Survival Benefits of Chemotherapy for Patients with Advanced Pancreatic Cancer in A Clinical Real-World Cohort
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
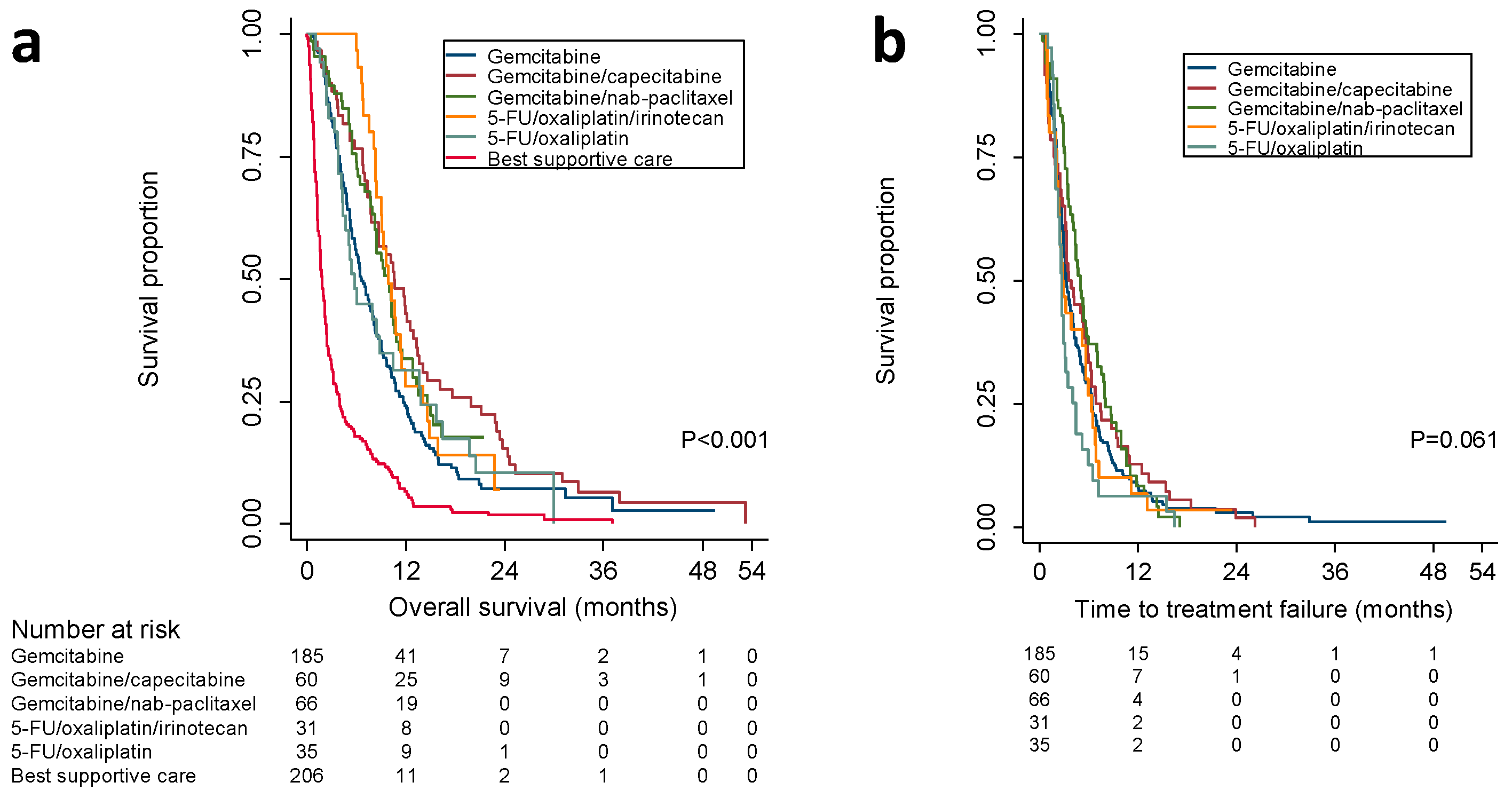
2.2. Overall Survival According to First-Line Treatment
2.3. Time to Treatment-Failure, Evaluation at the End of Treatment, and Protocol Adherence
2.4. Second-Line treatment
2.5. Adverse Events
3. Discussion
4. Materials and Methods
4.1. Patient Population
4.2. Patient and Tumor Characteristics
4.3. Chemotherapy Protocols
4.4. Outcome Measures
4.5. Dose Modifications and Protocol Adherence
4.6. Adverse Events
4.7. Statistical Analysis
4.8. Ethics Approval and Consent to Participate
4.9. Availability of Data and Material
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malvezzi, M.; Bertuccio, P.; Rosso, T.; Rota, M.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2015: Does lung cancer have the highest death rate in EU women? Ann. Oncol. 2015, 26, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta. Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Burris 3rd, H.; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Louvet, C.; Labianca, R.; Hammel, P.; Lledo, G.; Zampino, M.G.; Andre, T.; Zaniboni, A.; Ducreux, M.; Aitini, E.; Taieb, J.; et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J. Clin. Oncol. 2005, 23, 3509–3516. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Chau, I.; Stocken, D.D.; Valle, J.W.; Smith, D.; Steward, W.; Harper, P.G.; Dunn, J.; Tudur-Smith, C.; West, J.; et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. 2009, 27, 5513–5518. [Google Scholar] [CrossRef]
- Herrmann, R.; Bodoky, G.; Ruhstaller, T.; Glimelius, B.; Bajetta, E.; Schuller, J.; Saletti, P.; Bauer, J.; Figer, A.; Pestalozzi, B.; et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: A randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J. Clin. Oncol. 2007, 25, 2212–2217. [Google Scholar] [CrossRef]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.A.; et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Peixoto, R.D.; Ho, M.; Renouf, D.J.; Lim, H.J.; Gill, S.; Ruan, J.Y.; Cheung, W.Y. Eligibility of metastatic pancreatic cancer patients for first-line palliative intent nab-paclitaxel plus gemcitabine versus FOLFIRINOX. Am. J. Clin. Oncol. 