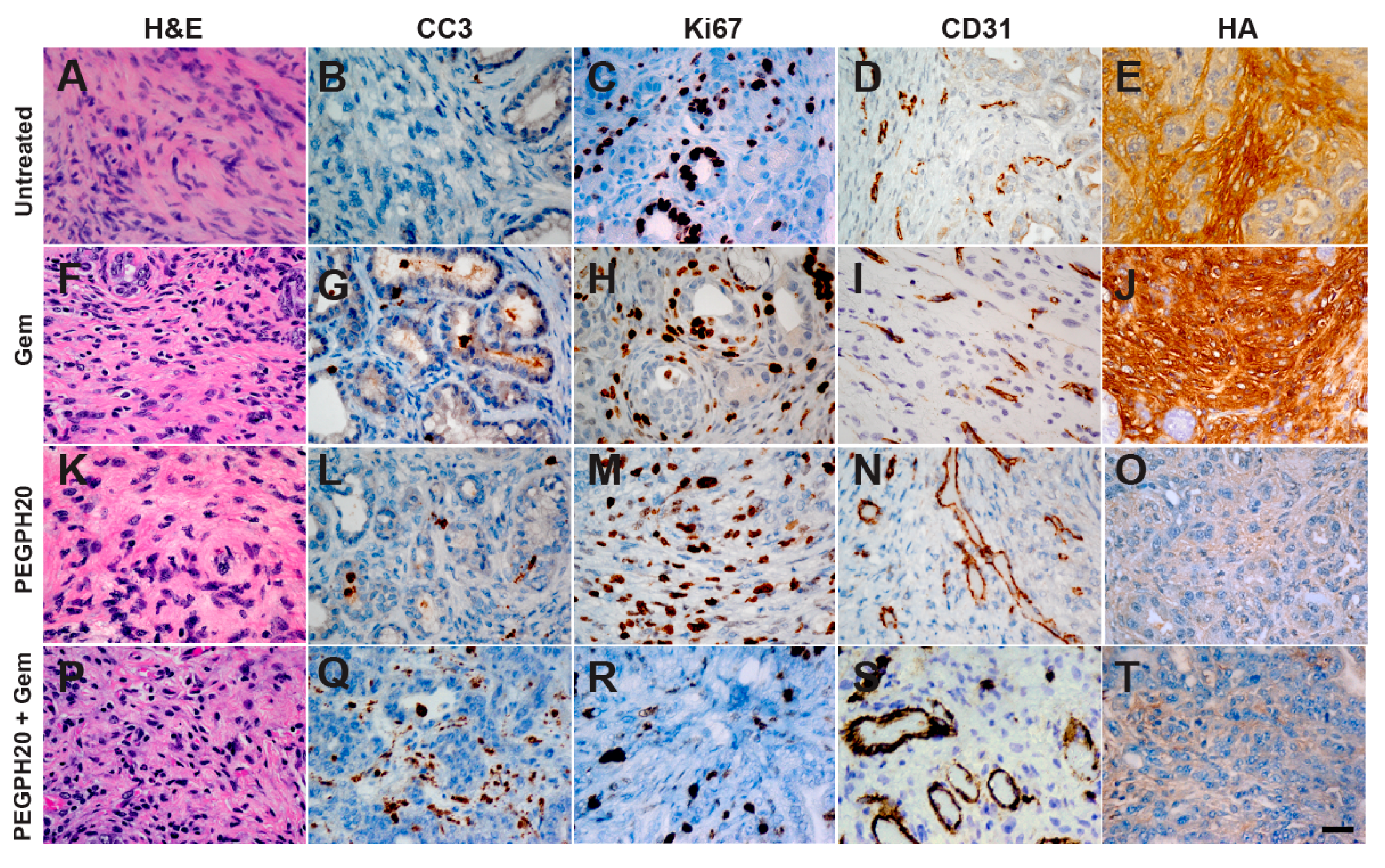
2. Results
2.1. Apparent Diffusion Coefficient (ADC) Values of PDA
2.2. GagCEST Imaging of PDA
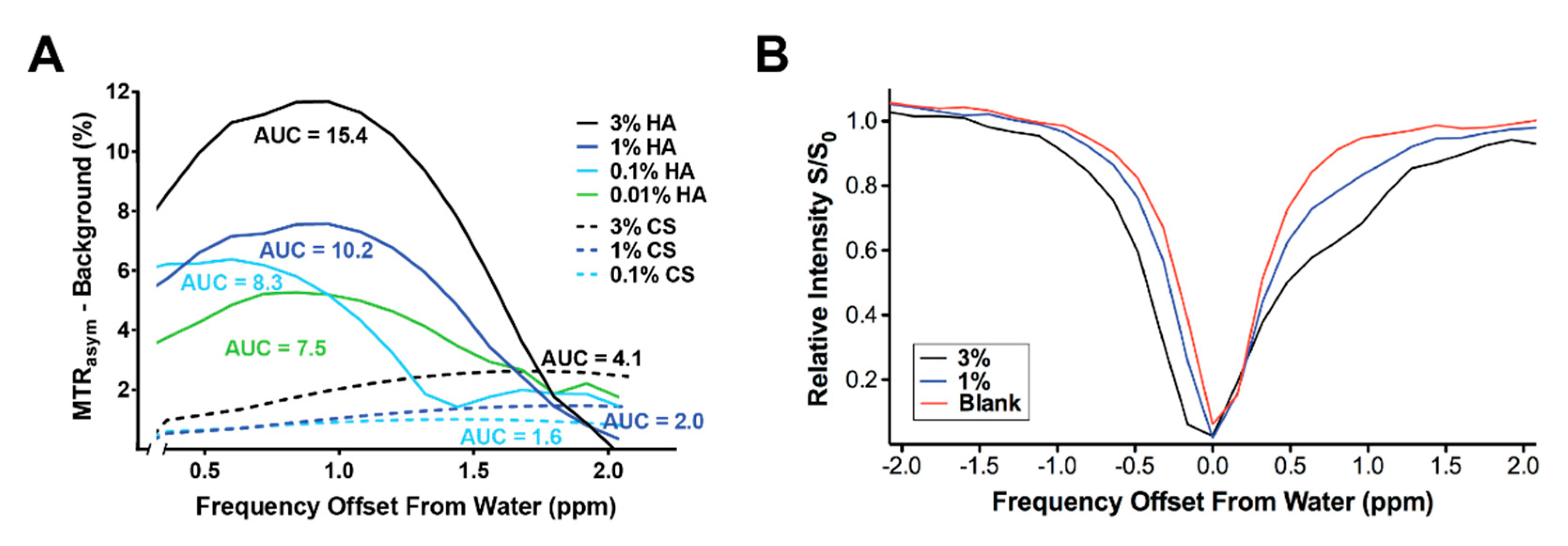
2.3. GagCEST Imaging of Phantom Tumors
3. Discussion
4. Materials and Methods
4.1. Mouse Strains
4.2. MRI Protocol
4.3. MRI Map Creation and Analysis
4.4. Histology and Immunohistochemistry
4.5. Phantom Imaging
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laurent, T.C.; Fraser, J.R.E. Hyaluronan. Faseb J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Hingorani, S.R. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br. J. Cancer 2013, 108, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DuFort, C.C.; DelGiorno, K.E.; Hingorani, S.R. Mounting pressure in the microenvironment: fluids, solids, and cells in pancreatic ductal adenocarcinoma. Gastroenterology 2016, 150, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Harris, W.P.; Beck, J.T.; Berdov, B.A.; Wagner, S.A.; Pshevlotsky, E.M.; Tjulandin, S.A.; Gladkov, O.A.; Holcombe, R.F.; Korn, R.; et al. Phase Ib Study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin. Cancer Res. 2016, 22, 2848–2854. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Zheng, L.; Bullock, A.J.; Seery, T.E.; Harris, W.P.; Sigal, D.S.; Braiteh, F.; Ritch, P.S.; Zalupski, M.M.; Bahary, N.; et al. HALO 202: Randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2018, 36, 359–366. [Google Scholar] [CrossRef]
- Sala, E.; Mema, E.; Himoto, Y.; Veeraraghavan, H.; Brenton, J.D.; Snyder, A.; Weigelt, B.; Vargas, H.A. Unravelling tumour heterogeneity using next-generation imaging: radiomics, radiogenomics, and habitat imaging. Clin. Radiol. 2017, 72, 3–10. [Google Scholar] [CrossRef]
- Stromnes, I.M.; DelGiorno, K.E.; Greenberg, P.D.; Hingorani, S.R. Stromal reengineering to treat pancreas cancer. Carcinogenesis 2014, 35, 1451–1460. [Google Scholar] [CrossRef]
- Kim, S.; Decarlo, L.; Cho, G.Y.; Jensen, J.H.; Sodickson, D.K.; Moy, L.; Formenti, S.; Schneider, R.J.; Goldberg, J.D.; Sigmund, E.E. Interstitial fluid pressure correlates with intravoxel incoherent motion imaging metrics in a mouse mammary carcinoma model. NMR Biomed. 2012, 25, 787–794. [Google Scholar] [CrossRef]
