Abstract
Background: The impact of the anterior commissure (AC) involvement on prognosis in laryngeal cancer remains a topic of discussion with inconsistent results in the literature. This review examines AC involvement as a prognostic factor in patients with early glottic cancer (Tis–T2) treated with radiotherapy or transoral laser microsurgery (TLM). Methods: A systematic literature search was performed. Due to the heterogeneity of the data, no meta-analysis was implemented. Weighted averages were calculated if the appropriate data were extractable. Results: Thirty-four studies on radiotherapy and 23 on TLM fit the inclusion criteria. The majority of studies for both radiotherapy (67.7%) and TLM (75.0%) did not report a significant impact on oncological outcomes. Weighted averages were slightly lower in patients with AC involvement. The two studies that applied a more detailed classification showed a significant impact on the amount of AC involvement. Conclusions: Binary variables (yes/no) for AC involvement lead to inconsistent results. Studies that use more detailed classifications of the AC show that there is a significant impact on the outcome. To further elucidate the role of the AC, detailed stratification of tumors involving the AC need to be investigated in further studies for both treatment modalities.
1. Introduction
Although it is widely acknowledged that the involvement of the anterior commissure (AC) in early glottic cancer (Tis–T2) can have negative impacts on outcomes, the extent of the impact remains a topic of discussion with inconsistent results reported in the literature. Some studies show a significant association between the AC and a higher recurrence rate, whereas others do not.
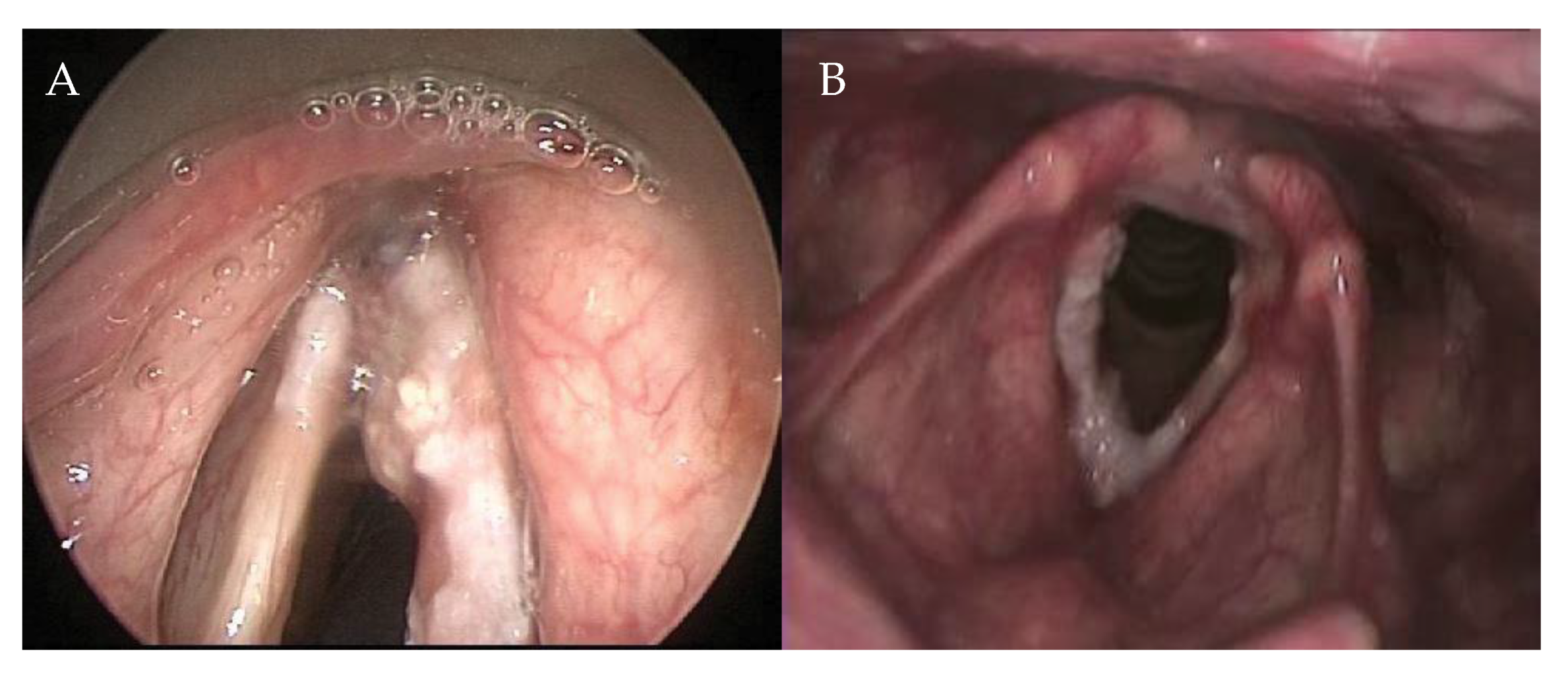
The AC is a complex anatomical subsite of the larynx, which encompasses different structures such as Broyles ligament, membranes, muscles, perichondrium, and the thyroid cartilage, and has a close relationship with the visceral structures surrounding it. Therefore, the AC has to be considered as a 3D structure and not as a point location (Figure 1). Rucci et al. defined the AC—on the basis of embryonic development—as the area of the glottis situated anteriorly between the vocal folds that extends in a vertical direction, both upwards and downwards [1]. It is rarely the site of origin of glottic cancer [1], but it is often involved in anterior lesions spreading from left to right, and from inferior to superior. Furthermore, due to its close proximity to the visceral spaces of the larynx (pre-epiglottic space, paraglottic space, and cricothyroid membrane), it has been argued that microscopic spread to these spaces may affect local control [2,3,4].
