Mucosal Injury during Anti-Cancer Treatment: From Pathobiology to Bedside
Abstract
:1. Introduction
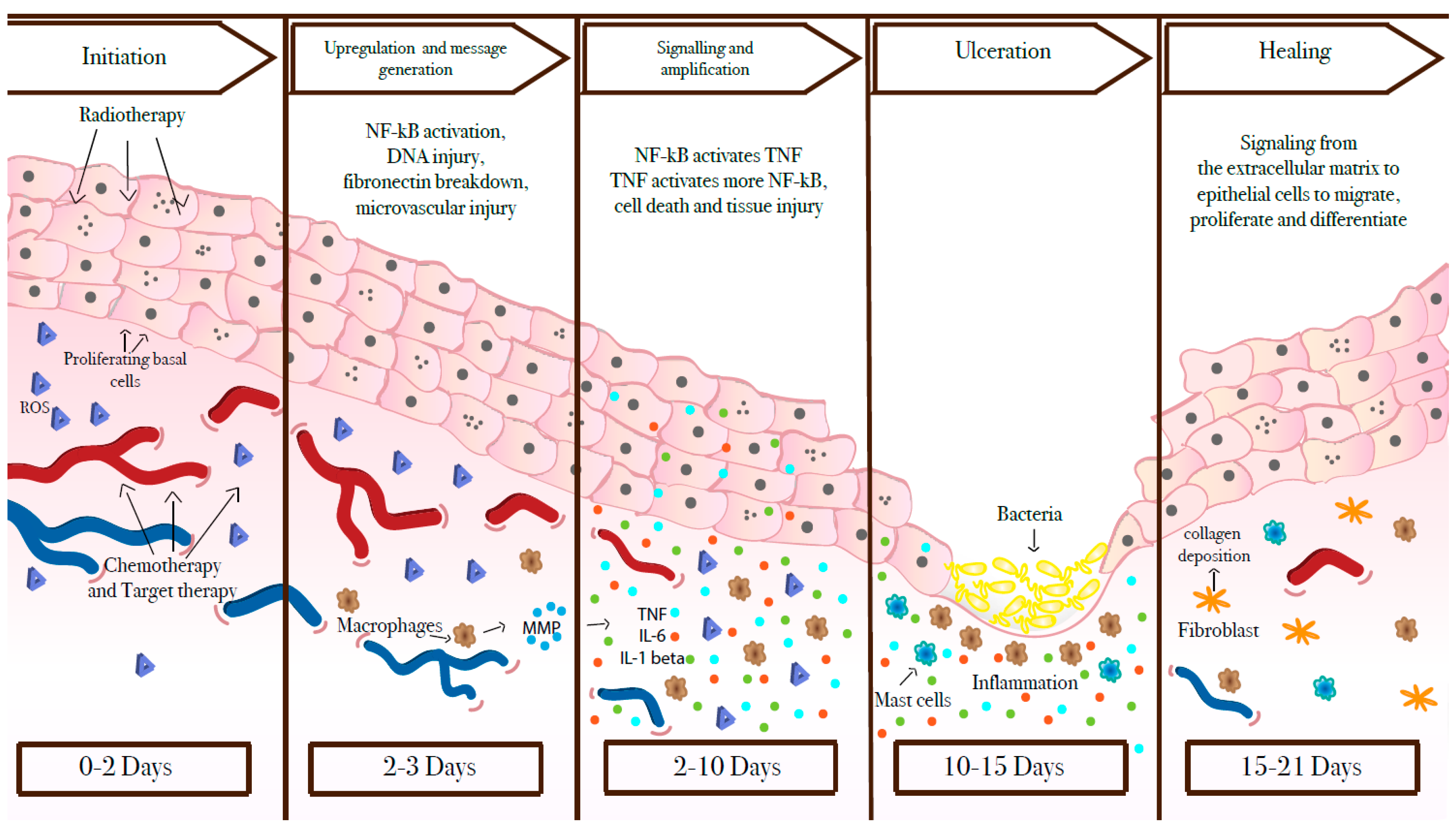
2. What Lies Beneath?
3. Risk Factors
3.1. Anti-Cancer Treatment
3.2. Genetic Polymorphisms
3.3. Role of Microbial Flora
3.4. Role of the Innate and Adaptive Response in Mucosal Injury
4. Prophylaxis
4.1. Basic Oral Care and Topic Therapies for OM and mTOR-Induced-Mucositis Prophylaxis
4.2. Physical Therapies for OM Prophylaxis
4.3. Systemic Therapies for OM and GIM Prophylaxis
5. Across Old and New Anti-Cancer Treatments
5.1. Chemotherapy Induced Mucositis
5.2. Radiotherapy-Induced Mucositis
5.3. Targeted Therapy-Induced Mucosal Injury
5.3.1. mTOR Inhibitors
5.3.2. EGFR/HER-1 Inhibitors
5.3.3. Anti-HER2 Agents
5.3.4. VEGF/VEGFR Inhibitors
5.3.5. BRAF Inhibitors
5.3.6. CDK4/6 Inhibitors
5.4. Immunotherapy
6. A Comprehensive Management
6.1. Pain Management
6.2. Treatment of Infections
6.3. Nutritional Assessment and Implementation
7. Conclusions
Funding
Conflicts of Interest
References
- Pico, J.-L.; Avila-Garavito, A.; Naccache, P. Mucositis: Its occurrence, consequences, and treatment in the oncology setting. Oncologist 1998, 3, 446–451. [Google Scholar]
- Bensinger, W.; Schubert, M.; Ang, K.K.; Brizel, D.; Brown, E.; Eilers, J.G.; Elting, L.; Mittal, B.B.; Schattner, M.A.; Speilberg, R.; et al. NCCN Task Force Report—Prevention and management of mucositis in cancer care. J. Natl. Compr. Cancer Netw. 2008, 6, S1–S21. [Google Scholar]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B.; et al. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, H.R.; Overgaard, J.; Specht, L.; Overgaard, M.; Johansen, J.; Evensen, J.F.; Andersen, L.J.; Andersen, E.; Grau, C. Prevalence and peak incidence of acute and late normal tissue morbidity in the DAHANCA 6&7 randomised trial with accelerated radiotherapy for head and neck cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2012, 103, 69–75. [Google Scholar]
- Blijlevens, N.; Schwenkglenks, M.; Bacon, P.; D’Addio, A.; Einsele, H.; Maertens, J.; Niederwieser, D.; Rabitsch, W.; Roosaar, A.; Ruutu, T.; et al. Prospective oral mucositis audit: Oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy--European Blood and Marrow Transplantation Mucositis Advisory Group. J. Clin. Oncol. 2008, 26, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Chaveli-López, B. Oral toxicity produced by chemotherapy: A systematic review. J. Clin. Exp. Dent. 2014, 6, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Villa, A.; Sonis, S.T. Mucositis: Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.C.; Modaffari, C.; Decembrino, N.; Zhou, F.X.; Zecca, M.; Defabianis, P. Preliminary study in a new protocol for the treatment of oral mucositis in pediatric patients undergoing hematopoietic stem cell transplantation (HSCT) and chemotherapy (CT). Lasers Med. Sci. 2017, 32, 1423–1428. [Google Scholar] [CrossRef]
- Lino, M.D.M.da.C.; de Carvalho, F.B.; de Oliveira, L.R.; Magalhães, E.B.; Pinheiro, A.L.B.; Ramalho, L.M.P. Laser phototherapy as a treatment for radiotherapy-induced oral mucositis. Braz. Dent. J. 2011, 22, 162–165. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, G.M.; Awde, J.D.; Ghandi, H.; Vincent, M.; Kocha, W.I. Risk factors associated with mucositis in cancer patients receiving 5-fluorouracil. Oral Oncol. 1998, 34, 484–490. [Google Scholar] [CrossRef]
- Vokurka, S.; Bystrická, E.; Koza, V.; Scudlová, J.; Pavlicová, V.; Valentová, D.; Visokaiová, M.; Misaniová, L. Higher incidence of chemotherapy induced oral mucositis in females: A supplement of multivariate analysis to a randomized multicentre study. Support. Care Cancer 2006, 14, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.M. Oral complications and management strategies for patients undergoing cancer therapy. ScientificWorldJournal 2014, 2014, 581795. [Google Scholar] [CrossRef] [PubMed]
- Elting, L.S.; Cooksley, C.D.; Chambers, M.S.; Garden, A.S. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Blijlevens, N.; Sonis, S. Palifermin (recombinant keratinocyte growth factor-1): A pleiotropic growth factor with multiple biological activities in preventing chemotherapy- and radiotherapy-induced mucositis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2007, 18, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.M.; Gibson, R.J.; Bowen, J.M.; Stringer, A.M.; Sonis, S.T.; Keefe, D.M.K. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: Implications for the pathobiology of mucositis. Cancer Chemother. Pharmacol. 2008, 62, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Boers-Doets, C.B.; Epstein, J.B.; Raber-Durlacher, J.E.; Ouwerkerk, J.; Logan, R.M.; Brakenhoff, J.A.; Lacouture, M.E.; Gelderblom, H. Oral adverse events associated with tyrosine kinase and mammalian target of rapamycin inhibitors in renal cell carcinoma: A structured literature review. Oncologist 2012, 17, 135–144. [Google Scholar] [CrossRef]
