Whole-blood DNA Methylation Markers for Risk Stratification in Colorectal Cancer Screening: A Systematic Review
Abstract
1. Introduction
2. Results
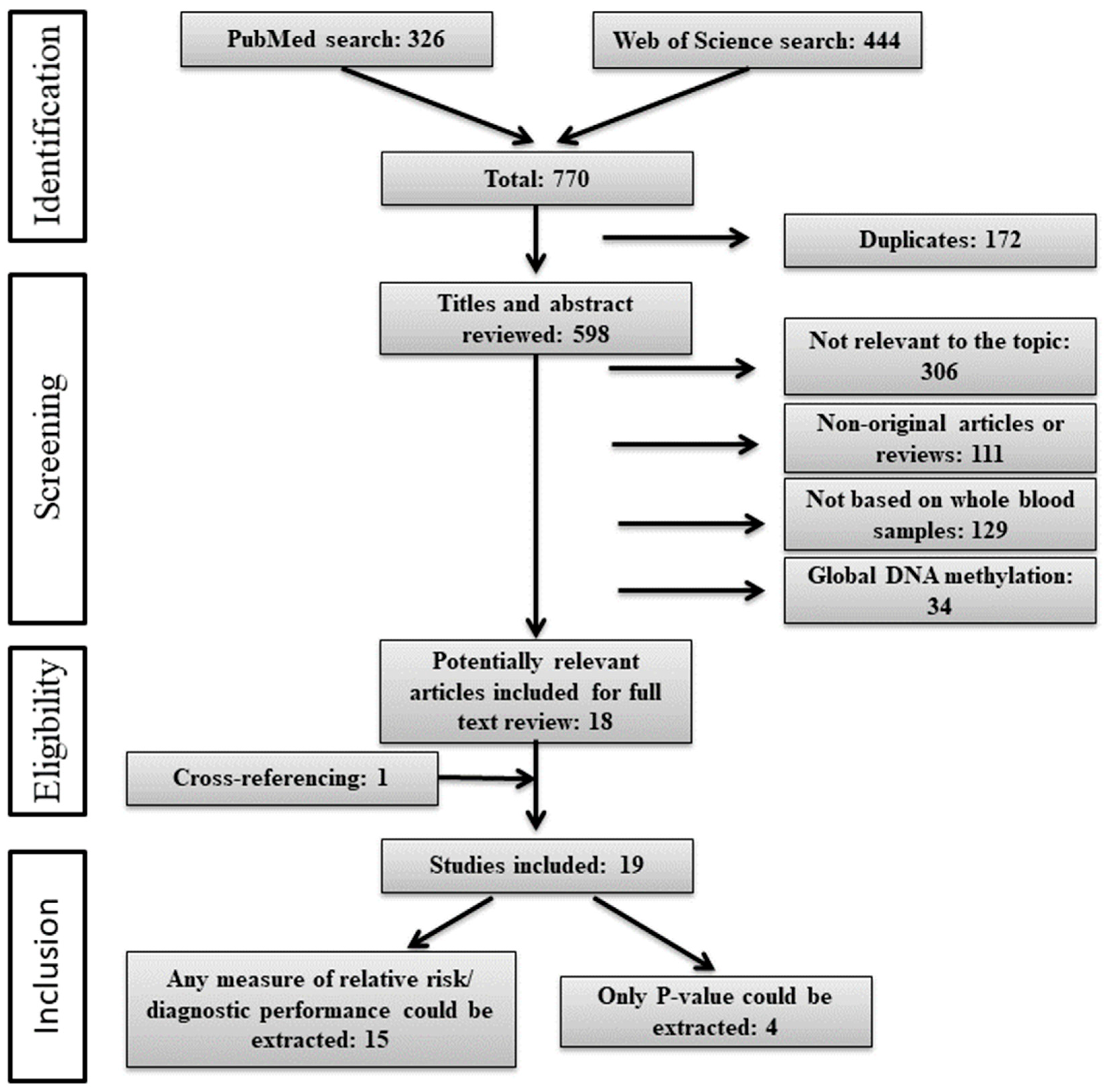
2.1. Study Selection
2.2. Study Characteristics
2.3. Overview of Whole-Blood based DNA Methylation Markers
2.4. Associations of Methylation Markers with Colorectal Neoplasms
2.5. DNA Methylation Panels
2.6. Quality Assessment of Studies
3. Discussion
4. Methods
4.1. Systematic Literature Search
4.2. Eligibility Criteria
4.3. Data Extraction and Quality Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Stock, C.; Hoffmeister, M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014, 348, g2467. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, R.; Wu, K.; Lochhead, P.; Morikawa, T.; Liao, X.; Qian, Z.R.; Inamura, K.; Kim, S.A.; Kuchiba, A.; Yamauchi, M.; et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. New Engl. J. Med. 2013, 369, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Mongin, S.J.; Geisser, M.S.; Lederle, F.A.; Bond, J.H.; Mandel, J.S.; Church, T.R. Long-term mortality after screening for colorectal cancer. New Engl. J. Med. 2013, 369, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Khalid-de Bakker, C.; Jonkers, D.; Smits, K.; Mesters, I.; Masclee, A.; Stockbrugger, R. Participation in colorectal cancer screening trials after first-time invitation: A systematic review. Endoscopy 2011, 43, 1059–1086. [Google Scholar] [CrossRef]
- Sabatino, S.A.; White, M.C.; Thompson, T.D.; Klabunde, C.N. Cancer screening test use—United States, 2013. Morb. Mortal. Wkly. Rep. 2015, 64, 464–468. [Google Scholar]
- Klabunde, C.N.; Vernon, S.W.; Nadel, M.R.; Breen, N.; Seeff, L.C.; Brown, M.L. Barriers to colorectal cancer screening: A comparison of reports from primary care physicians and average-risk adults. Med Care 2005, 43, 939–944. [Google Scholar] [CrossRef]
- Jones, R.M.; Devers, K.J.; Kuzel, A.J.; Woolf, S.H. Patient-reported barriers to colorectal cancer screening: A mixed-methods analysis. Am. J. Prev. Med. 2010, 38, 508–516. [Google Scholar] [CrossRef]
- Muliira, J.K.; D’Souza, M.S.; Ahmed, S.M.; Al-Dhahli, S.N.; Al-Jahwari, F.R. Barriers to colorectal cancer screening in primary care settings: Attitudes and knowledge of nurses and physicians. Asia-Pac. J. Oncol. Nurs. 2016, 3, 98–107. [Google Scholar] [CrossRef]
- Schroy, P.C., 3rd; Duhovic, E.; Chen, C.A.; Heeren, T.C.; Lopez, W.; Apodaca, D.L.; Wong, J.B. Risk stratification and shared decision making for colorectal cancer screening: A randomized controlled trial. Med Decis. Mak. Int. J. Soc. Med Decis. Mak. 2016, 36, 526–535. [Google Scholar] [CrossRef]
- Pancione, M.; Remo, A.; Colantuoni, V. Genetic and epigenetic events generate multiple pathways in colorectal cancer progression. Pathol. Res. Int. 2012, 2012, 509348. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.O.; Broaddus, R.R.; Houlihan, P.S.; Issa, J.P.; Hamilton, S.R.; Rashid, A. Cpg island methylation in aberrant crypt foci of the colorectum. Am. J. Pathol. 2002, 160, 1823–1830. [Google Scholar] [CrossRef]
- Kim, Y.H.; Petko, Z.; Dzieciatkowski, S.; Lin, L.; Ghiassi, M.; Stain, S.; Chapman, W.C.; Washington, M.K.; Willis, J.; Markowitz, S.D.; et al. Cpg island methylation of genes accumulates during the adenoma progression step of the multistep pathogenesis of colorectal cancer. Genes Chromosomes Cancer 2006, 45, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Shen, L.; Morris, J.S.; Issa, J.P.; Hamilton, S.R. Cpg island methylation in colorectal adenomas. Am. J. Pathol. 2001, 159, 1129–1135. [Google Scholar] [CrossRef]
