Targeted Radionuclide Therapy for Patients with Metastatic Pheochromocytoma and Paraganglioma: From Low-Specific-Activity to High-Specific-Activity Iodine-131 Metaiodobenzylguanidine
Abstract
1. Introduction
2. I-131-MIBG
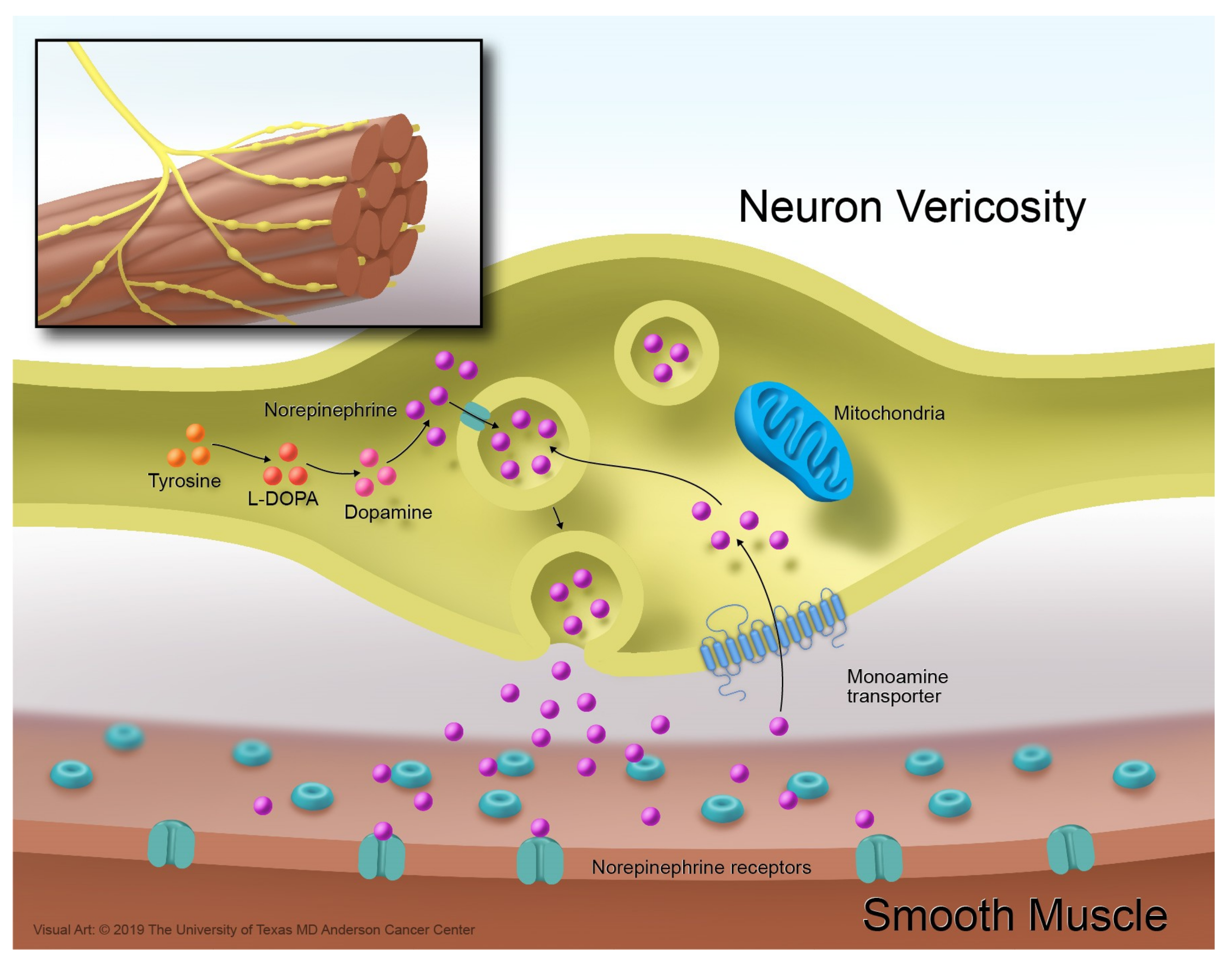
3. The Norepinephrine Transporter
4. Classification of MIBG Therapy Based on Manufacturing Aspects
5. Patient Preparation
6. LSA-I-131-MIBG for the Treatment of Patients with MPPG
6.1. Retrospective Studies with Low Doses of LSA-I-131-MIBG
6.2. Retrospective Studies with Intermediate Doses of LSA-I-131-MIBG
6.3. Prospective Clinical Trial with High-Dose LSA-I-131-MIBG
6.4. Remarks on the Retrospective and Prospective Studies with LSA-I-131-MIBG
7. HSA-I-131-MIBG for the Treatment of Patients with MPPG
7.1. Phase 1 Clinical Trials with HSA-I-131-MIBG
7.2. Phase 2 Clinical Trial
7.3. Biodistribution, Dosimetry, and Therapeutic Administration
7.4. LSA-I-131-MIBG vs. HSA-I-131-MIBG in the US
8. Future Directions
8.1. Surgery and I-131-MIBG
8.2. Chemotherapy and I-131-MIBG
8.3. Tyrosine Kinase Inhibitors and I-131-MIBG
8.4. Other Radiopharmaceutical Agents and I-131-MIBG
8.5. Immunotherapy and I-131-MIBG
8.6. Retreatment with I-131-MIBG
8.7. Other Radiopharmaceuticals that Target the NET
8.8. Other Indications for I-131-MIBG
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lam, A.K. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr. Pathol. 2017, 28, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yao, Z.; Zhu, X.; Li, Z.; Jiang, Y.; Wang, R.; Wen, N. Risk factors for postoperative cardiovascular morbidity after pheochromocytoma surgery: A large single center retrospective analysis. Endocr. J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yao, Z.; Zhu, X.; Li, Z.; Jiang, Y.; Wang, R.; Wu, B. Risk factors for postoperative severe morbidity after pheochromocytoma surgery: A single center retrospective analysis of 262 patients. Int. J. Surg. 2018, 60, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Thosani, S.; Ayala-Ramirez, M.; Roman-Gonzalez, A.; Zhou, S.; Thosani, N.; Bisanz, A.; Jimenez, C. Constipation: An overlooked, unmanaged symptom of patients with pheochromocytoma and sympathetic paraganglioma. Eur. J. Endocrinol. 2015, 173, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.W.; Poste, J.; Kunal, M.; Schwarcz, M.; Weiss, I. Cardiovascular Manifestations of Pheochromocytoma. Cardiol. Rev. 2017, 25, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Manger, W.M. The protean manifestations of pheochromocytoma. Horm. Metab. Res. 2009, 41, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Buffet, A.; Ben Aim, L.; Leboulleux, S.; Drui, D.; Vezzosi, D.; Libe, R.; Ajzenberg, C.; Bernardeschi, D.; Cariou, B.; Chabolle, F.; et al. Positive Impact of Genetic Test on the Management and Outcome of Patients With Paraganglioma and/or Pheochromocytoma. J. Clin. Endocrinol. Metab. 2019, 104, 1109–1118. [Google Scholar] [CrossRef]
