Observations on Solitary Versus Multiple Isolated Pancreatic Metastases of Renal Cell Carcinoma: Another Indication of a Seed and Soil Mechanism?
Abstract
:1. Introduction
2. Results
2.1. History and Literature Compilation
2.2. Epidemiology and Pathology of isPM
2.2.1. Histology
2.2.2. Grading
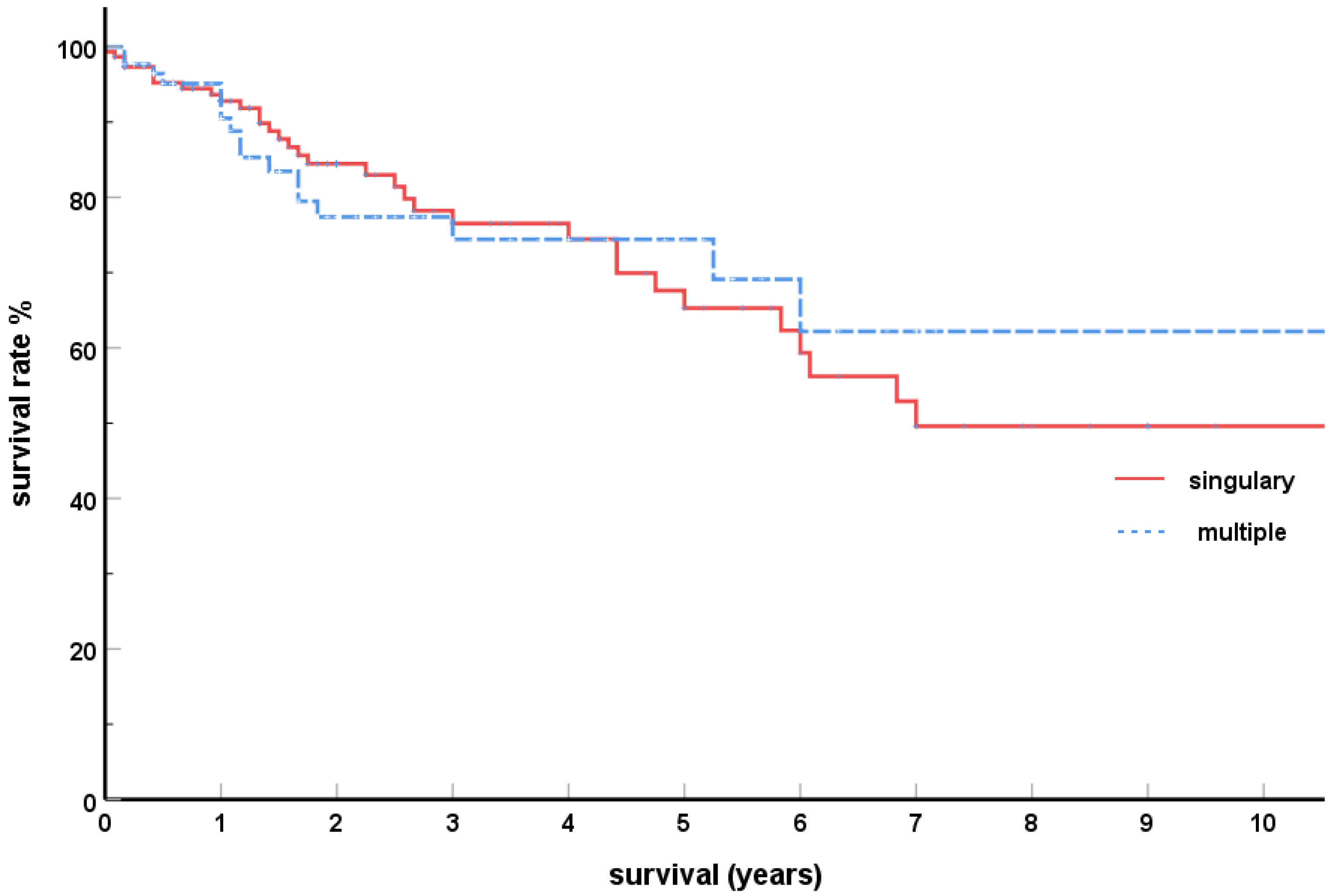
2.2.3. Singular-Multiple Pancreas Metastases
2.3. Arguments for a SSM in isPM
2.3.1. Metastasis Route and SSM
- A local venous spread route, where pre-existent, porto-renal anastomoses [106,157,200,201], or draining, collateral veins of hyper-vascularized tumors [27,48,88,101,106,168]—enabling a tumor cell embolism in the pancreas—and that independently of whether there is a renal vein thrombosis or not [48].
