Exploring Tumor Heterogeneity Using PET Imaging: The Big Picture
Abstract
:1. Introduction
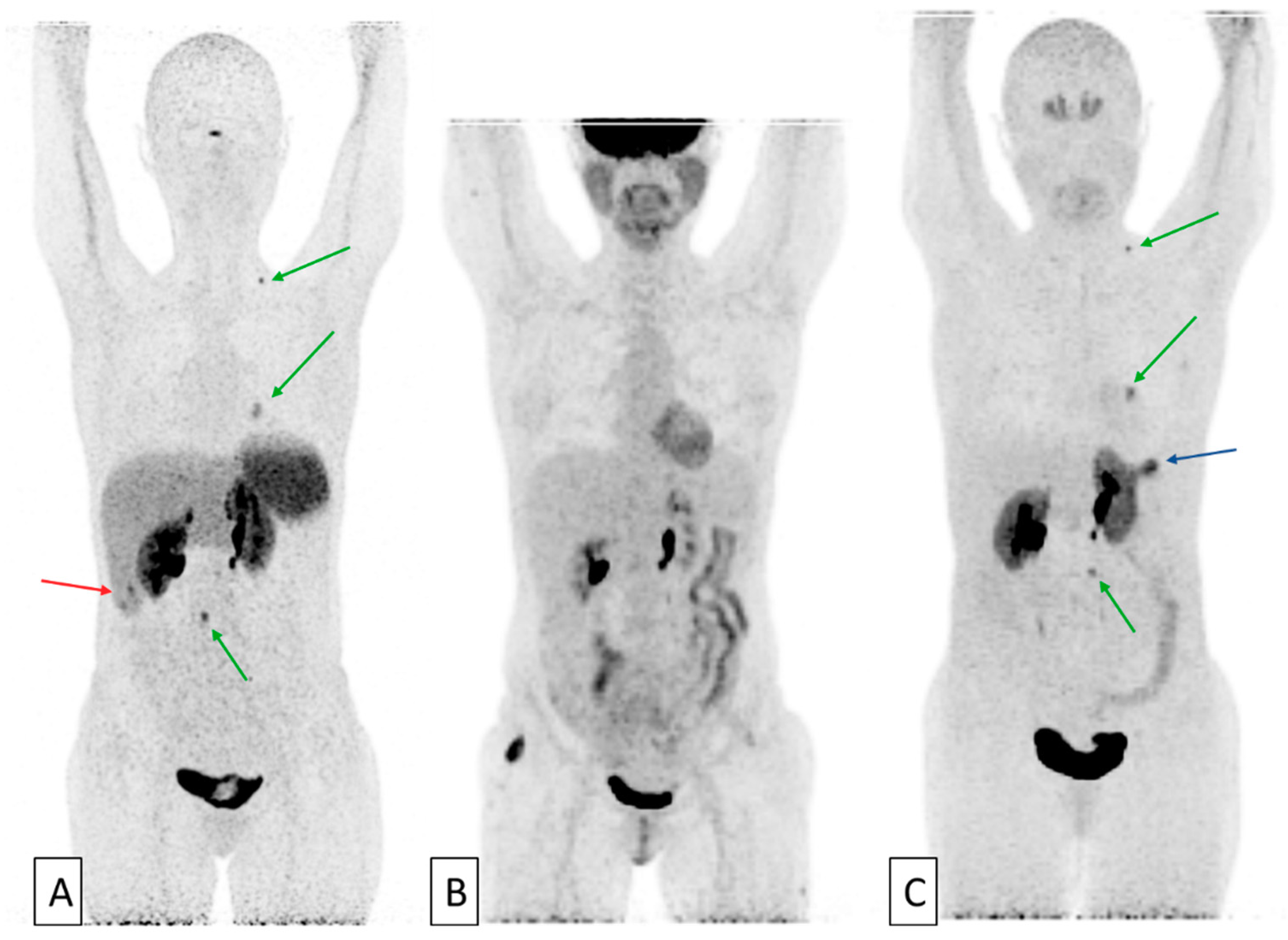
2. Inter- and Intra-patient Tumor Heterogeneity Exploration through Multiple Tracers PET Imaging
3. Intrapatient Tumor Heterogeneity Exploration through Quantitative Analysis of PET Imaging
4. Intratumor Heterogeneity Exploration through Quantitative Analysis of PET Imaging
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tabassum, D.P.; Polyak, K. Tumorigenesis: It takes a village. Nat. Rev. Cancer 2015, 15, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; Van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Cavalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Bensch, F.; Van Der Veen, E.L.; Hooge, M.N.L.-D.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; Van Der Wekken, A.J.; et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.-Y.; Huang, J.-A.; Chen, Y.; Chen, C.; Zhang, X.-G. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med. Oncol. 2011, 28, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Han, X. Anti–PD-1/PD-L1 therapy of human cancer: Past, present, and future. J. Clin. Investig. 2015, 125, 3384–3391. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Jimeno, A. Atezolizumab: A novel PD-L1 inhibitor in cancer therapy with a focus in bladder and non-small cell lung cancers. Drugs Today 2017, 53, 217–237. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Jadvar, H.; Delgado-Bolton, R.; Nadel, H.; Rohren, E.; Zukotynski, K.; Kauffman, J.; Ahuja, S.; Colletti, P.M.; Esposito, G.; Krause, B.J.; et al. Appropriate Use Criteria for 18 F-FDG PET/CT in Restaging and Treatment Response Assessment of Malignant Disease. J. Nucl. Med. 2017, 58, 2026–2037. [Google Scholar] [CrossRef]
- Lopci, E.; Nanni, C.; Castellucci, P.; Montini, G.C.; Allegri, V.; Rubello, D.; Chierichetti, F.; Ambrosini, V.; Fanti, S. Imaging with non-FDG PET tracers: Outlook for current clinical applications. Insights Imaging 2010, 1, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Chudgar, A.V.; Mankoff, D.A. Molecular Imaging and Precision Medicine in Breast Cancer. PET Clin. 2017, 12, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koleva-Kolarova, R.G.; Greuter, M.J.; Feenstra, T.L.; Vermeulen, K.M.; De Vries, E.F.; Parkin, D.; Buskens, E.; De Bock, G.H. Molecular imaging with positron emission tomography and computed tomography (PET/CT) for selecting first-line targeted treatment in metastatic breast cancer: A cost-effectiveness study. Oncotarget 2018, 9, 19836–19846. [Google Scholar] [CrossRef] [PubMed]
- Kurland, B.F.; Peterson, L.M.; Lee, J.H.; Schubert, E.K.; Currin, E.R.; Link, J.M.; Krohn, K.A.; Mankoff, D.A.; Linden, H.M. Estrogen receptor binding (FES PET) and glycolytic activity (FDG PET) predict progression-free survival on endocrine therapy in patients with ER+ breast cancer. Clin. Cancer Res. 2017, 23, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.E.; Dehdashti, F.; Siegel, B.A.; Katzenellenbogen, J.A.; Fracasso, P.; Welch, M.J. Positron emission tomography with 2-[18F]Fluoro-2-deoxy-D-glucose and 16alpha-[18F]fluoro-17beta-estradiol in breast cancer: Correlation with estrogen receptor status and response to systemic therapy. Clin. Cancer Res. 1996, 2, 933–939. [Google Scholar]
- Liao, G.J.; Clark, A.S.; Schubert, E.K.; Mankoff, D.A. 18F-Fluoroestradiol PET: Current Status and Potential Future Clinical Applications. J. Nucl. Med. 2016, 57, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Van Kruchten, M.; Glaudemans, A.W.J.M.; De Vries, E.F.J.; Beets-Tan, R.G.H.; Schröder, C.P.; Dierckx, R.A.; De Vries, E.G.E.; Hospers, G.A.P. PET Imaging of Estrogen Receptors as a Diagnostic Tool for Breast Cancer Patients Presenting with a Clinical Dilemma. J. Nucl. Med. 2012, 53, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Dijkers, E.C.; Kosterink, J.G.; Rademaker, A.P.; Perk, L.R.; Van Dongen, G.A.; Bart, J.; De Jong, J.R.; De Vries, E.G.; Hooge, M.N.L.-D. Development and Characterization of Clinical-Grade 89Zr-Trastuzumab for HER2/neu ImmunoPET Imaging. J. Nucl. Med. 2009, 50, 974–981. [Google Scholar] [CrossRef]