2017, 40, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Vinci, A.; Schober, M.; Krug, S.; Javed, M.A.; Kohlmann, T.; Sund, M.; Neesse, A.; Beyer, G. Real-World Clinical Practice of Intensified Chemotherapies for Metastatic Pancreatic Cancer: Results from a Pan-European Questionnaire Study. Digestion 2016, 94, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Rahma, O.E.; Duffy, A.; Liewehr, D.J.; Steinberg, S.M.; Greten, T.F. Second-line treatment in advanced pancreatic cancer: A comprehensive analysis of published clinical trials. Ann. Oncol. 2013, 24, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Oettle, H.; Riess, H.; Stieler, J.M.; Heil, G.; Schwaner, I.; Seraphin, J.; Gorner, M.; Molle, M.; Greten, T.F.; Lakner, V.; et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: Outcomes from the CONKO-003 trial. J. Clin. Oncol. 2014, 32, 2423–2429. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Ko, Y.J.; Cripps, C.; Beaudoin, A.; Dhesy-Thind, S.; Zulfiqar, M.; Zalewski, P.; Do, T.; Cano, P.; Lam, W.Y.; et al. PANCREOX: A Randomized Phase III Study of 5-Fluorouracil/Leucovorin With or Without Oxaliplatin for Second-Line Advanced Pancreatic Cancer in Patients Who Have Received Gemcitabine-Based Chemotherapy. J. Clin. Oncol. 2016, 34, 3914–3920. [Google Scholar] [CrossRef] [PubMed]
- Wang-Gillam, A.; Li, C.-P.; Bodoky, G.; Dean, A.; Shan, Y.-S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Burns, W.R.; Frankel, T.L.; Cho, C.S.; Nathan, H. Validation of the American Joint Commission on Cancer (AJCC) staging system for patients with pancreatic adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) analysis. Ann. Surg. Oncol. 2017, 24, 2023–2030. [Google Scholar] [CrossRef]
- Booth, C.M.; Karim, S.; Mackillop, W.J. Real-world data: Towards achieving the achievable in cancer care. Nat. Rev. Clin. Oncol. 2019, 16, 312–325. [Google Scholar] [CrossRef]
- Cartwright, T.H.; Parisi, M.; Espirito, J.L.; Wilson, T.W.; Pelletier, C.; Patel, M.; Babiker, H.M. Clinical Outcomes with First-Line Chemotherapy in a Large Retrospective Study of Patients with Metastatic Pancreatic Cancer Treated in a US Community Oncology Setting. Drugs Real World Outcomes 2018, 5, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Hwang, I.; Yoo, C.; Kim, K.-p.; Jeong, J.H.; Chang, H.-M.; Lee, S.S.; Park, D.H.; Song, T.J.; Seo, D.W.; et al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: Retrospective analysis. Invest. New Drugs 2018, 36, 732–741. [Google Scholar] [CrossRef]