- Thompson, C.B.; Shepard, H.M.; O’Connor, P.M.; Kadhim, S.; Jiang, P.; Osgood, R.J.; Bookbinder, L.H.; Li, X.; Sugarman, B.J.; Connor, R.J.; et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol. Cancer Ther. 2010, 9, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Regatte, R.R.; Navon, G.; Jerschow, A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc. Natl. Acad. Sci. USA 2008, 105, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Warnock, G.; Zaiss, M.; Lin, C.; Chen, M.; Zhou, Z.; Mu, L.; Nanz, D.; Tuura, R.; Delso, G. An overview of CEST MRI for non-MR physicists. EJNMMI Phys. 2016, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Maloney, E.; DuFort, C.C.; Carlson, M.A.; Vohra, R.; Park, J.; Hingorani, S.R.; Lee, D. Department of Radiology, University of Washington, Seattle, WA. Material not intended for publication. 2019. [Google Scholar]
- Jacobetz, M.A.; Chan, D.S.; Neesse, A.; Bapiro, T.E.; Cook, N.; Frese, K.K.; Feig, C.; Nakagawa, T.; Caldwell, M.E.; Zecchini, H.I.; et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013, 62, U112–U153. [Google Scholar] [CrossRef] [PubMed]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- DuFort, C.C.; DelGiorno, K.E.; Carlson, M.A.; Osgood, R.J.; Zhao, C.; Huang, Z.; Thompson, C.B.; Connor, R.J.; Thanos, C.D.; Scott Brockenbrough, J.; et al. Interstitial pressure in pancreatic ductal adenocarcinoma is dominated by a gel-fluid phase. Biophys. J. 2016, 110, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.A. Macromolecular basis of globular protein exclusion and of swelling pressure in loose connective tissue (umbilical cord). Biochim. Biophys. Acta 1983, 755, 388–399. [Google Scholar] [CrossRef]
- Meyer, F.A.; Laver-Rudich, Z.; Tanenbaum, R. Evidence for a mechanical coupling of glycoprotein microfibrils with collagen fibrils in Wharton’s jelly. Biochim. Biophys. Acta 1983, 755, 376–387. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Tsara, M.E.; Papageorgacopoulou, N.; Karavias, D.D.; Theocharis, D.A. Pancreatic carcinoma is characterized by elevated content of hyaluronan and chondroitin sulfate with altered disaccharide composition. Biochim. Biophys. Acta 2000, 1502, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Farr, N.; Wang, Y.N.; D’Andrea, S.; Gravelle, K.M.; Hwang, J.H.; Lee, D. Noninvasive characterization of pancreatic tumor mouse models using magnetic resonance imaging. Cancer Med. 2017, 6, 