- Baum, R.P.; Prasad, V.; Schuchardt, C.; Orlova, A.; Wennborg, A.; Tolmachev, V.; Feldwisch, J.; Müller, D. Molecular Imaging of HER2-Expressing Malignant Tumors in Breast Cancer Patients Using Synthetic 111In- or 68Ga-Labeled Affibody Molecules. J. Nucl. Med. 2010, 51, 892–897. [Google Scholar] [CrossRef]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-Trastuzumab PET Imaging in Patients with HER2-Positive Breast Cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef]
- Jauw, Y.W.S.; Oordt, C.W.M.-V.D.H.V.; Hoekstra, O.S.; Hendrikse, N.H.; Vugts, D.J.; Zijlstra, J.M.; Huisman, M.C.; Van Dongen, G.A.M.S. Immuno-Positron Emission Tomography with Zirconium-89-Labeled Monoclonal Antibodies in Oncology: What Can We Learn from Initial Clinical Trials? Front. Pharmacol. 2016, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.S.; DeMichele, A.; Mankoff, D. HER2 imaging in the ZEPHIR study. Ann. Oncol. 2016, 27, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Maguire, L.H.; Thomas, A.R.; Goldstein, A.M. Tumors of the neural crest: Common themes in development and cancer: Tumors of the Neural Crest. Dev. Dyn. 2015, 244, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sever, Z.; Biassoni, L.; Shulkin, B.; Kong, G.; Hofman, M.S.; Lopci, E.; Manea, I.; Koziorowski, J.; Castellani, R.; Boubaker, A.; et al. Guidelines on nuclear medicine imaging in neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2009–2024. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.S.; Dezarn, W.A.; McNeillie, P.; Coldwell, D.; Nutting, C.; Carter, D.; Murthy, R.; Rose, S.; Warner, R.R.P.; Liu, D.; et al. Radioembolization for Unresectable Neuroendocrine Hepatic Metastases Using Resin 90Y-Microspheres: Early Results in 148 Patients. Am. J. Clin. Oncol. 2008, 31, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-L.; Lu, M.-Y.; Chang, H.-H.; Lu, C.-C.; Lin, D.-T.; Jou, S.-T.; Yang, Y.-L.; Lee, Y.-L.; Huang, S.-F.; Jeng, Y.-M.; et al. Diagnostic FDG and FDOPA positron emission tomography scans distinguish the genomic type and treatment outcome of neuroblastoma. Oncotarget 2016, 7, 18774–18786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.L.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yu, J.; Li, J.; Shen, L.; Li, N.; Zhu, H.; Zhai, S.; Zhang, Y.; Yang, Z.; Lu, M. Clinical and Prognostic Value of PET/CT Imaging with Combination of 68Ga-DOTATATE and 18F-FDG in Gastroenteropancreatic Neuroendocrine Neoplasms. Contrast Media Mol. Imaging 2018, 2018, 2340389. [Google Scholar] [CrossRef]
- Cistaro, A.; Quartuccio, N.; Caobelli, F.; Piccardo, A.; Paratore, R.; Coppolino, P.; Sperandeo, A.; Arnone, G.; Ficola, U. 124I-MIBG: A new promising positron-emitting radiopharmaceutical for the evaluation of neuroblastoma. Nucl. Med. Rev. 2015, 18, 102–106. [Google Scholar] [CrossRef]
- Mankoff, D.A.; Dehdashti, F. Imaging Tumor Phenotype: 1 Plus 1 Is More than 2. J. Nucl. Med. 2009, 50, 1567–1569. [Google Scholar] [CrossRef] [PubMed]
- Waseem, N.; Aparici, C.M.; Kunz, P.L.; Waseem, N.L. Evaluating the Role of Theranostics in Grade 3 Neuroendocrine Neoplasms. J. Nucl. Med. 2019, 60, 882–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Ansell, S.; Schwartz, L.; Gordon, L.I.; Advani, R.; Jacene, H.A.; Hoos, A.; Barrington, S.F.; Armand, P. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 2016, 128, 2489–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, F.-L.; Ford, R.J.; Yang, D.J. Managing Lymphoma with Non-FDG Radiotracers: Current Clinical and Preclinical Applications. Available online: https://www.hindawi.com/journals/bmri/2013/626910/ (accessed on 20 August 2019).
