Near-Infrared Fluorescent Sorbitol Probe for Targeted Photothermal Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
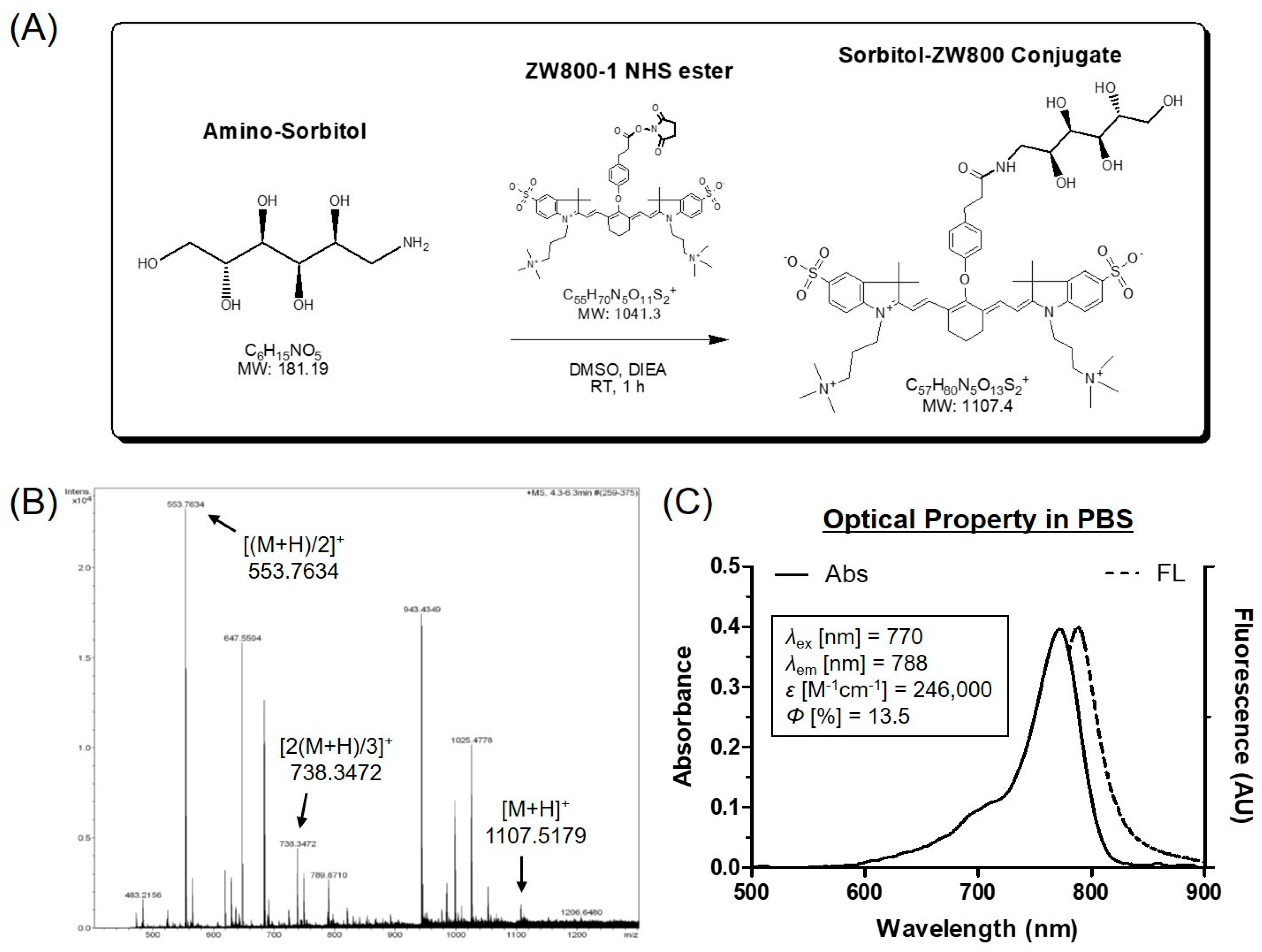
2.2. Conjugation of Amino-Sorbitol to ZW800-1 NIR Fluorophore (sorbitol–ZW800)
2.3. Optical Property Analysis
2.4. HT-29 Xenograft Mouse Model
2.5. In Vivo Tumor Imaging
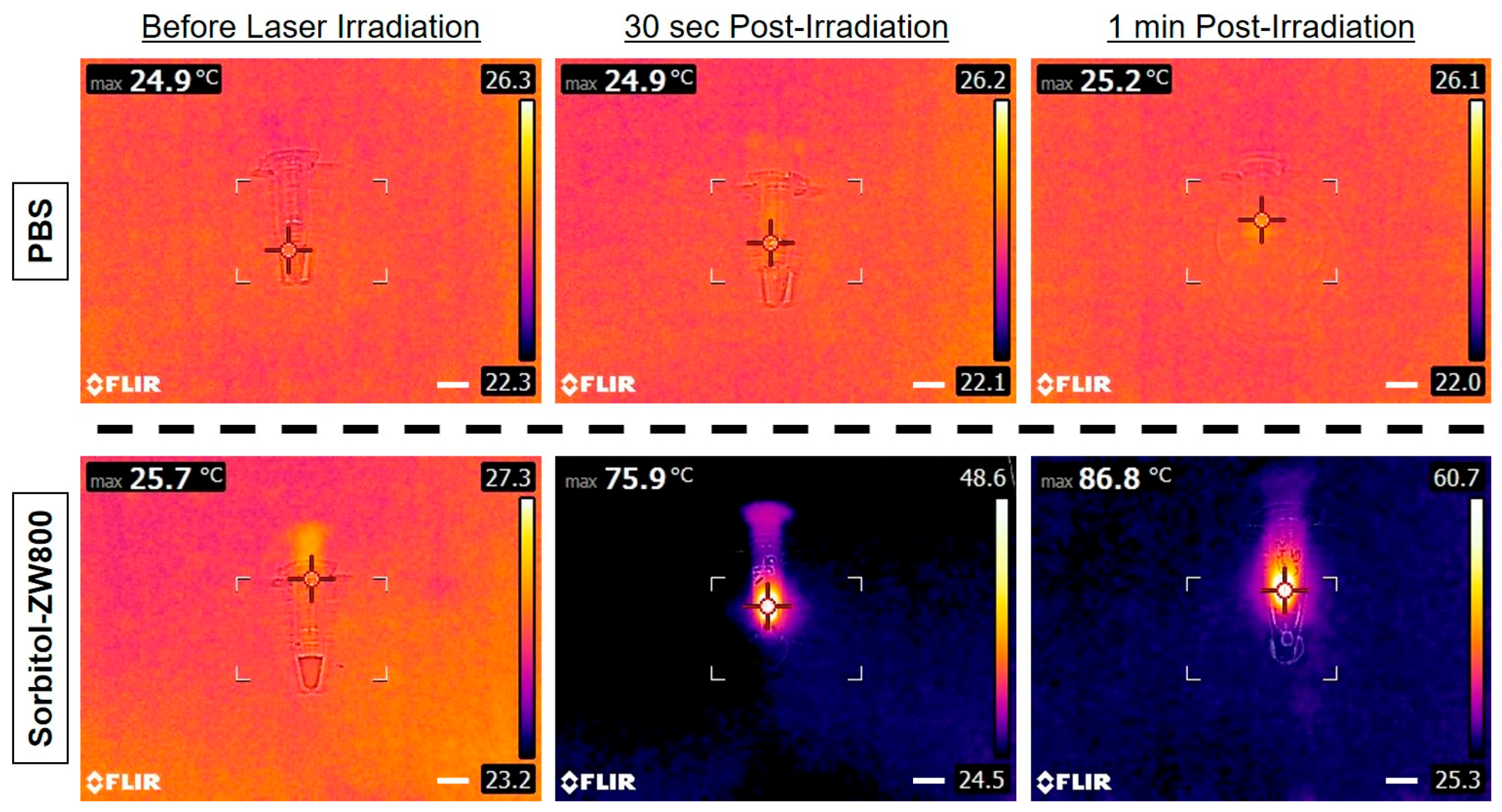
2.6. In Vivo Photothermal Effect Assessment
2.7. Statistical Analyses
2.8. Histological Analysis
3. Results and Discussion
3.1. Preparation and Characterization of sorbitol–ZW800 Conjugate
3.2. Assessment of In Vitro Photothermal Effect
3.3. In Vivo NIR Fluorescence Imaging for Tumor Targetability
3.4. Assessment of In Vivo Photothermal Effect
3.5. In Vivo PTT Efficacy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, J.; Choi, J.; Bang, D.; Kim, E.; Lim, E.K.; Park, H.; Suh, J.S.; Lee, K.; Yoo, K.H.; Kim, E.K.; et al. Convertible organic nanoparticles for near-infrared photothermal ablation of cancer cells. Angew. Chem. Int. Ed. 2011, 50, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, C.; Wang, S.; Li, Z.; Hu, H.; Wan, Y.; Yang, X. Hydroxyethyl starch-based nanoparticles featured with redox-sensitivity and chemo-photothermal therapy for synergized tumor eradication. Cancers 2019, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, S.P.; Dai, H. Multifunctional FeCo-graphitic carbon nanocrystals for combined imaging, drug delivery and tumor-specific photothermal therapy in mice. Nano Res. 2011, 4, 1248–1260. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Hu, X.; Xie, Z.; Jing, X. Cyanine-curcumin assembling nanoparticles for near-infrared imaging and photothermal therapy. ACS Biomater. Sci. Eng. 2016, 2, 1942–1950. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Y.; Niu, G.; Lan, M.; Jia, Q.; Liang, Q. Near-infrared organic dye-based nanoagent for the photothermal therapy of cancer. ACS Appl. Mater. Interfaces 2016, 8, 29899–29905. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Wada, H.; Bao, K.; Gravier, J.; Yadav, Y.; Laramie, M.; Henary, M.; Frangioni, J.V.; Choi, H.S. Phosphonated near-infrared fluorophores for biomedical imaging of bone. Angew. Chem. Int. Ed. 2014, 53, 10668–10672. [Google Scholar] [CrossRef]
- Hyun, H.; Owens, E.A.; Park, M.H.; Wada, H.; Frangioni, J.V.; Henary, M.; Choi, H.S. Cartilage-specific near-infrared fluorophores for biomedical imaging. Angew. Chem. Int. Ed. 2015, 54, 8648–8652. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Owens, E.A.; Wada, H.; Henary, M.; Handgraaf, H.J.M.; Vahrmeijer, A.L.; Frangioni, J.V.; Choi, H.S. Structure-inherent targeting of near-infrared fluorophores for parathyroid and thyroid gland imaging. Nat. Med. 2015, 21, 192–197. [Google Scholar] [CrossRef]
- Park, M.H.; Lim, W.; Jo, D.; Jung, J.S.; Kim, S.; Kim, J.; Lim, H.S.; Lee, J.S.; Min, J.J.; Hyun, H. Rapid differential diagnosis of breast microcalcification using targeted near-infrared fluorophores. Adv. Healthc. Mater. 2018, 7, e1701062. [Google Scholar] [CrossRef]
- Jo, D.; Hyun, H. Structure-inherent targeting of near-infrared fluorophores for image-guided surgery. Chonnam Med. J. 2017, 53, 95–102. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Ke, H.; Zhu, A.; Wang, Y.; Wang, J.; Shen, J.; Liu, G.; Chen, C.; Zhao, Y.; et al. Smart albumin-biomineralized nanocomposites for multimodal imaging and photothermal tumor ablation. Adv. Mater. 2015, 27, 3874–3882. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cui, Z.; Yu, P.; Guo, C.; Feng, B.; Jiang, T.; Wang, S.; Yin, Q.; Zhong, D.; Yang, X.; et al. pH- and NIR light-responsive micelles with hyperthermia-triggered tumor penetration and cytoplasm drug release to reverse doxorubicin resistance in breast cancer. Adv. Funct. Mater. 2015, 25, 2489–2500. [Google Scholar] [CrossRef]
