Novel Antibody-Drug Conjugate with Anti-CD26 Humanized Monoclonal Antibody and Transcription Factor IIH (TFIIH) Inhibitor, Triptolide, Inhibits Tumor Growth via Impairing mRNA Synthesis
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of Triptolide and TR-1 against MM and Leukemia Cell Lines
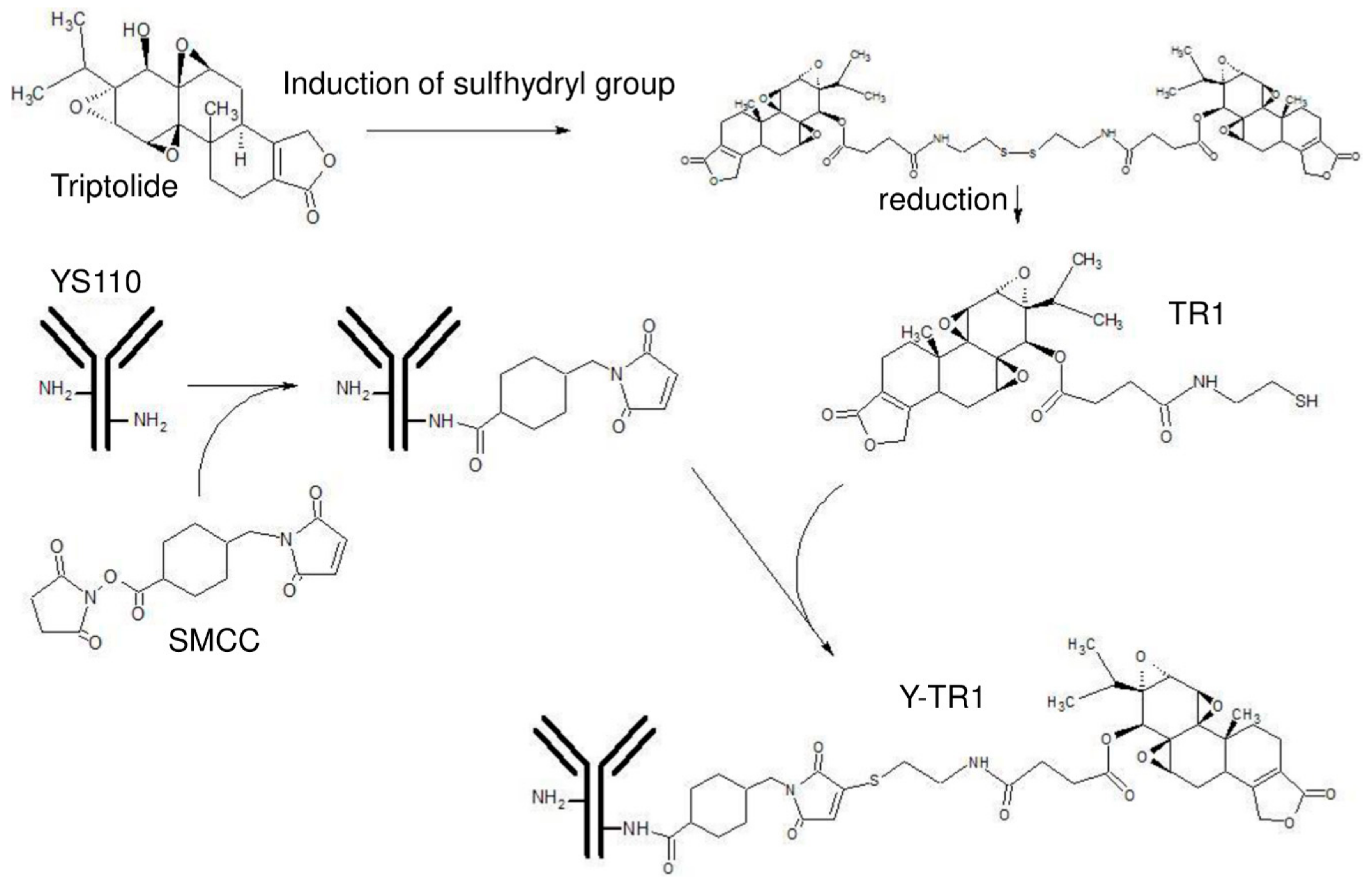
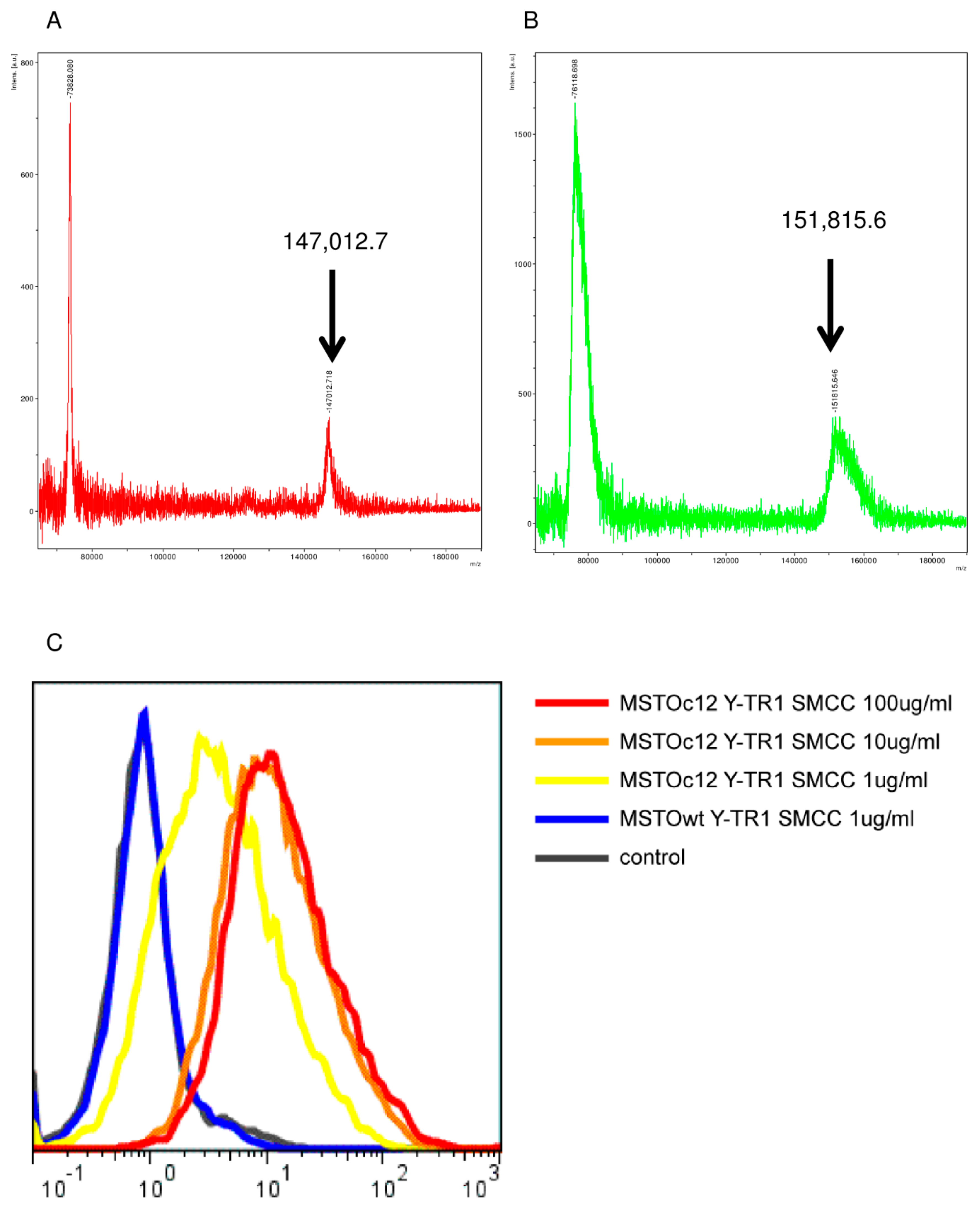
2.2. Conjugation of YS110 and TR-1, the Drug Antibody Ratio of Y-TR, and the Binding Activity of Y-TR1 to CD26-Positive Cell Line
2.3. In Vitro Cytotoxicity of Y-TR1 against MM and Leukemia Cell Lines
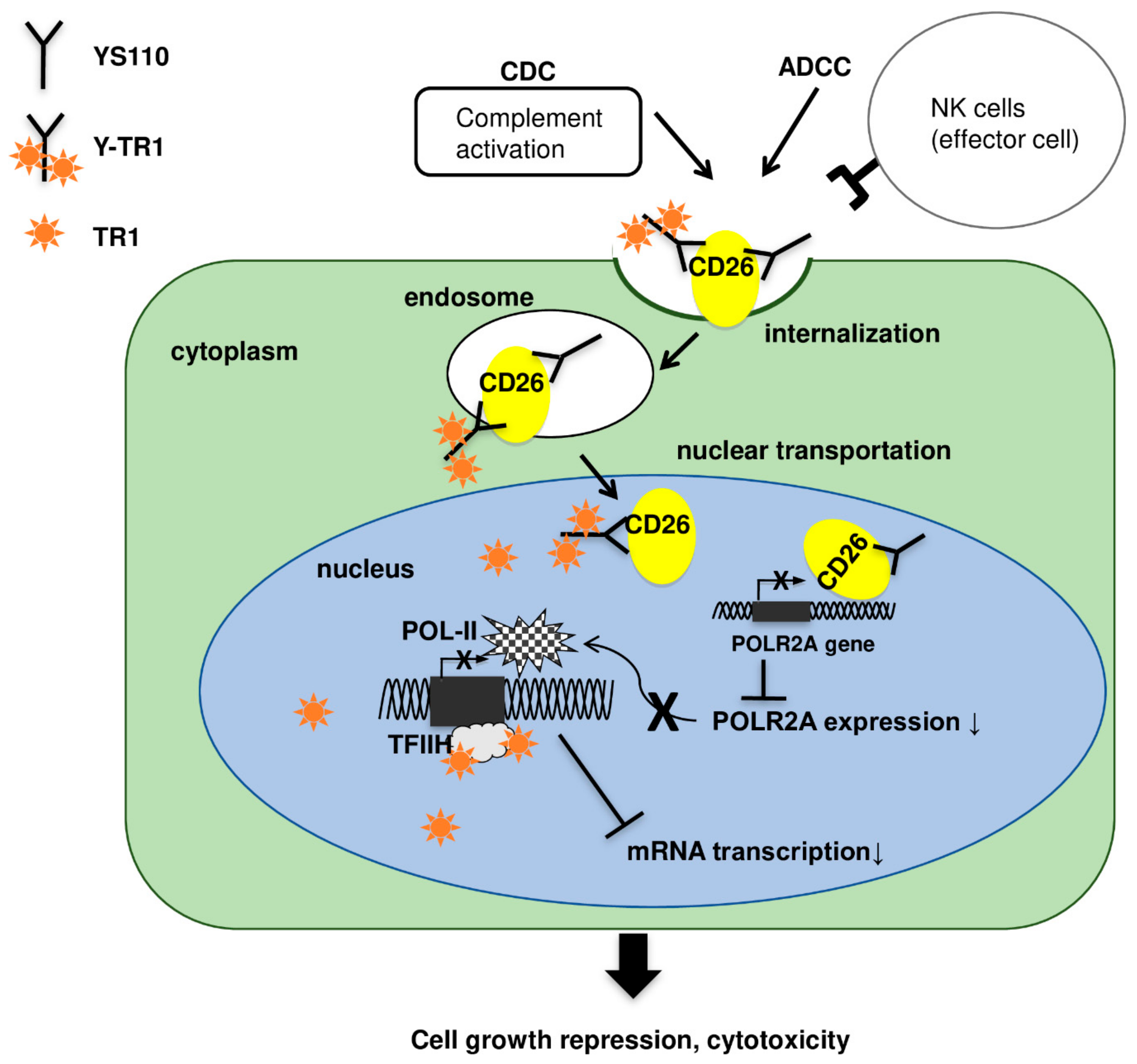
2.4. Nucler Translocation of Y-TR1
2.5. Apoptosis Assay
2.6. Effects of Triptolide and Y-TR1 on Heat Induction of HSP70 in CD26-Positive MM Cells
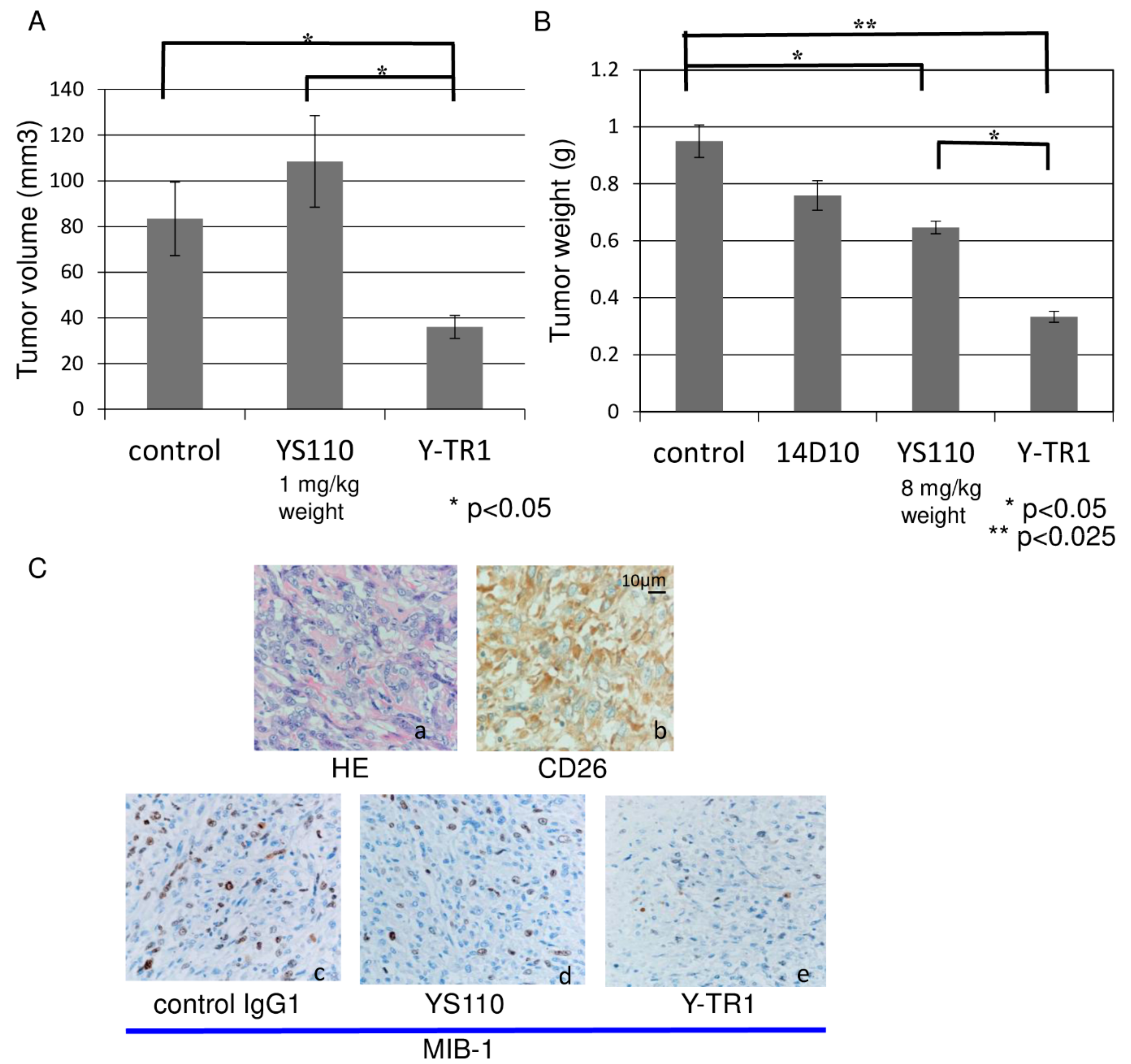
2.7. In Vivo Anti-Tumor Effect of Y-TR1
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Conjugation Protocols
4.3. Cell Culture
4.4. Mass Spectrometry Assay
4.5. Binding Assay
4.6. Cytotoxicity Assay
4.7. Western Blotting
4.8. Immunofluorescence Staining
4.9. Apoptosis Assay
4.10. Heat Induction of HSP70 and Real Time PCR Assay
4.11. In Vivo Efficacy Assay and Toxicity Study
4.12. Statistics
4.13. Study Approval
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rasmussen, H.B.; Branner, S.; Wiberg, F.C.; Wagtmann, N. Crystal structure of human dipeptidyl peptidase IV/CD26 in complex with a substrate analog. Nat. Struct. Biol. 2003, 10, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Havre, P.A.; Abe, M.; Urasaki, Y.; Ohnuma, K.; Morimoto, C.; Dang, N.H. The role of CD26/dipeptidyl peptidase IV in cancer. Front. Biosci. 