- Buck, A.K.; Bommer, M.; Stilgenbauer, S.; Juweid, M.; Glatting, G.; Schirrmeister, H.; Mattfeldt, T.; Tepsic, D.; Bunjes, D.; Mottaghy, F.M.; et al. Molecular Imaging of Proliferation in Malignant Lymphoma. Cancer Res. 2006, 66, 11055–11061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuutinen, J.; Leskinen, S.; Lindholm, P.; Söderström, K.-O.; Någren, K.; Huhtala, S.; Minn, H. Use of carbon-11 methionine positron emission tomography to assess malignancy grade and predict survival in patients with lymphomas. Eur. J. Nucl. Med. 1998, 25, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Minamimoto, R.; Fayad, L.; Advani, R.; Vose, J.; Macapinlac, H.; Meza, J.; Hankins, J.; Mottaghy, F.; Juweid, M.; Quon, A. Diffuse Large B-Cell Lymphoma: Prospective Multicenter Comparison of Early Interim FLT PET/CT versus FDG PET/CT with IHP, EORTC, Deauville, and PERCIST Criteria for Early Therapeutic Monitoring. Radiology 2016, 280, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, K.; Buck, A.K.; Schuster, T.; Abbrederis, K.; Blümel, C.; Santi, I.; Rudelius, M.; Wester, H.-J.; Peschel, C.; Schwaiger, M.; et al. Week one FLT-PET response predicts complete remission to R-CHOP and survival in DLBCL. Oncotarget 2014, 5, 4050–4059. [Google Scholar] [CrossRef]
- Chantepie, S.; Hovhannisyan, N.; Guillouet, S.; Pelage, J.-P.; Ibazizene, M.; Bodet-Milin, C.; Carlier, T.; Gac, A.-C.; Reboursière, E.; Vilque, J.-P.; et al. 18F-Fludarabine PET for Lymphoma Imaging: First-in-Humans Study on DLBCL and CLL Patients. J. Nucl. Med. 2018, 59, 1380–1385. [Google Scholar] [CrossRef]
- Gourni, E.; Demmer, O.; Schottelius, M.; D’Alessandria, C.; Schulz, S.; Dijkgraaf, I.; Schumacher, U.; Schwaiger, M.; Kessler, H.; Wester, H.-J. PET of CXCR4 Expression by a 68Ga-Labeled Highly Specific Targeted Contrast Agent. J. Nucl. Med. 2011, 52, 1803–1810. [Google Scholar] [CrossRef]
- Herrmann, K.; Schottelius, M.; Lapa, C.; Osl, T.; Poschenrieder, A.; Hänscheid, H.; Lückerath, K.; Schreder, M.; Bluemel, C.; Knott, M.; et al. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in Advanced-Stage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J. Nucl. Med. 2016, 57, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Hindié, E. The NETPET Score: Combining FDG and Somatostatin Receptor Imaging for Optimal Management of Patients with Metastatic Well-Differentiated Neuroendocrine Tumors. Theranostics 2017, 7, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Gains, J.E.; Sebire, N.J.; Moroz, V.; Wheatley, K.; Gaze, M.N. Immunohistochemical evaluation of molecular radiotherapy target expression in neuroblastoma tissue. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Gains, J.E.; Bomanji, J.B.; Fersht, N.L.; Sullivan, T.; D’Souza, D.; Sullivan, K.P.; Aldridge, M.; Waddington, W.; Gaze, M.N. 177Lu-DOTATATE Molecular Radiotherapy for Childhood Neuroblastoma. J. Nucl. Med. 2011, 52, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Kayano, D.; Kinuya, S. Iodine-131 Metaiodobenzylguanidine Therapy for Neuroblastoma: Reports So Far and Future Perspective. Sci. World J. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Deubzer, H.; Hundsdoerfer, P.; Fuchs, J.; Prasad, V.; Timmermann, B.; Astrahantseff, K.; Berthold, F.; Simon, T.; Hero, B.; Schulte, J.H.; et al. 2017 GPOH Guidelines for Diagnosis and Treatment of Patients with Neuroblastic Tumors. Klin. Pädiatr. 2017, 229, 147–167. [Google Scholar]