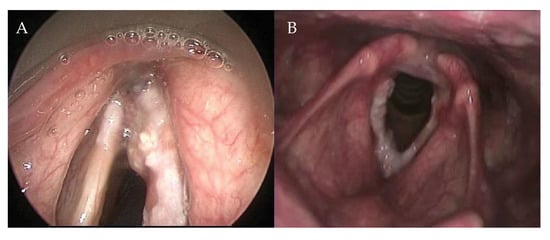
Figure 1.
Extension in the anterior commissure. (A) Fiber endoscopic view during outpatient examination; (B) Endoscopic examination of the same patient in anesthesia.
The purpose of this study was to perform a systematic review of studies that have investigated the involvement of the AC as a prognostic factor, with the aim of answering the following question: Is the involvement of the AC a prognostic factor in patients with early glottic cancer (Tis–T2) treated with radiotherapy or transoral CO2 laser microsurgery (TLM)?
2. Methods
2.1. Search
A systematic search was performed on 7 January 2019 on PubMed. The search strategy was conducted with a combination of the following keywords: laryngeal cancer, radiotherapy, and transoral laser microsurgery. For these keywords, all synonyms were used.
2.2. Inclusion Criteria and Data Extraction
For studies to be included, they had to be on adult patients with glottic squamous cell carcinoma staged as Tis, T1, or T2, treated with radiotherapy or TLM, to investigate the involvement of the AC as a prognostic factor, and be published between 1998 and 2018 in English. Also, a clear distinction had to be made, within the studies, between tumors that did and those that did not involve the AC to test this variable. Studies concerned with recurrent cases and studies reporting on less than 10 patients were excluded. Full-text versions of the included studies were reviewed for oncological outcomes. The primary endpoint was 5-year local control (LC) of tumors, with or without the involvement of the AC, calculated by the Kaplan-Meier or Cox regression method. Other oncological outcomes of interest were overall survival (OS), disease-specific survival (DSS), and laryngeal preservation (LP). During the extraction of data, papers that did not report LC were excluded. After the full-text screening, all papers were checked for relevant citations.
2.3. Statistical Analyses
Due to the heterogeneity of the data, no meta-analysis was performed. If data were extractable, weighted averages of the data were calculated for the separate tumor groups: T1, T2, and T1–T2.
3. Results
3.1. Search
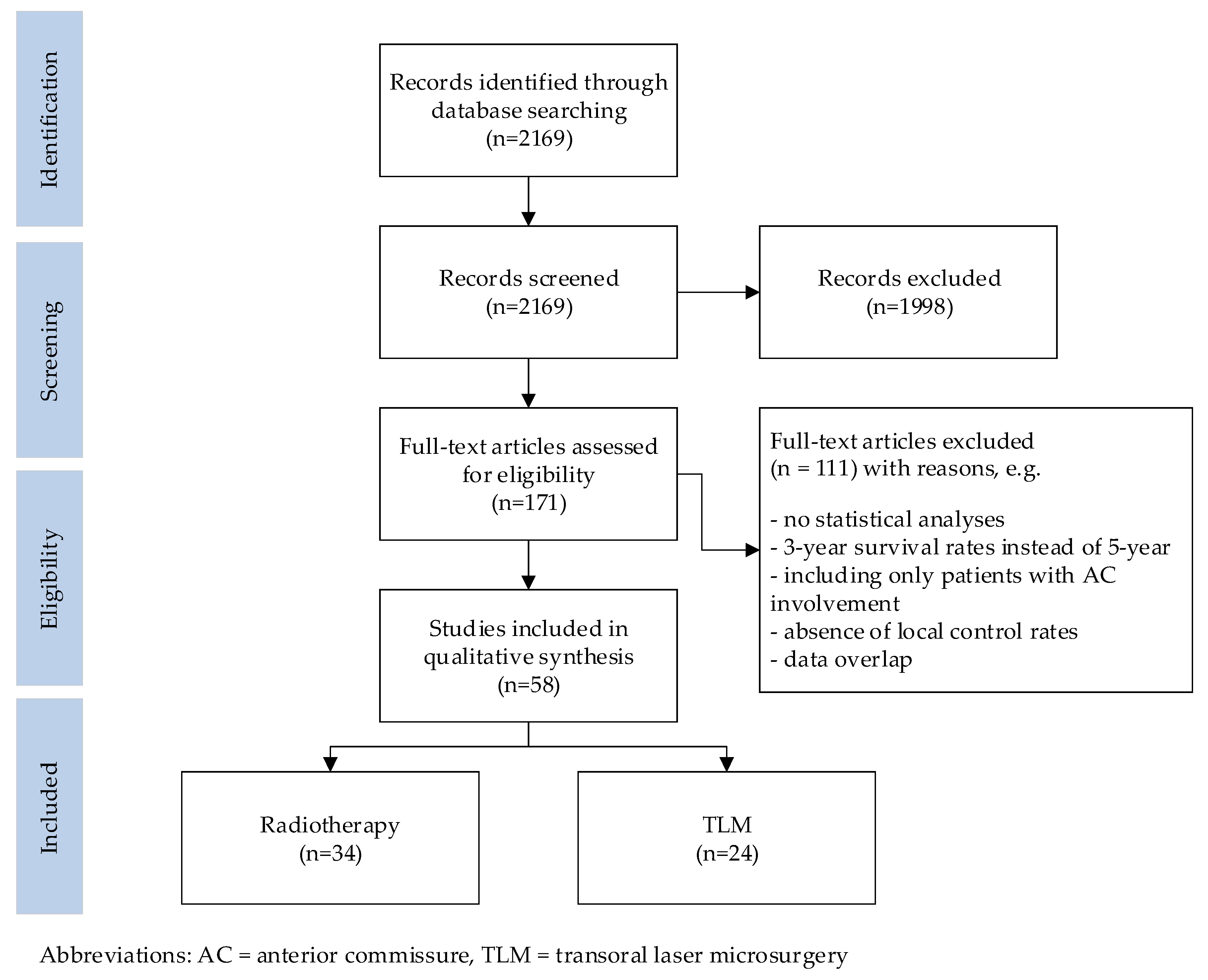
The results of the search are summarized in Figure 2. The initial literature search yielded 2169 citations, of which the title and abstract were screened. This identified 171 publications that underwent a full-text review. Of these, 34 publications on radiotherapy and 24 on TLM met the inclusion criteria. Reference cross-checking did not identify additional papers.