- The systemic haematogenic metastasis route
2.3.2. Histology, Grading, and SSM
2.3.3. Multiple Pancreas Metastases and SSM
3. Discussion
3.1. Pathomechanism
3.1.1. Genetic/Epigenetic Alterations and isPM
3.1.2. Organotropism and SSM
4. Materials and Methods
4.1. Data Sources
4.2. Inclusion and Exclusion Criteria
4.3. Statistics
5. Conclusions
Funding
Conflicts of Interest
References
- Chambers, A.F.; Groom, A.C.; McDonald, I.C. Metastasis: Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Shibue, T.; Weinberg, R.A. Metastatic colonization: Settlement, adaptation and propagation of tumor cells in a foreign tissue environment. Semin. Cancer Biol. 2011, 21, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Fidler, I.J. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010, 70, 5649–5669. [Google Scholar] [CrossRef]
- Schluter, K.; Gassmann, P.; Enns, A.; Korb, T.; Hemping-Bovenkerk, A.; Hölzen, J.; Haier, J. Organ specific tumor cell adhesion and extravasion of colon carcinoma cells with different metastatic potential. Am. J. Pathol. 2006, 169, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. Organ specificity of tumor metastasis: Role of preferential adhesion, invasion and growth of malignant cells at specific secondary sites. Cancer Metastasis Rev. 1988, 7, 143–188. [Google Scholar] [CrossRef] [PubMed]
- Strell, C.; Entschladen, F. Extravasation of leukocytes in comparison to tumor cells. Cell. Commun. Signal. 2008, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Miles, F.L.; Pruitt, F.L.; van Golen, K.L.; Cooper, C.R. Stepping out the flow: Capillary extravasion in cancer metastasis. Clin. Exp. Metastasis 2008, 25, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Gandalovičová, A.; Rosel, D.; Fernandes, M.; Veselý, P.; Heneberg, P.; Čermák, V.; Petruželka, L.; Kumar, S.; Sanz-Moreno, V.; Brábek, J. Migrastatics—Anti-Metastatic and anti-invasion drugs: Promises and challenges. Trends Cancer 2017, 3, 391–406. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, M.; Shen, J.; Gerhold, L.M.; Hoffman, R.M.; Xing, H.R. The role of the intravascular microenvironment in spontaneous metastasis development. Int. J. Cancer 2010, 126, 2534–2541. [Google Scholar] [CrossRef]
- Clark, A.; Vignjevic, D. Modes of cancer cell invasion and the role of the microenvironment. Curr. Opin. Cell Biol. 2015, 36, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Sidik, S.M.; Mahmud, R.; Stanlas, J. Molecular targets in the discovery and development of novel antimetastatic agents: Current progress and future prospects. Clin. Exp. Pharmacol. Physiol. 2013, 40, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Hedley, B.D.; Chambers, A.F. Tumor dormancy and metastasis. Cancer Res. 2009, 102, 67–101. [Google Scholar]
- Luzzi, K.J.; MacDonald, I.C.; Schmidt, E.E.; Kerkvliet, N.; Morris, V.L.; Chambers, A.F.; Groom, A.C. Multistep nature of metastatic efficiency: Dormancy of solitary cells after successful extravasation and limited survival of early micrometastasis. Am. J. Pathol. 1998, 153, 865–873. [Google Scholar] [CrossRef]
- Naumov, G.N.; MacDonald, I.C.; Weinmeister, P.M.; Kerkvliet, N.; Nadkarni, K.V.; Wilson, S.M.; Morris, V.L.; Groom, A.C.; Chambers, A.F. Persistence of solitary mammary carcinoma cells in a secondary site: A possible contributor to dormancy. Cancer Res. 2002, 62, 2162–2168. [Google Scholar] [PubMed]
- Goss, P.; Allan, A.L.; Rodenhiser, D.I.; Foster, P.J.; Chambers, A.F. New clinical and experimental approaches for studying tumor dormancy: Does tumor dormancy offer a therapeutic target? APMIS 2008, 116, 552–568. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis. Annu. Rev. Med. 2006, 57, 1–18. [Google Scholar] [CrossRef]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Killion, J.J.; Fidler, I.J. Therapy of cancer metastasis by tumoricidal activation of tissue macrophages using liposome-encapsulated immunomodulators. Pharmacol. Ther. 1998, 78, 141–154. [Google Scholar] [CrossRef]
- Fidler, I.J.; Gersten, D.M.; Hart, I.R. The biology of cancer invasion and metastasis. Adv. Cancer Res. 1978, 28, 149–250. [Google Scholar]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 1, 99–101. [Google Scholar] [CrossRef]