- Falhammar, H.; Kjellman, M.; Calissendorff, J. Initial clinical presentation and spectrum of pheochromocytoma: A study of 94 cases from a single center. Endocr. Connect. 2018, 7, 186–192. [Google Scholar] [CrossRef]
- Ayala-Ramirez, M.; Feng, L.; Johnson, M.M.; Ejaz, S.; Habra, M.A.; Rich, T.; Busaidy, N.; Cote, G.J.; Perrier, N.; Phan, A.; et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: Primary tumor size and primary tumor location as prognostic indicators. J. Clin. Endocrinol. Metab. 2011, 96, 717–725. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: Basel, Switzerland, 2017. [Google Scholar]
- Hescot, S.; Curras-Freixes, M.; Deutschbein, T.; Van Berkel, A.; Vezzosi, D.; Amar, L.; De La Fouchardiere, C.; Valdes, N.; Riccardi, N.; Do Cao, C.; et al. Prognosis of malignant pheochromocytoma and paraganglioma (MAPP-Prono study): An ENS@T retrospective study. J. Clin. Endocrinol. Metab. 2019, 104, 2367–2374. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Kwak, M.K.; Lee, S.E.; Ahn, S.H.; Kim, H.; Suh, S.; Kim, B.J.; Song, K.H.; Koh, J.M.; Kim, J.H.; et al. A clinical prediction model to estimate the metastatic potential of pheochromocytoma/paraganglioma: ASES score. Surgery 2018, 164, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: A clinicopathologic and immunophenotypic study of 100 cases. Am. J. Surg. Pathol. 2002, 26, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Tischler, A.S.; Lloyd, R.V.; DeLellis, R.A.; de Krijger, R.; van Nederveen, F.; Nose, V. Observer variation in the application of the Pheochromocytoma of the Adrenal Gland Scaled Score. Am. J. Surg. Pathol. 2009, 33, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ramirez, M.; Palmer, J.L.; Hofmann, M.C.; de la Cruz, M.; Moon, B.S.; Waguespack, S.G.; Habra, M.A.; Jimenez, C. Bone metastases and skeletal-related events in patients with malignant pheochromocytoma and sympathetic paraganglioma. J. Clin. Endocrinol. Metab. 2013, 98, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Rohren, E.; Habra, M.A.; Rich, T.; Jimenez, P.; Ayala-Ramirez, M.; Baudin, E. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr. Oncol. Rep. 2013, 15, 356–371. [Google Scholar] [CrossRef]
- Hescot, S.; Leboulleux, S.; Amar, L.; Vezzosi, D.; Borget, I.; Bournaud-Salinas, C.; De La Fouchardiere, C.; Libe, R.; Do Cao, C.; Niccoli, P.; et al. One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J. Clin. Endocrinol. Metab. 2013, 98, 4006–4012. [Google Scholar] [CrossRef]
- Hamidi, O.; Young, W.F., Jr.; Iniguez-Ariza, N.M.; Kittah, N.E.; Gruber, L.; Bancos, C.; Tamhane, S.; Bancos, I. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. J. Clin. Endocrinol. Metab. 2017, 102, 3296–3305. [Google Scholar] [CrossRef]