- Wang, Y.; Camateros, P.; Cheung, W.Y. A Real-World Comparison of FOLFIRINOX, Gemcitabine Plus nab-Paclitaxel, and Gemcitabine in Advanced Pancreatic Cancers. J. Gastrointest Cancer 2017, 50, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Palmer, D.; Jackson, R.; Cox, T.; Neoptolemos, J.P.; Ghaneh, P.; Rawcliffe, C.L.; Bassi, C.; Stocken, D.D.; Cunningham, D.; et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: Ongoing lessons from the ESPAC-3 study. J. Clin. Oncol. 2014, 32, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.; El-Maraghi, R.H.; Hammel, P.; Heinemann, V.; Kunzmann, V.; Sastre, J.; Scheithauer, W.; Siena, S.; Tabernero, J.; Teixeira, L. Nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long-term survival from a phase III trial. J. Natl. Cancer Inst. 2015, 107, dju413. [Google Scholar] [CrossRef] [PubMed]
- Humphris, J.L.; Chang, D.K.; Johns, A.L.; Scarlett, C.J.; Pajic, M.; Jones, M.D.; Colvin, E.K.; Nagrial, A.; Chin, V.T.; Chantrill, L.A.; et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann. Oncol. 2012, 23, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Eshuis, W.J.; van der Gaag, N.A.; Rauws, E.A.; van Eijck, C.H.; Bruno, M.J.; Kuipers, E.J.; Coene, P.P.; Kubben, F.J.; Gerritsen, J.J.; Greve, J.W.; et al. Therapeutic delay and survival after surgery for cancer of the pancreatic head with or without preoperative biliary drainage. Ann. Surg. 2010, 252, 840–849. [Google Scholar] [CrossRef]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zulke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. (Eds.) Exocrine and endocrine pancreas. In AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010; pp. 241–249. [Google Scholar]
- Trotti, A.; Colevas, A.D.; Setser, A.; Basch, E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J. Clin. Oncol. 2007, 25, 5121–5127. [Google Scholar] [CrossRef]




| Characteristic | Gemcitabine (n = 185) | Gemcitabine/ Capecitabine (n = 60) | Gemcitabine/Nab-Paclitaxel (n = 66) | 5-FU/Oxaliplatin/Irinotecan (n = 31) | 5-FU/Oxaliplatin (n = 35) | Other (n = 12) | Best Supportive Care (n = 206) | All Patients (n = 595) | p-Value¶ |
|---|---|---|---|---|---|---|---|---|---|
| Sex, no. (%) | |||||||||
| Female | 94 (50.8) | 25 (41.7) | 33 (50) | 12 (38.7) | 14 (40) | 6 (50) | 94 (45.6) | 278 (46.7) | 0.713 |
| Male | 91 (49.2) | 35 (58.3) | 33 (50) | 19 (61.3) | 21 (60) | 6 (50) | 112 (54.4) | 317 (53.3) | |
| Age at diagnosis, years | |||||||||
| Mean (range) | 70.6 (39.7–83.8) | 66.3 (38–81.7) | 64.9 (40.5–79.5) | 59.4 (39.7–71.9) | 65.7 (46.6–76.4) | 65.5 (44.2–76.1) | 73.1 (51–95.2) | 69.4 (38–95.2) | 0.000 |
| Body mass index, no. (%) * | |||||||||
| ≤18.4 | 17 (9.2) | 6 (10) | 3 (4.5) | – | 1 (2.9) | – | 26 (12.6) | 53 (8.9) | 0.194 |
| 18.5–29.9 | 157 (84.9) | 52 (86.7) | 60 (90.9) | 30 (96.8) | 32 (91.4) | 12 (100) | 155 (75.2) | 498 (83.7) | |