2008, 13, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, T.; Yamada, T.; Ohnuma, K.; Kina, S.; Takahashi, N.; Yamochi, T.; Inamoto, S.; Katsuoka, Y.; Hosono, O.; Tanaka, H.; et al. Humanized anti-CD26 monoclonal antibody as a treatment for malignant mesothelioma tumors. Clin. Cancer Res. 2007, 13, 4191–4200. [Google Scholar] [CrossRef] [PubMed]
- Pang, R.; Law, W.L.; Chu, A.C.; Poon, J.T.; Lam, C.S.; Chow, A.K.; Ng, L.; Cheung, L.W.; Lan, X.R.; Lan, H.Y.; et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell 2010, 6, 603–615. [Google Scholar] [CrossRef]
- Ghani, F.I.; Yamazaki, H.; Iwata, S.; Okamoto, T.; Aoe, K.; Okabe, K.; Mimura, Y.; Fujimoto, N.; Kishimoto, T.; Yamada, T.; et al. Identification of cancer stem cell markers in human malignant mesothelioma cells. Biochem. Biophys. Res. Commun. 2011, 404, 735–742. [Google Scholar] [CrossRef]
- Aoe, K.; Amatya, V.J.; Fujimoto, N.; Ohnuma, K.; Hosono, O.; Hiraki, A.; Fujii, M.; Yamada, T.; Dang, N.H.; Takeshima, Y.; et al. CD26 overexpression is associated with prolonged survival and enhanced chemosensitivity in malignant pleural mesothelioma. Clin. Cancer Res. 2012, 18, 1447–1456. [Google Scholar] [CrossRef]
- Amatya, V.J.; Takeshima, Y.; Kushitani, K.; Yamada, T.; Morimoto, C.; Inai, K. Overexpression of CD26/DPPIV in mesothelioma tissue and mesothelioma cell lines. Oncol. Rep. 2011, 26, 1369–1375. [Google Scholar] [CrossRef] [Green Version]