- Kong, G.; Hofman, M.S.; Murray, W.K.; Wilson, S.; Wood, P.; Downie, P.; Super, L.; Hogg, A.; Eu, P.; Hicks, R.J. Initial Experience with Gallium-68 DOTA-Octreotate PET/CT and Peptide Receptor Radionuclide Therapy for Pediatric Patients with Refractory Metastatic Neuroblastoma. J. Pediatr. Hematol. 2016, 38, 1–96. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Navalkissoor, S.; Flux, G.; Bomanji, J. Molecular radiotheranostics for neuroendocrine tumours. Clin. Med. 2017, 17, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.T.; Kulkarni, H.R.; Singh, A.; Baum, R.P. Theranostics of Neuroendocrine Tumors. Visc. Med. 2017, 33, 358–366. [Google Scholar] [CrossRef]
- Bailly, C.; Cléry, P.-F.; Faivre-Chauvet, A.; Bourgeois, M.; Guérard, F.; Haddad, F.; Barbet, J.; Chérel, M.; Kraeber-Bodéré, F.; Carlier, T.; et al. Immuno-PET for Clinical Theranostic Approaches. Int. J. Mol. Sci. 2016, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Giesen, D.; Jalving, M.; Moek, K.L.; Kok, I.C.; De Groot, D.J.A.; Fehrmann, R.S.; Hooge, M.N.L.-D.; Brouwers, A.H.; De Vries, E.G. Theranostics Using Antibodies and Antibody-Related Therapeutics. J. Nucl. Med. 2017, 58, 83–90. [Google Scholar]
- Lamberts, L.E.; Williams, S.P.; Van Scheltinga, A.G.T.; Hooge, M.N.L.-D.; Schroder, C.P.; Gietema, J.A.; Brouwers, A.H.; De Vries, E.G. Antibody Positron Emission Tomography Imaging in Anticancer Drug Development. J. Clin. Oncol. 2015, 33, 1491–1504. [Google Scholar] [CrossRef] [PubMed]
- Kraeber-Bodere, F.; Bailly, C.; Chérel, M.; Chatal, J.-F. ImmunoPET to help stratify patients for targeted therapies and to improve drug development. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2166–2168. [Google Scholar] [CrossRef] [PubMed]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring HER2-Receptor Expression In Metastatic Breast Cancer Using [68Ga]ABY-025 Affibody PET/CT. Theranostics 2016, 6, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.W.; Djulbegovic, B.; Soares, H.P.; Siegel, B.A.; Lowe, V.J.; Lyman, G.H.; Coleman, R.E.; Wahl, R.; Paschold, J.C.; Avril, N.; et al. Recommendations on the Use of 18F-FDG PET in Oncology. J. Nucl. Med. 2008, 49, 480–508. [Google Scholar] [CrossRef]
- Czernin, J.; Allen-Auerbach, M.; Nathanson, D.; Herrmann, K. PET/CT in Oncology: Current Status and Perspectives. Curr. Radiol. Rep. 2013, 1, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Petersen, H.; Holdgaard, P.C.; Madsen, P.H.; Knudsen, L.M.; Gad, D.; Gravergaard, A.E.; Rohde, M.; Godballe, C.; Engelmann, B.E.; Bech, K.; et al. FDG PET/CT in cancer: Comparison of actual use with literature-based recommendations. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 695–706. [Google Scholar] [CrossRef]
- Thie, J.A. Understanding the standardized uptake value, its methods, and implications for usage. J. Nucl. Med. 2004, 45, 1431–1434. [Google Scholar]
- Carlier, T.; Bailly, C. State-Of-The-Art and Recent Advances in Quantification for Therapeutic Follow-Up in Oncology Using PET. Front. Med. 2015, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Keyes, J.W. SUV: Standard uptake or silly useless value? J. Nucl. Med. 1995, 36, 1836–1839. [Google Scholar] [PubMed]
- Okada, J.; Oonishi, H.; Yoshikawa, K.; Itami, J.; Uno, K.; Imaseki, K.; Arimizu, N. FDG-PET for predicting the prognosis of malignant lymphoma. Ann. Nucl. Med. 1994, 8, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Schöder, H.; Noy, A.; Gönen, M.; Weng, L.; Green, D.; Erdi, Y.E.; Larson, S.M.; Yeung, H.W.D. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J. Clin. Oncol. 2005, 23, 4643–4651. [Google Scholar] [CrossRef] [PubMed]
- Bodet-Milin, C.; Kraeber-Bodéré, F.; Moreau, P.; Campion, L.; Dupas, B.; Le Gouill, S.; Drouet, M.; Delaunay, C.; Grenier, N.; Garrigou, P.; et al. Investigation of FDG-PET/CT imaging to guide biopsies in the detection of histological transformation of indolent lymphoma. Haematologica 2008, 93, 471–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodet-Milin, C.; Bailly, C.; Meignan, M.; Beriollo-Riedinger, A.; Casasnovas, R.-O.; Devillers, A.; Lamy, T.; Santiago-Ribeiro, M.; Gyan, E.; Gallazzini-Crépin, C.; et al. Predictive Power of FDG-PET Parameters at Diagnosis and after Induction in Patients with Mantle Cell Lymphoma, Interim Results from the LyMa-PET Project, Conducted on Behalf of the Lysa Group. Blood 2015, 126, 335. [Google Scholar]