- Deng, L.; Cai, X.; Sheng, D.; Yang, Y.; Strohm, E.M.; Wang, Z.; Ran, H.; Wang, D.; Zheng, Y.; Li, P.; et al. A laser-activated biocompatible theranostic nanoagent for targeted multimodal imaging and photothermal therapy. Theranostics 2017, 7, 4410–4423. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Abbas, M.; Zhao, L.; Li, S.; Shen, G.; Yan, X. Biological photothermal nanodots based on self-assembly of peptide-porphyrin conjugates for antitumor therapy. J. Am. Chem. Soc. 2017, 139, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Shafirstein, G.; Baumler, W.; Hennings, L.J.; Siegel, E.R.; Friedman, R.; Moreno, M.A.; Webber, J.; Jackson, C.; Griffin, R.J. Indocyanine green enhanced near-infrared laser treatment of murine mammary carcinoma. Int. J. Cancer 2012, 130, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.X.; Liu, P.; Zheng, M.B.; Zhao, P.F.; Wang, Y.Q.; Ma, Y.F.; Cai, L.T. IR-780 dye loaded tumor targeting theranostic nanoparticles for NIR imaging and photothermal therapy. Biomaterials 2013, 34, 6853–6861. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; He, W.W.; Gong, H.; Wang, C.; Chen, Q.; Cheng, Z.P.; Liu, Z. PEGylated micelle nanoparticles encapsulating a non-fluorescent near-infrared organic dye as a safe and highly-effective photothermal agent for in vivo cancer therapy. Adv. Funct. Mater. 2013, 23, 5893–5902. [Google Scholar] [CrossRef]
- Choi, H.S.; Nasr, K.; Alyabyev, S.; Feith, D.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Hyun, H.; Patonay, G.; Strekowski, L.; et al. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores. Angew. Chem. Int. Ed. 2011, 50, 6258–6263. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Bordo, M.W.; Nasr, K.; Feith, D.