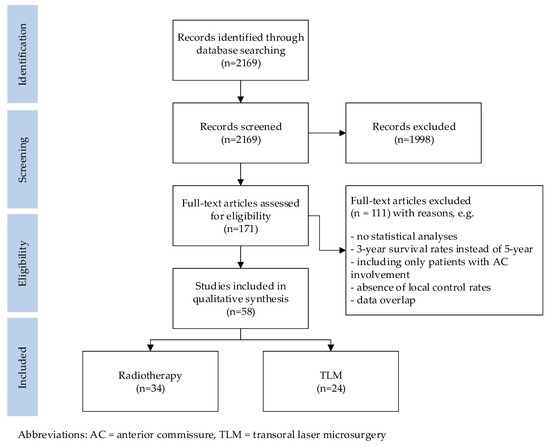
Figure 2.
Flow diagram illustrating the searching and selection procedure.
3.2. Study Characteristics
All 58 studies included in this review were published in peer-reviewed journals. Only one prospective randomized study, which was on radiation therapy, was identified [5]. All other publications had a level of evidence classified as B [6]. Most of the studies reported outcomes for early glottic cancer, grouping Tis–T2 tumors together, with only a few studies focusing on T1 or T2 tumors separately. The 34 radiotherapy studies included 9656 patients, of which 3930 patients (40.7%) had involvement of the AC. The 24 TLM studies included 3958 patients, of which 1169 patients (29.5%) had involvement of the AC.
In the radiotherapy studies, different treatment protocols were applied, and different techniques were used (conventional, accelerated, hyperfractionated, hypofractionated, and intensity-modulated radiation therapy), with doses varying between 60 and 78 Gy. Administration schedules varied from once daily, five times per week to twice daily, six times per week. Some studies applied elective neck irradiation, and some administrated a bolus in patients with AC involvement. In 14 studies (41.2%) LC rates were not presented for the AC separately [7,8,9,10,11,12,13,14,15,16,17,18,19,20]. These studies only presented p-values, hazard ratios (HR), or odds ratios.
Most studies on TLM classified resections according to the European Laryngological Society (ELS) classification system [21,22]. Two studies [23,24] performed resections according to the principles proposed by Steiner and Ambrosch [25], and in three studies, the resections were not further specified [26,27,28]. In six studies (25.0%), LC rates were not presented for the AC separately [26,29,30,31,32,33].
In both the radiotherapy and TLM studies, the follow-up time varied. In radiotherapy studies, the follow-up time ranged between a median of 33 and 147 months, and in TLM studies follow-up time ranged between a mean of 24.2 and 84 months. Characteristics of the included studies are presented in Table 1 for radiotherapy and in Table 2 for TLM.

Table 1.
Oncological Outcomes of Patients after Treatment with Radiotherapy for Involvement with or without the AC.

Table 2.
Oncological Outcomes of Patients after Treatment with TLM for Involvement with or Without the AC.
3.3. Local Control
In 23 out of 34 (67.6%) studies in the radiotherapy group, AC involvement did not have a significant impact on LC [5,7,8,9,11,13,14,15,16,17,18,20,34,39,40,41,42,44,46,48,49,51], and in 10 studies (29.4%), it did have a significant impact [10,19,35,36,37,43,45,47,50,52]. One study (2.9%) concluded that the AC was a predictive factor for LC in T1 tumors, but not in T2 tumors [38]. In the TLM studies, 18 out of 24 (75.0%) studies did not identify the AC involvement as a significant factor for LC [3,23,27,29,30,32,33,53,55,56,57,58,60,61,62,63,64,65], and two studies (8.3%) did [24,26]. One study (4.3%) concluded that the involvement of the AC was a predictive factor for LC in T1a tumors, although this was not the case in T1b or T2 tumors [28]. Three studies (12.5%) presented a more detailed classification of the AC involvement and concluded that the AC involvement had a significant impact on the AC [31,54,59]. One of these studies showed that in its binary approach (yes/no), the AC involvement did not have a significant impact on the AC, whereas it did in its more detailed classification [54].
Table 3 summarizes the weighted averages for the different tumor stages (T1, T2, T1–T2). Nineteen radiotherapy (55.8%) studies and 9 TLM (37.5%) studies could be included in the weighted averages. In T2 tumors treated with TLM, patients with involvement of the AC had a slightly higher 5-year LC rate than patients without involvement of the AC. In all the other groups, tumors with involvement of the AC resulted in a lower 5-year LC rate.

Table 3.
Weighted Averages for 5-Year Local Control Classified by Tumor Group.
3.4. Overall Survival, Disease Specific Survival, and Larynx Preservation
Four of the radiotherapy studies (11.8%) presented the 5-year OS [8,20,42,43]. The involvement of the AC did not have a statistically significant impact on any of these studies. Seven studies (20.6%) presented the 5-year DSS [8,9,10,16,20,35,38]. In two of these, the involvement of the AC had a statistically significant impact [10,38]. None of the radiotherapy studies presented the 5-year LP rates.