- Sisson, J.; Shapiro, B.; Beierwaltes, W.H.; Nakajo, M.; Glowniak, J.; Mangner, T.; Carey, J.E.; Swanson, D.P.; Copp, J.; Satterlee, W.; et al. Treatment of malignant pheochromocytoma with a new radiopharmaceutical. Trans. Assoc. Am. Physicians 1983, 96, 209–217. [Google Scholar]
- Coleman, R.E.; Stubbs, J.B.; Barrett, J.A.; De La Guardia, M.; Lafrance, N.; Babich, J.W. Radiation dosimetry, pharmacokinetics, and safety of ultratrace Iobenguane I-131 in patients with malignant pheochromocytoma/paraganglioma or metastatic carcinoid. Cancer Biother. Radiopharm. 2009, 24, 469–475. [Google Scholar] [CrossRef]
- Owens, J.; Bolster, A.A.; Prosser, J.E.; Cunningham, S.; Mairs, R.J.; Neilly, J.B.; Reed, N.S.; Hilditch, T.E. No-carrier-added 123I-MIBG: An initial clinical study in patients with phaeochromocytoma. Nucl. Med. Commun. 2000, 21, 437–440. [Google Scholar] [CrossRef]
- Pryma, D.A.; Chin, B.B.; Noto, R.B.; Dillon, J.S.; Perkins, S.; Solnes, L.; Kostakoglu, L.; Serafini, A.N.; Pampaloni, M.H.; Jensen, J.; et al. Efficacy and Safety of High-Specific-Activity I-131 MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. J. Nucl. Med. 2018, 60, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Wieland, D.M.; Wu, J.; Brown, L.E.; Mangner, T.J.; Swanson, D.P.; Beierwaltes, W.H. Radiolabeled adrenergi neuron-blocking agents: Adrenomedullary imaging with [131I]iodobenzylguanidine. J. Nucl. Med. 1980, 21, 349–353. [Google Scholar] [PubMed]
- Wieland, D.M.; Swanson, D.P.; Brown, L.E.; Beierwaltes, W.H. Imaging the adrenal medulla with an I-131-labeled antiadrenergic agent. J. Nucl. Med. 1979, 20, 155–158. [Google Scholar] [PubMed]
- Sisson, J.C.; Wieland, D.M. Radiolabeled meta-iodobenzylguanidine: Pharmacology and clinical studies. Am. J. Physiol. Imaging 1986, 1, 96–103. [Google Scholar] [PubMed]
- Wieland, D.M.; Brown, L.E.; Tobes, M.C.; Rogers, W.L.; Marsh, D.D.; Mangner, T.J.; Swanson, D.P.; Beierwaltes, W.H. Imaging the primate adrenal medulla with [123I] and [131I] meta-iodobenzylguanidine: Concise communication. J. Nucl. Med. 1981, 22, 358–364. [Google Scholar] [PubMed]
- Carrasquillo, J.A.; Pandit-Taskar, N.; Chen, C.C. I-131 Metaiodobenzylguanidine Therapy of Pheochromocytoma and Paraganglioma. Semin. Nucl. Med. 2016, 46, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Rangarajan, V.; Shah, S.; Puranik, A.; Purandare, N. MIBG (metaiodobenzylguanidine) theranostics in pediatric and adult malignancies. Br. J. Radiol. 2018, 91, 20180103. [Google Scholar] [CrossRef]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-Ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients from 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest (Version 1.4). Available online: http://physics.nist.gov/xaamdi (accessed on 4 June 2019).