- Inamoto, T.; Yamochi, T.; Ohnuma, K.; Iwata, S.; Kina, S.; Inamoto, S.; Tachibana, M.; Katsuoka, Y.; Dang, N.H.; Morimoto, C. Anti-CD26 monoclonal antibody-mediated G1-S arrest of human renal clear cell carcinoma Caki-2 is associated with retinoblastoma substrate dephosphorylation, cyclin-dependent kinase 2 reduction, p27(kip1) enhancement, and disruption of binding to the extracellular matrix. Clin. Cancer Res. 2006, 12, 3470–3477. [Google Scholar]
- Ho, L.; Aytac, U.; Stephens, L.C.; Ohnuma, K.; Mills, G.B.; McKee, K.S.; Neumann, C.; LaPushin, R.; Cabanillas, F.; Abbruzzese, J.L.; et al. In vitro and in vivo antitumor effect of the anti-CD26 monoclonal antibody 1F7 on human CD30+ anaplastic large cell T-cell lymphoma Karpas 299. Clin. Cancer Res. 2001, 7, 2031–2040. [Google Scholar]
- Angevin, E.; Isambert, N.; Trillet-Lenoir, V.; You, B.; Alexandre, J.; Zalcman, G.; Vielh, P.; Farace, F.; Valleix, F.; Podoll, T.; et al. First-in-human phase 1 of YS110, a monoclonal antibody directed against CD26 in advanced CD26-expressing cancers. Br. J. Cancer 2017, 116, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hayashi, M.; Du, W.; Ohnuma, K.; Sakamoto, M.; Morimoto, C.; Yamada, T. Localization of CD26/DPPIV in nucleus and its nuclear translocation enhanced by anti-CD26 monoclonal antibody with anti-tumor effect. Cancer Cell Int. 2009, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hayashi, M.; Madokoro, H.; Nishida, H.; Du, W.; Ohnuma, K.; Sakamoto, M.; Morimoto, C.; Yamada, T. Nuclear Localization of CD26 Induced by a Humanized Monoclonal Antibody Inhibits Tumor Cell Growth by Modulating of POLR2A Transcription. PLoS ONE 2013, 8, e62304. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.L.; Yang, Y.X.; Ding, J.; Li, Y.C.; Miao, Z.H. Triptolide: Structural modifications, structure-activity relationships, bioactivities, clinical development and mechanisms. Nat. Prod. Rep. 2012, 29, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gao, X.; Shilatifard, A. Stably paused genes revealed through inhibition of transcription initiation by the TFIIH inhibitor triptolide. Genes Dev. 2015, 29, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titov, D.V.; Gilman, B.; He, Q.L.; Bhat, S.; Low, W.K.; Dang, Y.; Smeaton, M.; Demain, A.L.; Miller, P.S.; Kugel, J.F.; et al. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat. Chem. Biol. 2011, 7, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, P.A.; Dudeja, V.; McCarroll, J.A.; Borja-Cacho, D.; Dawra, R.K.; Grizzle, W.E.; Vickers, S.M.; Saluja, A.K. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007, 67, 9407–9416. [Google Scholar] [CrossRef]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef]
- Wintzerith, M.; Acker, J.; Vicaire, S.; Vigneron, M.; Kedinger, C. Complete sequence of the human RNA polymerase II largest subunit. Nucleic Acids Res. 1992, 20, 910. [Google Scholar] [CrossRef]
- Mook, O.R.; Baas, F.; de Wissel, M.B.; Fluiter, K. Allele-specific cancer cell killing in vitro and in vivo targeting a single-nucleotide polymorphism in POLR2A. Cancer Gene Ther. 2009, 16, 532–538. [Google Scholar] [CrossRef] [Green Version]