- Ahlquist, T.; Lind, G.E.; Costa, V.L.; Meling, G.I.; Vatn, M.; Hoff, G.S.; Rognum, T.O.; Skotheim, R.I.; Thiis-Evensen, E.; Lothe, R.A. Gene methylation profiles of normal mucosa, and benign and malignant colorectal tumors identify early onset markers. Mol. Cancer 2008, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Azuara, D.; Rodriguez-Moranta, F.; de Oca, J.; Soriano-Izquierdo, A.; Mora, J.; Guardiola, J.; Biondo, S.; Blanco, I.; Peinado, M.A.; Moreno, V.; et al. Novel methylation panel for the early detection of colorectal tumors in stool DNA. Clin. Colorectal Cancer 2010, 9, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Gao, Y.T.; Chow, W.H.; Shu, X.O.; Yang, G.; Ji, B.T.; Wen, W.; Rothman, N.; Li, H.L.; Morrow, J.D.; et al. Prospective study of urinary prostaglandin e2 metabolite and colorectal cancer risk. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 5010–5016. [Google Scholar] [CrossRef]
- Harada, T.; Yamamoto, E.; Yamano, H.O.; Nojima, M.; Maruyama, R.; Kumegawa, K.; Ashida, M.; Yoshikawa, K.; Kimura, T.; Harada, E.; et al. Analysis of DNA methylation in bowel lavage fluid for detection of colorectal cancer. Cancer Prev. Res. 2014, 7, 1002–1010. [Google Scholar] [CrossRef]
- Heiss, J.A.; Brenner, H. Epigenome-wide discovery and evaluation of leukocyte DNA methylation markers for the detection of colorectal cancer in a screening setting. Clin. Epigenet. 2017, 9, 24. [Google Scholar] [CrossRef]
- Adler, A.; Geiger, S.; Keil, A.; Bias, H.; Schatz, P.; deVos, T.; Dhein, J.; Zimmermann, M.; Tauber, R.; Wiedenmann, B. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in germany. BMC Gastroenterol. 2014, 14, 183. [Google Scholar] [CrossRef]
- Liles, E.G.; Coronado, G.D.; Perrin, N.; Harte, A.H.; Nungesser, R.; Quigley, N.; Potter, N.T.; Weiss, G.; Koenig, T.; deVos, T. Uptake of a colorectal cancer screening blood test is higher than of a fecal test offered in clinic: A randomized trial. Cancer Treat. Res. Commun. 2017, 10, 27–31. [Google Scholar] [CrossRef]
- Bergheim, J.; Semaan, A.; Gevensleben, H.; Groening, S.; Knoblich, A.; Dietrich, J.; Weber, J.; Kalff, J.C.; Bootz, F.; Kristiansen, G.; et al. Potential of quantitative sept9 and shox2 methylation in plasmatic circulating cell-free DNA as auxiliary staging parameter in colorectal cancer: A prospective observational cohort study. Br. J. Cancer 2018, 118, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Jiang, X.; Li, Q.; Sun, Z.; Quan, W.; Duan, Y.; Li, D.; Chen, T. Diagnostic value of methylated septin9 for colorectal cancer detection. Front. Oncol. 2018, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Gormally, E.; Caboux, E.; Vineis, P.; Hainaut, P. Circulating free DNA in plasma or serum as biomarker of carcinogenesis: Practical aspects and biological significance. Mutat. Res. 2007, 635, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Choi, J.Y.; Lee, K.M.; Sung, H.; Park, S.K.; Oze, I.; Pan, K.F.; You, W.C.; Chen, Y.X.; Fang, J.Y.; et al. DNA methylation in peripheral blood: A potential biomarker for cancer molecular epidemiology. J. Epidemiol. 2012, 22, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Page, K.; Guttery, D.S.; Zahra, N.; Primrose, L.; Elshaw, S.R.; Pringle, J.H.; Blighe, K.; Marchese, S.D.; Hills, A.; Woodley, L.; et al. Influence of plasma processing on recovery and analysis of circulating nucleic acids. PLoS ONE 2013, 8, e77963. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Killian, K.; Zhang, H.; Yu, K.; Li, Q.Z.; Weinstein, S.; Virtamo, J.; Tucker, M.; Taylor, P.; Albanes, D.; et al. Leukocyte DNA methylation and colorectal cancer among male smokers. World J. Gastrointest. Oncol. 2012, 4, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Kaaks, R.; Stattin, P.; Villar, S.; Poetsch, A.R.; Dossus, L.; Nieters, A.; Riboli, E.; Palmqvist, R.; Hallmans, G.; Plass, C.; et al. Insulin-like growth factor-ii methylation status in lymphocyte DNA and colon cancer risk in the northern sweden health and disease cohort. Cancer Res. 2009, 69, 5400–5405. [Google Scholar] [CrossRef] [PubMed]
- Marsit, C.; Christensen, B. Blood-derived DNA methylation markers of cancer risk. Adv. Exp. Med. Biol. 2013, 754, 233–252. [Google Scholar]