| ≥30.0 | 10 (5.4) | 2 (3.3) | 3 (4.5) | 1 (3.2) | 2 (5.7) | – | 15 (7.3) | 33 (5.5) | |
| ECOG PS, no. (%) * | |||||||||
| 0 | 40 (21.6) | 26 (43.3) | 21 (31.8) | 17 (54.8) | 12 (34.3) | 3 (25) | 22 (10.7) | 141 (23.7) | 0.000 |
| 1 | 88 (47.6) | 23 (38.3) | 39 (59.1) | 12 (38.7) | 17 (48.6) | 5 (41.7) | 49 (23.8) | 233 (39.2) | |
| 2 | 48 (25.9) | 7 (11.7) | 4 (6.1) | – | 3 (8.6) | 3 (25) | 44 (21.4) | 109 (18.3) | |
| ≥3 | 8 (4.3) | 1 (1.7) | – | 2 (6.5) | 2 (5.7) | 1 (8.3) | 44 (21.4) | 58 (9.7) | |
| Diabetes, no. (%) * † | |||||||||
| yes | 61 (33) | 4 (6.7) | 17 (25.8) | 7 (22.6) | 9 (25.7) | 2 (16.7) | 70 (34) | 170 (28.6) | 0.002 |
| no | 123 (66.5) | 56 (93.3) | 48 (72.7) | 24 (77.4) | 26 (74.3) | 10 (83.3) | 133 (64.6) | 420 (70.6) | |
| Alcohol abuse, no. (%) * † | |||||||||
| yes | 10 (5.4) | 2 (3.3) | 1 (1.5) | – | 2 (5.7) | – | 18 (8.7) | 33 (5.5) | 0.414 |
| no | 159 (85.9) | 53 (88.3) | 54 (81.8) | 26 (83.9) | 33 (94.3) | 9 (75) | 150 (72.8) | 484 (81.3) | |
| former | 6 (3.2) | 3 (5) | 2 (3) | 1 (3.2) | – | – | 6 (2.9) | 18 (3) | |
| Smoking, no. (%) * † | |||||||||
| yes | 48 (25.9) | 12 (20) | 9 (13.6) | 1 (3.2) | 3 (8.6) | – | 34 (16.5) | 107 (18) | 0.003 |
| no | 67 (36.2) | 24 (40) | 23 (34.8) | 19 (61.3) | 17 (48.6) | 4 (33.3) | 104 (50.5) | 258 (43.4) | |
| former | 61 (33) | 21 (35) | 27 (40.9) | 10 (32.3) | 15 (42.9) | 5 (41.7) | 52 (25.2) | 191 (32.1) | |
| Primary tumor location, no. (%) * | |||||||||
| Head | 114 (61.6) | 36 (60) | 29 (43.9) | 15 (48.4) | 22 (62.9) | 5 (41.7) | 100 (48.5) | 321 (53.9) | 0.046 |
| Body | 28 (15.1) | 8 (13.3) | 14 (21.2) | 9 (29) | 7 (20) | 2 (16.7) | 22 (10.7) | 90 (15.1) | |
| Tail | 21 (11.4) | 4 (6.7) | 7 (10.6) | 2 (6.5) | 3 (8.6) | 2 (16.7) | 33 (16) | 72 (12.1) | |
| Overlapping | 18 (9.7) | 8 (13.3) | 12 (18.2) | 3 (9.7) | – | 2 (16.7) | 33 (16) | 76 (12.8) | |
| Ampulla of Vater | 2 (1.1) | 3 (5) | 3 (4.5) | 2 (6.5) | 3 (8.6) | 1 (8.3) | 10 (4.9) | 24 (4) | |
| Metastasization, no. (%) ‡ | |||||||||
| Non-local lymph nodes | 12 (6.5) | 3 (5) | 8 (12.1) | 1 (3.2) | 1 (2.9) | 2 (16.7) | 15 (7.3) | 42 (7.1) | 0.380 |
| Liver | 93 (50.3) | 27 (45) | 37 (56.1) | 14 (45.2) | 10 (28.6) | 6 (50) | 119 (57.8) | 306 (51.4) | 0.047 |
| Lung | 31 (16.8) | 7 (11.7) | 11 (16.7) | 1 (3.2) | 9 (25.7) | 2 (16.7) | 29 (14.1) | 90 (15.1) | 0.258 |
| Peritoneum | 20 (10.8) | 6 (10) | 18 (27.3) | 4 (12.9) | 7 (20) | 4 (33.3) | 41 (19.9) | 100 (16.8) | 0.012 |
| Other | 11 (5.9) | – | 3 (4.5) | – | 5 (14.3) | 2 (16.7) | 14 (6.8) | 35 (5.9) | 0.040 |
| No. of metastatic sites, no. (%) ‡ | |||||||||