- Sonis, S.T. The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2002, 13, 380–389. [Google Scholar] [CrossRef]
- Logan, R.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. Nuclear factor-kappaB (NF-κB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol. 2007, 43, 395–401. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. Serum levels of NFκB and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol. Ther. 2008, 7, 1139–1145. [Google Scholar] [CrossRef]
- Boers-Doets, C.B.; Raber-Durlacher, J.E.; Treister, N.S.; Epstein, J.B.; Arends, A.B.P.; Wiersma, D.R.; Lalla, R.V.; Logan, R.M.; van Erp, N.P.; Gelderblom, H. Mammalian target of rapamycin inhibitor-associated stomatitis. Future Oncol. Lond. Engl. 2013, 9, 1883–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellani, P.; Balza, E.; Rubartelli, A. Inflammation, DAMPs, tumor development, and progression: A vicious circle orchestrated by redox signaling. Antioxid. Redox Signal. 2014, 20, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Yeoh, A.S.-J.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treat. Rev. 2007, 33, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Al-Dasooqi, N.; Gibson, R.J.; Bowen, J.M.; Logan, R.M.; Stringer, A.M.; Keefe, D.M. Matrix metalloproteinases are possible mediators for the development of alimentary tract mucositis in the dark agouti rat. Exp. Biol. Med. Maywood NJ 2010, 235, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, S.; Zecha, J.A.E.M.; Barasch, A.; de Lange, J.; Rozema, F.R.; Raber-Durlacher, J.E. Oral Mucositis Induced by Anticancer Therapies. Curr. Oral Health Rep. 2015, 2, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.A.; Goldberg, R.M.; Sargent, D.J.; Vargas-Chanes, D.; Nair, S.; Cha, S.S.; Novotny, P.J.; Poon, M.A.; O’Connell, M.J.; Loprinzi, C.L. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J. Clin. Oncol. 2002, 20, 1491–1498. [Google Scholar] [CrossRef]
- Chansky, K.; Benedetti, J.; Macdonald, J.S. Differences in toxicity between men and women treated with 5-fluorouracil therapy for colorectal carcinoma. Cancer 2005, 103, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.; Zanger, U.M.; Marx, C.; Schaeffeler, E.; Klein, K.; Dippon, J.; Kerb, R.; Blievernicht, J.; Fischer, J.; Hofmann, U.; et al. Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: A prospective clinical trial by the German 5-FU Toxicity Study Group. J. Clin. Oncol. 2008, 26, 2131–2138. [Google Scholar] [CrossRef]
- Sonis, S.T.; Sonis, A.L.; Lieberman, A. Oral complications in patients receiving treatment for malignancies other than of the head and neck. J. Am. Dent. Assoc. 1939 1978, 97, 468–472. [Google Scholar] [CrossRef]
- Zalcberg, J.; Kerr, D.; Seymour, L.; Palmer, M. Haematological and non-haematological toxicity after 5-fluorouracil and leucovorin in patients with advanced colorectal cancer is significantly associated with gender, increasing age and cycle number. Tomudex International Study Group. Eur. J. Cancer Oxf. Engl. 1998, 34, 1871–1875. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Baracos, V.E.; McCargar, L.J.; Mourtzakis, M.; Mulder, K.E.; Reiman, T.; Butts, C.A.; Scarfe, A.G.; Sawyer, M.B. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin. Cancer Res. 2007, 13, 3264–3268. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, U.; Henricks, L.M.; Offer, S.M.; Barbarino, J.; Schellens, J.H.M.; Swen, J.J.; Klein, T.E.; McLeod, H.L.; Caudle, K.E.; Diasio, R.B.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin. Pharmacol. Ther. 2018, 103, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Park, Y.S.; Kang, W.K.; Kim, J.-W.; Lee, S.-Y. Thymidylate synthase (TYMS) and dihydropyrimidine dehydrogenase (DPYD) polymorphisms in the Korean population for prediction of 5-fluorouracil-associated toxicity. Ther. Drug Monit. 2007, 29, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Robien, K.; Schubert, M.M.; Bruemmer, B.; Lloid, M.E.; Potter, J.D.; Ulrich, C.M. Predictors of oral mucositis in patients receiving hematopoietic cell transplants for chronic myelogenous leukemia. J. Clin. Oncol. 