- Jimenez, C. Treatment for Patients With Malignant Pheochromocytomas and Paragangliomas: A Perspective From the Hallmarks of Cancer. Front. Endocrinol. 2018, 9, 277. [Google Scholar] [CrossRef]
- Bennett, M.R.; Cheung, A.; Brain, K.L. Sympathetic neuromuscular transmission at a varicosity in a syncytium. Microsc. Res. Tech. 1998, 42, 433–450. [Google Scholar] [CrossRef]
- Dodge, J.T.; Bevan, R.D.; Bevan, J.A. Comparison of density of sympathetic varicosities and their closeness to smooth muscle cells in rabbit middle cerebral and ear arteries and their branches. Circ. Res. 1994, 75, 916–925. [Google Scholar] [CrossRef]
- Furness, J.B.; Marshall, J.M. Correlation of the directly observed responses of mesenteric vessles of the rat to nerve stimulation and noradrenaline with the distribution of adrenergic nerves. J. Physiol. 1974, 239, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Takiyyuddin, M.A.; Brown, M.R.; Dinh, T.Q.; Cervenka, J.H.; Braun, S.D.; Parmer, R.J.; Kennedy, B.; O’Connor, D.T. Sympatho-adrenal secretion in humans: Factors governing catecholamine and storage vesicle peptide co-release. J. Auton. Pharm. 1994, 14, 187–200. [Google Scholar] [CrossRef]
- Aggarwal, S.; Mortensen, O.V. Overview of Monoamine Transporters. Curr. Protoc. Pharm. 2017, 79, 12. [Google Scholar] [CrossRef]
- Rudnick, G.; Kramer, R.; Blakely, R.D.; Murphy, D.L.; Verrey, F. The SLC6 transporters: Perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflug. Arch. 2014, 466, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. The synaptic vesicle cycle: A cascade of protein-protein interactions. Nature 1995, 375, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Castellani, M.R.; Seregni, E.; Maccauro, M.; Chiesa, C.; Aliberti, G.; Orunesu, E.; Bombardieri, E. MIBG for diagnosis and therapy of medullary thyroid carcinoma: Is there still a role? Q. J. Nucl. Med. Mol. Imaging 2008, 52, 430–440. [Google Scholar] [PubMed]
- Ezziddin, S.; Sabet, A.; Logvinski, T.; Alkawaldeh, K.; Yong-Hing, C.J.; Ahmadzadehfar, H.; Grunwald, F.; Biersack, H.J. Long-term outcome and toxicity after dose-intensified treatment with 131I-MIBG for advanced metastatic carcinoid tumors. J. Nucl. Med. 2013, 54, 2032–2038. [Google Scholar] [CrossRef][Green Version]
- Sisson, J.C.; Frager, M.S.; Valk, T.W.; Gross, M.D.; Swanson, D.P.; Wieland, D.M.; Tobes, M.C.; Beierwaltes, W.H.; Thompson, N.W. Scintigraphic localization of pheochromocytoma. N. Engl. J. Med. 1981, 305, 12–17. [Google Scholar] [CrossRef]
- Tan, T.H.; Hussein, Z.; Saad, F.F.; Shuaib, I.L. Diagnostic Performance of (68)Ga-DOTATATE PET/CT, (18)F-FDG PET/CT and (131)I-MIBG Scintigraphy in Mapping Metastatic Pheochromocytoma and Paraganglioma. Nucl. Med. Mol. Imaging 2015, 49, 143–151. [Google Scholar] [CrossRef]
- Maurice, J.B.; Troke, R.; Win, Z.; Ramachandran, R.; Al-Nahhas, A.; Naji, M.; Dhillo, W.; Meeran, K.; Goldstone, A.P.; Martin, N.M.; et al. A comparison of the performance of (6)(8)Ga-DOTATATE PET/CT and (1)(2)(3)I-MIBG SPECT in the diagnosis and follow-up of phaeochromocytoma and paraganglioma. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1266–1270. [Google Scholar] [CrossRef]
- Van Berkel, A.; Rao, J.U.; Lenders, J.W.; Pellegata, N.S.; Kusters, B.; Piscaer, I.; Hermus, A.R.; Plantinga, T.S.; Langenhuijsen, J.F.; Vriens, D.; et al. Semiquantitative 123I-Metaiodobenzylguanidine Scintigraphy to Distinguish Pheochromocytoma and Paraganglioma from Physiologic Adrenal Uptake and Its Correlation with Genotype-Dependent Expression of Catecholamine Transporters. J. Nucl. Med. 2015, 56, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Gonias, S.; Goldsby, R.; Matthay, K.K.; Hawkins, R.; Price, D.; Huberty, J.; Damon, L.; Linker, C.; Sznewajs, A.; Shiboski, S.; et al. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J. Clin. Oncol. 2009, 27, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Bomanji, J.; Levison, D.A.; Flatman, W.D.; Horne, T.; Bouloux, P.M.; Ross, G.; Britton, K.E.; Besser, G.M. Uptake of iodine-123 MIBG by pheochromocytomas, paragangliomas, and neuroblastomas: A histopathological comparison. J. Nucl. Med. 1987, 28, 973–978. [Google Scholar] [PubMed]