Ten of the TLM studies (41.7%) presented the 5-year OS [23,24,26,28,32,54,58,59,61,65]. The involvement of the AC had a statistically significant impact for one of these for patients with T1b and T2 tumors, but not for T1a tumors [23]. In seven studies, no significant impact was found [24,26,28,32,58,59,61], and in two studies, the impact on OS was not reported [23,54]. Six studies (25.0%) presented the 5-year DSS. Two of these studies showed a statistically significant impact of AC involvement on DSS [53,58], and three studies did not [3,26,59]. In one study, the impact of the AC was not reported [54]. Ten studies (41.7%) presented the 5-year LP rate. Six studies did not show a statistically significant impact of AC involvement on laryngeal preservation [3,26,53,56,59,64], whereas one study did [58]. In two studies, the impact of AC involvement on LP was not reported [23,28]. One study presented a binary approach (yes/no) as well as a more detailed classification. The first showed no significant impact on the AC, whereas the latest identified a significant impact on the involvement of the AC related to the amount of involvement of the AC [54].
4. Discussion
Both radiotherapy and TLM are well–established treatment modalities for early glottic cancer involving the AC. Although it is widely acknowledged that involvement of the AC can have a negative impact on outcome, results reported in the literature on the impact of AC involvement have been inconsistent. In this review, we found that most studies—both for radiotherapy and TLM—do not report a significant impact of AC involvement on LC, OS, DSS, and LP.
Although the results and the manner of reporting in the included studies were too heterogeneous to perform a formal meta-analysis, we did calculate weighted averages for T1 and T2 tumors separately and for T1–T2 tumors together from the papers that provided 5-year LC rates for the tumors with or without the involvement of the AC. On this basis, only 19 radiotherapy (55.9%) studies and 9 TLM (37.5%) studies could be included in the weighted averages. These weighted averages showed that the involvement of the AC leads to a slightly higher recurrence rate after treatment with both RT and TLM. However, as stated, this is no formal meta-analysis, and, therefore, no definite conclusions can be drawn from these calculations. The varying results in the literature can be explained by variations in the clinical definition of the AC area, and in the detail of the clinical, endoscopic, and radiologic evaluation of the lesion in the preoperative setting, the distinctive features and limitations of each therapeutic modality, the biological behavior of the tumor, and variations in the rigor of the follow-up policy. Due to these factors, combined with the complicated anatomy of the AC, the involvement of this subsite may very well be too complex to be included as a simple binary variable (yes/no) as it is in most publications. To try to draw some conclusions from the existing literature, it is, therefore, necessary to take a closer look at the data of individual publications and at the definition of involvement of the AC. In 1996, Rucci et al. proposed a new staging system of the anterior commissure, as there was no consideration of the AC involvement in the T stage of the TNM classification (Union for International Cancer Control-American Joint Committee on Cancer [66,67]). Rucci et al. classified the AC into four subgroups: AC0: patients without any involvement of the AC region; AC1: patients with involvement of the AC region on only one side of the midline, AC2: patients with involvement of the AC region that crosses the midline on only one part of the longitudinal extension of this region; AC3: patients with involvement of the whole AC region, on both sides of the midline [67]. They found that LC was significantly lower with the increase of the AC classification. They concluded that this AC classification was more reflective of prognosis than the TNM classification [67]. Since then, to our knowledge, every study utilizing this, or a similar classification of AC involvement into subgroups, has found a prognostic impact of increasing levels of AC involvement, with wider involvement leading to lower rates of LC or LP. Carta et al. did not show a statistically significant difference in LC rates between involvement and no involvement of the AC in patients with Tis–T2 tumors; however, they did find a statistically significant lower 5-year recurrence-free survival in the AC3 group when using Rucci et al.’s classification system. The AC3 group also showed a statistically lower 5-year LP rate [54]. Hoffmann et al. also found a significant difference in the 5-year disease-free survival in the AC3 group. However, they did not find a significant difference between the AC groups in terms of LP or DSS [59].
Recently, another classification was proposed by Piazza et al. [68]. They stratified six isoprognostic zones in early-intermediate tumors (T1–T3) treated with TLM according to the location and the extent of the tumor, describing different growth patterns and possible pathways of recurrence, and defined the role and limits of TLM as a single treatment modality. They concluded that the vertical extension across the AC leads to a decreased LC rate and lower LP rates in patients treated with TLM and that this location—with or without the involvement of the pre-epiglottic space (PES)—should be considered as a risk factor for TLM [68].
The classification of Piazza et al. regarding the AC is in line with earlier publications differentiating between the horizontal and vertical extension of the tumor [68]. In a recent review, Peretti et al. highlighted the importance of differentiating between tumors of the vocal cord affecting the AC in the horizontal plane against the vertical plane [69]. They defined several requirements when treating tumors involving the AC with TLM, such as complete exposure of the tumor, proper assessment tools with a suitable diagnostic workup, and having an experienced surgeon performing the procedure on this subsite of the larynx [69,70]. This is also suggested by the study of Vilaseca et al. [71], which investigated the impact on the AC involvement in patients with T1–T4a that were treated with TLM. They found that AC involvement was an independent factor for local recurrence. Half of their patients with recurrence were finally salvaged with TLM alone, suggesting that surgical experience could have played a role in local recurrence as a large proportion of patients were still amenable to TLM [71]. Tumors involving the AC and growing in the vertical plane are more difficult to expose due to a narrow angle, and the v-shaped configuration of the thyroid cartilage [54]. Difficult or incomplete surgical exposure has a tendency toward incomplete resection [61], which can subsequently lead to a higher recurrence rate. Several authors argue that tumors with vertical extension to the supra– and/or subglottic areas have a higher risk of local failure due to their narrow relationship with, and therefore the risk of (minor)spread into, the underlying visceral spaces [2,3].