2004, 22, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Oka, M.; Yoshino, S.; Hazama, S.; Abe, T.; Okayama, N.; Hinoda, Y. Relation between cytokine promoter gene polymorphism and toxicity of 5-fluorouracil plus cisplatin chemotherapy. Oncol. Rep. 2006, 16, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonis, S.T. Regimen-related gastrointestinal toxicities in cancer patients. Curr. Opin. Support. Palliat. Care 2010, 4, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Bachour, P.C.; Sonis, S.T. Predicting mucositis risk associated with cytotoxic cancer treatment regimens: Rationale, complexity, and challenges. Curr. Opin. Support. Palliat. Care 2018, 12, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Stringer, A.M.; Logan, R.M. The role of oral flora in the development of chemotherapy-induced oral mucositis. J. Oral Pathol. Med. 2015, 44, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, M.A. A gastroenterologist’s guide to probiotics. Clin. Gastroenterol. Hepatol. 2012, 10, 960–968. [Google Scholar] [CrossRef]
- Zelia, M.G.; Raquel, D.; Caio, T.F.; Danielle, G.S. Mechanisms Underlying Chemotherapy-Associated Mucositis: The Role of Inflammatory Mediators and Potential Therapeutic Targets. Eur. Med. J. 2018, 7, 82–91. [Google Scholar]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley des Varannes, S.; Le Vacon, F.; de La Cochetière, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, G.; Freeze, H.H. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia 2009, 11, 615–628. [Google Scholar] [CrossRef]
- Sonis, S.T. Oral mucositis. Anticancer Drugs 2011, 22, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Wong, D.V.T.; Lima-Júnior, R.C.P.; Carvalho, C.B.M.; Borges, V.F.; Wanderley, C.W.S.; Bem, A.X.C.; Leite, C.A.V.G.; Teixeira, M.A.; Batista, G.L.P.; Silva, R.L.; et al. The Adaptor Protein Myd88 Is a Key Signaling Molecule in the Pathogenesis of Irinotecan-Induced Intestinal Mucositis. PLoS ONE 2015, 10, e0139985. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Wanderley, C.W.S.; Wong, D.V.T.; Mota, J.M.S.C.; Leite, C.A.V.G.; Souza, M.H.L.P.; Cunha, F.Q.; Lima-Júnior, R.C.P. Irinotecan- and 5-fluorouracil-induced intestinal mucositis: Insights into pathogenesis and therapeutic perspectives. Cancer Chemother. Pharmacol. 2016, 78, 881–893. [Google Scholar] [CrossRef]
- Lima-Júnior, R.C.P.; Freitas, H.C.; Wong, D.V.T.; Wanderley, C.W.S.; Nunes, L.G.; Leite, L.L.; Miranda, S.P.; Souza, M.H.L.P.; Brito, G.A.C.; Magalhães, P.J.C.; et al. Targeted inhibition of IL-18 attenuates irinotecan-induced intestinal mucositis in mice. Br. J. Pharmacol. 2014, 171, 2335–2350. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Wanderley, C.W.S.; Silva, C.M.S.; Muniz, H.A.; Teixeira, M.A.; Souza, N.R.P.; Cândido, A.G.F.; Falcão, R.B.; Souza, M.H.L.P.; Almeida, P.R.C.; et al. Role of regulatory T cells in irinotecan-induced intestinal mucositis. Eur. J. Pharm. Sci. Eur. Fed. Pharm. Sci. 2018, 115, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Guabiraba, R.; Besnard, A.G.; Menezes, G.B.; Secher, T.; Jabir, M.S.; Amaral, S.S.; Braun, H.; Lima-Junior, R.C.P.; Ribeiro, R.A.; Cunha, F.Q.; et al. IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. Mucosal Immunol. 2014, 7, 1079–1093. [Google Scholar] [CrossRef] [Green Version]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, D.E.; Boers-Doets, C.B.; Bensadoun, R.J.; Herrstedt, J. ESMO Guidelines Committee Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann. Oncol. 2015, 26, 139–151. [Google Scholar] [CrossRef]
- Rugo, H.S.; Seneviratne, L.; Beck, J.T.; Glaspy, J.A.; Peguero, J.A.; Pluard, T.J.; Dhillon, N.; Hwang, L.C.; Nangia, C.; Mayer, I.A.; et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): A single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 654–662. [Google Scholar] [CrossRef]
- Culy, C.R.; Spencer, C.M. Amifostine: An update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome. Drugs 2001, 61, 641–684. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Benson, R.; Rath, G.K. Radiation induced oral mucositis: A review of current literature on prevention and management. Eur. Arch. Oto-Rhino-Laryngol 2016, 273, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Silverman, S.; Paggiarino, D.A.; Crockett, S.; Schubert, M.M.; Senzer, N.N.; Lockhart, P.B.; Gallagher, M.J.; Peterson, D.E.; Leveque, F.G. Benzydamine HCl for prophylaxis of radiation-induced oral mucositis: Results from a multicenter, randomized, double-blind, placebo-controlled clinical trial. Cancer 2001, 92, 875–885. [Google Scholar] [CrossRef]
- Mahood, D.J.; Dose, A.M.; Loprinzi, C.L.; Veeder, M.H.; Athmann, L.M.; Therneau, T.M.; Sorensen, J.M.; Gainey, D.K.; Mailliard, J.A.; Gusa, N.L. Inhibition of fluorouracil-induced stomatitis by oral cryotherapy. J. Clin. Oncol. 1991, 9, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Cascinu, S.; Fedeli, A.; Fedeli, S.L.; Catalano, G. Oral cooling (cryotherapy), an effective treatment for the prevention of 5-fluorouracil-induced stomatitis. Eur. J. Cancer. B. Oral Oncol. 1994, 30, 234–236. [Google Scholar] [CrossRef]