- Sabbioni, S.; Miotto, E.; Veronese, A.; Sattin, E.; Gramantieri, L.; Bolondi, L.; Calin, G.A.; Gafa, R.; Lanza, G.; Carli, G.; et al. Multigene methylation analysis of gastrointestinal tumors: Tpef emerges as a frequent tumor-specific aberrantly methylated marker that can be detected in peripheral blood. Mol. Diagn. J. Devoted Underst. Hum. Dis. Clin. Appl. Mol. Biol. 2003, 7, 201–207. [Google Scholar]
- Miotto, E.; Sabbioni, S.; Veronese, A.; Calin, G.A.; Gullini, S.; Liboni, A.; Gramantieri, L.; Bolondi, L.; Ferrazzi, E.; Gafa, R.; et al. Frequent aberrant methylation of the cdh4 gene promoter in human colorectal and gastric cancer. Cancer Res. 2004, 64, 8156–8159. [Google Scholar] [CrossRef] [PubMed]
- Ravegnini, G.; Zolezzi Moraga, J.M.; Maffei, F.; Musti, M.; Zenesini, C.; Simeon, V.; Sammarini, G.; Festi, D.; Hrelia, P.; Angelini, S. Simultaneous analysis of sept9 promoter methylation status, micronuclei frequency, and folate-related gene polymorphisms: The potential for a novel blood-based colorectal cancer biomarker. Int. J. Mol. Sci. 2015, 16, 28486–28497. [Google Scholar] [CrossRef]
- Ally, M.S.; Al-Ghnaniem, R.; Pufulete, M. The relationship between gene-specific DNA methylation in leukocytes and normal colorectal mucosa in subjects with and without colorectal tumors. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Koessler, T.; Ibrahim, A.E.; Rai, S.; Vowler, S.L.; Abu-Amero, S.; Silva, A.L.; Maia, A.T.; Huddleston, J.E.; Uribe-Lewis, S.; et al. Somatically acquired hypomethylation of igf2 in breast and colorectal cancer. Hum. Mol. Genet. 2008, 17, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Miroglio, A.; Jammes, H.; Tost, J.; Ponger, L.; Gut, I.G.; El Abdalaoui, H.; Coste, J.; Chaussade, S.; Arimondo, P.B.; Lamarque, D.; et al. Specific hypomethylated cpgs at the igf2 locus act as an epigenetic biomarker for familial adenomatous polyposis colorectal cancer. Epigenomics 2010, 2, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Nüsgen, N.; Goering, W.; Dauksa, A.; Biswas, A.; Jamil, M.A.; Dimitriou, I.; Sharma, A.; Singer, H.; Fimmers, R.; Fröhlich, H.; et al. Inter-locus as well as intra-locus heterogeneity in line-1 promoter methylation in common human cancers suggests selective demethylation pressure at specific cpgs. Clin. Epigenet. 2015, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.L.; Wang, X.; Sun, H.R.; Zhou, J.D.; Lin, S.Q.; Xing, Y.H.; Zhu, L.; Zhou, H.B.; Zhao, Y.S.; Chi, Q.; et al. Methylation status of transcriptional modulatory genes associated with colorectal cancer in northeast china. Gut Liver 2018, 12, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cui, G.; Yao, Y.L.; Wang, Q.C.; Gu, H.G.; Li, X.N.; Zhang, H.; Feng, W.M.; Shi, Q.L.; Cui, W.W. Value of cnrip1 promoter methylation in colorectal cancer screening and prognosis assessment and its influence on the activity of cancer cells. Arch. Med Sci. 2017, 13, 1281–1294. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Hu, F.; Sun, H.; Zhang, Z.; Wang, X.; Luo, X.; Zhu, L.; Huang, R.; Li, Y.; et al. Multiple gene-specific DNA methylation in blood leukocytes and colorectal cancer risk: A case-control study in china. Oncotarget 2017, 8, 61239–61252. [Google Scholar] [CrossRef]
- Luo, X.; Huang, R.; Sun, H.; Liu, Y.; Bi, H.; Li, J.; Yu, H.; Sun, J.; Lin, S.; Cui, B.; et al. Methylation of a panel of genes in peripheral blood leukocytes is associated with colorectal cancer. Sci. Rep. 2016, 6, 29922. [Google Scholar] [CrossRef]
- Xiao, W.; Zhao, H.; Dong, W.; Li, Q.; Zhu, J.; Li, G.; Zhang, S.; Ye, M. Quantitative detection of methylated ndrg4 gene as a candidate biomarker for diagnosis of colorectal cancer. Oncol. Lett. 2015, 9, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, D.; Pham, D.N.; Levesque, N.; Truongcao, M.; Foulkes, W.D.; Sapienza, C.; Rozen, R. Oncogenic role of pdk4 in human colon cancer cells. Br. J. Cancer 2017, 116, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Burch, J.B.; Steck, S.E.; Chen, C.F.; Hurley, T.G.; Cavicchia, P.; Shivappa, N.; Guess, J.; Zhang, H.; Youngstedt, S.D.; et al. Case-control study of candidate gene methylation and adenomatous polyp formation. Int. J. Colorectal Dis. 2017, 32, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Begum, R.; Akhgar, A.; Smoot, D.T.; Elbedawi, M.; Daremipouran, M.; Zhao, A.; Momen, B.; Giardiello, F.M. Folate status and risk of colorectal polyps in african americans. Dig. Dis. Sci. 2007, 52, 1462–1470. [Google Scholar] [CrossRef]