- Gassmann, P.; Haier, J. The tumor cell-host interface in the early onset of metastatic organ colonisation. Clin. Exp. Metastasis 2008, 25, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Tarin, D.; Price, J.E.; Kettlewell, M.G.; Souter, R.G.; Vass, A.C.; Crossley, B. Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunt. Cancer Res. 1984, 44, 3584–3592. [Google Scholar] [PubMed]
- Sellner, F.; Tykalsky, N.; De Santis, M.; Pont, J.; Klimpfinger, M. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: An indication for pancreatic surgery. Ann. Surg. Oncol. 2006, 13, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Sellner, F. Isolated pancreatic metastases from renal cell carcinoma: An outcome of a special metastatic pathway or of a specific tumor cell selection? Clin. Exp. Metastasis 2018, 35, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, E.A. Metastatic hypernephroma to the pancreas. Acta Chir. Scand. 1952, 104, 177–180. [Google Scholar] [PubMed]
- Py, J.M.; Arnaud, J.P.; Cinqualbre, J.; Adloff, M.; Bollack, C. Pancreatic metastases of nephro-epitheliomas. Apropos of 2 cases. Acta Chir. Belg. 1984, 84, 117–121. [Google Scholar] [PubMed]
- Skaarup, P.; Jorgensen, T.; Larsen, S. Asynchronous metastasizing renal cell carcinoma associated with progressive immune complex glomerulonephritis and proteinuria. Scand. J. Urol. Nephrol. 1984, 18, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Tomida, T.; Iwasa, M.; Takahashi, K.; Kaneda, M.; Tamaki, H. Solitary pancreatic metastasis, occuring 8 years after nephrectomy for renal cell carcinoma. Inter. J. Pancreatol. 1996, 19, 145–153. [Google Scholar]
- Lawson, L.I.; Holt, L.P.; Rooke, H.W. Recurrent duodenal haemorrhage from renal carcinoma. Br. J. Urol. 1966, 38, 133–137. [Google Scholar] [CrossRef]
- Franciosi, R.A.; Russo, J. Renal cell carcinoma metastatic to the pancreas thirteen years following nephrectomy. Mil. Med. 1969, 134, 200–203. [Google Scholar] [CrossRef]
- Marquand, J.; Giraud, B.; Maliakas, S. Pancreatic metastasis revealing a kidney neoplasm. J. Urol. Nephrol. 1971, 77, 595–601. [Google Scholar]
- Guttman, F.M.; Ross, M.; Lachance, C. Pancreatic metastasis of renal cell carcinoma treated by total pancreatectomy. Arch. Surg. 1972, 105, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Gillet, M.; Camelit, G.; Runser, G.; Clement, D. Duodenopancreatic metastasis from a carcinoma of the kidney revealed by digestive haemorrhage treated by cephalic duodeno-pancreatectomy. Chirurgie 1974, 100, 226–230. [Google Scholar] [PubMed]
- Hermanutz, K.D.; Sonnenberg, G.E. Late metastasis of a hypernephroid kidney carcinoma to the pancreas with tumor invasion to the duodenum. Fortschr. Röntgenstr. 1977, 127, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Saxon, A.; Gottesman, J.; Doolas, A. Bilateral hypernephroma with solitary pancreatic metastasis. J. Surg. Oncol. 1980, 13, 317–322. [Google Scholar] [CrossRef]
- Yazaki, T.; Ishikawa, S.; Ogawa, Y.; Takahashi, S.; Nemoto, S.; Rinsho, K.; Kanoh, S.; Kitagawa, R. Silent pancreatic metastasis from renal cell carcinoma. Acta Urol. Jpn. 1981, 27, 1517–1522. [Google Scholar]
- Audisio, R.A.; La Monica, G. Solitary pancreatic metastasis occuring 20 years after nephrectomy for carcinoma of the kidney. Tumori J. 1985, 71, 197–200. [Google Scholar] [CrossRef]
- Kishimoto, H.; Niumra, Y.; Okamoto, K.; Tsuchie, K.; Yamase, H.; Maeda, S.; Kamija, J.; Hasegawa, H.; Hayakawa, N.; Yamamoto, M. A case of resected renal cell carcinoma with massive pancreatic metastasis. Jpn. J. Cancer Clin. 1985, 31, 91–96. [Google Scholar]
- Amamiya, H.; Iizumi, T.; Yazaki, T.; Waku, M.; Yasuda, H.; Takada, T.; Shikata, J.; Nagai, J. A solitary pancreatic metastasis from renal cell carcinoma. Hinyouki Geka 1988, 2, 167–170. [Google Scholar]
- Carini, M.; Selli, C.; Barbanti, G.; Bianchi, S.; Muraro, G. Pancreatic late recurrence of bilateral renal cell carcinoma after conservative surgery. Eur. Urol. 1988, 14, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Douden, K.; Bantou, H.; Sakatoku, M.; Saitoh, H.; Tachikawa, H.; Takizawa, T.; Horigami, K.; Kameya, T.; Nagai, T.; et al. Solitary pancreatic metastasis occuring 10 years after nephrectomy for carcinoma of the kidney. Tan Sui 1988, 9, 233–237. [Google Scholar]
- Sharma, S.K.; Kumar, A.; Madhusoodnan, P.; Banerjee, C.; Suri, S.; Dhar, M. Solitary pancreatic metastasis from renal cell carcinoma. A rare metastatic site. Indian J. Cancer 1988, 25, 29–32. [Google Scholar] [PubMed]