- Sorensen, J.B.; Skovsgaard, T.; Bork, E.; Damstrup, L.; Ingeberg, S. Double-blind, placebo-controlled, randomized study of chlorhexidine prophylaxis for 5-fluorouracil-based chemotherapy-induced oral mucositis with nonblinded randomized comparison to oral cooling (cryotherapy) in gastrointestinal malignancies. Cancer 2008, 112, 1600–1606. [Google Scholar] [CrossRef]
- Riley, P.; Glenny, A.-M.; Worthington, H.V.; Littlewood, A.; Clarkson, J.E.; McCabe, M.G. Interventions for preventing oral mucositis in patients with cancer receiving treatment: Oral cryotherapy. Cochrane Database Syst. Rev. 2015, 23, CD011552. [Google Scholar]
- Barasch, A.; Peterson, D.E.; Tanzer, J.M.; D’Ambrosio, J.A.; Nuki, K.; Schubert, M.M.; Franquin, J.C.; Clive, J.; Tutschka, P. Helium-neon laser effects on conditioning-induced oral mucositis in bone marrow transplantation patients. Cancer 1995, 76, 2550–2556. [Google Scholar] [CrossRef]
- Cowen, D.; Tardieu, C.; Schubert, M.; Peterson, D.; Resbeut, M.; Faucher, C.; Franquin, J.C. Low energy Helium-Neon laser in the prevention of oral mucositis in patients undergoing bone marrow transplant: Results of a double blind randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 697–703. [Google Scholar] [CrossRef]
- Schubert, M.M.; Eduardo, F.P.; Guthrie, K.A.; Franquin, J.-C.; Bensadoun, R.-J.J.; Migliorati, C.A.; Lloid, C.M.E.; Eduardo, C.P.; Walter, N.-F.; Marques, M.M.; et al. A phase III randomized double-blind placebo-controlled clinical trial to determine the efficacy of low level laser therapy for the prevention of oral mucositis in patients undergoing hematopoietic cell transplantation. Support. Care Cancer 2007, 15, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.; da Motta Silveira, F.M.; de Orange, F.A. Low-level laser therapy prevents severe oral mucositis in patients submitted to hematopoietic stem cell transplantation: A randomized clinical trial. Support. Care Cancer 2016, 24, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Hashemi, S.; Epstein, J.B.; Nair, R.G.; Raber-Durlacher, J.E. Could the biological robustness of low level laser therapy (Photobiomodulation) impact its use in the management of mucositis in head and neck cancer patients. Oral Oncol. 2016, 54, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Van Sebille, Y.Z.A.; Stansborough, R.; Wardill, H.R.; Bateman, E.; Gibson, R.J.; Keefe, D.M. Management of Mucositis During Chemotherapy: From Pathophysiology to Pragmatic Therapeutics. Curr. Oncol. Rep. 2015, 17, 50. [Google Scholar] [CrossRef]
- Arbabi-kalati, F.; Arbabi-kalati, F.; Deghatipour, M.; Ansari Moghadam, A. Evaluation of the efficacy of zinc sulfate in the prevention of chemotherapy-induced mucositis: A double-blind randomized clinical trial. Arch. Iran. Med. 2012, 15, 413–417. [Google Scholar]
- Koukourakis, M.I.; Maltezos, E. Amifostine administration during radiotherapy for cancer patients with genetic, autoimmune, metabolic and other diseases. Anticancer. Drugs 2006, 17, 133–138. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Sarri, T.; Bowen, J.; Di Palma, M.; Kouloulias, V.E.; Niscola, P.; Riesenbeck, D.; Stokman, M.; Tissing, W.; Yeoh, E.; et al. Systematic review of amifostine for the management of oral mucositis in cancer patients. Support. Care Cancer 2013, 21, 357–364. [Google Scholar] [CrossRef]
- Prisciandaro, L.D.; Geier, M.S.; Butler, R.N.; Cummins, A.G.; Howarth, G.S. Probiotic factors partially improve parameters of 5-fluorouracil-induced intestinal mucositis in rats. Cancer Biol. Ther. 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Rahman, O.; ElHalawani, H.; Essam-Eldin, S. S-1-based regimens and the risk of oral and gastrointestinal mucosal injury: A meta-analysis with comparison to other fluoropyrimidines. Expert Opin. Drug Saf. 2016, 15, 5–20. [Google Scholar] [CrossRef]
- Wardill, H.R.; Bowen, J.M.; Al-Dasooqi, N.; Sultani, M.; Bateman, E.; Stansborough, R.; Shirren, J.; Gibson, R.J. Irinotecan disrupts tight junction proteins within the gut: Implications for chemotherapy-induced gut toxicity. Cancer Biol. Ther. 2014, 15, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Mancini, M.; Sonis, S.T.; Fernandez-Martinez, J.; Liu, J.; Cohen, E.E.W.; Toback, F.G. A Novel Peptide for Simultaneously Enhanced Treatment of Head and Neck Cancer and Mitigation of Oral Mucositis. PLoS ONE 2016, 11, e0152995. [Google Scholar] [CrossRef] [PubMed]