- Sudo, H.; Tsuji, A.B.; Sugyo, A.; Kohda, M.; Sogawa, C.; Yoshida, C.; Harada, Y.N.; Hino, O.; Saga, T. Knockdown of COPA, identified by loss-of-function screen, induces apoptosis and suppresses tumor growth in mesothelioma mouse model. Genomics 2010, 95, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Nitta, M.; Kuninaka, S.; Zhang, D.; Fujiwara, T.; Taya, Y.; Nakao, M.; Saya, H. Transcriptional blockade induces p53-dependent apoptosis associated with translocation of p53 to mitochondria. J. Biol. Chem. 2005, 280, 19166–19176. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Madokoro, H.; Yamada, K.; Nishida, H.; Morimoto, C.; Sakamoto, M.; Yamada, T. A humanized anti-CD26 monoclonal antibody inhibits cell growth of malignant mesothelioma via retarded G2/M cell cycle transition. Cancer Cell Int. 2016, 16, 35. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Han, C.; Wan, G.; Huang, X.; Ivan, C.; Jiang, D.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Rao, P.H.; et al. TP53 loss creates therapeutic vulnerability in colorectal cancer. Nature 2015, 520, 697–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letschert, K.; Faulstich, H.; Keller, D.; Keppler, D. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol. Sci. 2006, 91, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauer, G.; Salnikov, A.V.; Lüttgau, S.; Herr, I.; Anderl, J.; Faulstich, H. Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma. J. Natl. Cancer Inst. 2012, 104, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.F.; Mairinger, F.D.; Ting, S.; Vollbrecht, C.; Mairinger, T.; Theegarten, D.; Christoph, D.C.; Schmid, K.W.; Wohlschlaeger, J. MDM2 is an important prognostic and predictive factor for