- Ho, V.; Ashbury, J.E.; Taylor, S.; Vanner, S.; King, W.D. Gene-specific DNA methylation of dnmt3b and mthfr and colorectal adenoma risk. Mutat. Res. 2015, 782, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bretthauer, M.; Kaminski, M.F.; Loberg, M.; Zauber, A.G.; Regula, J.; Kuipers, E.J.; Hernan, M.A.; McFadden, E.; Sunde, A.; Kalager, M.; et al. Population-based colonoscopy screening for colorectal cancer: A randomized clinical trial. JAMA Intern. Med. 2016, 176, 894–902. [Google Scholar] [CrossRef]
- Oines, M.; Helsingen, L.M.; Bretthauer, M.; Emilsson, L. Epidemiology and risk factors of colorectal polyps. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 419–424. [Google Scholar] [CrossRef]
- Fraser, H.B.; Lam, L.L.; Neumann, S.M.; Kobor, M.S. Population-specificity of human DNA methylation. Genome Biol. 2012, 13, R8. [Google Scholar] [CrossRef]
- Xia, Y.Y.; Ding, Y.B.; Liu, X.Q.; Chen, X.M.; Cheng, S.Q.; Li, L.B.; Ma, M.F.; He, J.L.; Wang, Y.X. Racial/ethnic disparities in human DNA methylation. Biochim. Biophys. Acta 2014, 1846, 258–262. [Google Scholar] [CrossRef]
- Reinius, L.E.; Acevedo, N.; Joerink, M.; Pershagen, G.; Dahlen, S.E.; Greco, D.; Soderhall, C.; Scheynius, A.; Kere, J. Differential DNA methylation in purified human blood cells: Implications for cell lineage and studies on disease susceptibility. PloS ONE 2012, 7, e41361. [Google Scholar] [CrossRef]
- Terry, M.B.; Delgado-Cruzata, L.; Vin-Raviv, N.; Wu, H.C.; Santella, R.M. DNA methylation in white blood cells: Association with risk factors in epidemiologic studies. Epigenetics 2011, 6, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Heiss, J.A.; Brenner, H. Impact of confounding by leukocyte composition on associations of leukocyte DNA methylation with common risk factors. Epigenomics 2017, 9, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Mikeska, T.; Candiloro, I.L.; Dobrovic, A. The implications of heterogeneous DNA methylation for the accurate quantification of methylation. Epigenomics 2010, 2, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, T. Detection and interpretation of altered methylation patterns in cancer cells. Nat. Rev. Cancer 2005, 5, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Delaney, C.; Garg, S.K.; Yung, R. Analysis of DNA methylation by pyrosequencing. Methods Mol. Biol. 2015, 1343, 249–264. [Google Scholar] [PubMed]
- Tost, J.; Gut, I.G. DNA methylation analysis by pyrosequencing. Nat. Protoc. 2007, 2, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Huang, T.H.; Wang, L.S. Profiling DNA methylomes from microarray to genome-scale sequencing. Technol. Cancer Res. Treat. 2010, 9, 139–147. [Google Scholar] [CrossRef]
- Yong, W.S.; Hsu, F.M.; Chen, P.Y. Profiling genome-wide DNA methylation. Epigenet. Chromatin 2016, 9, 26. [Google Scholar] [CrossRef]
- Pidsley, R.; Zotenko, E.; Peters, T.J.; Lawrence, M.G.; Risbridger, G.P.; Molloy, P.; Van Djik, S.; Muhlhausler, B.; Stirzaker, C.; Clark, S.J. Critical evaluation of the illumina methylationepic beadchip microarray for whole-genome DNA methylation profiling. Genome Biol. 2016, 17, 208. [Google Scholar] [CrossRef]
- Herreros-Villanueva, M.; Duran-Sanchon, S.; Martin, A.C.; Perez-Palacios, R.; Vila-Navarro, E.; Marcuello, M.; Diaz-Centeno, M.; Cubiella, J.; Diez, M.S.; Bujanda, L.; et al. Plasma microrna signature validation for early detection of colorectal cancer. Clin. Transl. Gastroenterol. 2019, 10, e00003. [Google Scholar] [CrossRef]