- Bodet-Milin, C.; Touzeau, C.; Leux, C.; Sahin, M.; Moreau, A.; Maisonneuve, H.; Morineau, N.; Jardel, H.; Gallazini-Crépin, C.; Gries, P.; et al. Prognostic impact of 18F-fluoro-deoxyglucose positron emission tomography in untreated mantle cell lymphoma: A retrospective study from the GOELAMS group. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Carlier, T.; Touzeau, C.; Arlicot, N.; Kraeber-Bodéré, F.; Le Gouill, S.; Bodet-Milin, C. Interest of FDG-PET in the Management of Mantle Cell Lymphoma. Front. Med. 2019, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C.; Carlier, T.; Berriolo-Riedinger, A.; Casasnovas, O.; Gyan, E.; Meignan, M.; Moreau, A.; Burroni, B.; Djaileb, L.; Gressin, R.; et al. Prognostic value of FDG-PET in patients with mantle cell lymphoma: Results from the LyMa-PET Project. Haematologica 2019. [Google Scholar] [CrossRef]
- Eisenhauer, E.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; Van Oosterom, A.T.; Christian, M.C.; et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, L.H.; Litiere, S.; De Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and Clarification: From the RECIST Committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hajj, M.; Becker, M.W.; Wicha, M.; Weissman, I.; Clarke, M.F. Therapeutic implications of cancer stem cells. Curr. Opin. Genet. Dev. 2004, 14, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Huff, C.A.; Matsui, W.; Smith, B.D.; Jones, R.J. The paradox of response and survival in cancer therapeutics. Blood 2006, 107, 431–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Itti, E.; Haioun, C.; Petegnief, Y.; Luciani, A.; Dupuis, J.; Paone, G.; Talbot, J.-N.; Rahmouni, A.; Meignan, M. Early 18F-FDG PET for Prediction of Prognosis in Patients with Diffuse Large B-Cell Lymphoma: SUV-Based Assessment versus Visual Analysis. J. Nucl. Med. 2007, 48, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.L.; Zasadny, K.; Helvie, M.; Hutchins, G.D.; Weber, B.; Cody, R. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: Initial evaluation. J. Clin. Oncol. 1993, 11, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Carlier, T.; Jamet, B.; Eugene, T.; Touzeau, C.; Attal, M.; Hulin, C.; Facon, T.; Leleu, X.; Perrot, A.; et al. Interim PET Analysis in First-Line Therapy of Multiple Myeloma: Prognostic Value of ΔSUVmax in the FDG-Avid Patients of the IMAJEM Study. Clin. Cancer Res. 2018, 24, 5219–5224. [Google Scholar] [CrossRef] [PubMed]
- Matsui, W.; Huff, C.A.; Wang, Q.; Malehorn, M.T.; Barber, J.; Tanhehco, Y.; Smith, B.D.; Civin, C.I.; Jones, R.J. Characterization of clonogenic multiple myeloma cells. Blood 2004, 103, 2332–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasche, L.; Chavan, S.S.; Stephens, O.W.; Patel, P.H.; Tytarenko, R.; Ashby, C.; Bauer, M.; Stein, C.; Deshpande, S.; Wardell, C.; et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. Commun. 2017, 8, 268. [Google Scholar] [CrossRef]
- Rasche, L.; Kortüm, K.M.; Raab, M.S.; Weinhold, N. The Impact of Tumor Heterogeneity on Diagnostics and Novel Therapeutic Strategies in Multiple Myeloma. Int. J. Mol. Sci. 2019, 20, 1248. [Google Scholar] [CrossRef]
