Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer
Abstract
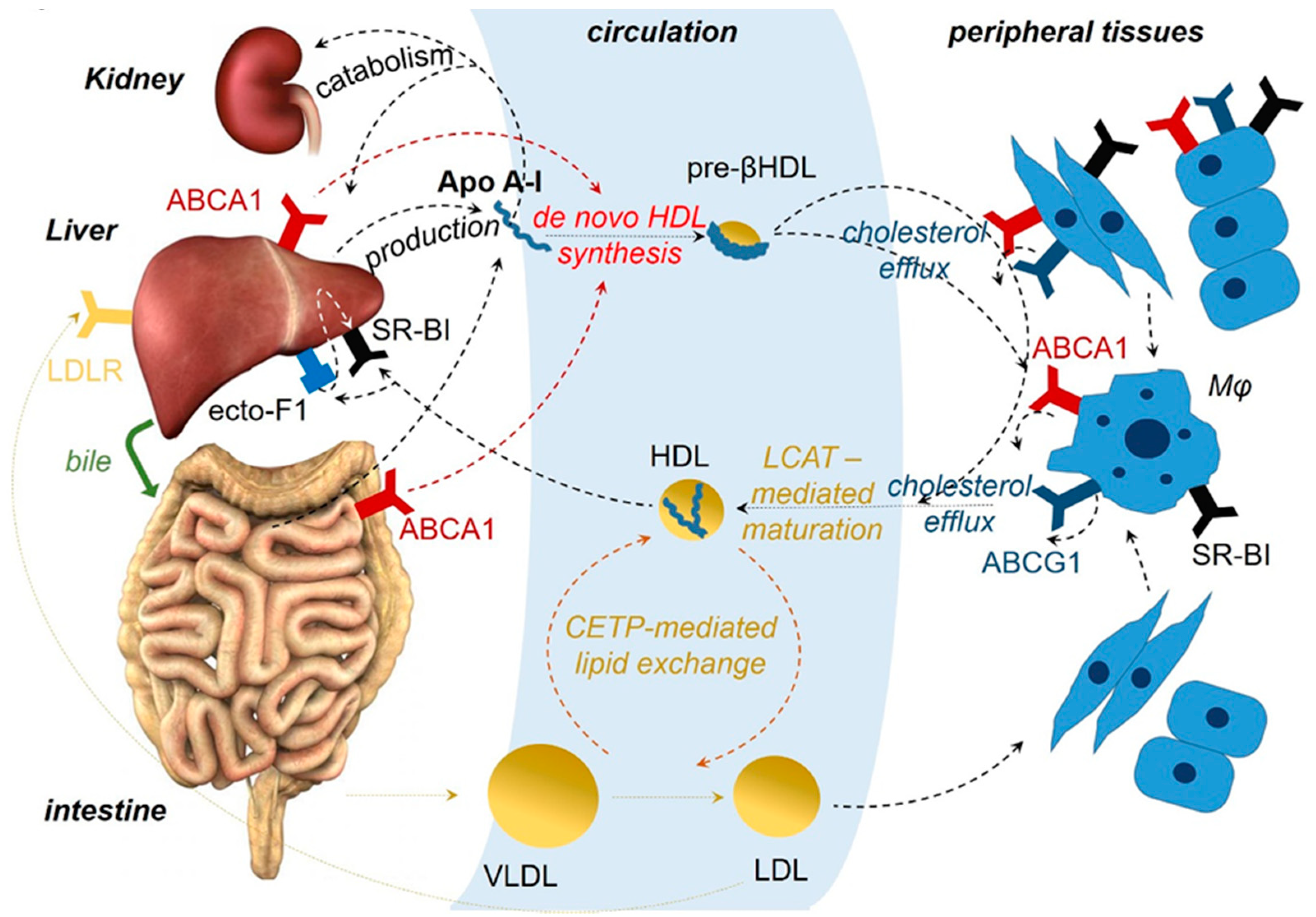
1. Introduction
2. ApoA-I, Immunity, and Inflammation
3. A Potential Protective Role of ApoA-I against Cancer: Evidence by Association
4. ApoA-I Exhibits Tumor Suppressive Activity: Evidence from In Vitro Studies
5. The Tumor Suppressive Function of ApoA-I: Evidence from Animal Studies
6. Anti-Inflammatory and Immune-Modulating Mechanisms Are Involved in the Tumor Suppressive Activity of ApoA-I
7. Tools for Therapeutic Targeting of ApoA-I
8. Open Questions for Future Research
9. Conclusions
Author Contributions
Funding
Acknowledgments

Conflicts of Interest
References
- Gordon, S.M.; Hofmann, S.; Askew, D.S.; Davidson, W.S. High density lipoprotein: It’s not just about lipid transport anymore. Trends Endocrinol. Metab. 2011, 22, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Puzio-Kuter, A.M. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 2010, 330, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Zamanian-Daryoush, M.; DiDonato, J.A. Apolipoprotein A-I and Cancer. Front. Pharmacol. 2015, 6, 265. [Google Scholar] [CrossRef] [PubMed]
- Barker, W.C.; Dayhoff, M.O. Evolution of lipoproteins deduced from protein sequence data. Comp. Biochem. Physiol. B Comp. Biochem. 1977, 57, 309–315. [Google Scholar] [CrossRef]
- Fitch, W.M. Phylogenies constrained by the crossover process as illustrated by human hemoglobins and a thirteen-cycle, eleven-amino-acid repeat in human apolipoprotein A-I. Genetics 1977, 86, 623–644. [Google Scholar] [PubMed]
- Li, W.H.; Tanimura, M.; Luo, C.C.; Datta, S.; Chan, L. The apolipoprotein multigene family: Biosynthesis, structure, structure-function relationships, and evolution. J. Lipid Res. 1988, 29, 245–271. [Google Scholar] [PubMed]
- McLachlan, A.D. Repeated helical pattern in apolipoprotein-A-I. Nature 1977, 267, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Bashtovyy, D.; Jones, M.K.; Anantharamaiah, G.M.; Segrest, J.P. Sequence conservation of apolipoprotein A-I affords novel insights into HDL structure-function. J. Lipid Res. 2011, 52, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Kardassis, D.; Mosialou, I.; Kanaki, M.; Tiniakou, I.; Thymiakou, E. Metabolism of HDL and its regulation. Curr. Med. Chem. 2014, 21, 2864–2880. [Google Scholar] [CrossRef]
- Halley, P.; Kadakkuzha, B.M.; Faghihi, M.A.; Magistri, M.; Zeier, Z.; Khorkova, O.; Coito, C.; Hsiao, J.; Lawrence, M.; Wahlestedt, C. Regulation of the apolipoprotein gene cluster by a long noncoding RNA. Cell Rep. 2014, 6, 222–230. [Google Scholar] [CrossRef]
- Azrolan, N.; Odaka, H.; Breslow, J.L.; Fisher, E.A. Dietary fat elevates hepatic apoA-I production by increasing the fraction of apolipoprotein A-I mRNA in the translating pool. J. Biol. Chem. 1995, 270, 19833–19838. [Google Scholar] [CrossRef] [PubMed]
- Bloedon, L.T.; Dunbar, R.; Duffy, D.; Pinell-Salles, P.; Norris, R.; DeGroot, B.J.; Movva, R.; Navab, M.; Fogelman, A.M.; Rader, D.J. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 2008, 49, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; Navab, M.; Anantharamaiah, G.M.; Fogelman, A.M. Apolipoprotein A-I mimetics. Curr. Opin. Lipidol. 2014, 25, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; Navab, M.; Anantharamaiah, G.M.; Fogelman, A.M. Searching for a successful HDL-based treatment strategy. Biochim. Biophys. Acta 2014, 1841, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Tan, L.; Long, J.L.; Davidson, W.S. Proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 2013, 54, 2575–2585. [Google Scholar] [CrossRef]
- Shao, B.; Heinecke, J.W. Quantifying HDL proteins by mass spectrometry: How many proteins are there and what are their functions? Expert Rev. Proteom. 2018, 15, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Duong, P.T.; Weibel, G.L.; Lund-Katz, S.; Rothblat, G.H.; Phillips, M.C. Characterization and properties of pre beta-HDL particles formed by ABCA1-mediated cellular lipid efflux to apoA-I. J. Lipid Res. 2008, 49, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B., Jr.; Chapman, M.J.; Fazio, S.; Hussain, M.M.; Kontush, A.; Krauss, R.M.; Otvos, J.D.; Remaley, A.T.; Schaefer, E.J. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem. 2011, 57, 392–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Silver, D.L.; Costet, P.; Tall, A.R. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 2000, 275, 33053–33058. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.Q.; Rye, K.A.; Barter, P.J. Cycling of apolipoprotein A-I between lipid-associated and lipid-free pools. Biochim. Biophys. Acta 1995, 1257, 31–37. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Oram, J.F.; Asztalos, B.F.; Vaughan, A.M.; Lund-Katz, S.; Adorni, M.P.; Phillips, M.C.; Rothblat, G.H. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J. Lipid Res. 2009, 50, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Rye, K.A.; Hime, N.J.; Barter, P.J. Evidence that cholesteryl ester transfer protein-mediated reductions in reconstituted high density lipoprotein size involve particle fusion. J. Biol. Chem. 1997, 272, 3953–3960. [Google Scholar] [CrossRef] [PubMed]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Krieger, M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996, 271, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Kozarsky, K.F.; Donahee, M.H.; Rigotti, A.; Iqbal, S.N.; Edelman, E.R.; Krieger, M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature 1997, 387, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.O.; Jacquet, S.; Esteve, J.P.; Rolland, C.; Cabezon, E.; Champagne, E.; Pineau, T.; Georgeaud, V.; Walker, J.E.; Terce, F.; et al. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature 2003, 421, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.O.; Najib, S.; Perret, B.; Cabou, C.; Lichtenstein, L. Ecto-F1-ATPase/P2Y pathways in metabolic and vascular functions of high density lipoproteins. Atherosclerosis 2015, 238, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Gburek, J. Protein reabsorption in renal proximal tubule-function and dysfunction in kidney pathophysiology. Pediatr. Nephrol. 2004, 19, 714–721. [Google Scholar] [CrossRef]
- Glass, C.; Pittman, R.C.; Weinstein, D.B.; Steinberg, D. Dissociation of tissue uptake of cholesterol ester from that of apoprotein A-I of rat plasma high density lipoprotein: Selective delivery of cholesterol ester to liver, adrenal, and gonad. Proc. Natl. Acad. Sci. USA 1983, 80, 5435–5439. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Pirillo, A.; Bonacina, F.; Norata, G.D. HDL in innate and adaptive immunity. Cardiovasc. Res. 2014, 103, 372–383. [Google Scholar] [CrossRef]
- Biedzka-Sarek, M.; Metso, J.; Kateifides, A.; Meri, T.; Jokiranta, T.S.; Muszynski, A.; Radziejewska-Lebrecht, J.; Zannis, V.; Skurnik, M.; Jauhiainen, M. Apolipoprotein A-I exerts bactericidal activity against Yersinia enterocolitica serotype O:3. J. Biol. Chem. 2011, 286, 38211–38219. [Google Scholar] [CrossRef]