- Toiyama, Y.; Takahashi, M.; Hur, K.; Nagasaka, T.; Tanaka, K.; Inoue, Y.; Kusunoki, M.; Boland, C.R.; Goel, A. Serum mir-21 as a diagnostic and prognostic biomarker in colorectal cancer. J. Natl. Cancer Inst. 2013, 105, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Weigl, K.; Thomsen, H.; Balavarca, Y.; Hellwege, J.N.; Shrubsole, M.J.; Brenner, H. Genetic risk score is associated with prevalence of advanced neoplasms in a colorectal cancer screening population. Gastroenterology 2018, 155, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qian, J.; Werner, S.; Cuk, K.; Knebel, P.; Brenner, H. Development and validation of a panel of five proteins as blood biomarkers for early detection of colorectal cancer. Clin. Epidemiol. 2017, 9, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zucknick, M.; Werner, S.; Knebel, P.; Brenner, H. Head-to-head comparison and evaluation of 92 plasma protein biomarkers for early detection of colorectal cancer in a true screening setting. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 3318–3326. [Google Scholar] [CrossRef] [PubMed]
- Balavarca, Y.; Weigl, K.; Thomsen, H.; Brenner, H. Performance of individual and joint risk stratification by an environmental risk score and a genetic risk score in a colorectal cancer screening setting. Int. J. Cancer 2019. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.; Jeon, J.; Brenner, H.; Gruber, S.B.; Schoen, R.E.; Berndt, S.I.; Chan, A.T.; Chang-Claude, J.; Du, M.; Gong, J.; et al. A model to determine colorectal cancer risk using common genetic susceptibility loci. Gastroenterology 2015, 148, 1330–1339. [Google Scholar] [CrossRef]
- Jeon, J.; Du, M.; Schoen, R.E.; Hoffmeister, M.; Newcomb, P.A.; Berndt, S.I.; Caan, B.; Campbell, P.T.; Chan, A.T.; Chang-Claude, J.; et al. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental, and genetic factors. Gastroenterology 2018, 154, 2152–2164. [Google Scholar] [CrossRef]
- Schumacher, F.R.; Schmit, S.L.; Jiao, S.; Edlund, C.K.; Wang, H.; Zhang, B.; Hsu, L.; Huang, S.C.; Fischer, C.P.; Harju, J.F.; et al. Genome-wide association study of colorectal cancer identifies six new susceptibility loci. Nat. Commun. 2015, 6, 7138. [Google Scholar] [CrossRef]
- Peng, L.; Weigl, K.; Boakye, D.; Brenner, H. Risk scores for predicting advanced colorectal neoplasia in the average-risk population: A systematic review and meta-analysis. Am. J. Gastroenterol. 2018, 113, 1788–1800. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
| Gene/LINE-1 locus | Chromosome | Gao, 2018 [37] | Zhang, 2017 a [38] | Liu, 2017 [39] | Leclerc, 2017 [42] | Heiss, 2017 [19] | Alexander, 2017 b [43] | Luo, 2016 [40] | Xiao, 2015 [41] | Ravegnini, 2015 [32] | Nüsgen, 2015 [36] | Ho, 2015 b [45] | Gao, 2012 [27] | Miroglio, 2010 [35] | Kaaks, 2009 [28] | Ally, 2009 a [33] | Ito, 2008 [34] | Ashktorab, 2007 b [44] | Miotto, 2004 [31] | Sabbioni, 2003 [30] | Report Frequency | Frequency of Significant Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CITED4 | 1 | ⬆ | 1 | 1 | ||||||||||||||||||
| GSTM2 | 1 | ○ c | 1 | 0 | ||||||||||||||||||
| MTHFR | 1 | ○ | 1 | 0 | ||||||||||||||||||
| PARP1 | 1 | ○ c | 1 | 0 | ||||||||||||||||||
| PER3 | 1 | ⬇ | 1 | 1 | ||||||||||||||||||
| PTCH2 | 1 | ○ c | 1 | 0 | ||||||||||||||||||
| AOX-1 | 2 | ⬆ | 1 | 1 | ||||||||||||||||||
| CNRIP1 | 2 | ⬆ | 1 | 1 | ||||||||||||||||||
| L1C2 | 2 | ○ | 1 | 0 | ||||||||||||||||||
| PER2 | 2 | ○ | 1 | 0 | ||||||||||||||||||
| TMEFF2 | 2 | ○ | ○ | 2 | 0 | |||||||||||||||||
| ADAMTS9 | 3 | ⬆ | 1 | 1 | ||||||||||||||||||
| MLH1 | 3 | ○ | ○ | 2 | 0 | |||||||||||||||||
| MME_ | 3 | ○ c | 1 | 0 | ||||||||||||||||||
| RARB2 | 3 | ○ | 1 | 0 | ||||||||||||||||||
| SEMA3F | 3 | ○ c | 1 | 0 | ||||||||||||||||||
| APC | 5 | ○ | ○ | ○ | 3 | 0 | ||||||||||||||||
| FLT4 | 5 | ○ c | 1 | 0 | ||||||||||||||||||
| NEUROG1 | 5 | ⬆ | 1 | 1 | ||||||||||||||||||
| DSP | 6 | ○ c | 1 | 0 | ||||||||||||||||||
| ESR1 | 6 | ○ | ○ c | 2 | 0 | |||||||||||||||||
| IRF4 | 6 | ⬆ | 1 | 1 | ||||||||||||||||||
| L1C6 | 6 | ○ | 1 | 0 | ||||||||||||||||||
| PLAGL1 | 6 | ○ c | 1 | 0 | ||||||||||||||||||