- Barrett, J.A.; Joyal, J.L.; Hillier, S.M.; Maresca, K.P.; Femia, F.J.; Kronauge, J.F.; Boyd, M.; Mairs, R.J.; Babich, J.W. Comparison of high-specific-activity ultratrace 123/131I-MIBG and carrier-added 123/131I-MIBG on efficacy, pharmacokinetics, and tissue distribution. Cancer Biother. Radiopharm. 2010, 25, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; Zalutsky, M.R. No-carrier-added synthesis of meta-[131I]iodobenzylguanidine. Appl. Radiat. Isot. 1993, 44, 621–628. [Google Scholar] [CrossRef]
- Bombardieri, E.; Giammarile, F.; Aktolun, C.; Baum, R.P.; Bischof Delaloye, A.; Maffioli, L.; Moncayo, R.; Mortelmans, L.; Pepe, G.; Reske, S.N.; et al. 131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: Procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Baudin, E.; Habra, M.A.; Deschamps, F.; Cote, G.; Dumont, F.; Cabanillas, M.; Arfi-Roufe, J.; Berdelou, A.; Moon, B.; Al Ghuzlan, A.; et al. Therapy of endocrine disease: Treatment of malignant pheochromocytoma and paraganglioma. Eur. J. Endocrinol. 2014, 171, R111–R122. [Google Scholar] [CrossRef]
- Fischer, M.; Vetter, W.; Winterberg, B.; Zidek, W.; Hengstmann, J.; Vetter, H. Diagnosis and treatment of phaeochromocytoma with 131I-metaiodobenzylguanidine. J. Hypertens Suppl. 1984, 2, S187–S189. [Google Scholar]
- Loh, K.C.; Fitzgerald, P.A.; Matthay, K.K.; Yeo, P.P.; Price, D.C. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): A comprehensive review of 116 reported patients. J. Endocrinol. Investig. 1997, 20, 648–658. [Google Scholar] [CrossRef]
- Charbonnel, B.; Chatal, J.F.; Brendel, A.J.; Lanehche, B.; Lumbroso, J.; Marchandise, X.; Mornex, R.; Schlumberger, M.; Wemeau, J.L. [Treatment of malignant pheochromocytoma by 131-I-metaiodobenzylguanidine]. Ann. Endocrinol. 1988, 49, 344–347. [Google Scholar]
- Krempf, M.; Lumbroso, J.; Mornex, R.; Brendel, A.J.; Wemeau, J.L.; Delisle, M.J.; Aubert, B.; Carpentier, P.; Fleury-Goyon, M.C.; Gibold, C.; et al. Use of m-[131I]iodobenzylguanidine in the treatment of malignant pheochromocytoma. J. Clin. Endocrinol. Metab. 1991, 72, 455–461. [Google Scholar] [CrossRef]
- Lewington, V.J.; Zivanovic, M.A.; Tristam, M.; McEwan, A.J.; Ackery, D.M. Radiolabelled metaiodobenzylguanidine targeted radiotherapy for malignant phaeochromocytoma. J. Nucl. Biol. Med. 1991, 35, 280–283. [Google Scholar] [PubMed]
- Schlumberger, M.; Gicquel, C.; Lumbroso, J.; Tenenbaum, F.; Comoy, E.; Bosq, J.; Fonseca, E.; Ghillani, P.P.; Aubert, B.; Travagli, J.P.; et al. Malignant pheochromocytoma: Clinical, biological, histologic and therapeutic data in a series of 20 patients with distant metastases. J. Endocrinol. Investig. 1992, 15, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Sakahara, H.; Endo, K.; Saga, T.; Hosono, M.; Kobayashi, H.; Konishi, J. 131I-metaiodobenzylguanidine therapy for malignant pheochromocytoma. Ann. Nucl. Med. 1994, 8, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Gedik, G.K.; Hoefnagel, C.A.; Bais, E.; Olmos, R.A. 131I-MIBG therapy in metastatic phaeochromocytoma and paraganglioma. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 725–733. [Google Scholar] [CrossRef]
- Shilkrut, M.; Bar-Deroma, R.; Bar-Sela, G.; Berniger, A.; Kuten, A. Low-dose iodine-131 metaiodobenzylguanidine therapy for patients with malignant pheochromocytoma and paraganglioma: Single center experience. Am. J. Clin. Oncol. 2010, 33, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Taki, J.; Inaki, A.; Nakamura, A.; Kayano, D.; Fukuoka, M.; Matsuo, S.; Nakajima, K.; Kinuya, S. Prognostic values of initial responses to low-dose (131)I-MIBG therapy in patients with malignant pheochromocytoma and paraganglioma. Ann. Nucl. Med. 2013, 27, 839–846. [Google Scholar] [CrossRef]
- Safford, S.D.; Coleman, R.E.; Gockerman, J.P.; Moore, J.; Feldman, J.M.; Leight, G.S., Jr.; Tyler, D.S.; Olson, J.A., Jr. Iodine-131 metaiodobenzylguanidine is an effective treatment for malignant pheochromocytoma and paraganglioma. Surgery 2003, 134, 956–962. [Google Scholar] [CrossRef]
- Castellani, M.R.; Seghezzi, S.; Chiesa, C.; Aliberti, G.L.; Maccauro, M.; Seregni, E.; Orunesu, E.; Luksch, R.; Bombardieri, E. (131)I-MIBG treatment of pheochromocytoma: Low versus intermediate activity regimens of therapy. Q. J. Nucl. Med. Mol. Imaging 2010, 54, 100–113. [Google Scholar]