To the best of our knowledge, no studies that treated patients with radiotherapy have used detailed stratification of the involvement of the AC. Therefore, although AC involvement, particularly in the vertical plane, may be a risk factor in TLM, it may well be the same for other treatment modalities. More studies are needed to investigate these factors in other treatment modalities to ascertain the relative benefits of different approaches.
Limitations
The main limitation of this review is the heterogeneity of the studies that were included with regard to factors such as the clinical definition of the AC area, diagnostic protocols, and treatment protocols. Also, the majority of studies could not be included in the calculation of the weighted averages, as they did not present LC rates for patients with or without AC separately. Therefore, the weighted averages that were calculated should be interpreted with caution.
5. Conclusions
This review shows that the use of a binary variable (yes/no) for the involvement of the AC leads to conflicting results due to variability in definition, work-up, and treatment parameters of the AC area. However, weighted averages indicate that LC may be lower in tumors with involvement than tumors without involvement in the AC. Furthermore, all studies that use specific, detailed classifications of the AC show that there is a significant impact on outcome related to the amount of involvement of the AC. All in all, these findings point to a negative impact of AC involvement that may not be evident in simple binary (yes/no) studies of AC involvement. To further elucidate the role of the AC, detailed stratification of tumors involving the AC should be applied in future studies. To the best of our knowledge, no studies of patients treated with radiotherapy have used detailed stratification of the involvement of the AC. Therefore, to further elucidate the impact of AC involvement in these patients, stratifications need to be employed in these populations as well.
Funding
This research received no external funding.
Conflicts of interest
The authors declare no conflict interest.
References
- Rucci, L.; Gammarota, L.; Borghi Cirri, M.B. Carcinoma of the anterior commissure of the larynx: I. Embryological and anatomic considerations. Ann. Otol. Rhinol. Laryngol. 1996, 105, 303–308. [Google Scholar] [PubMed]
- Sjögren, E. Transoral Laser Microsurgery in Early Glottic Lesions. Curr. Otorhinolaryngol. Rep. 2017, 5, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.; Piazza, C.; Cocco, D.; De, B.L.; Del, B.F.; Redaelli De Zinis, L.O.; Nicolai, P. Transoral CO2 laser treatment for Tis–T3 glottic cancer: The University of Brescia experience on 595 patients. Head Neck 2010, 32, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Prades, J.M.; Peoc’h, M.; Petcu, C.; Karkas, A.; Dumollard, J.M.; Gavid, M. The anterior commissure of the human larynx revisited. Surg. Radiol. Anat. 2017, 39, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Nishiyama, K.; Tanaka, E.; Koizumi, M.; Chatani, M. Radiotherapy for early glottic carcinoma (T1N0M0): Results of prospective randomized study of radiation fraction size and overall treatment time. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Siwek, J.; Slawson, D.C.; Health, V.; Shaughnessy, A.F.; D, P.; Family, H.; Residency, P.; Gourlay, M. How to write an evidence–based clinical review article. Am. Fam. Physician 2002, 65, 251–259. [Google Scholar] [PubMed]
- Al–Mamgani, A.; van Rooij, P.H.; Mehilal, R.; Verduijn, G.M.; Tans, L.; Kwa, S.L. Radiotherapy for T1a glottic cancer: The influence of smoking cessation and fractionation schedule of radiotherapy. Eur. Arch. Otorhinolaryngol. 2014, 271, 125–132. [Google Scholar] [CrossRef]
- Berwouts, D.; Swimberghe, M.; Duprez, F.; Boterberg, T.; Bonte, K.; Deron, P.; De, G.W.; De, N.W.; Madani, I. Intensity–modulated radiotherapy for early–stage glottic cancer. Head Neck 2016, 38 (Suppl. 1), E179–E184. [Google Scholar] [CrossRef]
- Raitiola, H.; Wigren, T.; Pukander, J. Radiotherapy outcome and prognostic factors in early glottic carcinoma. Auris Nasus Larynx 2000, 27, 153–159. [Google Scholar] [CrossRef]
- Smee, R.I.; Meagher, N.S.; Williams, J.R.; Broadley, K.; Bridger, G.P. Role of radiotherapy in early glottic carcinoma. Head Neck 2010, 32, 850–859. [Google Scholar] [CrossRef]
- Sommat, K.; Yit, N.L.; Kwok, L.L. Comparison between 4–MV and 6–MV radiotherapy in T1N0 glottic cancer. Laryngoscope 2017, 127, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Thariat, J.; Bruchon, Y.; Bonnetain, F.; Barillot, I.; Truc, G.; Peignaux, K.; Horiot, J.C.; Maingon, P. Conservative treatment of early glottic carcinomas with exclusive radiotherapy. Cancer Radiother. 2004, 8, 288–296. [Google Scholar] [CrossRef]
- Bignardi, M.; Antognoni, P.; Sanguineti, G.; Magli, A.; Molteni, M.; Merlotti, A.; Richetti, A.; Tordiglione, M.; Conte, L.; Magno, L. Hyperfractionated radiotherapy for T2N0 glottic carcinoma: A retrospective analysis at 10 years follow-up in a series of 60 consecutive patients. Tumori J. 2004, 90, 317–323. [Google Scholar] [CrossRef]