- Perez-Morga, D.; Vanhollebeke, B.; Paturiaux-Hanocq, F.; Nolan, D.P.; Lins, L.; Homble, F.; Vanhamme, L.; Tebabi, P.; Pays, A.; Poelvoorde, P.; et al. Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science 2005, 309, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Chopra, A.K.; Coppenhaver, D.H.; Ananatharamaiah, G.M.; Baron, S. Lipoproteins account for part of the broad non-specific antiviral activity of human serum. Antivir. Res. 1999, 42, 211–218. [Google Scholar] [CrossRef]
- Jiao, Y.L.; Wu, M.P. Apolipoprotein A-I diminishes acute lung injury and sepsis in mice induced by lipoteichoic acid. Cytokine 2008, 43, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Wurfel, M.M.; Kunitake, S.T.; Lichenstein, H.; Kane, J.P.; Wright, S.D. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J. Exp. Med. 1994, 180, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.M.; Parker, T.S.; Donnelly, T.M.; Walsh, A.; Rubin, A.L. In vivo protection against endotoxin by plasma high density lipoprotein. Proc. Natl. Acad. Sci. USA 1993, 90, 12040–12044. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Wu, G.; Shen, L.; Chen, B. Effect of lipid-bound apoA-I cysteine mutants on lipopolysaccharide-induced endotoxemia in mice. J. Lipid Res. 2008, 49, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Morin, E.E.; Guo, L.; Schwendeman, A.; Li, X.A. HDL in sepsis - risk factor and therapeutic approach. Front. Pharmacol. 2015, 6, 244. [Google Scholar] [CrossRef] [PubMed]
- Wendel, M.; Paul, R.; Heller, A.R. Lipoproteins in inflammation and sepsis. II. Clinical aspects. Intensive Care Med. 2007, 33, 25–35. [Google Scholar] [CrossRef]
- Bottazzi, B.; Doni, A.; Garlanda, C.; Mantovani, A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu. Rev. Immunol. 2010, 28, 157–183. [Google Scholar] [CrossRef]
- Norata, G.D.; Marchesi, P.; Pirillo, A.; Uboldi, P.; Chiesa, G.; Maina, V.; Garlanda, C.; Mantovani, A.; Catapano, A.L. Long pentraxin 3, a key component of innate immunity, is modulated by high-density lipoproteins in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 925–931. [Google Scholar] [CrossRef]
- Cabana, V.G.; Siegel, J.N.; Sabesin, S.M. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J. Lipid Res. 1989, 30, 39–49. [Google Scholar] [PubMed]
- Van Leeuwen, H.J.; Heezius, E.C.; Dallinga, G.M.; Van Strijp, J.A.; Verhoef, J.; Van Kessel, K.P. Lipoprotein metabolism in patients with severe sepsis. Crit. Care Med. 2003, 31, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Van Lenten, B.J.; Hama, S.Y.; De Beer, F.C.; Stafforini, D.M.; McIntyre, T.M.; Prescott, S.M.; La Du, B.N.; Fogelman, A.M.; Navab, M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J. Clin. Investig. 1995, 96, 2758–2767. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.K.; Zhao, J.; Sims, P.J. Interaction between apolipoproteins A-I and A-II and the membrane attack complex of complement. Affinity of the apoproteins for polymeric C9. J. Biol. Chem. 1993, 268, 3632–3638. [Google Scholar] [PubMed]
- Doni, A.; D’Amico, G.; Morone, D.; Mantovani, A.; Garlanda, C. Humoral innate immunity at the crossroad between microbe and matrix recognition: The role of PTX3 in tissue damage. Semin Cell Dev. Biol. 2017, 61, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Pagler, T.; Gautier, E.L.; Avagyan, S.; Siry, R.L.; Han, S.; Welch, C.L.; Wang, N.; Randolph, G.J.; Snoeck, H.W.; et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 2010, 328, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Fessler, M.B.; Parks, J.S. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J. Immunol. 2011, 187, 1529–1535. [Google Scholar] [CrossRef]
- Gupta, N.; DeFranco, A.L. Lipid rafts and B cell signaling. Semin Cell Dev. Biol. 2007, 18, 616–626. [Google Scholar] [CrossRef]
- Kabouridis, P.S.; Jury, E.C. Lipid rafts and T-lymphocyte function: Implications for autoimmunity. FEBS Lett. 2008, 582, 3711–3718. [Google Scholar] [CrossRef]
- Murphy, A.J.; Woollard, K.J.; Suhartoyo, A.; Stirzaker, R.A.; Shaw, J.; Sviridov, D.; Chin-Dusting, J.P. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1333–1341. [Google Scholar] [CrossRef]
- Wang, S.H.; Yuan, S.G.; Peng, D.Q.; Zhao, S.P. HDL and ApoA-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis 2012, 225, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.D.; Lim, H.Y.; Lee, H.G.; Yoon, D.Y.; Choe, Y.K.; Choi, I.; Paik, S.G.; Kim, Y.S.; Yang, Y.; Lim, J.S. Apolipoprotein A-I induces IL-10 and PGE2 production in human monocytes and inhibits dendritic cell differentiation and maturation. Biochem. Biophys. Res. Commun. 2005, 338, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Tiniakou, I.; Drakos, E.; Sinatkas, V.; Van Eck, M.; Zannis, V.I.; Boumpas, D.; Verginis, P.; Kardassis, D. High-density lipoprotein attenuates Th1 and Th17 autoimmune responses by modulating dendritic cell maturation and function. J. Immunol. 2015, 194, 4676–4687. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, D.; Labzin, L.I.; Kono, H.; Seki, R.; Schmidt, S.V.; Beyer, M.; Xu, D.; Zimmer, S.; Lahrmann, C.; Schildberg, F.A.; et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014, 15, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Thacker, S.G.; Zarzour, A.; Chen, Y.; Alcicek, M.S.; Freeman, L.A.; Sviridov, D.O.; Demosky, S.J., Jr.; Remaley, A.T. High-density lipoprotein reduces inflammation from cholesterol crystals by inhibiting inflammasome activation. Immunology 2016, 149, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Westerterp, M. Inflammasomes, neutrophil extracellular traps, and cholesterol. J. Lipid Res. 2019, 60, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, M.; Fotakis, P.; Ouimet, M.; Bochem, A.E.; Zhang, H.; Molusky, M.M.; Wang, W.; Abramowicz, S.; La Bastide-van Gemert, S.; Wang, N.; et al. Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis, and Atherogenesis. Circulation 2018, 138, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, M.; Gautier, E.L.; Ganda, A.; Molusky, M.M.; Wang, W.; Fotakis, P.; Wang, N.; Randolph, G.J.; D’Agati, V.D.; Yvan-Charvet, L.; et al. Cholesterol Accumulation in Dendritic Cells Links the Inflammasome to Acquired Immunity. Cell Metab. 2017, 25, 1294–1304. [Google Scholar] [CrossRef]
- Wilhelm, A.J.; Zabalawi, M.; Owen, J.S.; Shah, D.; Grayson, J.M.; Major, A.S.; Bhat, S.; Gibbs, D.P., Jr.; Thomas, M.J.; Sorci-Thomas, M.G. Apolipoprotein A-I modulates regulatory T cells in autoimmune LDLr-/-, ApoA-I-/- mice. J. Biol. Chem. 2010, 285, 36158–36169. [Google Scholar] [CrossRef]
- Castella, B.; Kopecka, J.; Sciancalepore, P.; Mandili, G.; Foglietta, M.; Mitro, N.; Caruso, D.; Novelli, F.; Riganti, C.; Massaia, M. The ATP-binding cassette transporter A1 regulates phosphoantigen release and Vgamma9Vdelta2 T cell activation by dendritic cells. Nat. Commun. 2017, 8, 15663. [Google Scholar] [CrossRef]
- Gkouskou, K.K.; Ioannou, M.; Pavlopoulos, G.A.; Georgila, K.; Siganou, A.; Nikolaidis, G.; Kanellis, D.C.; Moore, S.; Papadakis, K.A.; Kardassis, D.; et al. Apolipoprotein A-I inhibits experimental colitis and colitis-propelled carcinogenesis. Oncogene 2016, 35, 2496–2505. [Google Scholar] [CrossRef] [PubMed]