| PDK4 | 7 | ⬇ | 1 | 1 | ||||||||||||||||||
| PODXL | 7 | ○ c | 1 | 0 | ||||||||||||||||||
| SEMA3C | 7 | ○ c | 1 | 0 | ||||||||||||||||||
| SFRP4 | 7 | ○ | 1 | 0 | ||||||||||||||||||
| SGCE | 7 | ○ c | 1 | 0 | ||||||||||||||||||
| CRH | 8 | ○ | 1 | 0 | ||||||||||||||||||
| APBA1 | 9 | ⬇ | 1 | 1 | ||||||||||||||||||
| CDKN2A | 9 | ○ | ○ | 2 | 0 | |||||||||||||||||
| DAPK1 | 9 | ⬆ | 1 | 1 | ||||||||||||||||||
| FOXE-1 | 9 | ○ | 1 | 0 | ||||||||||||||||||
| L1C10 | 10 | ○ | 1 | 0 | ||||||||||||||||||
| MGMT | 10 | ○ | ○ | 2 | 0 | |||||||||||||||||
| SFRP5 | 10 | ○ | 1 | 0 | ||||||||||||||||||
| H19 | 11 | ○ | 1 | 0 | ||||||||||||||||||
| IGF2 | 11 | ⬆ | ○ | ⬇ | ○ | ○ | 5 | 2 | ||||||||||||||
| IL18BP | 11 | ○ c | 1 | 0 | ||||||||||||||||||
| KIAA1549L | 11 | ⬆ c | 1 | 1 | ||||||||||||||||||
| KCNK4 | 11 | ○ c | 1 | 0 | ||||||||||||||||||
| L1C11 | 11 | ○ | 1 | 0 | ||||||||||||||||||
| WT1 | 11 | ⬆ | 1 | 1 | ||||||||||||||||||
| CYP27B1 | 12 | ○ | 1 | 0 | ||||||||||||||||||
| PDE1B_ | 12 | ○ c | 1 | 0 | ||||||||||||||||||
| RERG | 12 | ⬆ | 1 | 1 | ||||||||||||||||||
| TMEM132D | 12 | ○ | 1 | 0 | ||||||||||||||||||
| WIF1 | 12 | ⬆ | ○ | 2 | 1 | |||||||||||||||||
| WNT1 | 12 | ○ c | 1 | 0 | ||||||||||||||||||
| GJB2 | 13 | ○ c | 1 | 0 | ||||||||||||||||||
| MLH3 | 14 | ○ c | 1 | 0 | ||||||||||||||||||
| SNRPN | 15 | ○ c | 1 | 0 | ||||||||||||||||||
| CDH1 | 16 | ○ | 1 | 0 | ||||||||||||||||||
| NDRG4 | 16 | ○ | 1 | 0 | ||||||||||||||||||
| BRCA1 | 17 | ○ | 1 | 0 | ||||||||||||||||||
| CA10 | 17 | ⬆ | 1 | 1 | ||||||||||||||||||
| HIC1 | 17 | ○ c | 1 | 0 | ||||||||||||||||||
| NGFR | 17 | ○ | 1 | 0 | ||||||||||||||||||
| PER1 | 17 | ⬇ | 1 | 1 | ||||||||||||||||||
| SEPT9 | 17 | ○ | ⬇ | 2 | 1 | |||||||||||||||||
| BCL2 | 18 | ⬆ c | 1 | 1 | ||||||||||||||||||
| INSR | 19 | ○ c | 1 | 0 | ||||||||||||||||||
| CDH4 | 20 | ○ | 1 | 0 | ||||||||||||||||||
| DNMT3B | 20 | ⬆ | 1 | 1 | ||||||||||||||||||
| HCK | 20 | ○ c | 1 | 0 | ||||||||||||||||||
| L1C20 | 20 | ○ | 1 | 0 | ||||||||||||||||||
| B3GALT5 | 21 | ○ c | 1 | 0 | ||||||||||||||||||
| COL18A1 | 21 | ○ c | 1 | 0 | ||||||||||||||||||
| TIMP3 | 22 | ○ | 1 | 0 | ||||||||||||||||||
| DKC1 | X | ○ c | 1 | 0 | ||||||||||||||||||
| GLA | X | ○ c | 1 | 0 | ||||||||||||||||||
| L1X1 | X | ○ | 1 | 0 | ||||||||||||||||||
| L1X3 | X | ○ | 1 | 0 | ||||||||||||||||||
| L1X4a | X | ○ | 1 | 0 | ||||||||||||||||||
| L1X5bd | X | ○ | 1 | 0 | ||||||||||||||||||
| L1X6b | X | ○ | 1 | 0 | ||||||||||||||||||
| L1X8 | X | ⬆ c | 1 | 1 | ||||||||||||||||||
| HBII | ○ c | 1 | 0 | |||||||||||||||||||
| MINT31 | ⬆ | 1 | 1 |
| Gene | First Author, Year [Ref NO.] | Country | No. Cases/Controls | Age (Years) Cases/Controls | DNAm Assay | OR (95%CI) | p-Value * |
|---|---|---|---|---|---|---|---|
| Hypomethylation a | |||||||
| PER1 (promoter) | Alexander, 2017 [43] | USA | 38/69 | -- | MS-PCR | 2.9 (1.1–7.7) b | 0.03 |
| APBA1 (promoter) | Alexander, 2017 [43] | USA | 38/69 | -- | MS-PCR | 5.3 (1.0–28.2) c | 0.05 |
| PER3 (promoter) | Alexander, 2017 [43] | USA | 38/69 | -- | MS-PCR | 11.1 (1.6–78.5) d | 0.02 |
| Hypermethylation | |||||||
| AOX-1 | Luo, 2016 [40] | China | 421/506 | 59.5/56.6 | MS-HRM | 1.72 (1.30–2.27) e | 0.00 |
| ADAMTS9 | Luo, 2016 [40] | China | 421/506 | 59.5/56.6 | MS-HRM | 1.85 (1.37–2.49) e | 0.00 |
| RERG | Luo, 2016 [40] | China | 421/506 | 59.5/56.6 | MS-HRM | 2.08 (1.56–2.77) e | 0.00 |
| WIF1 | Liu, 2017 [39] | China | 428/428 | 59.4/59.4 | MS-HRM | 2.44 (1.53–3.87) f | <0.0001 |
| IGF2 | Liu, 2017 [39] | China | 428/428 | 59.4/59.4 | MS-HRM | 2.54 (1.65–3.92) f | <0.0001 |
| NEUROG1 | Liu, 2017 [39] | China | 428/428 | 59.4/59.4 | MS-HRM | 2.57 (1.55–4.25) f | <0.0001 |
| WT1 | Gao, 2018 [37] | China | 466/507 | 60.1/56.7 | MS-HRM | 2.59 (1.73–3.88) g | 0.00 |
| DAPK1 | Liu, 2017 [39] | China | 428/428 | 59.4/59.4 | MS-HRM | 2.95 (1.94–4.49) f | <0.0001 |
| CITED4 | Gao, 2018 [37] | China | 466/507 | 60.1/56.7 | MS-HRM | 2.96 (1.68–5.24) g | 0.00 |