- Guyenne, C.; Rat, P.; Haas, O.; Baudet, J.G.; Favre, J.P. Triple metastase pancreatique d´un cancer du rein traitee par duodenopancreatectomie subtotale. Presse Med. 1989, 18, 231. [Google Scholar] [PubMed]
- Iwanami, M.; Nakayoshi, A.; Yagi, H.; Shimizu, K.; Kimura, K.; Suzuki, K.; Matsumoto, K.; Kai, Y.; Ueno, M.; Sagawa, F. A resected case of the asymptomatic pancreatic metastasis in the body and tail of the pancreas from renal cell carcinoma. J. Jpn. Panc. Soc. 1989, 4, 100–106. [Google Scholar]
- Roland, C.F.; Van Heerden, J.A. Nonpancreatic primary tumors with metastasis to the pancreas. Surg. Gynec. Obstet. 1989, 168, 345–347. [Google Scholar]
- Simpson, N.S.; Mulholland, C.; Lioe, T.; Spence, R. Late solitary metastatic renal carcinoma in the pancreas. Ulst. Med. J. 1989, 58, 198–199. [Google Scholar]
- Strijk, S.P. Pancreatic metastases of renal cell carcinoma: Report of two cases. Gastrointest. Radiol. 1989, 14, 123–126. [Google Scholar] [CrossRef]
- Temellini, F.; Bavosi, M.; Lamarra, M.; Quagliarini, P.; Giuliani, F. Pancreatic metastasis 25 years after nephrectomy for renal cancer. Tumori J. 1989, 75, 503–504. [Google Scholar] [CrossRef]
- Gohji, K.; Matsumoto, O.; Kamidono, S. Solitary pancreatic metastasis from renal cell carcinoma. Hinyokika Kiyo 1990, 36, 677–681. [Google Scholar]
- Terashima, M.; Abe, H.; Suga, K.; Matsuya, F.; Kobayashi, K.; Itoh, S.; Sasaki, R.; Kanno, S.; Tomichi, N. Two cases of renal cell carcinoma metastasized to the pancreas and to the gallbladder. Jpn. J. Gastroenterol. Surg. 1990, 23, 1952–1956. [Google Scholar] [CrossRef]
- Furukawa, T.; Hattori, R.; Ohtake, H.; Souma, T.; Kinukawa, T.; Hirai, A.; Kimura, J.; Sakata, T.; Ishii, M.; Hayashi, N.; et al. A resectable case of pancreatic head metastasis from renal cell carcinoma. Hinyouki Geka 1991, 4, 111–114. [Google Scholar]
- Kubo, K.; Morita, J.; Mizoe, J.; Ogawa, H.; Irie, G. Renal cell carcinoma metastatic to the pancreas 8 years following nephrectomy. Jpn. J. Clin. Radiol. 1991, 36, 509–512. [Google Scholar]
- Nishida, O.; Matsunaga, Y.; Dekigai, H.; Um, S.H.; Hsieh, C.C.; Kimura, F.; Yoshioka, H.; Murakami, M.; Inoue, R.; Murai, A. Three elderly cases of renal cell carcinoma with pancreatic metastasis. Jpn. J. Geriatr. 1991, 28, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.; Hatayama, T.; Taki, Y.; Ueyama, H.; Hida, S.; Noguchi, M. A resected case of renal cell carcinoma with metastasis to pancreas. Hinyokika Kiyo 1991, 37, 1531–1534. [Google Scholar] [PubMed]
- Tabata, T.; Kuroda, Y.; Nishimatsu, S.; Satoh, Y. A resected case of pancreatic tumor metastasized from renal cell carcinoma. J. Jpn. Panc. Soc. 1991, 6, 245–250. [Google Scholar]
- Yamamoto, S.; Tobinaga, K.; Taketomi, K.; Kimino, K.; Ashizuka, S.; Kishikawa, M. Pancreatic metastasis of renal cell carcinoma occuring 17 years after nephrectomy. J. Jpn. Soc. Clin. Surg. 1991, 52, 3006–3011. [Google Scholar]
- Fujii, M.; Kogawa, T.; Matsuyama, K.; Yamamoto, H.; Kawahito, Y.; Iinuma, S.; Kokura, S.; Takemura, S.; Yoshikawa, T.; Kondo, M.; et al. A case of metastatic renal cell carcinoma to pancreas ten years after nephrectomy. J. Kyoto Pref. Univ. Med. 1992, 101, 589–596. [Google Scholar]
- Melo, C.R.; Melo, I.S.; Monteiro, A.Z.; de Mello, E.S. Pancreatic metastasis from renal cell carcinoma. Arq. Gastroenterol. 1992, 29, 110–112. [Google Scholar]
- Nakagawa, K.; Tsuchiya, T.M.; Momono, S.; Sasaki, Y.; Sato, T. A case of pancreatic metastasis of renal cell carcinoma. Jpn. J. Gastroenterol. Surg. 1992, 25, 2200–2204. [Google Scholar] [CrossRef]
- Rypens, F.; Gansbeke, V.; Lambiliotte, J.; Regemorter, V.; Verhesi, A.; Struyven, J. Pancreatic metastasis from renal cell carcinoma. Br. J. Radiol. 1992, 65, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Stankard, C.E.; Karl, R.C. The treatment of isolated pancreatic metastases from renal cell carcinoma: A surgical review. Am. J. Gastroenterol. 1992, 87, 1658–1660. [Google Scholar] [PubMed]
- Aikou, S.; Tokura, Y.; Yamafuji, K.; Takahashi, T.; Yoshihide, O.; Kishii, K.; Fujii, S.; Katsumata, K.; Tamiya, M. A resected case of pancreatic metastasis from renal cell carcinoma presenting with acute duodenal bleeding. J. Jpn. Soc. Clin. Surg. 1993, 54, 2666–2672. [Google Scholar] [CrossRef]
- Calmes, J.M.; Meyer, A. Pancreatic hypernephroma manifested by a duodenal hemorrhage. Rev. Med. Suisse Romande 1993, 113, 629–631. [Google Scholar] [PubMed]