| 1 | 76 (41.1) | 30 (50) | 44 (66.7) | 11 (35.5) | 12 (34.3) | 5 (41.7) | 88 (42.7) | 266 (44.7) | 0.002 |
| 2 | 23 (12.4) | 5 (8.3) | 8 (12.1) | 2 (6.5) | 4 (11.4) | 2 (16.7) | 34 (16.5) | 78 (13.1) | |
| ≥3 | 7 (4.3) | – | 2 (4.5) | 1 (3.2) | – | 1 (8.3) | 8 (4.4) | 19 (3.7) | |
| Morphology, no. (%) * | |||||||||
| Adenocarcinoma | 166 (89.7) | 50 (83.3) | 56 (84.8) | 23 (74.2) | 33 (94.3) | 11 (91.7) | 152 (73.8) | 491 (82.5) | 0.169 |
| Other | – | 1 (1.7) | 1 (1.5) | 1 (3.2) | 2 (5.7) | 1 (8.3) | 5 (2.4) | 11 (1.8) | |
| CA 19–9, kE/l * § | |||||||||
| Median (IQR) | 764 (124–5828) | 817.5 (76.5–3855.3) | 1390 (267–7620) | 626 (66–2330) | 142 (34.5–881) | 170 (79.3–2927.5) | 1309.5 (139.3–8975) | 908 (106–5814.5) | 0.002 |
| Surgery | |||||||||
| Tumor resection, no. (%) | 31 (16.8) | 5 (8.3) | 8 (12.1) | 2 (6.5) | 30 (85.7) | 7 (58.3) | 62 (30.1) | 145 (24.4) | 0.000 |
| Median time to relapse, mo. (IQR) | 7.8 (3.9–14.5) | 15.7 (3.2–17.3) | 4 (2.2–12.9) | 14.2 (11.7–16.6) | 12.2 (9.8–15.9) | 11.5 (9.7–15.6) | 8.4 (5.8–12.8) | 9.4 (5.8–14.2) | 0.069 |
| Adjuvant treatment, no. (%) ‖ | |||||||||
| Total | 13 (7) | 4 (6.7) | 5 (7.6) | 2 (6.5) | 30 (85.7) | 7 (58.3) | 32 (15.5) | 93 (15.6) | 0.000 |
| Completed | 7 (3.8) | 3 (5) | 3 (4.5) | 2 (6.5) | 24 (68.6) | 3 (25) | 12 (5.8) | 54 (9.1) | |
| Interrupted | 6 (3.2) | 1 (1.7) | 2 (3) | – | 6 (17.1) | 4 (33.3) | 20 (9.7) | 39 (6.6) | |
| Interventions, no. (%) | |||||||||
| ERCP/PTC | 84 (45.4) | 23 (38.3) | 28 (42.4) | 20 (64.5) | 18 (51.4) | 6 (50) | 88 (42.7) | 267 (44.9) | 0.266 |
| Exploration | 18 (9.7) | 6 (10) | 9 (13.6) | 7 (22.6) | 1 (2.9) | – | 6 (2.9) | 47 (7.9) | 0.000 |
| Outcome | Gemcitabine (n = 185) | Gemcitabine/ Capecitabine (n = 60) | Gemcitabine/ Nab-Paclitaxel (n = 66) | 5-FU/Oxaliplatin/ Irinotecan (n = 31) | 5-FU/Oxaliplatin (n = 35) | Other (n = 12) | Best Supportive Care (n = 206) | p-Value |
|---|---|---|---|---|---|---|---|---|
| OS | ||||||||
| Median OS, mo. (95% CI) | 6.6 (5.5–7.7) | 10.6 (7.8–13.3) | 9.8 (7.9–11.8) | 9.9 (8.1–11.7) | 5.8 (4.5–7) | 7.9 (2.2–13.7) | 1.8 (1.5–2.1) | 0.0001 |
| Univariate HR, (95% CI) | 1 (ref) | 0.67 (0.49–0.91) | 0.72 (0.53–0.99) | 0.7 (0.46–1.06) | 0.95 (0.65–1.41) | – | 2.69 (2.18–3.32) | |
| HR adjusted for co-variables *, (95% CI) | 1 (ref) | 0.57 (0.41–0.8) | 0.54 (0.38–0.76) | 0.5 (0.31–0.81) | 1.33 (0.84–2.1) | – | 2.4 (1.85–3.12) | |
| Median OS in RCT of first-line treatment, mo. (95% CI) | ||||||||
| Burris et al. [3] | 5.7 (–) | – | – | – | – | – | – | |
| Cunningham et al. [6] | 6.2 (5.5–7.2) | 7.1 (6.2–7.8) | – | – | – | – | – | |