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Moffitt, L.A.; Rosenberg, M.; Henary, M.; et al. cGMP-Compatible preparative scale synthesis of near-infrared fluorophores. Contrast Media Mol. Imaging 2012, 7, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Gibbs, S.L.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Liu, F.; Hyun, H.; Park, G.; Xie, Y.; Bae, S.; et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat. Biotechnol. 2013, 31, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Teoh, C.L.; Samanta, A.; Kang, N.Y.; Park, S.J.; Chang, Y.T. The development of a highly photostable and chemically stable zwitterionic near-infrared dye for imaging applications. Chem. Commun. 2015, 51, 3989–3992. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lim, W.; Jo, D.; Jung, J.S.; Kim, S.; Jo, G.; Min, J.J.; Choi, E.Y.; Hyun, H. Near-infrared fluorescent sorbitol probe for tumor diagnosis in vivo. J. Ind. Eng. Chem. 2018, 64, 80–84. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Jung, J.S.; Jo, G.; Yang, D.H.; Koh, Y.S.; Hyun, H. Near-Infrared Fluorescent Sorbitol Probe for Targeted Photothermal Cancer Therapy. Cancers 2019, 11, 1286. https://doi.org/10.3390/cancers11091286
Lee S, Jung JS, Jo G, Yang DH, Koh YS, Hyun H. Near-Infrared Fluorescent Sorbitol Probe for Targeted Photothermal Cancer Therapy. Cancers. 2019; 11(9):1286. https://doi.org/10.3390/cancers11091286
Chicago/Turabian StyleLee, Sungsu, Jin Seok Jung, Gayoung Jo, Dae Hyeok Yang, Yang Seok Koh, and Hoon Hyun. 2019. "Near-Infrared Fluorescent Sorbitol Probe for Targeted Photothermal Cancer Therapy" Cancers 11, no. 9: 1286. https://doi.org/10.3390/cancers11091286
APA StyleLee, S., Jung, J. S., Jo, G., Yang, D. H., Koh, Y. S., & Hyun, H. (2019). Near-Infrared Fluorescent Sorbitol Probe for Targeted Photothermal Cancer Therapy. Cancers, 11(9), 1286. https://doi.org/10.3390/cancers11091286