- Ishikawa, T.; Horimi, T.; Majima, K. A resected case of pancreatic tumor metastasized from renal cell carcinoma. A review of 11 cases in the Japanese and 13 cases in the foreign literature. J. Jpn. Soc. Clin. Surg. 1993, 51, 1642–1647. [Google Scholar]
- Marcote-Valdivieso, E.; Arlandis, F.; Baltasar, A.; Martinez-Castro, R.; Vierna-Garcia, J. Synchronous pancreatic metastasis of renal carcinoma. Rev. Esp. Enferm. Dig. 1993, 83, 471–473. [Google Scholar] [PubMed]
- Nan, Y.; Kuno, N.; Kurimoto, K.; Nakamura, T.; Kobayashi, S. A resected case of pancreatic tumor metastasized from renal cell carcinoma diagnosed by endoscopic biopsy through the main pancreatic duct. Gastroenterol. Endosc. 1993, 35, 1380–1385. [Google Scholar]
- Sauvanet, A.; Barthes, T.; Levy, P.; Flejou, J.F.; Delcenserie, R.; Bernades, P.; Belghiti, J. Late pancreatic metastasis from renal cell carcinoma. Pancreas 1993, 8, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Konaga, E.; Harano, M.; Watanabe, K.; Takeuchi, Y.; Hara, M.; Mano, S. Solitary pancreatic metastasis from renal cell carcinoma. Acta Med. Okayama 1993, 47, 63–66. [Google Scholar] [PubMed]
- Vergara, V.; Marucci, M.; Marcarino, C.; Brunello, F.; Capussotti, L. Metastatic involvement of the pancreas from renal cell carcinoma treated by surgery. Ital. J. Gastroenterol. 1993, 25, 388–390. [Google Scholar]
- Yanagisawa, T.; Nakayama, K.; Kashiwagi, M.; Tanaka, J.; Kashiwagi, T.; Mizusaki, K.; Itoh, A.; Akimoto, H.; Takahashi, T.; Aoki, T.; et al. Three cases of resectable pancreatic metastases from renal cell carcinoma. Geka Shinryo 1993, 35, 651–655. [Google Scholar]
- Zugel, N.; Leipprand, F.; Weckermann, D.; Witte, J. Solitary metastasis to the head of the pancreas in hypernephroid carcinoma. Fortschr. Med. 1994, 112, 388–390. [Google Scholar] [PubMed]
- Dousset, B.; Andant, C.; Guimbaud, R.; Roseau, G.; Tulliez, M.; Gaudric, M.; Palazzo, L.; Chaussade, S.; Chapuis, Y. Late pancreatic metastasis from renal cell carcinoma diagnosed by endoscopic ultrasonography. Surgery 1995, 117, 591–594. [Google Scholar] [CrossRef]
- Fabre, J.M.; Rouanet, P.; Dagues, F.; Blanc, F.; Baumel, H.; Domergue, J. Various features and surgical approach of solitary pancreatic metastasis from renal cell carcinoma. Eur. J. Surg. Oncol. 1995, 21, 683–686. [Google Scholar] [CrossRef]
- Onishi, T.; Ohishi, Y.; Iizuka, N.; Suzuki, Y.; Shirakawa, H.; Hatano, T.; Tom, M. Clinical characteristics of 7 renal cell carcinoma patients developing a solitary pancreatic metastasis after nephrectomy. Jpn. J. Urol. 1995, 86, 1538–1542. [Google Scholar] [CrossRef] [PubMed]
- Orita, M.; Morita, N.; Hiraoka, H.; Noshima, S.; Takaimashi, T.; Esato, K. A case of resected pancreatic metastasis from renal cell carcinoma 14 years after radical nephrectomy. J. Jpn. Pancreas Soc. 1995, 10, 63–68. [Google Scholar]
- Takashi, M.; Takagi, Y.; Sakata, T.; Shimoji, T.; Miyake, K. Surgical treatment of renal cell carcinoma metastases: Prognostic significance. Int. Urol. Nephrol. 1995, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barras, J.P.; Baer, H.; Stenzl, A.; Czerniak, A. Isolated late metastasis of a renal cell cancer treated by radical distal pancreatectomy. HPB Surg. 1996, 10, 51–53. [Google Scholar] [CrossRef]
- Palazzo, L.; Borotto, E.; Cellier, C.; Roseau, G.; Chaussade, S.; Couturier, D.; Paolaggi, J. Endosonographic features of pancreatic metastases. Gastrointest. Endosc. 1996, 44, 433–436. [Google Scholar] [CrossRef]
- Paz, A.; Koren, R.; Gal, R.; Wolloch, Y. Late solitary pancreatic metastasis from renal cell carcinoma. Isr. J. Med. Sci. 1996, 32, 1319–1321. [Google Scholar]
- Chambers, T.P.; Fishman, E.K.; Hruban, R.H. Pancreatic metastases from renal cell carcinoma in von Hippel-Lindau disease. Clin. Imaging 1997, 21, 40–42. [Google Scholar] [CrossRef]
- Robbins, E.G.; Franceschi, D.; Barkin, J.S. Solitary metastatic tumors to the pancreas: A case report and review of the literature. Am. J. Gastroenterol. 1996, 91, 2414–2417. [Google Scholar]
- Harrison, L.; Merchant, N.; Cohen, A.; Brennan, M. Pancreaticoduodenectomy for nonperiampullary primary tumors. Am. J. Surg. 1997, 174, 393–395. [Google Scholar] [CrossRef]
- Adem, C.; Chetritt, J.; Guymar, S.; Bellil, K.; Ladouch-Badre, A.; Benlagha, N.; Bedossa, P. Pancreatic metastasis of a renal adenocarcinoma. Apropos on 2 cases. Ann. Pathol. 1998, 18, 481–483. [Google Scholar]