- Mayo, B.J.; Stringer, A.M.; Bowen, J.M.; Bateman, E.H.; Keefe, D.M. Irinotecan-induced mucositis: The interactions and potential role of GLP-2 analogues. Cancer Chemother. Pharmacol. 2017, 79, 233–249. [Google Scholar] [CrossRef]
- Keefe, D.M.; Elting, L.S.; Nguyen, H.T.; Grunberg, S.M.; Aprile, G.; Bonaventura, A.; Selva-Nayagam, S.; Barsevick, A.; Koczwara, B.; Sonis, S.T. Risk and outcomes of chemotherapy-induced diarrhea (CID) among patients with colorectal cancer receiving multi-cycle chemotherapy. Cancer Chemother. Pharmacol. 2014, 74, 675–680. [Google Scholar] [CrossRef]
- Hartmann, J.T.; Lipp, H.-P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef]
- Aprile, G.; Rihawi, K.; De Carlo, E.; Sonis, S.T. Treatment-related gastrointestinal toxicities and advanced colorectal or pancreatic cancer: A critical update. World J. Gastroenterol. 2015, 21, 11793–11803. [Google Scholar] [CrossRef] [PubMed]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Worthington, H.V.; Clarkson, J.E.; Bryan, G.; Furness, S.; Glenny, A.-M.; Littlewood, A.; McCabe, M.G.; Meyer, S.; Khalid, T. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst. Rev. 2011, 13, CD000978. [Google Scholar] [CrossRef]
- Dodd, M.J.; Dibble, S.L.; Miaskowski, C.; MacPhail, L.; Greenspan, D.; Paul, S.M.; Shiba, G.; Larson, P. Randomized clinical trial of the effectiveness of 3 commonly used mouthwashes to treat chemotherapy-induced mucositis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 90, 39–47. [Google Scholar] [CrossRef]
- Ala, S.; Saeedi, M.; Janbabai, G.; Ganji, R.; Azhdari, E.; Shiva, A. Efficacy of Sucralfate Mouth Wash in Prevention of 5-fluorouracil Induced Oral Mucositis: A Prospective, Randomized, Double-Blind, Controlled Trial. Nutr. Cancer 2016, 68, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Arash, A.; Somayeh, A.; Pardis, P.; Ahmad, R.M. Efficacy of Topical and Systemic Vitamin E in Preventing Chemotherapy-Induced Oral Mucositis. Rep. Radiother. Oncol. 2015, 2, e796. [Google Scholar]
- Cascinu, S.; Fedeli, A.; Fedeli, S.L.; Catalano, G. Octreotide versus loperamide in the treatment of fluorouracil-induced diarrhea: A randomized trial. J. Clin. Oncol. 1993, 11, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Goumas, P.; Naxakis, S.; Christopoulou, A.; Chrysanthopoulos, C.; Nikolopoulou, V.; Kalofonos, H.P. Octreotide acetate in the treatment of fluorouracil-induced diarrhea. Oncologist 1998, 3, 50–53. [Google Scholar]
- Cascinu, S.; Fedeli, A.; Fedeli, S.L.; Catalano, G. Control of chemotherapy-induced diarrhea with octreotide. A randomized trial with placebo in patients receiving cisplatin. Oncology 1994, 51, 70–73. [Google Scholar] [CrossRef]
- Zidan, J.; Haim, N.; Beny, A.; Stein, M.; Gez, E.; Kuten, A. Octreotide in the treatment of severe chemotherapy-induced diarrhea. Ann. Oncol. 2001, 12, 227–229. [Google Scholar] [CrossRef]
- Barbounis, V.; Koumakis, G.; Vassilomanolakis, M.; Demiri, M.; Efremidis, A.P. Control of irinotecan-induced diarrhea by octreotide after loperamide failure. Support. Care Cancer 2001, 9, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Giralt, J.; Harari, P.M.; Baselga, J.; Spencer, S.; Bell, D.; Raben, D.; Liu, J.; Schulten, J.; Ang, K.K.; et al. Association of human papillomavirus and p16 status with mucositis and dysphagia for head and neck cancer patients treated with radiotherapy with or without cetuximab: Assessment from a phase 3 registration trial. Eur. J. Cancer Oxf. Engl. 1990 2016, 64, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossi, P.; Bergamini, C.; Miceli, R.; Cova, A.; Orlandi, E.; Resteghini, C.; Locati, L.; Alfieri, S.; Imbimbo, M.; Granata, R.; et al. Salivary Cytokine Levels and Oral Mucositis in Head and Neck Cancer Patients Treated with Chemotherapy and Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 959–966. [Google Scholar] [CrossRef]
- Vera-Llonch, M.; Oster, G.; Hagiwara, M.; Sonis, S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer 2006, 106, 329–336. [Google Scholar] [CrossRef]
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef]
- McGuire, D.B.; Fulton, J.S.; Park, J.; Brown, C.G.; Correa, M.E.P.; Eilers, J.; Elad, S.; Gibson, F.; Oberle-Edwards, L.K.; Bowen, J.; et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients. Support. Care Cancer 2013, 21, 3165–3177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brizel, D.M.; Wasserman, T.H.; Henke, M.; Strnad, V.; Rudat, V.; Monnier, A.; Eschwege, F.; Zhang, J.; Russell, L.; Oster, W.; et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J. Clin. Oncol. 2000, 18, 3339–3345. [Google Scholar] [CrossRef] [PubMed]