- Porzig, A.; Matthay, K.K.; Dubois, S.; Pampaloni, M.; Damon, L.; Hawkins, R.; Goldsby, R.; Hollinger, F.; Fitzgerald, P. Proteinuria in metastatic pheochromocytoma is associated with an increased risk of Acute Respiratory Distress Syndrome, spontaneously or after therapy with 131I-meta-iodobenzylguanidine (131I-MIBG). Horm. Metab. Res. 2012, 44, 539–542. [Google Scholar] [CrossRef]
- Plouin, P.F.; Fitzgerald, P.; Rich, T.; Ayala-Ramirez, M.; Perrier, N.D.; Baudin, E.; Jimenez, C. Metastatic pheochromocytoma and paraganglioma: Focus on therapeutics. Horm. Metab. Res. 2012, 44, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Van Hulsteijn, L.T.; Niemeijer, N.D.; Dekkers, O.M.; Corssmit, E.P. (131)I-MIBG therapy for malignant paraganglioma and phaeochromocytoma: Systematic review and meta-analysis. Clin. Endocrinol. 2014, 80, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Szalat, A.; Fraenkel, M.; Doviner, V.; Salmon, A.; Gross, D.J. Malignant pheochromocytoma: Predictive factors of malignancy and clinical course in 16 patients at a single tertiary medical center. Endocrine 2011, 39, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, L.; Bonner, L.; Torigian, D.A.; Nathanson, K.L.; Cohen, D.L.; Pryma, D.; Cengel, K.A. External beam radiation therapy (EBRT) for patients with malignant pheochromocytoma and non-head and -neck paraganglioma: Combination with 131I-MIBG. Horm. Metab. Res. 2012, 44, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Mairs, R.J.; Cunningham, S.H.; Russell, J.; Armour, A.; Owens, J.; McKellar, K.; Gaze, M.N. No-carrier-added iodine-131-MIBG: Evaluation of a therapeutic preparation. J. Nucl. Med. 1995, 36, 1088–1095. [Google Scholar] [PubMed]
- Mairs, R.J.; Russell, J.; Cunningham, S.; O’Donoghue, J.A.; Gaze, M.N.; Owens, J.; Vaidyanathan, G.; Zalutsky, M.R. Enhanced tumour uptake and in vitro radiotoxicity of no-carrier-added [131I]meta-iodobenzylguanidine: Implications for the targeted radiotherapy of neuroblastoma. Eur. J. Cancer 1995, 31A, 576–581. [Google Scholar] [CrossRef]
- Noto, R.B.; Pryma, D.A.; Jensen, J.; Lin, T.; Stambler, N.; Strack, T.; Wong, V.; Goldsmith, S.J. Phase 1 Study of High-Specific-Activity I-131 MIBG for Metastatic and/or Recurrent Pheochromocytoma or Paraganglioma. J. Clin. Endocrinol. Metab. 2018, 103, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chin, B.B.; Kronauge, J.F.; Femia, F.J.; Chen, J.; Maresca, K.P.; Hillier, S.; Petry, N.A.; James, O.G.; Oldan, J.D.; Armor, T.; et al. Phase-1 clinical trial results of high-specific-activity carrier-free 123I-iobenguane. J. Nucl. Med. 2014, 55, 765–771. [Google Scholar] [CrossRef]
- Musini, V.M.; Tejani, A.M.; Bassett, K.; Puil, L.; Wright, J.M. Pharmacotherapy for hypertension in adults 60 years or older. Cochrane. Database Syst. Rev. 2019, 6, CD000028. [Google Scholar] [CrossRef]
- Jimenez, C.; Chin, B.B.; Noto, R.B.; Dillon, J.S.; Solnes, L.; Jensen, J.; Lin, T.; White, T.; Stambler, N.; Apfel, S.; et al. Azedra® (iobenguane I 131) in Patients with Metastatic and/or Recurrent and/or Unresectable Pheochromocytoma or Paraganglioma: Biochemical Tumor Marker Results of a Multicenter, Open-Label Pivotal Phase 2b Study. In Proceedings of the ENDO, Chicago, IL, USA, 17 March 2018; Available online: https://www.endocrine.org/meetings/endo-annual-meetings/abstract-details?ID=43345 (accessed on 4 June 2019).
- Monsieurs, M.; Brans, B.; Bacher, K.; Dierckx, R.; Thierens, H. Patient dosimetry for 131I-MIBG therapy for neuroendocrine tumours based on 123I-MIBG scans. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1581–1587. [Google Scholar] [CrossRef]
- Roman-Gonzalez, A.; Zhou, S.; Ayala-Ramirez, M.; Shen, C.; Waguespack, S.G.; Habra, M.A.; Karam, J.A.; Perrier, N.; Wood, C.G.; Jimenez, C. Impact of Surgical Resection of the Primary Tumor on Overall Survival in Patients With Metastatic Pheochromocytoma or Sympathetic Paraganglioma. Ann. Surg. 2018, 268, 172–178. [Google Scholar] [CrossRef]
- Strajina, V.; Dy, B.M.; Farley, D.R.; Richards, M.L.; McKenzie, T.J.; Bible, K.C.; Que, F.G.; Nagorney, D.M.; Young, W.F.; Thompson, G.B. Surgical Treatment of Malignant Pheochromocytoma and Paraganglioma: Retrospective Case Series. Ann. Surg. Oncol. 2017, 24, 1546–1550. [Google Scholar] [CrossRef]