- Altschuler, E.L.; Ray, A. Spontaneous regression of a pancreatic metastasis of a renal cell carcinoma. Arch. Fam. Med. 1998, 7, 516–517. [Google Scholar] [CrossRef]
- Butturini, G.; Bassi, C.; Falconi, M.; Salvia, R.; Caldiron, E.; Iannucci, A.; Zamboni, G.; Graziani, R.; Procacci, C.; Pederzoli, P. Surgical treatment of pancreatic metastases from renal cell carcinomas. Dig. Surg. 1998, 15, 241–246. [Google Scholar] [CrossRef]
- Gupta, R.K.; Lallu, S.; Delahunt, B. Fine-needle aspiration cytology of metastatic clear-cell renal carcinoma presenting as a solitary mass in the head of the pancreas. Diagn. Cytopathol. 1998, 19, 194–197. [Google Scholar] [CrossRef]
- Hashimoto, M.; Watanabe, G.; Matsuda, M.; Dohi, T.; Tsurumaru, M. Management of the pancreatic metastases from renal cell carcinoma: Report of four resected cases. Hepatogastroenterology 1998, 45, 1150–1154. [Google Scholar]
- Jingu, K.; Watanabe, K.; Yamamoto, H.; Fujita, Y.; Honda, I.; Watanabe, S.; Nagata, M.; Sugimoto, K.; Watanabe, Y. Surgical treatment of a solitary pancreatic metastasis from renal cell carcinoma: Report of a case. Surg. Today 1998, 28, 91–94. [Google Scholar] [CrossRef]
- Merkle, E.M.; Boaz, T.; Kolokythas, O.; Haaga, J.R.; Lewin, J.S.; Brambs, H.J. Metastases to the pancreas. Br. J. Radiol. 1998, 71, 1208–1214. [Google Scholar] [CrossRef]
- Sahin, M.; Foulis, A.A.; Poon, F.W.; Imrie, C.W. Late focal pancreatic metastasis of renal cell carcinoma. Dig. Surg. 1998, 15, 72–74. [Google Scholar] [CrossRef]
- Z’graggen, K.; Fernandez-del Castillo, C.; Rattner, D.W.; Sigala, H.; Warshaw, A.L. Metastases to the pancreas and their surgical extirpation. Arch. Surg. 1998, 133, 418–419. [Google Scholar]
- Augustin, H.; Bacher, H.; Uggowitzer, M.; Ott, A.; Hubmer, G.; Mischinger, H.J. Pancreatic metastases from renal cell carcinoma mimicking insulinomas. BJU Int. 1999, 83, 140–141. [Google Scholar] [CrossRef]
- Carucci, L.R.; Siegelman, E.S.; Feldman, M.D. Pancreatic metastasis from clear cell renal carcinoma: Diagnosis with chemical shift MRI. J. Comput. Assist. Tomogr. 1999, 23, 934–936. [Google Scholar] [CrossRef]
- Erigushi, N.; Aoyagi, S.; Hara, M.; Miyazaki, T.; Hashino, K.; Imamura, I.; Jimi, A.; Naito, H. A resected case of pancreatic metastasis from primary renal cell carcinoma. Kurume Med. J. 1999, 46, 119–122. [Google Scholar] [CrossRef]
- Ng, C.S.; Loyer, E.M.; Iyer, R.B.; David, C.L.; DuBrow, R.A.; Charnsangavej, C. Metastases to the pancreas from renal cell carcinoma: Findings on three-phase contrast-enhanced CT. Am. J. Roentgenol. 1999, 172, 1555–1559. [Google Scholar] [CrossRef]
- Sugiyama, M.; Katsura, M.; Yamamoto, K.; Nouchi, W.; Abe, N.; Hatano, N.; Atomi, Y. Pancreatic metastasis from renal cell carcinoma causing massive gastrointestinal bleeding in von Hippel-Lindau disease. Hepatogastroenterology 1999, 46, 1199–1201. [Google Scholar]
- Yavascaoglu, I.; Korun, N.; Oktay, B.; Simsek, U.; Ozyurt, M. Renal cell carcinoma with solitary synchronous pancreaticoduodenal and metachronous periprostatic metastases: Report of a case. Surg. Today 1999, 29, 364–366. [Google Scholar] [CrossRef]
- Fricke, P.; Schulz, H.U.; Buhtz, P.; Lippert, H. The pancreas as a site of multiple metastases from renal cell carcinoma. Report of one case and review of the literature. Chirurg 2000, 71, 575–579. [Google Scholar] [CrossRef]
- Ghavamian, R.; Klein, K.A.; Stephens, D.H.; Welch, T.J.; LeRoy, A.J.; Richards, R.L.; Burch, P.A.; Zincke, H. Renal cell carcinoma metastatic to the pancreas: Clinical and radiological features. Mayo Clin. Proc. 2000, 75, 581–585. [Google Scholar] [CrossRef]
- Kassabian, A.; Stein, J.; Jabbour, N.; Parsa, K.; Skinner, B.; Parekh, D.; Cosenza, C.; Selby, R. Renal cell carcinoma metastatic to the pancreas: A single institution series and review of the literature. Urology 2000, 56, 211–215. [Google Scholar] [CrossRef]
- Le Borgne, J.; Partensky, C.; Glemain, P.; Dupas, B.; de Kerviller, B. Pancreaticoduodenectomy for metastatic ampullary and pancreatic tumors. Hepatogastroenterology 2000, 47, 540–544. [Google Scholar]
- Mehta, N.; Volpe, C.; Haley, T.; Balos, L.; Bradley, E.L.; Doerr, R.J. Pancreaticoduodenectomy for metastatic renal cell carcinoma: Report of a case. Surg. Today 2000, 30, 94–97. [Google Scholar] [CrossRef]