- Büntzel, J.; Küttner, K.; Fröhlich, D.; Glatzel, M. Selective cytoprotection with amifostine in concurrent radiochemotherapy for head and neck cancer. Ann. Oncol. 1998, 9, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Sonis, S. Emerging therapies for the prevention and treatment of oral mucositis. Expert Opin. Emerg. Drugs 2014, 19, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, T.; Yamamoto, Y.; Wasa, M.; Takenaka, Y.; Nakahara, S.; Takagi, T.; Tsugane, M.; Hayashi, N.; Maeda, K.; Inohara, H.; et al. L-glutamine decreases the severity of mucositis induced by chemoradiotherapy in patients with locally advanced head and neck cancer: A double-blind, randomized, placebo-controlled trial. Oncol. Rep. 2015, 33, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, E.B.; Peterson, D.E.; Schubert, M.; Keefe, D.; McGuire, D.; Epstein, J.; Elting, L.S.; Fox, P.C.; Cooksley, C.; Sonis, S.T.; et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 2004, 100, 2026–2046. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, R.; Patel, F.; Dhar, A.; Sharma, S.C.; Ayyagari, S.; Aggarwal, R.; Goenka, M.K.; Gupta, B.D.; Mehta, S.K. Radiation-induced proctosigmoiditis. Prospective, randomized, double-blind controlled trial of oral sulfasalazine plus rectal steroids versus rectal sucralfate. Dig. Dis. Sci. 1991, 36, 103–107. [Google Scholar] [CrossRef]
- Martins, F.; de Oliveira, M.A.; Wang, Q.; Sonis, S.; Gallottini, M.; George, S.; Treister, N. A review of oral toxicity associated with mTOR inhibitor therapy in cancer patients. Oral Oncol. 2013, 49, 293–298. [Google Scholar] [CrossRef]
- Tian, T.; Li, X.; Zhang, J. mTOR Signaling in Cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. Int. J. Mol. Sci. 2019, 20, 755. [Google Scholar] [CrossRef]
- Bachelot, T.; Bourgier, C.; Cropet, C.; Ray-Coquard, I.; Ferrero, J.-M.; Freyer, G.; Abadie-Lacourtoisie, S.; Eymard, J.-C.; Debled, M.; Spaëth, D.; et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: A GINECO study. J. Clin. Oncol. 2012, 30, 2718–2724. [Google Scholar] [CrossRef]
- Shameem, R.; Lacouture, M.; Wu, S. Incidence and risk of high-grade stomatitis with mTOR inhibitors in cancer patients. Cancer Invest. 2015, 33, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Vigarios, E.; Epstein, J.B.; Sibaud, V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support. Care Cancer 2017, 25, 1713–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonis, S.; Treister, N.; Chawla, S.; Demetri, G.; Haluska, F. Preliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patients. Cancer 2010, 116, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Paplomata, E.; Zelnak, A.; O’Regan, R. Everolimus: Side effect profile and management of toxicities in breast cancer. Breast Cancer Res. Treat. 2013, 140, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.J.; Eilers, J.; Harriman, A.; Cashavelly, B.J.; Maxwell, C. Putting evidence into practice: Evidence-based interventions for the management of oral mucositis. Clin. J. Oncol. Nurs. 2008, 12, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Nikolaidi, A.; Athanassiadis, I.; Papadopoulou, E.; Sonis, S. Oral ulcers in patients with advanced breast cancer receiving everolimus: A case series report on clinical presentation and management. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 110–116. [Google Scholar] [CrossRef]
- Zecha, J.A.E.M.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.J.; Genot, M.-T.; et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: Part 2: Proposed applications and treatment protocols. Support. Care Cancer 2016, 24, 2793–2805. [Google Scholar] [CrossRef]
- Price, T.J.; Peeters, M.; Kim, T.W.; Li, J.; Cascinu, S.; Ruff, P.; Suresh, A.S.; Thomas, A.; Tjulandin, S.; Zhang, K.; et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): A randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014, 15, 569–579. [Google Scholar] [CrossRef]
- Cunningham, D.; Humblet, Y.; Siena, S.; Khayat, D.; Bleiberg, H.; Santoro, A.; Bets, D.; Mueser, M.; Harstrick, A.; Verslype, C.; et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004, 351, 337–345. [Google Scholar] [CrossRef]
- Sibaud, V.; Niec, R.E.; Schindler, K.; Busam, K.J.; Roché, H.; Modi, S.; Delord, J.P.; Lacouture, M.E. Ado-trastuzumab emtansine-associated telangiectasias in metastatic breast cancer: A case series. Breast Cancer Res. Treat. 2014, 146, 451–456. [Google Scholar] [CrossRef]