- Haugen, B.R.; Sawka, A.M.; Alexander, E.K.; Bible, K.C.; Caturegli, P.; Doherty, G.M.; Mandel, S.J.; Morris, J.C.; Nassar, A.; Pacini, F.; et al. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force Review and Recommendation on the Proposed Renaming of Encapsulated Follicular Variant Papillary Thyroid Carcinoma Without Invasion to Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid 2017, 27, 481–483. [Google Scholar] [CrossRef]
- Niemeijer, N.D.; Alblas, G.; Van Hulsteijn, L.T.; Dekkers, O.M.; Corssmit, E.P. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: Systematic review and meta-analysis. Clin. Endocrinol. 2014, 81, 642–651. [Google Scholar] [CrossRef]
- Ayala-Ramirez, M.; Feng, L.; Habra, M.A.; Rich, T.; Dickson, P.V.; Perrier, N.; Phan, A.; Waguespack, S.; Patel, S.; Jimenez, C. Clinical benefits of systemic chemotherapy for patients with metastatic pheochromocytomas or sympathetic extra-adrenal paragangliomas: Insights from the largest single-institutional experience. Cancer 2012, 118, 2804–2812. [Google Scholar] [CrossRef]
- Yanik, G.A.; Villablanca, J.G.; Maris, J.M.; Weiss, B.; Groshen, S.; Marachelian, A.; Park, J.R.; Tsao-Wei, D.; Hawkins, R.; Shulkin, B.L.; et al. 131I-metaiodobenzylguanidine with intensive chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma. A new approaches to neuroblastoma therapy (NANT) phase II study. Biol. Blood Marrow. Transpl. 2015, 21, 673–681. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Bal, C. Concomitant (177)Lu-DOTATATE and capecitabine therapy in malignant paragangliomas. EJNMMI Res. 2019, 9, 13. [Google Scholar] [CrossRef]
- Ashwathanarayana, A.G.; Biswal, C.K.; Sood, A.; Parihar, A.S.; Kapoor, R.; Mittal, B.R. Imaging-Guided Use of Combined (177)Lu-DOTATATE and Capecitabine Therapy in Metastatic Mediastinal Paraganglioma. J. Nucl. Med. Technol. 2017, 45, 314–316. [Google Scholar] [CrossRef]
- Martiniova, L.; Perera, S.M.; Brouwers, F.M.; Alesci, S.; Abu-Asab, M.; Marvelle, A.F.; Kiesewetter, D.O.; Thomasson, D.; Morris, J.C.; Kvetnansky, R.; et al. Increased uptake of [(1)(2)(3)I]meta-iodobenzylguanidine, [(1)(8)F]fluorodopamine, and [(3)H]norepinephrine in mouse pheochromocytoma cells and tumors after treatment with the histone deacetylase inhibitors. Endocr. Relat. Cancer 2011, 18, 143–157. [Google Scholar] [CrossRef]
- Luzon-Toro, B.; Geerlings, A.; Hilfiker, S. Hydroxytyrosol increases norepinephrine transporter function in pheochromocytoma cells. Nucl. Med. Biol. 2008, 35, 801–804. [Google Scholar] [CrossRef]
- Karlsson, J.; Ora, I.; Porn-Ares, I.; Pahlman, S. Arsenic trioxide-induced death of neuroblastoma cells involves activation of Bax and does not require p53. Clin. Cancer Res. 2004, 10, 3179–3188. [Google Scholar] [CrossRef]
- Modak, S.; Zanzonico, P.; Carrasquillo, J.A.; Kushner, B.H.; Kramer, K.; Cheung, N.K.; Larson, S.M.; Pandit-Taskar, N. Arsenic Trioxide as a Radiation Sensitizer for 131I-Metaiodobenzylguanidine Therapy: Results of a Phase II Study. J. Nucl. Med. 2016, 57, 231–237. [Google Scholar] [CrossRef]
- Roman-Gonzalez, A.; Jimenez, C. Malignant pheochromocytoma-paraganglioma: Pathogenesis, TNM staging, and current clinical trials. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 174–183. [Google Scholar] [CrossRef]
- Makis, W.; McCann, K.; McEwan, A.J.; Sawyer, M.B. Combined Treatment With 131I-MIBG and Sunitinib Induces Remission in a Patient With Metastatic Paraganglioma Due to Hereditary Paraganglioma-Pheochromocytoma Syndrome From an SDHB Mutation. Clin. Nucl. Med. 2016, 41, 204–206. [Google Scholar] [CrossRef]
- Jimenez, P.; Tatsui, C.; Jessop, A.; Thosani, S.; Jimenez, C. Treatment for Malignant Pheochromocytomas and Paragangliomas: 5 Years of Progress. Curr. Oncol. Rep. 2017, 19, 83. [Google Scholar] [CrossRef]
- Jasim, S.; Suman, V.J.; Jimenez, C.; Harris, P.; Sideras, K.; Burton, J.K.; Worden, F.P.; Auchus, R.J.; Bible, K.C. Phase II trial of pazopanib in advanced/progressive malignant pheochromocytoma and paraganglioma. Endocrine 2017, 57, 220–225. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Meng, X.; Feng, R.; Yang, L.; Xing, L.; Yu, J. The Role of Radiation Oncology in Immuno-Oncology. Oncologist 2019, 24, S42–S52. [Google Scholar] [CrossRef]