- Pugachev, A.; Ruan, S.; Carlin, S.; Larson, S.M.; Campa, J.; Ling, C.C.; Humm, J.L. Dependence of FDG uptake on tumor microenvironment. Int. J. Radiat. Oncol. 2005, 62, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambin, P.; Leijenaar, R.T.; Deist, T.M.; Peerlings, J.; De Jong, E.E.; Van Timmeren, J.; Sanduleanu, S.; LaRue, R.T.; Even, A.J.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Hatt, M.; Tixier, F.; Visvikis, D.; Cheze Le Rest, C. Radiomics in PET/CT: More Than Meets the Eye? J. Nucl. Med. 2017, 58, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Hatt, M.; Tixier, F.; Pierce, L.; Kinahan, P.E.; Rest, C.C.L.; Visvikis, D. Characterization of PET/CT images using texture analysis: The past, the present … any future? Eur. J. Nucl. Med. Mol. Imaging 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vallières, M.; Zwanenburg, A.; Badic, B.; Cheze Le Rest, C.; Visvikis, D.; Hatt, M. Responsible Radiomics Research for Faster Clinical Translation. J. Nucl. Med. 2018, 59, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Berthon, B.; Spezi, E.; Galavis, P.; Shepherd, T.; Apte, A.; Hatt, M.; Fayad, H.; De Bernardi, E.; Soffientini, C.D.; Schmidtlein, C.R.; et al. Toward a standard for the evaluation of PET-Auto-Segmentation methods following the recommendations of AAPM task group No. 211: Requirements and implementation. Med Phys. 2017, 44, 4098–4111. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. Image biomarker standardisation initiative. arXiv 2016, arXiv:1612.07003. [Google Scholar]
- Tixier, F.; Le Rest, C.C.; Hatt, M.; Albarghach, N.M.; Pradier, O.; Metges, J.-P.; Corcos, L.; Visvikis, D. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J. Nucl. Med. 2011, 52, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.J.R.; Siddique, M.; Taylor, B.P.; Yip, C.; Chicklore, S.; Goh, V. Radiomics in PET: Principles and applications. Clin. Transl. Imaging 2014, 2, 269–276. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, S.M. Radiomics in Oncological PET/CT: Clinical Applications. Nucl. Med. Mol. Imaging 2018, 52, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Chalkidou, A.; O’Doherty, M.J.; Marsden, P.K. False Discovery Rates in PET and CT Studies with Texture Features: A Systematic Review. PLoS ONE 2015, 10, e0124165. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.L.; McIntosh, C.; Haibe-Kains, B.; Milosevic, M.F.; Wee, L.; Dekker, A.; Huang, S.H.; Purdie, T.G.; O’Sullivan, B.; Aerts, H.J.; et al. Vulnerabilities of radiomic signature development: The need for safeguards. Radiother. Oncol. 2019, 130, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucia, F.; Visvikis, D.; Vallières, M.; Desseroit, M.-C.; Miranda, O.; Robin, P.; Bonaffini, P.A.; Alfieri, J.; Masson, I.; Mervoyer, A.; et al. External validation of a combined PET and MRI radiomics model for prediction of recurrence in cervical cancer patients treated with chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Aguilera, T.; Shultz, D.; Gudur, M.; Rubin, D.L.; Loo, B.W.; Diehn, M.; Li, R. Early-Stage Non–Small Cell Lung Cancer: Quantitative Imaging Characteristics of 18F Fluorodeoxyglucose PET/CT Allow Prediction of Distant Metastasis. Radiology 2016, 281, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.; Leijenaar, R.T.H.; Troost, E.G.C.; van Timmeren, J.E.; Oberije, C.; van Elmpt, W.; de Geus-Oei, L.-F.; Bussink, J.; Lambin, P. 18F-fluorodeoxyglucose positron-emission tomography (FDG-PET)-Radiomics of metastatic lymph nodes and primary tumor in non-small cell lung cancer (NSCLC)—A prospective externally validated study. PLoS ONE 2018, 13, e0192859. [Google Scholar] [CrossRef] [PubMed]
- Ypsilantis, P.-P.; Siddique, M.; Sohn, H.-M.; Davies, A.; Cook, G.; Goh, V.; Montana, G. Predicting Response to Neoadjuvant Chemotherapy with PET Imaging Using Convolutional Neural Networks. PLoS ONE 2015, 10, e0137036. [Google Scholar] [CrossRef] [PubMed]