- Murano, T.; Najibi, M.; Paulus, G.L.C.; Adiliaghdam, F.; Valencia-Guerrero, A.; Selig, M.; Wang, X.; Jeffrey, K.; Xavier, R.J.; Lassen, K.G.; et al. Transcription factor TFEB cell-autonomously modulates susceptibility to intestinal epithelial cell injury in vivo. Sci. Rep. 2017, 7, 13938. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Cuchel, M.; De la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Ren, H.; Lv, X.; Zhao, Y.; Yu, B.; He, Y.; Ma, Y.; Niu, C.; Kong, J.; Yu, F.; et al. Hypochlorite-induced oxidative stress elevates the capability of HDL in promoting breast cancer metastasis. J. Transl. Med. 2012, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. Biological Consequences of Dysfunctional HDL. Curr. Med. Chem. 2019, 26, 1644–1664. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; DiDonato, J.A.; Levison, B.S.; Schmitt, D.; Li, L.; Wu, Y.; Buffa, J.; Kim, T.; Gerstenecker, G.S.; Gu, X.; et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat. Med. 2014, 20, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, A.; Zhao, Y.; Ying, W.; Sun, H.; Yang, X.; Xing, B.; Sun, W.; Ren, L.; Hu, B.; et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature 2019, 567, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Tan, Y.; Ying, W.; Hong, Y.; Liu, S.; Wu, M.; Qian, X.; Wang, H. Proteome analysis of hepatocellular carcinoma by laser capture microdissection. Proteomics 2006, 6, 538–546. [Google Scholar] [CrossRef]
- Mustafa, M.G.; Petersen, J.R.; Ju, H.; Cicalese, L.; Snyder, N.; Haidacher, S.J.; Denner, L.; Elferink, C. Biomarker discovery for early detection of hepatocellular carcinoma in hepatitis C-infected patients. Mol. Cell. Proteom. 2013, 12, 3640–3652. [Google Scholar] [CrossRef]
- Steel, L.F.; Shumpert, D.; Trotter, M.; Seeholzer, S.H.; Evans, A.A.; London, W.T.; Dwek, R.; Block, T.M. A strategy for the comparative analysis of serum proteomes for the discovery of biomarkers for hepatocellular carcinoma. Proteomics 2003, 3, 601–609. [Google Scholar] [CrossRef]
- Jiang, J.; Nilsson-Ehle, P.; Xu, N. Influence of liver cancer on lipid and lipoprotein metabolism. Lipids Health Dis. 2006, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lim, U.; Weinstein, S.J.; Schatzkin, A.; Hayes, R.B.; Virtamo, J.; Albanes, D. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Chandler, P.D.; Song, Y.; Lin, J.; Zhang, S.; Sesso, H.D.; Mora, S.; Giovannucci, E.L.; Rexrode, K.E.; Moorthy, M.V.; Li, C.; et al. Lipid biomarkers and long-term risk of cancer in the Women’s Health Study. Am. J. Clin. Nutr. 2016, 103, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Borgquist, S.; Butt, T.; Almgren, P.; Shiffman, D.; Stocks, T.; Orho-Melander, M.; Manjer, J.; Melander, O. Apolipoproteins, lipids and risk of cancer. Int. J. Cancer 2016, 138, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Bayerdorffer, E.; Mannes, G.A.; Richter, W.O.; Ochsenkuhn, T.; Seeholzer, G.; Kopcke, W.; Wiebecke, B.; Paumgartner, G. Decreased high-density lipoprotein cholesterol and increased low-density cholesterol levels in patients with colorectal adenomas. Ann. Intern. Med. 1993, 118, 481–487. [Google Scholar] [CrossRef]
- Jung, Y.S.; Ryu, S.; Chang, Y.; Yun, K.E.; Park, J.H.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; et al. Associations Between Parameters of Glucose and Lipid Metabolism and Risk of Colorectal Neoplasm. Dig. Dis. Sci. 2015, 60, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Alford, S.H.; Divine, G.; Chao, C.; Habel, L.A.; Janakiraman, N.; Wang, Y.; Feigelson, H.S.; Scholes, D.; Roblin, D.; Epstein, M.M.; et al. Serum cholesterol trajectories in the 10 years prior to lymphoma diagnosis. Cancer Causes Control. 2018, 29, 143–156. [Google Scholar] [CrossRef]
- Van Hemelrijck, M.; Walldius, G.; Jungner, I.; Hammar, N.; Garmo, H.; Binda, E.; Hayday, A.; Lambe, M.; Holmberg, L. Low levels of apolipoprotein A-I and HDL are associated with risk of prostate cancer in the Swedish AMORIS study. Cancer Causes Control. 2011, 22, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Gourin, C.G.; Zhi, W.; Adam, B.L. Proteomic identification of serum biomarkers for head and neck cancer surveillance. Laryngoscope 2009, 119, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wei, J.W.; Chen, K.; Zhang, S.; Han, F.; Lu, L.X.; Xiao, W.W.; Gao, Y.H. Apolipoprotein A-I Is a Prognosticator of Nasopharyngeal Carcinoma in the Era of Intensity-modulated Radiotherapy. J. Cancer 2018, 9, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yang, Z.H.; Luo, D.H.; Guo, L.; Sun, R.; Chen, Q.Y.; Huang, P.Y.; Qiu, F.; Zou, X.; Cao, K.J.; et al. Elevated apolipoprotein A-I levels are associated with favorable prognosis in metastatic nasopharyngeal carcinoma. Med Oncol. 2014, 31, 80. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Zhong, G.Z.; Hu, L.Y.; Chen, J.; Liang, Y.; Chen, Q.Y.; Liu, Q.; Rao, H.L.; Chen, K.L.; Cai, Q.Q. Serum apolipoprotein A-I is a novel prognostic indicator for non-metastatic nasopharyngeal carcinoma. Oncotarget 2015, 6, 44037–44048. [Google Scholar] [CrossRef]
- Zablocka-Slowinska, K.; Placzkowska, S.; Skorska, K.; Prescha, A.; Pawelczyk, K.; Porebska, I.; Kosacka, M.; Grajeta, H. Oxidative stress in lung cancer patients is associated with altered serum markers of lipid metabolism. PLoS ONE 2019, 14, e0215246. [Google Scholar] [CrossRef]
- Chang, Y.K.; Lai, Y.H.; Chu, Y.; Lee, M.C.; Huang, C.Y.; Wu, S. Haptoglobin is a serological biomarker for adenocarcinoma lung cancer by using the ProteomeLab PF2D combined with mass spectrometry. Am. J. Cancer Res. 2016, 6, 1828–1836. [Google Scholar] [PubMed]
- Cheng, T.; Dai, X.; Zhou, D.L.; Lv, Y.; Miao, L.Y. Correlation of apolipoprotein A-I kinetics with survival and response to first-line platinum-based chemotherapy in advanced non-small cell lung cancer. Med. Oncol. 2015, 32, 407. [Google Scholar] [CrossRef]
- Shi, H.; Huang, H.; Pu, J.; Shi, D.; Ning, Y.; Dong, Y.; Han, Y.; Zarogoulidis, P.; Bai, C. Decreased pretherapy serum apolipoprotein A-I is associated with extent of metastasis and poor prognosis of non-small-cell lung cancer. Onco Targets Ther. 2018, 11, 6995–7003. [Google Scholar] [CrossRef] [PubMed]
- Marchi, N.; Mazzone, P.; Fazio, V.; Mekhail, T.; Masaryk, T.; Janigro, D. ProApolipoprotein A1: A serum marker of brain metastases in lung cancer patients. Cancer 2008, 112, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xia, G.; Jianqing, Z.; Mei, Y.; Ge, B.; Li, Z. Serum differential protein identification of Xinjiang Kazakh esophageal cancer patients based on the two-dimensional liquid-phase chromatography and LTQ MS. Mol. Biol. Rep. 2014, 41, 2893–2905. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, X.H.; Zhang, L.; Lin, J.H.; Huang, H.; Kang, T.; Mao, M.J.; Chen, H.; Zheng, X. High level of serum apolipoprotein A-I is a favorable prognostic factor for overall survival in esophageal squamous cell carcinoma. BMC Cancer 2016, 16, 516. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wu, H.; Qu, K.; Sun, Q.; Li, F.; Shi, C.; Li, Y.; Xiong, X.; Qin, Q.; Yu, T.; et al. Identification of serum proteins AHSG, FGA and APOA-I as diagnostic biomarkers for gastric cancer. Clin. Proteom. 2018, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Cheng, C.C.; Wang, J.Y.; Wu, D.C.; Hsieh, J.S.; Lee, S.C.; Wang, W.M. Discovery of tumor markers for gastric cancer by proteomics. PLoS ONE 2014, 9, e84158. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.C.; Looi, M.L.; Zakaria, S.Z.; Sagap, I.; Rose, I.M.; Chin, S.F.; Jamal, R. Identification of Differentially Expressed Proteins in the Serum of Colorectal Cancer Patients Using 2D-DIGE Proteomics Analysis. Pathol. Oncol. Res. 2016, 22, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Van Duijnhoven, F.J.; Bueno-De-Mesquita, H.B.; Calligaro, M.; Jenab, M.; Pischon, T.; Jansen, E.H.; Frohlich, J.; Ayyobi, A.; Overvad, K.; Toft-Petersen, A.P.; et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut 2011, 60, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.W.; Liu, D.B.; Han, C.Z.; Du, L.L.; Jing, J.X.; Wang, Y. Lipid levels in serum and cancerous tissues of colorectal cancer patients. World J. Gastroenterol. 2014, 20, 8646–8652. [Google Scholar] [CrossRef] [PubMed]