| Conroy et al. [10] | 6.8 (5.5–7.8) | – | – | 11.1 (9–13.1) | – | – | – | |
| Von Hoff et al. [9] | 6.7 (6–7.2) | – | 8.5 (7.9–9.5) | – | – | – | – | |
| HR for death compared to gemcitabine in RCT, (95% CI) | ||||||||
| Cunningham et al. [6] | 1 (ref) | 0.86 (0.72–1.02) | – | – | – | – | – | |
| Conroy et al. [10] | 1 (ref) | – | – | 0.57 (0.45–0.73) | – | – | – | |
| Von Hoff et al. [9] | 1 (ref) | – | 0.72 (0.62–0.83) | – | – | – | – | |
| Time to treatment-failure | ||||||||
| TTF, mo. (95% CI) | 3.3 (2.8–3.8) | 3.7 (2.4–4.9) | 5.1 (4.1–6) | 2.9 (2–3.8) | 2.8 (2.4–3.1) | 3.5 (2.1–4.8) | – | 0.08 |
| Univariate HR, (95% CI) | 1 (ref) | 0.88 (0.66–1.18) | 0.78 (0.59–1.05) | 1.09 (0.74–1.62) | 1.43 (0.99–2.07) | – | – | |
| HR adjusted for co-variables *, (95% CI) | 1 (ref) | 0.86 (0.62–1.2) | 0.62 (0.44–0.87) | 0.95 (0.57–1.57) | 1.8 (1.09–2.98) | – | – | |
| Progression-free survival in RCT of first-line treatment, months (95% CI) | ||||||||
| Burris et al. [3] | 3.7 (–) | – | – | – | – | – | – | |
| Cunningham et al. [6] | 3.8 (2.9–4.8) | 5.3 (4.5–5.7) | – | – | – | – | – | |
| Conroy et al. [10] | 3.3 (2.2–3.6) | – | – | 6.4 (5.5–7.2) | – | – | – | |
| Von Hoff et al. [9] | 3.7 (3.6– 4) | – | 5.5 (4.5–5.9) | – | – | – | – | |
| HR for disease progression compared to gemcitabine in RCT, (95% CI) | ||||||||
| Cunningham et al. [6] | 1 (ref) | 0.78 (0.66–0.93) | – | – | – | – | – | |
| Conroy et al. [10] | 1 (ref) | – | – | 0.47 (0.37–0.59) | – | – | – | |
| Von Hoff et al. [9] | 1 (ref) | – | 0.69 (0.58–0.82) | – | – | – | – | |
| Clinical evaluation at end of treatment | ||||||||
| Progression, no. (%) | 68 (36.8) | 32 (53.3) | 35 (53) | 15 (48.4) | 16 (45.7) | – | – | 0.002 |
| Stable disease, no. (%) | 23 (12.4) | 4 (6.7) | 5 (7.6) | 3 (9.7) | 2 (5.7) | – | – | |
| Partial response, no. (%) | 7 (3.8) | 5 (8.3) | 8 (12.1) | 2 (6.5) | 3 (8.6) | – | – | |
| Mixed response, no. (%) | 1 (0.5) | 1 (1.7) | 3 (4.5) | 2 (6.5) | 5 (14.3) | – | – | |
| Death, no. (%) | 54 (29.2) | 5 (8.3) | 10 (15.2) | 0 (0) | 7 (20) | – | – | |
| Not evaluated, no. (%) | 32 (17.3) | 13 (21.7) | 5 (7.6) | 9 (29) | 5 (14.3) | – | – | |
| Adverse event (CTCAE 4.02) | Gemcitabine (n = 185) | Gemcitabine/ Capecitabine (n = 60) | Gemcitabine/ Nab-Paclitaxel (n = 66) | 5-FU/ Oxaliplatin/ Irinotecan (n = 31) | 5-FU/ Oxaliplatin (n = 35) | Other (n = 12) | All Treated Patients (n = 389) | p-Value |
|---|---|---|---|---|---|---|---|---|
| Hematological adverse events, no. (%) | ||||||||
| Anemia | ||||||||
| All grades | 15 (8.1) | 3 (5) | 17 (25.8) | 5 (16.1) | 2 (5.7) | – | 42 (10.8) | 0.000 |