| MINT31 | Liu, 2017 [39] | China | 428/428 | 59.4/59.4 | MS-HRM | 4.27 (1.52–12.05) f | 0.01 |
| CA10 | Gao, 2018 [37] | China | 466/507 | 60.1/56.7 | MS-HRM | 4.83 (2.82–8.28) g | 0.00 |
| IRF4 | Luo, 2016 [40] | China | 421/506 | 59.5/56.6 | MS-HRM | 16.96 (5.15–55.84) e | 0.00 |
| Gene | First Author, Year [Ref. No.] | No. Cases/ Controls | Age (Year) Cases/ Controls | DNA Methylation Assay | AUC | p-Value * | OR a Tertile 3 vs. 1 (95%CI) | OR b Quartile 4 vs. 1 (95%CI) | OR b per SD (95%CI) |
|---|---|---|---|---|---|---|---|---|---|
| BCL2 (gene-body, cg12459502) | Heiss, 2017 [19] | Screening Setting: 46/46 Clinical setting: 93/94 | 67/67 65/65 | HM 450K MassArray | 0.57 0.69 | <0.05 <0.05 | -- | -- | -- |
| B3GALT5 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0.01 c | 1.2 (1.06–1.37) | -- | -- | |
| COL18A1 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.2 (1.05–1.37) | -- | -- | |
| DKC1 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0.01 c | 1.2 (1.05–1.43) | -- | -- | |
| DNMT3B (GCA, 31351136) | Ho, 2015 [45] | 87/172 | -- | Sequenom EpiTYPER | 0.03 | -- | -- | 1.38 (1.03–1.86) | |
| DNMT3B (GCA, 31351260–31351263) | Ho, 2015 [45] | 87/172 | -- | Sequenom EpiTYPER | 0.03 | -- | 2.07 (0.88–4.86) | -- | |
| DSP (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.27 (1.12–1.43) | -- | -- | |
| FLT4 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.15 (0.99–1.32) | -- | -- | |
| GJB2 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.03 (0.9–1.18) | -- | -- | |
| GLA (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.45 (1.19–1.75) | -- | -- | |
| GSTM2 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.17 (1.03–1.34) | -- | -- | |
| HBII (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 0.87 (0.76–0.99) | -- | -- | |
| HCK (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.2 (1.03–1.39) | -- | -- | |
| HIC1 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0.01 c | 1.22 (1.07–1.41) | -- | -- | |
| IL18BP (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0.01 c | 1.07 (0.91–1.26) | |||
| INSR (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.24 (1.09–1.41) | -- | -- | |
| KCNK4 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.15 (1.01–1.31) | -- | -- | |
| KIAA1549L (promoter, cg04036920) | Heiss, 2017 [19] | Screening Setting: 46/46 Clinical setting: 93/94 | 67/67 65/65 | HM 450K MassArray | 0.67 0.70 | <0.05 <0.05 | -- | -- | -- |
| KIAA1549L (promoter, cg14472551) | Heiss, 2017 [19] | Screening Setting: 46/46 Clinical setting: 93/94 | 67/67 65/65 | HM 450K MassArray | 0.72 0.64 | <0.05 <0.05 | -- | -- | -- |
| L1X8 (intergenic, UBQLN2/ Centromer) | Nüsgen, 2015 [36] | 21/59 | -- | Pyrosequencing | 0.66 | <0.05 | -- | -- | -- |
| MLH3 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.27 (1.1–1.46) | -- | -- | |
| MME_ (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0.01 c | 1.17 (1.03–1.33) | -- | -- | |
| PARP1 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 0.87 (0.76–0.99) | -- | -- | |
| PDE1B_ (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0.01 c | 1.21 (1.05–1.41) | -- | -- | |
| PLAGL1 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 0.88 (0.76–1.01) | -- | -- | |
| PODXL (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0.01 c | 1.12 (0.99–1.28) | -- | -- | |
| PTCH2 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.26 (1.11–1.43) | -- | -- | |
| KIAA1549L (promoter, cg14472551) | Heiss, 2017 [19] | Screening Setting: 46/46 Clinical setting: 93/94 | 67/67 65/65 | HM 450K MassArray | 0.72 0.64 | <0.05 <0.05 | -- | -- | -- |
| L1X8 (intergenic, UBQLN2/ Centromer) | Nüsgen, 2015 [36] | 21/59 | -- | Pyrosequencing | 0.66 | <0.05 | -- | -- | -- |
| MLH3 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.27 (1.1–1.46) | -- | -- | |
| MME_ (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0.01 c | 1.17 (1.03–1.33) | -- | -- | |
| PARP1 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 0.87 (0.76–0.99) | -- | -- | |