- Sirnio, P.; Vayrynen, J.P.; Klintrup, K.; Makela, J.; Makinen, M.J.; Karttunen, T.J.; Tuomisto, A. Decreased serum apolipoprotein A1 levels are associated with poor survival and systemic inflammatory response in colorectal cancer. Sci. Rep. 2017, 7, 5374. [Google Scholar] [CrossRef] [PubMed]
- Sayagues, J.M.; Corchete, L.A.; Gutierrez, M.L.; Sarasquete, M.E.; Del Mar Abad, M.; Bengoechea, O.; Ferminan, E.; Anduaga, M.F.; Del Carmen, S.; Iglesias, M.; et al. Genomic characterization of liver metastases from colorectal cancer patients. Oncotarget 2016, 7, 72908–72922. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Yamamichi, N.; Tomida, S.; Takeuchi, C.; Kageyama-Yahara, N.; Takahashi, Y.; Shiogama, K.; Inada, K.I.; Ichinose, M.; Fujishiro, M.; et al. Identification of marker genes and pathways specific to precancerous duodenal adenomas and early stage adenocarcinomas. J. Gastroenterol. 2018, 54, 131–140. [Google Scholar] [CrossRef]
- Fye, H.K.; Wright-Drakesmith, C.; Kramer, H.B.; Camey, S.; Nogueira da Costa, A.; Jeng, A.; Bah, A.; Kirk, G.D.; Sharif, M.I.; Ladep, N.G.; et al. Protein profiling in hepatocellular carcinoma by label-free quantitative proteomics in two west African populations. PLoS ONE 2013, 8, e68381. [Google Scholar] [CrossRef]
- Qin, X.; Chen, Q.; Sun, C.; Wang, C.; Peng, Q.; Xie, L.; Liu, Y.; Li, S. High-throughput screening of tumor metastatic-related differential glycoprotein in hepatocellular carcinoma by iTRAQ combines lectin-related techniques. Med Oncol. 2013, 30, 420. [Google Scholar] [CrossRef]
- Ma, X.L.; Gao, X.H.; Gong, Z.J.; Wu, J.; Tian, L.; Zhang, C.Y.; Zhou, Y.; Sun, Y.F.; Hu, B.; Qiu, S.J.; et al. Apolipoprotein A1: A novel serum biomarker for predicting the prognosis of hepatocellular carcinoma after curative resection. Oncotarget 2016, 7, 70654–70668. [Google Scholar] [CrossRef]
- Mao, M.; Wang, X.; Sheng, H.; Liu, Y.; Zhang, L.; Dai, S.; Chi, P.D. A novel score based on serum apolipoprotein A-1 and C-reactive protein is a prognostic biomarker in hepatocellular carcinoma patients. BMC Cancer 2018, 18, 1178. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wei, X.; Ling, Q.; Cheng, J.; Zhou, B.; Xie, H.; Zhou, L.; Zheng, S. Identification of two portal vein tumor thrombosis associated proteins in hepatocellular carcinoma: Protein disulfide-isomerase A6 and apolipoprotein A-I. J. Gastroenterol. Hepatol. 2011, 26, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.; Rashid, A.; Wang, Y.; Jain, A.; Li, D.; Behari, A.; Kapoor, V.K.; Koay, E.J.; Chang, P.; Vauthey, J.N.; et al. RNA sequencing-based analysis of gallbladder cancer reveals the importance of the liver X receptor and lipid metabolism in gallbladder cancer. Oncotarget 2016, 7, 35302–35312. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, M.; Felix, K.; Hartmann, D.; Schnolzer, M.; Nees, M.; Vorderwulbecke, S.; Bogumil, R.; Buchler, M.W.; Friess, H. Identification of potential markers for the detection of pancreatic cancer through comparative serum protein expression profiling. Pancreas 2007, 34, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, W.; Wang, W.; Shen, H.; Liu, L.; Lou, W.; Wang, X.; Yang, P. A new panel of pancreatic cancer biomarkers discovered using a mass spectrometry-based pipeline. Br. J. Cancer 2017, 117, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Moore, K.; Phillips, L.; Boyle, F.M.; Marsh, D.J.; Baxter, R.C. Novel serum protein biomarker panel revealed by mass spectrometry and its prognostic value in breast cancer. Breast Cancer Res. 2014, 16, R63. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Esterni, B.; Bertucci, F.; Sauvan, R.; Chabannon, C.; Cubizolles, M.; Bardou, V.J.; Houvenaegel, G.; Jacquemier, J.; Granjeaud, S.; et al. Postoperative serum proteomic profiles may predict metastatic relapse in high-risk primary breast cancer patients receiving adjuvant chemotherapy. Oncogene 2006, 25, 981–989. [Google Scholar] [CrossRef] [PubMed]
- His, M.; Zelek, L.; Deschasaux, M.; Pouchieu, C.; Kesse-Guyot, E.; Hercberg, S.; Galan, P.; Latino-Martel, P.; Blacher, J.; Touvier, M. Prospective associations between serum biomarkers of lipid metabolism and overall, breast and prostate cancer risk. Eur. J. Epidemiol. 2014, 29, 119–132. [Google Scholar] [CrossRef]
- Lin, X.; Hong, S.; Huang, J.; Chen, Y.; Chen, Y.; Wu, Z. Plasma apolipoprotein A1 levels at diagnosis are independent prognostic factors in invasive ductal breast cancer. Discov. Med. 2017, 23, 247–258. [Google Scholar]
- Hamrita, B.; Ben Nasr, H.; Gabbouj, S.; Bouaouina, N.; Chouchane, L.; Chahed, K. Apolipoprotein A1 -75 G/A and +83 C/T polymorphisms: Susceptibility and prognostic implications in breast cancer. Mol. Biol. Rep. 2011, 38, 1637–1643. [Google Scholar] [CrossRef]
- Hsu, M.C.; Lee, K.T.; Hsiao, W.C.; Wu, C.H.; Sun, H.Y.; Lin, I.L.; Young, K.C. The dyslipidemia-associated SNP on the APOA1/C3/A5 gene cluster predicts post-surgery poor outcome in Taiwanese breast cancer patients: A 10-year follow-up study. BMC Cancer 2013, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Melnichouk, O.; Huszti, E.; Connelly, P.W.; Greenberg, C.V.; Minkin, S.; Boyd, N.F. Serum lipids, lipoproteins, and risk of breast cancer: A nested case-control study using multiple time points. J. Natl. Cancer Inst. 2015, 107, 32. [Google Scholar] [CrossRef] [PubMed]
- Zografos, E.; Anagnostopoulos, A.K.; Papadopoulou, A.; Legaki, E.; Zagouri, F.; Marinos, E.; Tsangaris, G.T.; Gazouli, M. Serum Proteomic Signatures of Male Breast Cancer. Cancer Genom. Proteom. 2019, 16, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Cine, N.; Baykal, A.T.; Sunnetci, D.; Canturk, Z.; Serhatli, M.; Savli, H. Identification of ApoA1, HPX and POTEE genes by omic analysis in breast cancer. Oncol. Rep. 2014, 32, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Yuan, Q.; Min, Y.L.; He, Y.; Xu, Q.H.; Li, B.; Shi, W.Q.; Lin, Q.; Li, Q.H.; Zhu, P.W.; et al. Apolipoprotein A1 and B as risk factors for development of intraocular metastasis in patients with breast cancer. Cancer Manag. Res. 2019, 11, 2881–2888. [Google Scholar] [CrossRef]
- Pendharkar, N.; Gajbhiye, A.; Taunk, K.; RoyChoudhury, S.; Dhali, S.; Seal, S.; Mane, A.; Abhang, S.; Santra, M.K.; Chaudhury, K.; et al. Quantitative tissue proteomic investigation of invasive ductal carcinoma of breast with luminal B HER2 positive and HER2 enriched subtypes towards potential diagnostic and therapeutic biomarkers. J. Proteom. 2016, 132, 112–130. [Google Scholar] [CrossRef]