- Thompson, L.D.; Heffess, C.S. Renal cell carcinoma to the pancreas in surgical pathology material. Cancer 2000, 89, 1076–1089. [Google Scholar] [CrossRef]
- Espinoza, R.; Rossi, R.; Rossi, R.; Rosenberg, H. Metachronous pancreatic metastasis of a renal cell carcinoma: 3 new cases. Rev. Med. Chile 2001, 129, 86–90. [Google Scholar]
- Faure, J.P.; Tuech, J.J.; Richer, J.P.; Pessaux, P.; Arnaud, J.P.; Carretier, M. Pancreatic metastasis of renal cell carcinoma: Presentation, treatment and survival. J. Urol. 2001, 165, 20–22. [Google Scholar] [CrossRef]
- Hashimoto, M.; Miura, Y.; Matsuda, M.; Watanabe, G. Concomitant duodenal and pancreatic metastases from renal cell carcinoma: Report of a case. Surg. Today 2001, 31, 180–183. [Google Scholar] [CrossRef]
- Marusch, F.; Koch, A.; Dietrich, F.; Hoschke, B.; Gastinger, I. A singular late metastasis of renal cell carcinoma in the pancreas. An uncommon pancreatic tumor. Zentralbl. Chir. 2001, 126, 391–395. [Google Scholar] [CrossRef]
- Ruibal Moldes, M.; Quintana de la Rosa, J.L.; Farina Perez, L.A.; Tardaguila, F.; Ortiz Rey, J.A.; Zungri Telo, E. Late pancreatic metastasis from renal carcinoma. Acta Urol. Esp. 2001, 25, 122–124. [Google Scholar] [CrossRef]
- Scatarige, J.C.; Horton, K.M.; Sheth, S.; Fishman, E.K. Pancreatic parenchymal metastases. Am. J. Roentgenol. 2001, 176, 695–699. [Google Scholar] [CrossRef]
- Sohn, T.A.; Yeo, C.J.; Cameron, Y.L.; Nakeeb, A.; Lillemoe, K.D. Renal cell carcinoma metastatic to the pancreas: Results of surgical management. J. Gastrointest. Surg. 2001, 5, 346–351. [Google Scholar] [CrossRef]
- Tada, T.; Kobayashi, G.; Noda, Y.; Kimura, K.; Ito, K.; Fujita, N. A resected case with multiple pancreatic metastasis of renal cell carcinoma. Jpn. J. Gastro Enterol. 2001, 98, 1368–1373. [Google Scholar]
- Bechade, D.; Palazzo, I.; Desrame, J.; Duvic, C.; Herody, M.; Didelot, F.; Coutant, G.; Algayres, J.P. Pancreatic metastasis of renal carcinoma: Report of three cases. Rev. Med. Intern. 2002, 23, 862–866. [Google Scholar]
- Chou, Y.H.; Chiou, H.J.; Hong, T.M.; Tiu, C.M.; Chiou, S.Y.; Su, C.H.; Tsay, S.H. Solitary metastasis from renal cell carcinoma presenting as diffuse pancreatic enlargement. J. Clin. Ultrasound 2002, 30, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Eloubeidi, M.A.; Jhala, D.; Chhieng, D.C.; Jhala, N.; Eltoum, I.; Wilcox, C.M. Multiple late asymptomatic pancreatic metastases from renal cell carcinoma: Diagnosis by endoscopic ultrasound-guided fine needle aspiration biopsy with immunocytochemical correlation. Dig. Dis. Sci. 2002, 47, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Hiotis, S.P.; Klimstra, D.S.; Conlon, K.C.; Brennan, M.F. Results after pancreatic resection for metastatic lesions. Ann. Surg. Oncol. 2002, 9, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Lisii, D.; Gaimant, A.; Sautereau, D.; Paraf, F.; Maubon, A. Duodenal bleeding revealing a renal cell carcinoma. Gastroenterol. Clin. Biol. 2002, 26, 1044–1046. [Google Scholar] [PubMed]
- Peschaud, F.; Cheynel, N.; Hagry, O.; Tremeaux, J.C.; Rat, P.; Favre, J.P. Surgical treatment of pancreatic metastases from renal carcinoma. Ann. Chir. 2002, 127, 527–531. [Google Scholar] [CrossRef]
- Roviello, F.; Nastri, G.; Hako, L.; Marrelli, D.; De Stefano, A.; Cioppa, T.; Pinto, E. Pancreatic metastasis from clear cell carcinoma. Chir. Ital. 2002, 54, 873–877. [Google Scholar]
- Yachida, S.; Fukushima, N.; Kanai, Y.; Nimura, S.; Shimada, K.; Yamamoto, J.; Sakamoto, M. Pancreatic metastasis from renal cell carcinoma extending into the main pancreatic duct: A case report. Jpn. J. Clin. Oncol. 2002, 32, 315–317. [Google Scholar] [CrossRef]
- Bassi, C.; Butturini, G.; Falconi, M.; Sargenti, W.; Mantovavi, W.; Pederzoli, P. High recurrence rate after atypical resection for pancreatic metastases from renal cell carcinoma. Br. J. Surg. 2003, 90, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Giulini, S.; Portolani, N.; Bonardelli, S.; Baiocchi, G.; Zampatti, M.; Coniglio, A.; Baronchelli, C. Distal pancreatic resection with splenic preservation for metastasis of renal carcinoma diagnosed 24 years later from nephrectomy. Ann. Ital. Chir. 2003, 74, 93–96. [Google Scholar] [PubMed]
- Hermandez, D.J.; Kavoussi, L.R.; Ellison, L.M. Laparoscopic pancreatectomy for metastatic renal cell carcinoma. Urology 2003, 62, 551. [Google Scholar] [CrossRef]