- Chera, B.S.; Amdur, R.J.; Morris, C.G.; Kirwan, J.M.; Mendenhall, W.M. T1N0 to T2N0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Kim, K.H.; Keum, K.C.; Koh, Y.W.; Kim, S.H.; Choi, E.C.; Lee, C.G. Radiotherapy versus Cordectomy in the Management of Early Glottic Cancer. Cancer Res. Treat 2017. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Sasaki, R.; Miyawaki, D.; Yoshida, K.; Nishimura, H.; Ejima, Y.; Kitajima, K.; Saito, M.; Otsuki, N.; Nibu, K. Treatment outcomes of the patients with early glottic cancer treated with initial radiotherapy and salvaged by conservative surgery. Jpn. J. Clin. Oncol. 2015, 45, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Mendenhall, C.M.; Kirwan, J.; Morris, C.G.; Donnan, A.; Holwerda, S.; Kraus, S.T.; Mann, C.J.; Grant, J.R.; Donnan, B.; et al. Radiation therapy for management of t1–t2 glottic cancer at a private practice. Am. J. Clin. Oncol. 2010, 33, 587–590. [Google Scholar] [CrossRef]
- Khan, M.K.; Koyfman, S.A.; Hunter, G.K.; Reddy, C.A.; Saxton, J.P. Definitive radiotherapy for early (T1–T2) glottic squamous cell carcinoma: A 20 year Cleveland Clinic experience. Radiat. Oncol. 2012, 7, 193. [Google Scholar] [CrossRef]
- Matsumoto, F.; Ohba, S.; Fujimaki, M.; Ikeda, K. The value of insulin–like growth factor–1 receptor for predicting early glottic carcinoma response to radiotherapy. Auris Nasus Larynx 2016, 43, 440–445. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Amdur, R.J.; Morris, C.G.; Hinerman, R.W. T1–T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. J. Clin. Oncol. 2001, 19, 4029–4036. [Google Scholar] [CrossRef]
- Remacle, M.; Eckel, H.E.; Antonelli, A.; Brasnu, D.; Chevalier, D.; Friedrich, G.; Olofsson, J.; Rudert, H.H.; Thumfart, W.; de Vincentiis, M.; et al. Endoscopic cordectomy. A proposal for a classification by the Working Committee, European Laryngological Society. Eur. Arch. Otorhinolaryngol. 2000, 257, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Remacle, M.; Van Haverbeke, C.; Eckel, H.; Bradley, P.; Chevalier, D.; Djukic, V.; de Vicentiis, M.; Friedrich, G.; Olofsson, J.; Peretti, G.; et al. Proposal for revision of the European Laryngological Society classification of endoscopic cordectomies. Eur. Arch. Otorhinolaryngol. 2007, 264, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Steiner, W.; Ambrosch, P.; Rodel, R.M.; Kron, M. Impact of anterior commissure involvement on local control of early glottic carcinoma treated by laser microresection. Laryngoscope 2004, 114, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Wolber, P.; Schwarz, D.; Stange, T.; Ortmann, M.; Balk, M.; Anagiotos, A.; Gostian, A.O. Surgical Treatment for Early Stage Glottic Carcinoma with Involvement of the Anterior Commissure. Otolaryngol. Head Neck Surg. 2018, 158, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Steiner, W.; Ambrosch, P. Endoscopic Laser Surgery of the Upper Aerodigestive Tract, 1st ed.; Thieme: Stuttgart, Germany, 2000; pp. 17–33. [Google Scholar]
- Hakeem, A.H.; Tubachi, J.; Pradhan, S.A. Significance of anterior commissure involvement in early glottic squamous cell carcinoma treated with trans–oral CO2 laser microsurgery. Laryngoscope 2013, 123, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Hsin, L.J.; Fang, T.J.; Chang, K.P.; Fang, K.H.; Tsang, N.M.; Chen, Y.L.; Jung, S.M.; Yeh, A.R.; Hao, S.P. Transoral endoscopic CO2 laser microsurgery for early laryngeal cancers. Chang Gung Med. J. 2009, 32, 517–525. [Google Scholar] [PubMed]
- Rodel, R.M.; Steiner, W.; Muller, R.M.; Kron, M.; Matthias, C. Endoscopic laser surgery of early glottic cancer: Involvement of the anterior commissure. Head Neck 2009, 31, 583–592. [Google Scholar] [CrossRef]
- Gallet, P.; Rumeau, C.; Nguyen, D.T.; Teixeira, P.A.; Baumann, C.; Toussaint, B. “Watchful observation” follow-up scheme after endoscopic CO2 laser treatment for small glottic carcinomas: A retrospective study of 93 cases. Clin. Otolaryngol. 2017, 42, 1193–1199. [Google Scholar] [CrossRef]
- Hartl, D.M.; De Mones, E.; Hans, S.; Janot, F.; Brasnu, D. Treatment of early–stage glottic cancer by transoral laser resection. Ann. Otol. Rhinol. Laryngol. 2007, 116, 832–836. [Google Scholar] [CrossRef]
- Rucci, L.; Romagnoli, P.; Scala, J. CO2 laser therapy in Tis and T1 glottic cancer: Indications and results. Head Neck 2010, 32, 392–398. [Google Scholar]
- Son, H.-J.; Lee, Y.S.; Ku, J.Y.; Roh, J.-L.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Radiological tumor thickness as a risk factor for local recurrence in early glottic cancer treated with laser cordectomy. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Mortuaire, G.; Francois, J.; Wiel, E.; Chevalier, D. Local recurrence after CO2 laser cordectomy for early glottic carcinoma. Laryngoscope 2006, 116, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Al–Mamgani, A.; van Rooij, P.H.; Woutersen, D.P.; Mehilal, R.; Tans, L.; Monserez, D.; Baatenburg de Jong, R.J. Radiotherapy for T1–2N0 glottic cancer: A multivariate analysis of predictive factors for the long–term outcome in 1050 patients and a prospective assessment of quality of life and voice handicap index in a subset of 233 patients. Clin. Otolaryngol. 