- Clarke, C.H.; Yip, C.; Badgwell, D.; Fung, E.T.; Coombes, K.R.; Zhang, Z.; Lu, K.H.; Bast, R.C., Jr. Proteomic biomarkers apolipoprotein A1, truncated transthyretin and connective tissue activating protein III enhance the sensitivity of CA125 for detecting early stage epithelial ovarian cancer. Gynecol. Oncol. 2011, 122, 548–553. [Google Scholar] [CrossRef]
- Kozak, K.R.; Amneus, M.W.; Pusey, S.M.; Su, F.; Luong, M.N.; Luong, S.A.; Reddy, S.T.; Farias-Eisner, R. Identification of biomarkers for ovarian cancer using strong anion-exchange ProteinChips: Potential use in diagnosis and prognosis. Proc. Natl. Acad. Sci. USA 2003, 100, 12343–12348. [Google Scholar] [CrossRef]
- Kozak, K.R.; Su, F.; Whitelegge, J.P.; Faull, K.; Reddy, S.; Farias-Eisner, R. Characterization of serum biomarkers for detection of early stage ovarian cancer. Proteomics 2005, 5, 4589–4596. [Google Scholar] [CrossRef]
- Wegdam, W.; Argmann, C.A.; Kramer, G.; Vissers, J.P.; Buist, M.R.; Kenter, G.G.; Aerts, J.M.; Meijer, D.; Moerland, P.D. Label-free LC-MSe in tissue and serum reveals protein networks underlying differences between benign and malignant serous ovarian tumors. PLoS ONE 2014, 9, e108046. [Google Scholar] [CrossRef]
- Cruz, I.N.; Coley, H.M.; Kramer, H.B.; Madhuri, T.K.; Safuwan, N.A.; Angelino, A.R.; Yang, M. Proteomics Analysis of Ovarian Cancer Cell Lines and Tissues Reveals Drug Resistance-associated Proteins. Cancer Genom. Proteom. 2017, 14, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Tuft Stavnes, H.; Nymoen, D.A.; Hetland Falkenthal, T.E.; Kaern, J.; Trope, C.G.; Davidson, B. APOA1 mRNA expression in ovarian serous carcinoma effusions is a marker of longer survival. Am. J. Clin. Pathol. 2014, 142, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Farias-Eisner, G.; Su, F.; Robbins, T.; Kotlerman, J.; Reddy, S.; Farias-Eisner, R. Validation of serum biomarkers for detection of early- and late-stage endometrial cancer. Am. J. Obstet. Gynecol. 2010, 202, 73. [Google Scholar] [CrossRef] [PubMed]
- Rizner, T.L. Discovery of biomarkers for endometrial cancer: Current status and prospects. Expert Rev. Mol. Diagn. 2016, 16, 1315–1336. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Kikuchi, Y.; Asakawa, T.; Goto, T.; Kita, T.; Kudoh, K.; Kigawa, J.; Sakuragi, N.; Sakamoto, M.; Sugiyama, T.; et al. Identification of potential serum markers for endometrial cancer using protein expression profiling. J. Cancer Res. Clin. Oncol. 2010, 136, 475–481. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, X.; Wang, Y.; Zhao, J.; Shi, H.; Zhang, H.; Wang, Y.; Wei, Y.; Xue, W.; Zhang, J. Proteomic Screening for Serum Biomarkers for Cervical Cancer and Their Clinical Significance. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hao, Y.; Kamilijiang, M.; Hasimu, A.; Yuan, J.; Wu, G.; Reyimu, H.; Kadeer, N.; Abudula, A. Potential predictive plasma biomarkers for cervical cancer by 2D-DIGE proteomics and Ingenuity Pathway Analysis. Tumour Biol. 2015, 36, 1711–1720. [Google Scholar] [CrossRef]
- Alaiya, A.A.; Al-Mohanna, M.; Aslam, M.; Shinwari, Z.; Al-Mansouri, L.; Al-Rodayan, M.; Al-Eid, M.; Ahmad, I.; Hanash, K.; Tulbah, A.; et al. Proteomics-based signature for human benign prostate hyperplasia and prostate adenocarcinoma. Int. J. Oncol. 2011, 38, 1047–1057. [Google Scholar] [CrossRef]
- Davalieva, K.; Kiprijanovska, S.; Komina, S.; Petrusevska, G.; Zografska, N.C.; Polenakovic, M. Proteomics analysis of urine reveals acute phase response proteins as candidate diagnostic biomarkers for prostate cancer. Proteome Sci. 2015, 13, 2. [Google Scholar] [CrossRef]
- Chen, C.L.; Lin, T.S.; Tsai, C.H.; Wu, C.C.; Chung, T.; Chien, K.Y.; Wu, M.; Chang, Y.S.; Yu, J.S.; Chen, Y.T. Identification of potential bladder cancer markers in urine by abundant-protein depletion coupled with quantitative proteomics. J. Proteom. 2013, 85, 28–43. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chen, C.L.; Chen, H.W.; Chung, T.; Wu, C.C.; Chen, C.D.; Hsu, C.W.; Chen, M.C.; Tsui, K.H.; Chang, P.L.; et al. Discovery of novel bladder cancer biomarkers by comparative urine proteomics using iTRAQ technology. J. Proteome Res. 2010, 9, 5803–5815. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, C.; Wu, H.; Zhang, T.; Wang, J.; Wang, S.; Chang, J. Identification of Apo-A1 as a biomarker for early diagnosis of bladder transitional cell carcinoma. Proteome Sci. 2011, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Wang, J.; Wang, X.; Yan, H.; Cui, B.; Jia, C.; Wang, Q.; Cui, X.; Li, J.; Ou, T. Preoperative serum apolipoprotein A-I levels predict long-term survival in non-muscle-invasive bladder cancer patients. Cancer Manag. Res. 2018, 10, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Chinello, C.; Stella, M.; Piga, I.; Smith, A.J.; Bovo, G.; Varallo, M.; Ivanova, M.; Denti, V.; Grasso, M.; Grasso, A.; et al. Proteomics of liquid biopsies: Depicting RCC infiltration into the renal vein by MS analysis of urine and plasma. J. Proteom. 2019, 191, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; He, X.; Chen, Q.; Yang, G.; Yao, K.; Dong, P.; Ye, Y.; Chen, D.; Zhang, Z.; Qin, Z.; et al. The Effect of Preoperative Apolipoprotein A-I on the Prognosis of Surgical Renal Cell Carcinoma: A Retrospective Large Sample Study. Medicine 2016, 95, e3147. [Google Scholar] [CrossRef] [PubMed]
- Halton, J.M.; Nazir, D.J.; McQueen, M.J.; Barr, R.D. Blood lipid profiles in children with acute lymphoblastic leukemia. Cancer 1998, 83, 379–384. [Google Scholar] [CrossRef]
- Scribano, D.; Baroni, S.; Pagano, L.; Zuppi, C.; Leone, G.; Giardina, B. Return to normal values of lipid pattern after effective chemotherapy in acute lymphoblastic leukemia. Haematologica 1996, 81, 343–345. [Google Scholar]
- Egler, R.A.; Li, Y.; Dang, T.A.; Peters, T.L.; Leung, E.; Huang, S.; Russell, H.V.; Liu, H.; Man, T.K. An integrated proteomic approach to identifying circulating biomarkers in high-risk neuroblastoma and their potential in relapse monitoring. Proteom. Clin. Appl. 2011, 5, 532–541. [Google Scholar] [CrossRef]
- Naru, J.; Aggarwal, R.; Mohanty, A.K.; Singh, U.; Bansal, D.; Kakkar, N.; Agnihotri, N. Identification of differentially expressed proteins in retinoblastoma tumors using mass spectrometry-based comparative proteomic approach. J. Proteom. 2017, 159, 77–91. [Google Scholar] [CrossRef]