1082–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hingorani, S.R.; Wang, L.F.; Multani, A.S.; Combs, C.; Deramaudt, T.B.; Hruban, R.H.; Rustgi, A.K.; Chang, S.; Tuveson, D.A. Trp53(R172H) and KraS(G12D) cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005, 7, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Stromnes, I.M.; Schmitt, T.M.; Hulbert, A.; Brockenbrough, J.S.; Nguyen, H.N.; Cuevas, C.; Dotson, A.M.; Tan, X.; Hotes, J.L.; Greenberg, P.D.; et al. T Cells engineered against a native antigen can surmount immunologic and physical barriers to treat pancreatic ductal adenocarcinoma. Cancer Cell 2015, 28, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Whittle, M.C.; Hingorani, S.R. Understanding disease biology and informing the management of pancreas cancer with preclinical model systems. Cancer J. 2017, 23, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Vohra, R.; Park, J.; Wang, Y.N.; Gravelle, K.; Whang, S.; Hwang, J.H.; Lee, D. Evaluation of pancreatic tumor development in KPC mice using multi-parametric MRI. Cancer Imaging 2018, 18, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmond, K.L.; Moosvi, F.; Stanisz, G.J. Mapping of amide, amine, and aliphatic peaks in the CEST spectra of murine xenografts at 7 T. Magn. Reson. Med. 2014, 71, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Wang, P.; Zong, X.; Kim, S.G. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magn. Reson. Med. 2013, 69, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Gillen, J.; Landman, B.A.; Zhou, J.; van Zijl, P.C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn. Reson. Med. 2009, 61, 1441–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Haris, M.; Singh, A.; Reddy, S.; Bagga, P.; Kneeland, J.B.; Tjoumakaris, F.P.; Hariharan, H.; Marincola, F.M.; Reddy, R. Characterization of viscosupplementation formulations using chemical exchange saturation transfer (ViscoCEST). J. Transl. Med. 2016, 14, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, B.; Zbyn, S.; Stelzeneder, D.; Jellus, V.; Paul, D.; Lauer, L.; Bachert, P.; Trattnig, S. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)Na MR imaging at 7 T. Radiology 2011, 260, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K. ImageJ Plugin “MRI Analysis Calculator”. Available online: https://imagej.nih.gov/ij/plugins/mri-analysis.html (accessed on 20 May 2019).