- Sibaud, V.; Vigarios, E.; Combemale, P.; Lamant, L.; Lacouture, M.E.; Lacaze, J.L.; Dalenc, F.; Delord, J.P. T-DM1-related telangiectasias: A potential role in secondary bleeding events. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; Troiano, G.; De Lillo, A.; Testa, N.F.; Lo Muzio, L. Stomatitis and VEGFR-Tyrosine Kinase Inhibitors (VR-TKIs): A Review of Current Literature in 4369 Patients. BioMed Res. Int. 2018, 2018, 5035217. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Kurtz, S.L.; Barysauskas, C.M.; Pilotte, A.P.; Wagner, A.J.; Treister, N.S. Oral adverse events in cancer patients treated with VEGFR-directed multitargeted tyrosine kinase inhibitors. Oral Oncol. 2015, 51, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Kollmannsberger, C.; Bjarnason, G.; Burnett, P.; Creel, P.; Davis, M.; Dawson, N.; Feldman, D.; George, S.; Hershman, J.; Lechner, T.; et al. Sunitinib in metastatic renal cell carcinoma: Recommendations for management of noncardiovascular toxicities. Oncologist 2011, 16, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Califano, R.; Tariq, N.; Compton, S.; Fitzgerald, D.A.; Harwood, C.A.; Lal, R.; Lester, J.; McPhelim, J.; Mulatero, C.; Subramanian, S.; et al. Expert Consensus on the Management of Adverse Events from EGFR Tyrosine Kinase Inhibitors in the UK. Drugs 2015, 75, 1335–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacouture, M.; Sibaud, V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am. J. Clin. Dermatol. 2018, 19, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krausova, M.; Korinek, V. Wnt signaling in adult intestinal stem cells and cancer. Cell. Signal. 2014, 26, 570–579. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Yu, S.; Liu, Q.; Yuan, X.; Mani, S.; Pestell, R.G.; Wu, K. Recent advances of highly selective CDK4/6 inhibitors in breast cancer. J. Hematol. Oncol. 2017, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2- Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef]
- Lasheen, S.; Shohdy, K.S.; Kassem, L.; Abdel-Rahman, O. Fatigue, alopecia and stomatitis among patients with breast cancer receiving cyclin-dependent kinase 4 and 6 inhibitors: A systematic review and meta-analysis. Expert Rev. Anticancer Ther. 2017, 17, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Thill, M.; Schmidt, M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther. Adv. Med. Oncol. 2018, 10, 1758835918793326. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Felice, K.M.D.; Loftus, E.V.; Khanna, S. Systematic review: Colitis associated with anti-CTLA-4 therapy. Aliment. Pharmacol. Ther. 2015, 42, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Horvat, T.Z.; Adel, N.G.; Dang, T.-O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, C.; Ribas, A.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Kefford, R.; Weber, J.S.; Joshua, A.M.; Hwu, W.-J.; Gangadhar, T.C.; et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet 2014, 384, 1109–1117. [Google Scholar] [CrossRef]
- Larkin, J.; Hodi, F.S.; Wolchok, J.D. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 1270–1271. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V.; Meyer, N.; Lamant, L.; Vigarios, E.; Mazieres, J.; Delord, J.P. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr. Opin. Oncol. 2016, 28, 254–263. [Google Scholar] [CrossRef]
- Beck, K.E.; Blansfield, J.A.; Tran, K.Q.; Feldman, A.L.; Hughes, M.S.; Royal, R.E.; Kammula, U.S.; Topalian, S.L.; Sherry, R.M.; Kleiner, D.; et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J. Clin. Oncol. 2006, 24, 2283–2289. [Google Scholar] [CrossRef]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. On behalf of the ESMO Guidelines Committee Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, 119–142. [Google Scholar] [CrossRef]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Göppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer Oxf. Engl. 1990 2016, 60, 190–209. [Google Scholar] [CrossRef]
- Collins, L.K.; Chapman, M.S.; Carter, J.B.; Samie, F.H. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr. Probl. Cancer 2017, 41, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Marthey, L.; Mateus, C.; Mussini, C.; Nachury, M.; Nancey, S.; Grange, F.; Zallot, C.; Peyrin-Biroulet, L.; Rahier, J.F.; Bourdier de Beauregard, M.; et al. Cancer Immunotherapy with Anti-CTLA-4 Monoclonal Antibodies Induces an Inflammatory Bowel Disease. J. Crohns Colitis 2016, 10, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.; Parker, S.M.; Siegel, J.; Chasalow, S.D.; Weber, J.; Galbraith, S.; Targan, S.R.; Wang, H.L. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010, 10, 11. [Google Scholar] [PubMed]