- Muntoni, S.; Atzori, L.; Mereu, R.; Satta, G.; Macis, M.D.; Congia, M.; Tedde, A.; Desogus, A.; Muntoni, S. Serum lipoproteins and cancer. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 218–225. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.L.; Wu, Y.T.; Wu, H.; Dai, W.; Arshad, B.; Xu, Z.; Li, H.; Wu, K.N.; Kong, L.Q. Status of lipid and lipoprotein in female breast cancer patients at initial diagnosis and during chemotherapy. Lipids Health Dis. 2018, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, C.; Zhang, G.; Wang, Y.; Zhang, Z.; Su, W.; Lyu, J. Association Between Pretreatment Serum Apolipoprotein A1 and Prognosis of Solid Tumors in Chinese Population: A Systematic Review and Meta-Analysis. Cell. Physiol. Biochem. 2018, 51, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X. Prognostic Significance of Pretreatment Apolipoprotein A-I as a Noninvasive Biomarker in Cancer Survivors: A Meta-Analysis. Dis. Markers 2018, 2018, 1034037. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.Q.; Wang, F.H.; Lei, X.F.; Yan, S.M.; Wang, D.S.; Zhang, F.; Xu, R.H.; Wang, L.Y.; Li, Y.H. Predictive value of chemotherapy-related high-density lipoprotein cholesterol (HDL) elevation in patients with colorectal cancer receiving adjuvant chemotherapy: An exploratory analysis of 851 cases. Oncotarget 2016, 7, 57290–57300. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, L.; Barchetti, A.; De Matteis, E.; Rossi, E.; Della Casa, L.; Marcheselli, L.; Tazzioli, G.; Lazzaretti, M.G.; Ficarra, G.; Federico, M.; et al. Identification of protein clusters predictive of response to chemotherapy in breast cancer patients. J. Proteome Res. 2009, 8, 4916–4933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, Y.; Hu, H.; Lan, P.; Wang, L.; Huang, M.; Kang, L.; Wu, X.; Wang, H.; Ling, J.; et al. Nomogram basing pre-treatment parameters predicting early response for locally advanced rectal cancer with neoadjuvant chemotherapy alone: A subgroup efficacy analysis of FOWARC study. Oncotarget 2016, 7, 5053–5062. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Weinschenk, T.; Stenzl, A.; Zdrojowy, R.; Pluzanska, A.; Szczylik, C.; Staehler, M.; Brugger, W.; Dietrich, P.Y.; Mendrzyk, R.; et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 2012, 18, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Guo, J.; Gu, J.; Wang, Z.; Wang, G.; Li, H.; Wang, J. Identifying the key genes and microRNAs in colorectal cancer liver metastasis by bioinformatics analysis and in vitro experiments. Oncol. Rep. 2019, 41, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Danilo, C.; Gutierrez-Pajares, J.L.; Mainieri, M.A.; Mercier, I.; Lisanti, M.P.; Frank, P.G. Scavenger receptor class B type I regulates cellular cholesterol metabolism and cell signaling associated with breast cancer development. Breast Cancer Res. 2013, 15, R87. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Pajares, J.L.; Ben Hassen, C.; Chevalier, S.; Frank, P.G. SR-BI: Linking Cholesterol and Lipoprotein Metabolism with Breast and Prostate Cancer. Front. Pharmacol. 2016, 7, 338. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Jin, H.; Pan, S.; Qian, Y.; Huang, C.; Zeng, Y.; Luo, Q.; Zeng, M.; Zhang, Z. Scavenger receptor B1 is a potential biomarker of human nasopharyngeal carcinoma and its growth is inhibited by HDL-mimetic nanoparticles. Theranostics 2013, 3, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Kozak, K.R.; Imaizumi, S.; Gao, F.; Amneus, M.W.; Grijalva, V.; Ng, C.; Wagner, A.; Hough, G.; Farias-Eisner, G.; et al. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 19997–20002. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, E.; Su, F.; Meriwether, D.; Devarajan, A.; Grijalva, V.; Gao, F.; Chattopadhyay, A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M.; et al. D-4F, an apoA-I mimetic peptide, inhibits proliferation and tumorigenicity of epithelial ovarian cancer cells by upregulating the antioxidant enzyme MnSOD. Int. J. Cancer 2012, 130, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.T.; Lu, H.; Pereira, S.A.; Monteiro, E.; Gabra, H.; Recchi, C. Anti-tumorigenic and Platinum-Sensitizing Effects of Apolipoprotein A1 and Apolipoprotein A1 Mimetic Peptides in Ovarian Cancer. Front. Pharmacol. 2018, 9, 1524. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Vasquez, S.X.; Su, F.; Roberts, S.; Shah, N.; Grijalva, V.; Imaizumi, S.; Chattopadhyay, A.; Ganapathy, E.; Meriwether, D.; et al. L-5F, an apolipoprotein A-I mimetic, inhibits tumor angiogenesis by suppressing VEGF/basic FGF signaling pathways. Integr. Biol. 2011, 3, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chattopadhyay, A.; Navab, M.; Grijalva, V.; Su, F.; Fogelman, A.M.; Reddy, S.T.; Farias-Eisner, R. Apolipoprotein A-I mimetic peptides inhibit expression and activity of hypoxia-inducible factor-1alpha in human ovarian cancer cell lines and a mouse ovarian cancer model. J. Pharmacol. Exp. Ther. 2012, 342, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Portoles, C.; Feliu, J.; Reglero, G.; Ramirez de Molina, A. ABCA1 overexpression worsens colorectal cancer prognosis by facilitating tumour growth and caveolin-1-dependent invasiveness, and these effects can be ameliorated using the BET inhibitor apabetalone. Mol. Oncol. 2018, 12, 1735–1752. [Google Scholar] [CrossRef]
- Su, F.; Grijalva, V.; Navab, K.; Ganapathy, E.; Meriwether, D.; Imaizumi, S.; Navab, M.; Fogelman, A.M.; Reddy, S.T.; Farias-Eisner, R. HDL mimetics inhibit tumor development in both induced and spontaneous mouse models of colon cancer. Mol. Cancer Ther. 2012, 11, 1311–1319. [Google Scholar] [CrossRef]
- Cedo, L.; Garcia-Leon, A.; Baila-Rueda, L.; Santos, D.; Grijalva, V.; Martinez-Cignoni, M.R.; Carbo, J.M.; Metso, J.; Lopez-Vilaro, L.; Zorzano, A.; et al. ApoA-I mimetic administration, but not increased apoA-I-containing HDL, inhibits tumour growth in a mouse model of inherited breast cancer. Sci. Rep. 2016, 6, 36387. [Google Scholar] [CrossRef]
- Peng, M.; Zhang, Q.; Cheng, Y.; Fu, S.; Yang, H.; Guo, X.; Zhang, J.; Wang, L.; Zhang, L.; Xue, Z.; et al. Apolipoprotein A-I mimetic peptide 4F suppresses tumor-associated macrophages and pancreatic cancer progression. Oncotarget 2017, 8, 99693–99706. [Google Scholar] [CrossRef]
- Zamanian-Daryoush, M.; Lindner, D.; Tallant, T.C.; Wang, Z.; Buffa, J.; Klipfell, E.; Parker, Y.; Hatala, D.; Parsons-Wingerter, P.; Rayman, P.; et al. The cardioprotective protein apolipoprotein A1 promotes potent anti-tumorigenic effects. J. Biol. Chem. 2013, 288, 21237–21252. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Yang, X.; Mukherjee, P.; Sulaiman, D.; Fogelman, H.R.; Grijalva, V.; Dubinett, S.; Wasler, T.C.; Paul, M.K.; Salehi-Rad, R.; et al. Treating the Intestine with Oral ApoA-I Mimetic Tg6F Reduces Tumor Burden in Mouse Models of Metastatic Lung Cancer. Sci. Rep. 2018, 8, 9032. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Grijalva, V.; Hough, G.; Su, F.; Mukherjee, P.; Farias-Eisner, R.; Anantharamaiah, G.M.; Faull, K.F.; Hwang, L.H.; Navab, M.; et al. Efficacy of tomato concentrates in mouse models of dyslipidemia and cancer. Pharmacol. Res. Perspect. 2015, 3, e00154. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Lin, Q.; Lai, R.; Chirieac, L.R.; Li, C.; Thomazy, V.A.; Grammatikakis, I.; Rassidakis, G.Z.; Zhang, W.; Fujio, Y.; Kunisada, K.; et al. Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines: Inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. Am. J. Pathol. 2005, 167, 969–980. [Google Scholar] [CrossRef]
- Cedo, L.; Reddy, S.T.; Mato, E.; Blanco-Vaca, F.; Escola-Gil, J.C. HDL and LDL: Potential New Players in Breast Cancer Development. J. Clin. Med. 2019, 8, 853. [Google Scholar] [CrossRef] [PubMed]
- Borlak, J.; Langer, F.; Chatterji, B. Serum proteome mapping of EGF transgenic mice reveal mechanistic biomarkers of lung cancer precursor lesions with clinical significance for human adenocarcinomas. Biochim. Et Biophys. Acta Mol. Basis Dis. 2018, 1864, 3122–3144. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.K.; Lee, H.; Zhou, J.; Liu, S.C.; Loh, M.C.; So, J.B.; Lim, K.H.; Yeoh, K.G.; Lim, Y.P. Reduced plasma APOA1 level is associated with gastric tumor growth in MKN45 mouse xenograft model. J. Proteom. 2010, 73, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Pope, I.; Sharma, A.; Matthias, M.; Doran, K.S.; Obonyo, M. Effect of myeloid differentiation primary response gene 88 on expression profiles of genes during the development and progression of Helicobacter-induced gastric cancer. BMC Cancer 2017, 17, 133. [Google Scholar] [CrossRef]