- Arimura, H.; Soufi, M.; Kamezawa, H.; Ninomiya, K.; Yamada, M. Radiomics with artificial intelligence for precision medicine in radiation therapy. J. Radiat. Res. 2019, 60, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Orlhac, F.; Boughdad, S.; Philippe, C.; Stalla-Bourdillon, H.; Nioche, C.; Champion, L.; Soussan, M.; Frouin, F.; Frouin, V.; Buvat, I. A Postreconstruction Harmonization Method for Multicenter Radiomic Studies in PET. J. Nucl. Med. 2018, 59, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Vallieres, M.; Dohan, A.; Levesque, I.R.; Ueno, Y.; Saif, S.; Reinhold, C.; Seuntjens, J. Creating Robust Predictive Radiomic Models for Data from Independent Institutions Using Normalization. IEEE Trans. Radiat. Plasma Med Sci. 2019, 3, 210–215. [Google Scholar] [CrossRef]
- Upadhaya, T.; Vallieres, M.; Chatterjee, A.; Lucia, F.; Bonaffini, P.A.; Masson, I.; Mervoyer, A.; Reinhold, C.; Schick, U.; Seuntjens, J.; et al. Comparison of Radiomics Models Built Through Machine Learning in a Multicentric Context with Independent Testing: Identical Data, Similar Algorithms, Different Methodologies. IEEE Trans. Radiat. Plasma Med Sci. 2019, 3, 192–200. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Fieguth, P.; Zhao, G.; Chellappa, R.; Pietikäinen, M. From BoW to CNN: Two Decades of Texture Representation for Texture Classification. Int. J. Comput. Vis. 2019, 127, 74–109. [Google Scholar] [CrossRef]
- Desseroit, M.-C.; Visvikis, D.; Tixier, F.; Majdoub, M.; Guillevin, R.; Perdrisot, R.; Le Rest, C.C.; Hatt, M. Development of a nomogram combining clinical staging with (18)F-FDG PET/CT image features in non-small-cell lung cancer stage I-III. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.-C.; Cheng, N.-M.; Hsieh, C.-H.; Ng, S.-H.; Lin, C.-Y.; Yen, T.-C.; Hsu, C.-L.; Wan, H.-M.; Liao, C.-T.; Chang, K.-P.; et al. Multiparametric imaging using 18F-FDG PET/CT heterogeneity parameters and functional MRI techniques: Prognostic significance in patients with primary advanced oropharyngeal or hypopharyngeal squamous cell carcinoma treated with chemoradiotherapy. Oncotarget 2017, 8, 62606–62621. [Google Scholar] [CrossRef] [PubMed]
- Vallières, M.; Freeman, C.R.; Skamene, S.R.; El Naqa, I. A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys. Med. Boil. 2015, 60, 5471–5496. [Google Scholar] [CrossRef] [PubMed]
- Bodet-Milin, C.; Bailly, C.; Meignan, M.; Berriolo-riedinger, A.; Devillers, A.; Hermine, O.; Carlier, T.; Hatt, M.; Kraeber-Bodéré, F.; Gouill, S.L. Prognosis value of quantitative indices derived from initial FDG PET/CT in untreated mantle cell lymphoma patients enrolled in the Lyma trial, a LYSA study. Preliminary results. J. Nucl. Med. 2015, 56, 659. [Google Scholar]
- Carlier, T.; Bailly, C.; Hatt, M.; Kraeber-Bodéré, F.; Visvikis, D.; Gouill, S.L.; Bodet-Milin, C. Quantification of intratumor heterogeneity derived from baseline FDG PET/CT in untreated mantle cell lymphoma patients enrolled in a prospective phase III trial of the LYSA group: Preliminary results. J. Nucl. Med. 2015, 56, 429. [Google Scholar]
- Mayerhoefer, M.E.; Riedl, C.C.; Kumar, A.; Gibbs, P.; Weber, M.; Tal, I.; Schilksy, J.; Schöder, H. Radiomic features of glucose metabolism enable prediction of outcome in mantle cell lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 1–10. [Google Scholar] [CrossRef]
- Lucia, F.; Visvikis, D.; Desseroit, M.-C.; Miranda, O.; Malhaire, J.-P.; Robin, P.; Pradier, O.; Hatt, M.; Schick, U. Prediction of outcome using pretreatment 18F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 768–786. [Google Scholar] [CrossRef]




| Tracer | Metabolic Process | Principal Oncological Indications |
|---|---|---|
| 11C-Methionine | Amino acid transport and protein synthesis | Diagnosis and grading of brain tumors |
| 18F-Choline (FCH) | Phosphatidylcholine metabolism and cellular membrane turnover | Biopsy guidance of prostate cancer recurrence/primary staging in high-risk prostate cancer before surgical procedures or planning external beam radiation |
| 18F-Fluoro-Deoxyglucose (FDG) | Glucose metabolism | Diagnosis/restaging of lung cancer, colorectal cancer, breast cancer, lymphoma, sarcoma, melanoma, head and neck cancer |