| PDE1B_ (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0.01 c | 1.21 (1.05–1.41) | -- | -- | |
| PLAGL1 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 0.88 (0.76–1.01) | -- | -- | |
| PODXL (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0.01 c | 1.12 (0.99–1.28) | -- | -- | |
| PTCH2 (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.26 (1.11–1.43) | -- | -- | |
| SEMA3C (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 0.88 (0.77–1) | -- | -- | |
| SEMA3F (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0 c | 1.16 (1.01–1.34) | -- | -- | |
| SGCE (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0.01 c | 1.23 (1.08–1.4) | -- | -- | |
| SNRPN (promoter) | Gao, 2012 [27] | 221/219 | 58/58 | GoldenGate Cancer Panel I | 0.01 c | 0.98 (0.87–1.11) | -- | -- |
| Gene Panel | First author, Year [Ref. No.] | No. Cases/Controls | Age(yrs) Cases/ Controls | DNAm Assay | AUC | Classification | OR (95%CI) | p- Value * |
|---|---|---|---|---|---|---|---|---|
| Hypomethylation | ||||||||
| PER1/PER3 | Alexander, 2017 [43] | 38/69 | -- | MS-PCR | -- | -- | 0.50 (0.22–1.15) a | 0.10 |
| PER1/APBA1 | Alexander, 2017 [43] | 38/69 | -- | MS-PCR | -- | -- | 0.59 (0.26–1.33) a | 0.20 |
| PER3/APBA1 | Alexander, 2017 [43] | 38/69 | -- | MS-PCR | -- | -- | 0.27 (0.09–0.81) a | 0.02 |
| PER1/PER3/APBA1 | Alexander, 2017 [43] | 38/69 | -- | MS-PCR | -- | -- | 0.57 (0.25–1.27) a | 0.17 |
| Hypermethylation | ||||||||
| MCSM (WT1, CA10, CITED4 & TMEM132D) | Gao, 2018 [37] | 466/507 | 60.1/56.7 | MS-HRM | -- | Non-MCSM | 1.00 b | |
| (no methylated gene) (ref) | ||||||||
| MCSM-L (≤2 methylated genes) | 1.43 (0.50–4.05) | 0.50 | ||||||
| MCSM-H (≥3 methylated genes) | 4.32 (1.53–12.2) | 0.01 | ||||||
| MCSM | 2.53 (0.92–6.94) | 0.07 | ||||||
| MRS_10 (APC+CDH1+ CDKN2A+DAPK1+ IGF2+MGMT+ MINT31+MLH1+ NEUROG1+WIF1) | Liu, 2017 [39] | 428/428 | 59.4/59.4 | MS-HRM | 0.69 (0.66–0.73), p < 0.0001 | Low predicted probability ≤0.5 (ref) | 1.00 c | |
| Medium | 3.85 (2.72–5.45) | <0.0001 | ||||||
| (0.5 < predicted probability ≤ 0.7) | ||||||||
| High | 6.51 (3.77–11.27) | <0.0001 | ||||||
| (predicted probability >0.7) | ||||||||
| Medium or High | 4.39 (3.19–6.05) | <0.0001 | ||||||
| Markers-only model (cg04036920, cg14472551 & cg12459502) | Heiss, 2017 [19] | SS: 46/46 CS: 93/94 | 67/67 65/65 | HM 450K | 0.69 (0.55, 0.82) d 0.73 (0.63, 0.83) d | |||
| Full model (combining sex, age, cg04036920, cg14472551 & cg12459502) | Heiss, 2017 [19] | SS: 46/46 CS: 93/94 | 67/67 65/65 | HM 450K | 0.69 (0.54, 0.83) d 0.73 (0.61, 0.82) d | |||
| MCSM (AOX-1, RARB2, RERG, ADAMTS9, IRF4, & FOXE-1) | Luo, 2016 [40] | 421/506 | 59.5/56.6 | MS-HRM | -- | Non-MCSM | 1.00 e | |
| (no methylated gene) (ref) | ||||||||
| MCSM-L (1 methylated gene) | 1.23 (0.87–1.75) | 0.24 | ||||||
| MCSM-H (≥2 methylated genes | 1.79 (1.28–2.52) | 0.00 | ||||||
| (except for RARB2)) | ||||||||
| MCSM | 1.50 (1.11–2.03) | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raut, J.R.; Guan, Z.; Schrotz-King, P.; Brenner, H. Whole-blood DNA Methylation Markers for Risk Stratification in Colorectal Cancer Screening: A Systematic Review. Cancers 2019, 11, 912. https://doi.org/10.3390/cancers11070912
Raut JR, Guan Z, Schrotz-King P, Brenner H. Whole-blood DNA Methylation Markers for Risk Stratification in Colorectal Cancer Screening: A Systematic Review. Cancers. 2019; 11(7):912. https://doi.org/10.3390/cancers11070912
Chicago/Turabian StyleRaut, Janhavi R., Zhong Guan, Petra Schrotz-King, and Hermann Brenner. 2019. "Whole-blood DNA Methylation Markers for Risk Stratification in Colorectal Cancer Screening: A Systematic Review" Cancers 11, no. 7: 912. https://doi.org/10.3390/cancers11070912
APA StyleRaut, J. R., Guan, Z., Schrotz-King, P., & Brenner, H. (2019). Whole-blood DNA Methylation Markers for Risk Stratification in Colorectal Cancer Screening: A Systematic Review. Cancers, 11(7), 912. https://doi.org/10.3390/cancers11070912