- Takaishi, S.; Wang, T.C. Gene expression profiling in a mouse model of Helicobacter-induced gastric cancer. Cancer Sci. 2007, 98, 284–293. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Dapito, D.H.; Mencin, A.; Gwak, G.Y.; Pradere, J.P.; Jang, M.K.; Mederacke, I.; Caviglia, J.M.; Khiabanian, H.; Adeyemi, A.; Bataller, R.; et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 2012, 21, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Fukata, M.; Chen, A.; Vamadevan, A.S.; Cohen, J.; Breglio, K.; Krishnareddy, S.; Hsu, D.; Xu, R.; Harpaz, N.; Dannenberg, A.J.; et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology 2007, 133, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Mineo, C.; Shaul, P.W. Novel biological functions of high-density lipoprotein cholesterol. Circ. Res. 2012, 111, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Liu, Y.; Kessler, P.S.; Vaughan, A.M.; Oram, J.F. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J. Biol. Chem. 2009, 284, 32336–32343. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Sci. 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Sag, D.; Cekic, C.; Wu, R.; Linden, J.; Hedrick, C.C. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat. Commun. 2015, 6, 6354. [Google Scholar] [CrossRef] [PubMed]
- Zamanian-Daryoush, M.; Lindner, D.J.; DiDonato, J.A.; Wagner, M.; Buffa, J.; Rayman, P.; Parks, J.S.; Westerterp, M.; Tall, A.R.; Hazen, S.L. Myeloid-specific genetic ablation of ATP-binding cassette transporter ABCA1 is protective against cancer. Oncotarget 2017, 8, 71965–71980. [Google Scholar] [CrossRef] [PubMed]
- Degoma, E.M.; Rader, D.J. Novel HDL-directed pharmacotherapeutic strategies. Nat. Rev. Cardiol. 2011, 8, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.N.; Hong, C.; Chen, M.; Joseph, S.B.; Wilpitz, D.C.; Wang, X.; Lusis, A.J.; Collins, A.; Hseuh, W.A.; Collins, J.L.; et al. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J. Clin. Investig. 2007, 117, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.B.; Bury, M.J.; Cheung, M.; Cichy-Knight, M.A.; Dowdell, S.E.; Dunn, A.K.; Lee, D.; Lieby, J.A.; Moore, M.L.; Scherzer, D.A.; et al. Discovery of potent, selective sulfonylfuran urea endothelial lipase inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Hoeg, J.M.; Santamarina-Fojo, S.; Berard, A.M.; Cornhill, J.F.; Herderick, E.E.; Feldman, S.H.; Haudenschild, C.C.; Vaisman, B.L.; Hoyt, R.F., Jr.; Demosky, S.J., Jr.; et al. Overexpression of lecithin:cholesterol acyltransferase in transgenic rabbits prevents diet-induced atherosclerosis. Proc. Natl. Acad. Sci. USA 1996, 93, 11448–11453. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Yonemori, F.; Wakitani, K.; Minowa, T.; Maeda, K.; Shinkai, H. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature 2000, 406, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Tsunoda, T.; Tuzcu, E.M.; Schoenhagen, P.; Cooper, C.J.; Yasin, M.; Eaton, G.M.; Lauer, M.A.; Sheldon, W.S.; Grines, C.L.; et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. J. Am. Med. Assoc. 2003, 290, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Torguson, R.; Kent, K.M.; Pichard, A.D.; Suddath, W.O.; Satler, L.F.; Martin, B.D.; Perlman, T.J.; Maltais, J.A.; Weissman, N.J.; et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 2010, 55, 2727–2735. [Google Scholar] [CrossRef]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; Fogelman, A.M. Apolipoprotein A-I mimetic peptides and their role in atherosclerosis prevention. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 540–547. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Navab, M.; Hough, G.; Gao, F.; Meriwether, D.; Grijalva, V.; Springstead, J.R.; Palgnachari, M.N.; Namiri-Kalantari, R.; Su, F.; et al. A novel approach to oral apoA-I mimetic therapy. J. Lipid Res. 2013, 54, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. The structure/function of apoprotein A-I mimetic peptides: An update. Curr. Opin. Endocrinol. Diabetesand Obes. 2014, 21, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Ditiatkovski, M.; D’Souza, W.; Kesani, R.; Chin-Dusting, J.; De Haan, J.B.; Remaley, A.; Sviridov, D. An apolipoprotein A-I mimetic peptide designed with a reductionist approach stimulates reverse cholesterol transport and reduces atherosclerosis in mice. PLoS ONE 2013, 8, e68802. [Google Scholar] [CrossRef] [PubMed]
- Karalis, I.; Jukema, J.W. HDL Mimetics Infusion and Regression of Atherosclerosis: Is It Still Considered a Valid Therapeutic Option? Curr. Cardiol. Rep. 2018, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Lin, X.J.; Bastian, I.N.; Brain, J.; Burt, A.D.; Aksenov, A.A.; Vrbanac, A.F.; Li, W.; Perkins, A.; Matsutani, T.; et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 2017, 551, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Lu, M.; Jung, K.H.; Park, J.H.; Yu, L.; Onuchic, J.N.; Kaipparettu, B.A.; Levine, H. Elucidating cancer metabolic plasticity by coupling gene regulation with metabolic pathways. Proc. Natl. Acad. Sci. USA 2019, 116, 3909–3918. [Google Scholar] [CrossRef] [PubMed]
- Korolainen, M.A.; Nyman, T.A.; Nyyssonen, P.; Hartikainen, E.S.; Pirttila, T. Multiplexed proteomic analysis of oxidation and concentrations of cerebrospinal fluid proteins in Alzheimer disease. Clin. Chem. 2007, 53, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Revilla, G.; Corcoy, R.; Moral, A.; Escola-Gil, J.C.; Mato, E. Cross-Talk between Inflammatory Mediators and the Epithelial Mesenchymal Transition Process in the Development of Thyroid Carcinoma. Int. J. Mol. Sci. 2019, 20, 2466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Prijic, S.; Urban, B.C.; Tisza, M.J.; Zuo, Y.; Li, L.; Tan, Z.; Chen, X.; Mani, S.A.; Chang, J.T. Candidate Antimetastasis Drugs Suppress the Metastatic Capacity of Breast Cancer Cells by Reducing Membrane Fluidity. Cancer Res. 2016, 76, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Tuaine, J.; McLaren, B.; Waters, D.L.; Black, K.; Jones, L.M.; McCormick, S.P. Chemotherapy Agents Alter Plasma Lipids in Breast Cancer Patients and Show Differential Effects on Lipid Metabolism Genes in Liver Cells. PLoS ONE 2016, 11, e0148049. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Grunfeld, C. The role of HDL in innate immunity. J. Lipid Res. 2011, 52, 1–3. [Google Scholar] [CrossRef] [PubMed]

| Organ | Type of Cancer | Association of ApoA-I Levels with: | References | ||||
|---|---|---|---|---|---|---|---|
| Risk for the Development of Cancer | Cancer at Primary Diagnosis | Cancer Progression/Metastasis | Cancer Prognosis | ||||
| head & neck | squamous cell cancer | + | [79] | ||||
| nasopharyngeal carcinoma | − | − | [80,81,82] | ||||
| lung | non-small cell carcinoma | − | − | − (+) | − | [74,83,84,85,86,87] | |
| esophagus | squamous cell carcinoma | − | − | [88,89] | |||
| stomach | gastric cancer | − (+) | [90,91] | ||||