| 18F-DOPA | Dopamine uptake and metabolism | Diagnosis of neuroendocrine tumors (NET)/documented NET metastasis in unknown primary |
| 68Ga-DOTA-Peptides | Somatostatin receptors | Identification of primary tumor in patients with documented NET metastasis/assessment of NET disease extent before treatment |
| 18F-Fluoroestradiol (FES) | Estrogen receptor | Status of tumor lesions to determine need for endocrine therapy in breast cancer |
| 18F-Fluorothymidine (FLT) | Cellular proliferation and | Differential diagnosis between benign and malignant lesions/lymphoma staging and therapeutic evaluation |
| 18-Sodium Fluoride (NaF) | Bone metabolism | Detection of bone involvement in tumors with elevated risk of bone metastasis |
| 68Ga-Prostate-Specific Membrane Antigen (PSMA) | PSMA expression | Localization of tumor tissue in recurrent prostate cancer |
| Order | Matrix | Name of the Parameter | Description of the Parameter |
|---|---|---|---|
| First Order | SUVmax | SUV value of the maximum intensity voxel within a region of interest (ROI) | |
| SUVpeak | Average SUV within a small ROI (usually, a 1-cm3 spherical volume) | ||
| Second Order | SUVmean | Average measure of SUV within a defined ROI | |
| Metabolic tumor volume (MTV) | Volume of a defined ROI | ||
| Total lesion glycolysis (TLG) | Product of SUVmean × MTV | ||
| Grey-Level Co-Occurrence Matrix (GLCM) | Contrast | Local variations in the GLCM | |
| Correlation | Joint probability occurrence of the specified pixel pairs | ||
| Entropy | Texture randomness or irregularity | ||
| Energy | Sum of squared elements in the GLCM | ||
| Homogeneity | Closeness of the distribution of elements to the diagonal | ||
| High Order | Gray-Level Run-Length Matrix (GLRLM) | Short run emphasis (SRE) | Distribution of short runs |
| Long run emphasis (LRE) | Distribution of long runs | ||
| High gray level run emphasis (HGRE) | Distribution of high grey level values runs | ||
| Grey-level non-uniformity (GLNU) | Similarity of grey level values throughout the image | ||
| Run percentage (RP) | Homogeneity and distribution of runs of an image in a specific direction | ||
| Gray-Level Zone Size Encoding Method (GLZSM) | High gray-level zone emphasis (HGZE) | Distribution of high grey level values zones | |
| Zone length non uniformity (ZLNU) | Similarity of zone length throughout the image | ||
| Zone percentage (ZP) | Homogeneity and distribution of zones of an image in a specific direction | ||
| Short zone emphasis (SZE) | Distribution of small zones | ||
| Neighborhood Grey Tone Difference Matrix (NGTDM) | Coarseness | Granularity within an image. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, C.; Bodet-Milin, C.; Bourgeois, M.; Gouard, S.; Ansquer, C.; Barbaud, M.; Sébille, J.-C.; Chérel, M.; Kraeber-Bodéré, F.; Carlier, T. Exploring Tumor Heterogeneity Using PET Imaging: The Big Picture. Cancers 2019, 11, 1282. https://doi.org/10.3390/cancers11091282
Bailly C, Bodet-Milin C, Bourgeois M, Gouard S, Ansquer C, Barbaud M, Sébille J-C, Chérel M, Kraeber-Bodéré F, Carlier T. Exploring Tumor Heterogeneity Using PET Imaging: The Big Picture. Cancers. 2019; 11(9):1282. https://doi.org/10.3390/cancers11091282
Chicago/Turabian StyleBailly, Clément, Caroline Bodet-Milin, Mickaël Bourgeois, Sébastien Gouard, Catherine Ansquer, Matthieu Barbaud, Jean-Charles Sébille, Michel Chérel, Françoise Kraeber-Bodéré, and Thomas Carlier. 2019. "Exploring Tumor Heterogeneity Using PET Imaging: The Big Picture" Cancers 11, no. 9: 1282. https://doi.org/10.3390/cancers11091282
APA StyleBailly, C., Bodet-Milin, C., Bourgeois, M., Gouard, S., Ansquer, C., Barbaud, M., Sébille, J. -C., Chérel, M., Kraeber-Bodéré, F., & Carlier, T. (2019). Exploring Tumor Heterogeneity Using PET Imaging: The Big Picture. Cancers, 11(9), 1282. https://doi.org/10.3390/cancers11091282