platin-pemetrexed therapy in malignant pleural mesotheliomas and deregulation of P14/ARF (encoded by CDKN2A) seems to contribute to an MDM2-driven inactivation of P53. Br. J. Cancer 2015, 112, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.P.; Weiner, L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Lien, H.C.; Xia, W.; Chen, I.F.; Lo, H.W.; Wang, Z.; Ali-Seyed, M.; Lee, D.F.; Bartholomeusz, G.; Ou-Yang, F.; et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell 2004, 6, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, R.J.; Sharma, S.K.; Springer, C.; Green, A.J.; Hope-Stone, L.D.; Sena, L.; Martin, J.; Adamson, K.L.; Robbins, A.; Gumbrell, L.; et al. A phase I trial of antibody directed enzyme prodrug therapy (ADEPT) in patients with advanced colorectal carcinoma or other CEA producing tumours. Br. J. Cancer 2002, 87, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J.; Wilson, W.H.; Bergeron, K.; Raggio, M.; Stetler-Stevenson, M.; FitzGerald, D.J.; Pastan, I. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N. Engl. J. Med. 2001, 345, 241–247. [Google Scholar] [CrossRef]
- Sievers, E.L.; Appelbaum, F.R.; Spielberger, R.T.; Forman, S.J.; Flowers, D.; Smith, F.O.; Shannon-Dorcy, K.; Berger, M.S.; Bernstein, I.D. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: A phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood 1999, 93, 3678–3684. [Google Scholar] [PubMed]