| colon | adenocarcinoma | − | − | − (+) | − | [76,92,93,94,95,96,97] | |
| liver | hepatocellular carcinoma | − | − | − | [69,70,98,99,100,101,102] [69,70,98,99,100,101,102] | ||
| gallbladder | adenocarcinoma | − | [103] | ||||
| pancreas | adenocarcinoma | − | [104,105] | ||||
| breast | adenocarcinoma | − (+) | − | − | − | [106,107,108,109,110,111,112,113,114,115,116] | |
| ovary | ovarian carcinoma | − | − | [117,118,119,120,121,122] | |||
| uterus | endometrial carcinoma | − | [123,124,125] | ||||
| cervix | cervical squamous cell carcinoma | − | − | [126,127] | |||
| prostate | adenocarcinoma | − | − | [78,128,129] | |||
| bladder | transitional cell carcinoma, | − (+) | − | − | [130,131,132,133] | ||
| kidney | renal cell carcinoma | − | [134,135] | ||||
| hematopoietic/lymphoid system | leukemia/lymphoma | − | − | − | [77,136,137] | ||
| neural tumors | neuroblastoma | − | − | [138] | |||
| retinoblastoma | + | [139] | |||||
| Type of Cancer | In Vitro System | Apo A-I Manipulation | Biologic Effect and Associated Mechanisms | Ref. |
|---|---|---|---|---|
| ovarian carcinoma (OC) | murine ovarian cell line ID8 | treatment with human ApoA-I or ApoA-I mimetics (L-5F and L-4F) | ↓ viability and proliferation | [152] |
| ↓ LPA-induced viability | ||||
| murine ovarian cell line ID8 | treatment with the ApoA-I mimetic D-4F | ↓ viability and proliferation | [153] | |
| ↓ oxidative stress | ||||
| ↑ MnSOD expression and activity | ||||
| cis-platinum–resistant human ovarian cell lines (OVCAR5, SKOV3, OV2008, and A2780) | treatment with the ApoA-I mimetic L-4F | ↓ viability and invasiveness | [154] | |
| ↓ AKT activation | ||||
| cis-platinum-resistant human ovarian cell lines (SKOV3, OV2008) | treatment with the ApoA-I mimetic L-5F | ↓ LPA-induced cell viability and VEGF production | [155] | |
| human ovarian cancer cell lines (OV2008, CAOV-3 and SKOV3) | treatment with the ApoA-I mimetics L-4F and L-5F | ↑ proteasome-dependent protein degradation of HIF 1α | [156] | |
| ↓ ROS production | ||||
| hepatocellular carcinoma (HCC) | human HCC cell lines (MHCC97H and Huh7) | treatment with recombinant ApoA-I | ↓ proliferation (cell cycle arrest) | [100] |
| ↑ apoptosis | ||||
| ↓ MMP2/9 | ||||
| ↓ VEGF inhibition of the MAPK signaling pathway | ||||
| colon adenocarcinoma (CA) | human CA cell lines (DLD-1 and Caco-2) overexpressing ABCA1 | transgenic overexpression of ApoA-I, treatment with recombinant ApoA-I or apabetalone (a BET inhibitor, inducer of ApoA-I production) | ↓ cell proliferation, migration and invasion | [157] |
| modulation of ABCA1 expression through COX-2 downregulation | ||||
| compensation for ABCA1-dependent excessive export of cholesterol | ||||
| murine CA cell line, CT26 | treatment with the ApoA-I mimetic L-4F | ↓ viability and proliferation | [158] | |
| ↓ cyclin D1 and cyclin A protein levels | ||||
| ↓ LPA-induced viability | ||||
| breast adenocarcinoma (BA) | human CA cell line, MCF-7 | treatment with the ApoA-I mimetic D-4F | ↓ oxLDL-induced proliferation | [159] |
| pancreatic adenocarcinoma (PA) | murine PA cell line P7 | treatment with the ApoA-I mimetic L-4F | none | [160] |
| Type of Cancer | Animal Model | Apo A-I Manipulation | Biologic Effect and Associated Alterations | Ref. |
|---|---|---|---|---|
| melanoma and non-small lung carcinoma | syngeneic murine melanoma (B16F10L), human melanoma (A375) and Lewis lung (murine) carcinoma cells engrafted subcutaneously or injected intravenously in a metastatic cancer mouse model | human ApoA-I transgenic overexpression or injection of human ApoA-I | ↓ tumor growth and metastasis | [161] |
| ↑ survival | ||||
| ↓ tumor angiogenesis | ||||
| ↓ MMP-9 | ||||
| ↓ surviving modulation of the tumor immune microenvironment: | ||||
| ↓ M2 Mφ | ||||
| ↑ M2 Mφ | ||||
| ↓ MDSCs | ||||
| ↑ TILs | ||||
| ApoA-I KO | the opposite effects | |||
| ovarian carcinoma | syngeneic murine ovarian carcinoma cells (ID-8) engrafted subcutaneously or injected intraperitoneally in mice | transgenic overexpression of human ApoA-I, or treatment with ApoA-I mimetic peptides (L-5F, L-4F, D-4F) | ↓ tumor growth | [152] |
| ↑ survival | ||||
| ovarian carcinoma | syngeneic murine ovarian carcinoma cells (ID-8) engrafted subcutaneously in mice | treatment with ApoA-I mimetic peptides (L-5F, L-4F, D-4F) | ↓ tumor growth | [152,153,155,156] |
| ↓ LPA serum levels | ||||
| ↓ tumor angiogenesis | ||||
| ↓ VEGF (L-5F) | ||||
| ↓ HIF-1α expression (L-4F) | ||||
| ↑ MnSOD (D-4F) | ||||
| ↓ oxidized phospholipids | ||||
| colon adenocarcinoma | AOM/DSS-induced murine colorectal adenocarcinomas | ApoA-I haploinsufficiency Apo A-I(+/−) | ↑ tumor growth and altered tumor distribution (proximal extension) | [61] |
| ↓ survival | ||||
| ↑ inflammation | ||||
| ↑ tumor cell proliferation | ||||
| ↑ IL-6, pSTAT3, NF-kB signaling | ||||
| colon adenocarcinoma | syngeneic murine colon adenocarcinoma cells CT26 engrafted subcutaneously in mice | treatment with the ApoA-I mimetic peptide L-4F | ↓ tumor growth | [158] |
| ↓ LPA serum levels | ||||
| a murine model for familial adenomatous polyposis (APC−/+) | ↓ number and size of colon polyps | |||
| colon adenocarcinoma and non-small lung carcinoma | syngeneic murine colon adenocarcinoma (CT26) and Lewis lung carcinoma cells injected intravenously in a metastatic lung mouse carcinoma model | treatment with a concentrate of transgenic tomatoes expressing the ApoA-I mimetic peptide 6F | ↓ number of tumors in the lung | [162] |
| ↓ Notch signaling | ||||
| ↓ oxidized phospholipids | ||||
| ↑ osteopontin | ||||
| ↓ MDSCs in lung and intestine tissues | ||||
| colon and ovarian adenocarcinoma | syngeneic murine ovarian carcinoma cells (ID-8) engrafted intraperitoneally and colon adenocarcinoma cells (CT26) injected intravenously in a metastatic lung carcinoma mouse model | treatment with a concentrate of transgenic tomatoes expressing the ApoA-I mimetic peptide 6F | ↓ tumor growth in the abdomen | [163] |
| ↓ number of tumors in the lung | ||||
| pancreatic adenocarcinoma | syngeneic murine pancreatic adenocarcinoma cells line P7 orthotopically engrafted in mice | treatment with the ApoA-I mimetic peptide L-4F | ↓ tumor growth in the abdomen | [160] |
| ↓ M2 Mφ in tumors | ||||
| breast adenocarcinoma | mammary tumour virus-polyoma middle T-antigen transgenic (PyMT) mice | treatment with the ApoA-I mimetic peptide D-4F | ↑ latency of tumor appearance | [159] |
| ↓ tumor growth | ||||
| ↓ oxidized LDL plasma levels | ||||
| transgenic overexpression of human ApoA-I in PyMT mice | none |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgila, K.; Vyrla, D.; Drakos, E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers 2019, 11, 1097. https://doi.org/10.3390/cancers11081097
Georgila K, Vyrla D, Drakos E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers. 2019; 11(8):1097. https://doi.org/10.3390/cancers11081097
Chicago/Turabian StyleGeorgila, Konstantina, Dimitra Vyrla, and Elias Drakos. 2019. "Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer" Cancers 11, no. 8: 1097. https://doi.org/10.3390/cancers11081097
APA StyleGeorgila, K., Vyrla, D., & Drakos, E. (2019). Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers, 11(8), 1097. https://doi.org/10.3390/cancers11081097