- Boyd, M.; Ross, S.C.; Dorrens, J.; Fullerton, N.E.; Tan, K.W.; Zalutsky, M.R.; Mairs, R.J. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with alpha-, beta-, and auger electron-emitting radionuclides. J. Nucl. Med. 2006, 47, 1007–1015. [Google Scholar]
- Vaidyanathan, G.; Strickland, D.K.; Zalutsky, M.R. Meta-[211At]astatobenzylguanidine: Further evaluation of a potential therapeutic agent. Int. J. Cancer 1994, 57, 908–913. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Zalutsky, M.R. 1-(m-[211At]astatobenzyl)guanidine: Synthesis via astato demetalation and preliminary in vitro and in vivo evaluation. Bioconjug. Chem. 1992, 3, 499–503. [Google Scholar] [CrossRef]
- Ohshima, Y.; Sudo, H.; Watanabe, S.; Nagatsu, K.; Tsuji, A.B.; Sakashita, T.; Ito, Y.M.; Yoshinaga, K.; Higashi, T.; Ishioka, N.S. Antitumor effects of radionuclide treatment using alpha-emitting meta-(211)At-astato-benzylguanidine in a PC12 pheochromocytoma model. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 999–1010. [Google Scholar] [CrossRef]
- Leung, A.; Shapiro, B.; Hattner, R.; Kim, E.; de Kraker, J.; Ghazzar, N.; Hartmann, O.; Hoefnagel, C.A.; Jamadar, D.A.; Kloos, R.; et al. Specificity of radioiodinated MIBG for neural crest tumors in childhood. J. Nucl. Med. 1997, 38, 1352–1357. [Google Scholar]
- Khan, M.U.; Morse, M.; Coleman, R.E. Radioiodinated metaiodobenzylguanidine in the diagnosis and therapy of carcinoid tumors. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 441–454. [Google Scholar]
- Troncone, L.; Riccardi, R.; Montemaggi, P.; Rufini, V.; Lasorella, A.; Mastrangelo, R. Treatment of neuroblastoma with 131I-metaiodobenzylguanidine. Med. Pediatr. Oncol. 1987, 15, 220–223. [Google Scholar] [CrossRef]
- Cottino, F.; Mussa, G.C.; Madon, E.; Favero, A.; Silvestro, L.; Grazia, G. 131I-metaiodobenzylguanidine treatment in neuroblastoma: Report of two cases. Med. Pediatr. Oncol. 1987, 15, 216–219. [Google Scholar] [CrossRef]
- Fischer, M.; Wehinger, H.; Kraus, C.; Ritter, J.; Schroter, W. Treatment of neuroblastoma with 131I-metaiodobenzylguanidine: Experience of the Munster/Kassel Group. Med. Pediatr. Oncol. 1987, 15, 196–198. [Google Scholar] [CrossRef]



| Characteristics | LSA-I-131-MIBG | HSA-I-131 MIBG |
|---|---|---|
| Manufacturing process | Simple isotope exchange methodology [47] | Solid phase precursor Ultratrace process [46] |
| Unlabeled MIBG in each dose | Large amount [46] | None [46] |
| Chemical mass of unlabeled amount of MIBG in a 500 mCi dose | ~12 mg [46] | ~0.2 mg [46] |
| Specific activity of final drug product | ~1.59 MBq/μg (low) [44,46] | ~92.5 MBq/μg (very high) [46] |
| Potential efficacy | Low levels of radioactivity delivered to tumor per dose [46] | High levels of radioactivity delivered to tumor per dose [46] |
| Potential safety | Excess cold MIBG and increased risk for cardiovascular issues [46] | No cold MIBG, low cardiovascular risk [46] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez, C.; Erwin, W.; Chasen, B. Targeted Radionuclide Therapy for Patients with Metastatic Pheochromocytoma and Paraganglioma: From Low-Specific-Activity to High-Specific-Activity Iodine-131 Metaiodobenzylguanidine. Cancers 2019, 11, 1018. https://doi.org/10.3390/cancers11071018
Jimenez C, Erwin W, Chasen B. Targeted Radionuclide Therapy for Patients with Metastatic Pheochromocytoma and Paraganglioma: From Low-Specific-Activity to High-Specific-Activity Iodine-131 Metaiodobenzylguanidine. Cancers. 2019; 11(7):1018. https://doi.org/10.3390/cancers11071018
Chicago/Turabian StyleJimenez, Camilo, William Erwin, and Beth Chasen. 2019. "Targeted Radionuclide Therapy for Patients with Metastatic Pheochromocytoma and Paraganglioma: From Low-Specific-Activity to High-Specific-Activity Iodine-131 Metaiodobenzylguanidine" Cancers 11, no. 7: 1018. https://doi.org/10.3390/cancers11071018
APA StyleJimenez, C., Erwin, W., & Chasen, B. (2019). Targeted Radionuclide Therapy for Patients with Metastatic Pheochromocytoma and Paraganglioma: From Low-Specific-Activity to High-Specific-Activity Iodine-131 Metaiodobenzylguanidine. Cancers, 11(7), 1018. https://doi.org/10.3390/cancers11071018