- Weiner, L.M.; Murray, J.C.; Shuptrine, C.W. Antibody-based immunotherapy of cancer. Cell 2012, 148, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.I.; Witzig, T.; Molina, A.; Czuczman, M.; Emmanouilides, C.; Joyce, R.; Vo, K.; Theuer, C.; Pohlman, B.; Bartlett, N.; et al. Yttrium 90-labeled ibritumomab tiuxetan radioimmunotherapy produces high response rates and durable remissions in patients with previously treated B-cell lymphoma. Clin. Lymphoma 2004, 5, 98–101. [Google Scholar] [CrossRef]
- Rakowicz-Szulczynska, E.M.; Koprowski, H. Nuclear uptake of monoclonal antibody to a surface glycoprotein and its effect on transcription. Arch. Biochem. Biophys. 1989, 271, 366–379. [Google Scholar] [CrossRef]
- Rakowicz-Szulczynska, E.M.; Steplewski, Z.; Koprowski, H. Nuclear translocation of monoclonal antibody directed against cell-surface carbohydrate Y determinant. Am. J. Pathol. 1992, 141, 937–947. [Google Scholar] [PubMed]
- Shao, S.; Tsai, M.H.; Lu, J.; Yu, T.; Jin, J.; Xiao, D.; Jiang, H.; Han, M.; Wang, M.; Wang, J. Site-specific and hydrophilic ADCs through disulfide-bridged linker and branched PEG. Bioorg. Med. Chem. Lett. 2018, 28, 1363–1370. [Google Scholar] [CrossRef]
- Xu, L.; Qiu, Y.; Xu, H.; Ao, W.; Lam, W.; Yang, X. Acute and subacute toxicity studies on triptolide and triptolide-loaded polymeric micelles following intravenous administration in rodents. Food Chem. Toxicol. 2013, 57, 371–379. [Google Scholar] [CrossRef]
- Tanaka, T.; Kameoka, J.; Yaron, A.; Schlossman, S.F.; Morimoto, C. The costimulatory activity of the CD26 antigen requires dipeptidyl peptidase IV enzymatic activity. Proc. Natl. Acad. Sci. USA 1993, 90, 4586–4590. [Google Scholar] [CrossRef]