- Weber, J.; Thompson, J.A.; Hamid, O.; Minor, D.; Amin, A.; Ron, I.; Ridolfi, R.; Assi, H.; Maraveyas, A.; Berman, D.; et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin. Cancer Res. 2009, 15, 5591–5598. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Gorsky, M.; Cabay, R.J.; Day, T.; Gonsalves, W. Screening for and diagnosis of oral premalignant lesions and oropharyngeal squamous cell carcinoma: Role of primary care physicians. Can. Fam. Physician Med. Fam. Can. 2008, 54, 870–875. [Google Scholar]
- Leenstra, J.L.; Miller, R.C.; Qin, R.; Martenson, J.A.; Dornfeld, K.J.; Bearden, J.D.; Puri, D.R.; Stella, P.J.; Mazurczak, M.A.; Klish, M.D.; et al. Doxepin rinse versus placebo in the treatment of acute oral mucositis pain in patients receiving head and neck radiotherapy with or without chemotherapy: A phase III, randomized, double-blind trial (NCCTG-N09C6 [Alliance]). J. Clin. Oncol. 2014, 32, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-P.; Wu, S.-G.; Zhou, J.; Feng, H.-X.; Li, F.-Y.; Wu, Y.-J.; Sun, J.-Y.; He, Z.-Y. Transdermal fentanyl for pain due to chemoradiotherapy-induced oral mucositis in nasopharyngeal cancer patients: Evaluating efficacy, safety, and improvement in quality of life. Drug Des. Devel. Ther. 2014, 8, 497–503. [Google Scholar] [PubMed]
- Orvain, C.; Moles-Moreau, M.P.; François, S.; Mercier, M.; Moal, F.; Hamel, J.F.; Parot-Schinkel, E.; Ifrah, N.; Hunault-Berger, M.; Tanguy-Schmidt, A. Miconazole mucoadhesive buccal tablet in high-dose therapy with autologous stem cell transplantation (HDT/ASCT)-induced mucositis. Support. Care Cancer 2015, 23, 359–364. [Google Scholar] [CrossRef]
- Rao, N.G.; Han, G.; Greene, J.N.; Tanvetyanon, T.; Kish, J.A.; De Conti, R.C.; Chuong, M.D.; Shridhar, R.; Biagioli, M.C.; Caudell, J.J.; et al. Effect of prophylactic fluconazole on oral mucositis and candidiasis during radiation therapy for head-and-neck cancer. Pract. Radiat. Oncol. 2013, 3, 229–233. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. Edinb. Scotl. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Caccialanza, R.; Pedrazzoli, P.; Cereda, E.; Gavazzi, C.; Pinto, C.; Paccagnella, A.; Beretta, G.D.; Nardi, M.; Laviano, A.; Zagonel, V. Nutritional Support in Cancer Patients: A Position Paper from the Italian Society of Medical Oncology (AIOM) and the Italian Society of Artificial Nutrition and Metabolism (SINPE). J. Cancer 2016, 7, 131–135. [Google Scholar] [CrossRef] [PubMed]



| Intervention | Aim | Treatment Setting | Ref. |
|---|---|---|---|
| Prevention of OM | |||
| Basic oral care protocol | Prevention of OM | All cancer treatments | [50,51] |
| Oral cryotherapy | Prevention of OM | Bolus 5-FU chemotherapy HD melphalan ± TB-RT for HSCT | [50,51] [50,51] |
| Benzydamine mouthwash | Prevention of OM | RT for HN cancer patients | [50,51] |
| Photobiomodulation (PBM) | Prevention of OM | HDCT ± TB-RT for HSCT RT for HN cancer patients | [50,51] [50,51] |
| Palifermin | Prevention of OM | HDCT and TB-RT for HSCT | [50,51] |
| Zinc supplements | Prevention of OM | RT or CT | [50,51] |
| Prevention of mIAS | |||
| Dexamethasone-containing mouthwashes | Prevention of mIAS | BC patients treated with everolimus | [52] |
| Prevention of GIM | |||
| Amifostine | Prevention of xerostomia | Post-operative RT for HN cancer patients | [53] |
| Prevention of proctitis | RT for pelvic malignancy | [50,51] | |
| Prevention of esophagitis | Concomitant CT-RT in NSCLC | [50,51] | |
| Sulfasalazine | Prevention of enteropathy | Pelvis RT | [50,51] |
| Probiotics | Prevention of diarrhea | CT and/or RT for pelvic malignancy | [50,51] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basile, D.; Di Nardo, P.; Corvaja, C.; Garattini, S.K.; Pelizzari, G.; Lisanti, C.; Bortot, L.; Da Ros, L.; Bartoletti, M.; Borghi, M.; et al. Mucosal Injury during Anti-Cancer Treatment: From Pathobiology to Bedside. Cancers 2019, 11, 857. https://doi.org/10.3390/cancers11060857
Basile D, Di Nardo P, Corvaja C, Garattini SK, Pelizzari G, Lisanti C, Bortot L, Da Ros L, Bartoletti M, Borghi M, et al. Mucosal Injury during Anti-Cancer Treatment: From Pathobiology to Bedside. Cancers. 2019; 11(6):857. https://doi.org/10.3390/cancers11060857
Chicago/Turabian StyleBasile, Debora, Paola Di Nardo, Carla Corvaja, Silvio Ken Garattini, Giacomo Pelizzari, Camilla Lisanti, Lucia Bortot, Lucia Da Ros, Michele Bartoletti, Matteo Borghi, and et al. 2019. "Mucosal Injury during Anti-Cancer Treatment: From Pathobiology to Bedside" Cancers 11, no. 6: 857. https://doi.org/10.3390/cancers11060857
APA StyleBasile, D., Di Nardo, P., Corvaja, C., Garattini, S. K., Pelizzari, G., Lisanti, C., Bortot, L., Da Ros, L., Bartoletti, M., Borghi, M., Gerratana, L., Lombardi, D., & Puglisi, F. (2019). Mucosal Injury during Anti-Cancer Treatment: From Pathobiology to Bedside. Cancers, 11(6), 857. https://doi.org/10.3390/cancers11060857