- Jadin, L.; Huang, L.; Wei, G.; Zhao, Q.P.; Gelb, A.B.; Frost, G.I.; Jiang, P.; Shepard, H.M. Characterization of a novel recombinant hyaluronan binding protein for tissue hyaluronan detection. J. Histochem. Cytochem. 2014, 62, 672–683. [Google Scholar] [CrossRef] [PubMed]


| Method | Sequence Type | TR/TE (ms) | Comments |
|---|---|---|---|
| T1 | RARE | 5500, 3000, 1500, 1000, 385.8/9.66 | NA = 1; FOV = 30 × 30 mm2; rare factor = 2, matrix size = 256 × 128 (reconstructed phase encoding steps = 128; acquisition phase encoding steps = 96); yielding spatial resolution of 0.117 × 0.234 mm/pixel. Approximately 9 min acquisition time. |
| T2 | MSME, fat suppressed | 4000/twelve echoes equally spaced from 6.28 to 75.4 | NA = 1; FOV = 30 × 30 mm2; matrix size = 256 × 128 (reconstructed phase encoding steps = 128; acquisition phase encoding steps = 91); spatial resolution of 0.117 × 0.234 mm/pixel. 10 contiguous slices were acquired with respiration gating to cover the entire abdomen. Approximately 6 min acquisition time. |
| ADC | EPI | 2500/17.7 | Echo train length = 16; Pulse duration = 3.0 ms; Diffusion time = 7.46 ms; NA = 1; FOV = 30 × 30 mm2; matrix size = 128 × 128; spatial resolution of 0.234 × 0.234 mm/pixel; 8 b values (7, 47, 81, 126, 180, 234, 340, 549) s/mm2. Ten contiguous slices were acquired to cover the entire abdomen. Approximately 2 min 40 s acquisition time. |
| GagCEST | (1) RARE (2) RARE (3) RARE | (1) 2200/7 (2) 5000/7 (3) 5000/7 | (1) Center frequency estimate: Continuous-wave block saturation pulse with B1 = 3 μT and duration = 1 s; 25 frequency offsets from −360 Hz to 360Hz with an interval of 0.5 ppm (WASSR approach). FOV = 30 × 30 mm2; Matrix size = 128 × 128; Flip angle = 180°; NA = 1. A single, 1 mm slice delineating the tumor was acquired. (2) Frequency shift saturation: Six frequency offsets at ± 0.5, ± 1.0. ± 1.5 ppm were acquired through the same single slice using respiration gating with an off-resonance RF pulse applied for 1 s at a power of 3 μT. Matrix = 128 × 128 (reconstructed phase encoding steps = 128; acquisition phase encoding steps = 96); FOV = 30 × 30 mm2; rare factor = 8. (3) Control image: A control image was acquired through the same slice using the same settings as #2, except with saturation offset at 300 ppm.~19 min total acquisition time. |
| MTR | GRE | 625/2 | Flip angle = 30°; off-resonance frequency 7000 Hz; saturation pulse block pulse shape = 50 ms width and 10 µT amplitude; FOV = 30 × 30 mm2; matrix size = 256 × 256; spatial resolution of 0.117 × 0.117 mm/pixel. Ten contiguous images were acquired to cover the entire abdomen. Approximately 3 min acquisition time. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maloney, E.; DuFort, C.C.; Provenzano, P.P.; Farr, N.; Carlson, M.A.; Vohra, R.; Park, J.; Hingorani, S.R.; Lee, D. Non-Invasive Monitoring of Stromal Biophysics with Targeted Depletion of Hyaluronan in Pancreatic Ductal Adenocarcinoma. Cancers 2019, 11, 772. https://doi.org/10.3390/cancers11060772
Maloney E, DuFort CC, Provenzano PP, Farr N, Carlson MA, Vohra R, Park J, Hingorani SR, Lee D. Non-Invasive Monitoring of Stromal Biophysics with Targeted Depletion of Hyaluronan in Pancreatic Ductal Adenocarcinoma. Cancers. 2019; 11(6):772. https://doi.org/10.3390/cancers11060772
Chicago/Turabian StyleMaloney, Ezekiel, Christopher C. DuFort, Paolo P. Provenzano, Navid Farr, Markus A. Carlson, Ravneet Vohra, Joshua Park, Sunil R. Hingorani, and Donghoon Lee. 2019. "Non-Invasive Monitoring of Stromal Biophysics with Targeted Depletion of Hyaluronan in Pancreatic Ductal Adenocarcinoma" Cancers 11, no. 6: 772. https://doi.org/10.3390/cancers11060772
APA StyleMaloney, E., DuFort, C. C., Provenzano, P. P., Farr, N., Carlson, M. A., Vohra, R., Park, J., Hingorani, S. R., & Lee, D. (2019). Non-Invasive Monitoring of Stromal Biophysics with Targeted Depletion of Hyaluronan in Pancreatic Ductal Adenocarcinoma. Cancers, 11(6), 772. https://doi.org/10.3390/cancers11060772