| Cell Line | Origin | CD26 | IC50 of Triptolide (nM) | IC50 of TR1 (nM) | IC50 of Y-TR1 (μg/mL) |
|---|---|---|---|---|---|
| MSTO wt | Mesothelioma | (-) | 10 | 250 | 35 |
| MSTO clone12 | Mesothelioma | (+) | 10 | 250 | 15 |
| JMN | Mesothelioma | (+) | 15 | ND | 30 |
| Jurkat (-) | Leukemia | (-) | >100 | ND | >100 |
| Jurkat CD26(+) | Leukemia | (+) | 6 | ND | 30 |
| Conjugate | IC50 (μg/mL) |
|---|---|
| Y-TR1 SPDP | 35 |
| Y-TR1 GMBS | 18 |
| Y-TR1 SMCC | 15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, M.; Madokoro, H.; Yamada, K.; Nishida, H.; Morimoto, C.; Sakamoto, M.; Yanagawa, H.; Yamada, T. Novel Antibody-Drug Conjugate with Anti-CD26 Humanized Monoclonal Antibody and Transcription Factor IIH (TFIIH) Inhibitor, Triptolide, Inhibits Tumor Growth via Impairing mRNA Synthesis. Cancers 2019, 11, 1138. https://doi.org/10.3390/cancers11081138
Hayashi M, Madokoro H, Yamada K, Nishida H, Morimoto C, Sakamoto M, Yanagawa H, Yamada T. Novel Antibody-Drug Conjugate with Anti-CD26 Humanized Monoclonal Antibody and Transcription Factor IIH (TFIIH) Inhibitor, Triptolide, Inhibits Tumor Growth via Impairing mRNA Synthesis. Cancers. 2019; 11(8):1138. https://doi.org/10.3390/cancers11081138
Chicago/Turabian StyleHayashi, Mutsumi, Hiroko Madokoro, Koji Yamada, Hiroko Nishida, Chikao Morimoto, Michiie Sakamoto, Hiroshi Yanagawa, and Taketo Yamada. 2019. "Novel Antibody-Drug Conjugate with Anti-CD26 Humanized Monoclonal Antibody and Transcription Factor IIH (TFIIH) Inhibitor, Triptolide, Inhibits Tumor Growth via Impairing mRNA Synthesis" Cancers 11, no. 8: 1138. https://doi.org/10.3390/cancers11081138