2013, 38, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Bron, L.P.; Soldati, D.; Zouhair, A.; Ozsahin, M.; Brossard, E.; Monnier, P.; Pasche, P. Treatment of early stage squamous–cell carcinoma of the glottic larynx: Endoscopic surgery or cricohyoidoepiglottopexy versus radiotherapy. Head Neck 2001, 23, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Cellai, E.; Frata, P.; Magrini, S.M.; Paiar, F.; Barca, R.; Fondelli, S.; Polli, C.; Livi, L.; Bonetti, B.; Vitali, E.; et al. Radical radiotherapy for early glottic cancer: Results in a series of 1087 patients from two Italian radiation oncology centers. I. The case of T1N0 disease. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Cheah, N.L.; Lupton, S.; Marshall, A.; Hartley, A.; Glaholm, J. Outcome of T1N0M0 squamous cell carcinoma of the larynx treated with short-course radiotherapy to a total dose of 50 Gy in 16 fractions: The Birmingham experience. Clin. Oncol. (R. Coll. Radiol.) 2009, 21, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-F.F.; Chang, J.T.-C.; Tsang, N.-M.M.; Liao, C.-T.T.; Chen, W.-C.C. Radiotherapy of early–stage glottic cancer: Analysis of factors affecting prognosis. Ann. Otol. Rhinol. Laryngol. 2003, 112, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Frata, P.; Cellai, E.; Magrini, S.M.; Bonetti, B.; Vitali, E.; Tonoli, S.; Buglione, M.; Paiar, F.; Barca, R.; Fondelli, S.; et al. Radical radiotherapy for early glottic cancer: Results in a series of 1087 patients from two Italian radiation oncology centers. II. The case of T2N0 disease. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Garden, A.S.; Forster, K.; Wong, P.F.; Morrison, W.H.; Schechter, N.R.; Ang, K.K. Results of radiotherapy for T2N0 glottic carcinoma: Does the “2” stand for twice–daily treatment? Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 322–328. [Google Scholar] [CrossRef]
- Gowda, R.V.; Henk, J.M.; Mais, K.L.; Sykes, A.J.; Swindell, R.; Slevin, N.J. Three weeks radiotherapy for T1 glottic cancer: The Christie and Royal Marsden Hospital Experience. Radiother. Oncol. 2003, 68, 105–111. [Google Scholar] [CrossRef]
- Gultekin, M.; Ozyar, E.; Cengiz, M.; Ozyigit, G.; Hayran, M.; Hosal, S.; Akyol, F. High daily fraction dose external radiotherapy for T1 glottic carcinoma: Treatment results and prognostic factors. Head Neck 2012, 34, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Liao, Z.; Gao, L.; Huang, X.; Xu, G. Analysis of prognostic factors for T(1)N(0)M(0) glottic cancer treated with definitive radiotherapy alone: Experience of the cancer hospital of Peking Union Medical College and the Chinese Academy Of Medical Sciences. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 471–478. [Google Scholar] [CrossRef]
- Laskar, S.G.; Baijal, G.; Murthy, V.; Chilukuri, S.; Budrukkar, A.; Gupta, T.; Agarwal, J.P. Hypofractionated Radiotherapy for T1N0M0 Glottic Cancer: Retrospective Analysis of Two Different Cohorts of Dose–fractionation Schedules from a Single Institution. Clin. Oncol. 2012, 24, e180–e186. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Wu, H.G.; Kwon, T.K.; Hah, J.H.; Sung, M.W.; Kim, K.H.; Park, C.I. Long–Term Outcome of Definitive Radiotherapy for Early Glottic Cancer: Prognostic Factors and Patterns of Local Failure. Cancer Res. Treat. 2015, 47, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Nishimura, R.; Baba, Y.; Furusawa, M.; Ogata, N.; Yumoto, E.; Yamashita, Y. Prognostic factors of glottic carcinomas treated with radiation therapy: Value of the adjacent sign on radiological examinations in the sixth edition of the UICC TNM staging system. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Furuta, M.; Murakami, Y.; Izawa, Y.; Iwasaki, N.; Takahashi, H.; Watanabe, K. Radiation therapy for T1 glottic cancer: Involvement of the anterior commissure. Anticancer Res. 2000, 20, 1121–1124. [Google Scholar] [PubMed]
- Robert, A.; Pointreau, Y.; Janoray, G.; Bardet, E.; Fesneau, M.; Garaud, P.; Chapet, S.; Lafond, C.; Dupuis, O.; Calais, G. A large French multicenter retrospective series of T1–T2N0 vocal cords carcinomas treated with exclusive irradiation Une grande serie retrospective francaise de carcinomes des cordes vocales de stade T1–T2N0 traites par irradiation exclusive. Cancer/Radiotherapie 2017, 21, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, E.V.; Wiggenraad, R.G.; Le, C.S.; Snijder, S.; Pomp, J.; Baatenburg de Jong, R.J. Outcome of radiotherapy in T1 glottic carcinoma: A population–based study. Eur. Arch. Otorhinolaryngol. 2009, 266, 735–744. [Google Scholar] [CrossRef][Green Version]
- Tong, C.C.; Au, K.H.; Ngan, R.K.; Chow, S.M.; Cheung, F.Y.; Fu, Y.T.; Au, J.S.; Law, S.C. Impact and relationship of anterior commissure and time–dose factor on the local control of T1N0 glottic cancer treated by 6 MV photons. Radiat. Oncol. 2011, 6, 53. [Google Scholar] [CrossRef]
- Warde, P.; O’Sullivan, B.; Bristow, R.G.; Panzarella, T.; Keane, T.J.; Gullane, P.J.; Witterick, I.P.; Payne, D.; Liu, F.F.; McLean, M.; et al. T1/T2 glottic cancer managed by external beam radiotherapy: The influence of pretreatment hemoglobin on local control. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 347–353. [Google Scholar] [CrossRef]