| Grade ≥3 | (0) | (0) | 3 (4.5) | 2 (6.5) | 1 (2.9) | – | (0) | |
| Platelet count decrease | ||||||||
| All grades | 20 (10.8) | 3 (5) | 14 (21.2) | 2 (6.5) | 1 (2.9) | – | 38 (9.8) | 0.000 |
| Grade ≥3 | 10 (5.4) | 1 (0.5) | 2 (1.1) | (0) | (0) | – | 13 (7) | |
| White blood cell decrease | ||||||||
| All grades | 11 (5.9) | 3 (5) | 14 (21.2) | 2 (6.5) | – | – | 38 (9.8) | 0.004 |
| Grade ≥3 | 2 (1.1) | 2 (1.1) | 2 (1.1) | – | – | – | 6 (3.2) | |
| Non-hematological adverse events, no. (%) | ||||||||
| Bile duct obstruction | ||||||||
| All grades | 6 (3.2) | 4 (6.7) | 3 (4.5) | 1 (3.2) | – | – | 14 (3.6) | 0.042 |
| Grade ≥3 | 6 (3.2) | 4 (6.7) | 3 (4.5) | 1 (3.2) | – | – | 14 (3.6) | |
| Diarrhea | ||||||||
| All grades | 6 (3.2) | 3 (5) | 4 (6.1) | 5 (16.1) | 3 (8.6) | 1 (8.3) | 22 (5.7) | 0.000 |
| Grade ≥3 | 2 (1.1) | 1 (1.7) | – | 2 (6.5) | 2 (5.7) | 1 (8.3) | 8 (2.1) | |
| Fatigue | ||||||||
| All grades | 19 (10.3) | 2 (3.3) | 2 (3) | 2 (6.5) | 2 (5.7) | 2 (16.7) | 29 (7.5) | 0.000 |
| Grade ≥3 | 5 (2.7) | – | – | – | – | – | 5 (1.3) | |
| Fever | ||||||||
| All grades | 8 (4.3) | 2 (3.3) | 5 (7.6) | 1 (3.2) | 1 (2.9) | – | 17 (4.4) | 0.04 |
| Grade ≥3 | 3 (1.6) | – | 2 (3) | – | – | – | 5 (1.3) | |
| Nausea | ||||||||
| All grades | 8 (4.3) | 3 (5) | 4 (6.1) | 1 (3.2) | 1 (2.9) | – | 17 (4.4) | 0.08 |
| Grade ≥3 | 4 (2.2) | 2 (3.3) | – | – | – | – | 6 (1.5) | |
| Peripheral sensory neuropathy | ||||||||
| All grades | – | – | 14 (21.2) | 2 (6.5) | 1 (2.9) | – | 38 (9.8) | 0.000 |
| Grade ≥3 | – | – | 3 (1.6) | – | – | – | 3 (1.6) | |
| Sepsis | ||||||||
| All grades (always Grade ≥4) | 15 (8.1) | – | 6 (9.1) | 2 (6.5) | 4 (11.4) | – | 27 (6.9) | 0.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordes, M.; Yu, J.; Malgerud, O.; Gustafsson Liljefors, M.; Löhr, J.-M. Survival Benefits of Chemotherapy for Patients with Advanced Pancreatic Cancer in A Clinical Real-World Cohort. Cancers 2019, 11, 1326. https://doi.org/10.3390/cancers11091326
Kordes M, Yu J, Malgerud O, Gustafsson Liljefors M, Löhr J-M. Survival Benefits of Chemotherapy for Patients with Advanced Pancreatic Cancer in A Clinical Real-World Cohort. Cancers. 2019; 11(9):1326. https://doi.org/10.3390/cancers11091326
Chicago/Turabian StyleKordes, Maximilian, Jingru Yu, Oscar Malgerud, Maria Gustafsson Liljefors, and J. -Matthias Löhr. 2019. "Survival Benefits of Chemotherapy for Patients with Advanced Pancreatic Cancer in A Clinical Real-World Cohort" Cancers 11, no. 9: 1326. https://doi.org/10.3390/cancers11091326