- Zouhair, A.; Azria, D.; Coucke, P.; Matzinger, O.; Bron, L.; Moeckli, R.; Do, H.P.; Mirimanoff, R.O.; Ozsahin, M. Decreased local control following radiation therapy alone in early–stage glottic carcinoma with anterior commissure extension. Strahlenther. Onkol. 2004, 180, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ansarin, M.; Cattaneo, A.; De, B.L.; Zorzi, S.; Lombardi, F.; Alterio, D.; Rocca, M.C.; Scelsi, D.; Preda, L.; Chiesa, F.; et al. Retrospective analysis of factors influencing oncologic outcome in 590 patients with early–intermediate glottic cancer treated by transoral laser microsurgery. Head Neck 2017, 39, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Bandino, F.; Olla, A.M.; Chuchueva, N.; Gerosa, C.; Puxeddu, R. Prognostic value of age, subglottic, and anterior commissure involvement for early glottic carcinoma treated with CO2 laser transoral microsurgery: A retrospective, single–center cohort study of 261 patients. Eur. Arch. Otorhinolaryngol. 2018, 275, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Chu, P.Y. Predictors of local recurrence of glottic cancer in patients after transoral laser microsurgery. J. Chin. Med. Assoc. 2017, 80, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Chone, C.T.; Yonehara, E.; Martins, J.E.F.; Altemani, A.; Crespo, A.N. Importance of anterior commissure in recurrence of early glottic cancer after laser endoscopic resection. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.J.; Courey, M.S.; Liao, C.T.; Yen, T.C.; Li, H.Y. Frozen margin analysis as a prognosis predictor in early glottic cancer by laser cordectomy. Laryngoscope 2013, 123, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Hans, S.; Sadoughi, B.; Brasnu, D. Identifying outcome predictors of transoral laser cordectomy for early glottic cancer. Head Neck 2016, 38, E411. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Cornu, N.; Hans, S.; Sadoughi, B.; Badoual, C.; Brasnu, D. Early glottic cancer involving the anterior commissure treated by transoral laser cordectomy. Laryngoscope 2016, 126, 1817–1822. [Google Scholar] [CrossRef]
- Ledda, G.P.; Grover, N.; Pundir, V.; Masala, E.; Puxeddu, R. Functional outcomes after CO2 laser treatment of early glottic carcinoma. Laryngoscope 2006, 116, 1007–1011. [Google Scholar] [CrossRef]
- Lee, H.S.; Chun, B.G.; Kim, S.W.; Kim, S.T.; Oh, J.H.; Hong, J.C.; Lee, K.D. Transoral laser microsurgery for early glottic cancer as one–stage single–modality therapy. Laryngoscope 2013, 123, 2670–2674. [Google Scholar] [CrossRef]
- Peretti, G.; Nicolai, P.; Redaelli De Zinis, L.O.; Berlucchi, M.; Bazzana, T.; Bertoni, F.; Antonelli, A.R. Endoscopic CO2 laser excision for tis, T1, and T2 glottic carcinomas: Cure rate and prognostic factors. Otolaryngol. Head Neck Surg. 2000, 123, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.; Nicolai, P.; Piazza, C.; Redaelli De Zinis, L.O.; Valentini, S.; Antonelli, A.R. Oncological results of endoscopic resections of Tis and T1 glottic carcinomas by carbon dioxide laser. Ann. Otol. Rhinol. Laryngol. 2001, 110, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.; Piazza, C.; Del, B.F.; Mora, R.; Grazioli, P.; Barbieri, D.; Mangili, S.; Nicolai, P. Function preservation using transoral laser surgery for T2–T3 glottic cancer: Oncologic, vocal, and swallowing outcomes. Eur. Arch. Otorhinolaryngol. 2013, 270, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Sachse, F.; Stoll, W.; Rudack, C. Evaluation of treatment results with regard to initial anterior commissure involvement in early glottic carcinoma treated by external partial surgery or transoral laser microresection. Head Neck 2009, 31, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz,, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 9–11. [Google Scholar]
- Rucci, L.; Gammarota, L.; Gallo, O. Carcinoma of the anterior commissure of the larynx: II. Proposal of a new staging system. Ann. Otol. Rhinol. Laryngol. 1996, 105, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Piazza, C.; Filauro, M.; Paderno, A.; Marchi, F.; Perotti, P.; Morello, R.; Taboni, S.; Parrinello, G.; Incandela, F.; Iandelli, A.; et al. Three-Dimensional Map of Isoprognostic Zones in Glottic Cancer Treated by Transoral Laser Microsurgery as a Unimodal Treatment Strategy. Front. Oncol. 2018, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.; Piazza, C.; Mora, F.; Garofolo, S.; Guastini, L. Reasonable limits for transoral laser microsurgery in laryngeal cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Blanch, J.L.; Vilaseca, I.; Caballero, M.; Moragas, M.; Berenguer, J.; Bernal–Sprekelsen, M. Outcome of transoral laser microsurgery for T2–T3 tumors growing in the laryngeal anterior commissure. Head Neck 2011, 33, 1252–1259. [Google Scholar] [CrossRef]
- Vilaseca, I.; Nogués-Sabaté, A.; Avilés-Jurado, F.X.; Berenguer, J.; Grau, J.J.; Verger, E.; Nadal, A.; Muxí, A.; Bernal–Sprekelsen, M.; Blanch, J.L. Factors of local recurrence and organ preservation with transoral laser microsurgery in laryngeal carcinomas; CHAID decision–tree analysis. Head Neck 2019, 41, 756–764. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).