- Law, C.H.; Wei, A.C.; Hanna, S.S.; Al-Zahrani, M.; Taylor, B.R.; Greig, P.D.; Langer, B.; Gallinger, S. Pancreatic resection for metastatic renal carcinoma: Presentation, treatment and outcome. Ann. Surg. Oncol. 2003, 10, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Nakagohri, T.; Konishi, M.; Inoue, K.; Nakamura, T.; Kinoshita, T. Partial pancreatic head resection for pancreatic metastasis from renal cell carcinoma. Hepatogastroenterology 2003, 50, 2236–2238. [Google Scholar] [PubMed]
- Pecchi, A.; Cesinaro, A.; Torricelli, P. Solitary pancreatic metastasis from renal cell carcinoma. Radiol. Med. 2003, 105, 386–390. [Google Scholar]
- Zacharoulis, D.; Asopa, V.; Karvounis, E.; Williamson, R. Resection of renal metastases to the pancreas: A surgical challenge. HPB 2003, 5, 137–141. [Google Scholar] [CrossRef]
- Moussa, A.; Mitry, E.; Hammel, P.; Sauvanet, A.; Nassif, T.; Palazzo, L. Pancreatic metastasis: A multicentric study of 22 patients. Gastroenterol. Clin. Biol. 2004, 28, 872–876. [Google Scholar] [CrossRef]
- Ninan, S.; Jain, P.; Paul, A.; Menon, K. Synchronous pancreatic metastases from asymptomatic renal cell carcinoma. JOP 2005, 6, 26–28. [Google Scholar]
- Sotiropoulos, G.C.; Lang, H.; Liu, C.; Brokalaki, E.I.; Molmenti, E.I.; Broelsch, C.E. Surgical treatment of pancreatic metastases of renal cell carcinoma. JOP 2005, 6, 339–343. [Google Scholar]
- Wente, M.N.; Kleeff, J.; Esposito, I.; Hartel, M.; Müller, M.W.; Fröhlich, B.E.; Büchler, M.W.; Friess, H. Renal cancer cell metastasis into the pancreas: A single center experience and overview of the literature. Pancreas 2005, 30, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Angelini, C.; Mussi, C.; Bonardi, C.; Romano, F.; Sartori, P.; Uggeri, F.; Bovo, G. Surgical treatment of metastatic tumors to the pancreas: A single center experience and review of the literature. World J. Surg. 2006, 30, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Köhler, K.; Haroske, G.; Ludwig, K. Management of pancreatic metastases from renal cell carcinoma. Report of five cases. Zentralbl. Chir. 2006, 131, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Shrikhande, S.V.; Büchler, P.; Esposito, I.; Loos, M.; Büchler, M.W.; Friess, H. Splenic and portal vein thrombosis in pancreatic metastasis from renal cell carcinoma. World J. Surg. Oncol. 2006, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Eidt, S.; Jergas, M.; Schmidt, R.; Siedek, M. Metastasis to the pancreas—An indication for pancreatic resection? Langenbecks Arch. Surg. 2007, 392, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.M.; McFadden, D.W. Pancreatic resection for metastatic renal cell cancer to the pancreas. Am. Surg. 2007, 73, 58–60. [Google Scholar]
- Maeda, H.; Okabayashi, T.; Nishimori, I.; Kobayashi, M.; Sugimoto, T.; Kohsaki, T.; Onishi, S.; Hanazaki, K. Duodenum-preserving pancreatic head resection for pancreatic metastasis from renal cell carcinoma: A case report. Langenbecks Arch. Surg. 2007, 392, 649–652. [Google Scholar] [CrossRef]
- Varker, K.A.; Muscarella, P.; Wall, K.; Ellison, C.; Bloomston, M. Pancreatectomy for non-pancreatic malignancies results in improved survival after R0 resection. World J. Surg. Oncol. 2007, 5, 145. [Google Scholar] [CrossRef]
- Aimoto, T.; Uchida, E.; Yamahatsu, K.; Yoshida, H.; Hiroi, M.; Tajiri, T. Surgical treatment for isolated multiple pancreatic metastases from renal cell carcinoma: Report of a case. J. Nippon Med. Sch. 2008, 75, 221–224. [Google Scholar] [CrossRef]
- Bahra, M.; Jacob, D.; Langrehr, J.M.; Glanemann, M.; Schumacher, G.; Lopez-Hänninen, E.; Neuhaus, P. Metastatic lesions to the pancreas. When is resection reasonable? Chirurg 2008, 79, 241–248. [Google Scholar] [CrossRef]
- Kawakami, H.; Kuwatani, M.; Yamato, H.; Shinada, K.; Hirano, S.; Kondo, S.; Yonemori, A.; Matsuno, Y.; Asaka, M. Pancreatic metastasis from renal cell carcinoma with intraportal tumor thrombus. Inter. Med. 2008, 47, 1967–1970. [Google Scholar] [CrossRef]
- Koide, N.; Yokoyama, Y.; Oda, K.; Nishio, H.; Ebata, T.; Abe, T.; Igami, T.; Nimura, Y.; Nagino, M. Pancreatic metastasis from renal cell carcinoma. Results of the surgical management and pathologic findings. Pancreas 2008, 37, 104–107. [Google Scholar] [CrossRef]
- Matsutani, T.; Sasajima, K.; Miyamoto, M.; Yokoyama, T.; Maruyama, H.; Yanagi, K.; Matsuda, A.; Kashiwabara, M.; Suzuki, S.; Tajiri, T. Resection of pancreatic metastasis from renal cell carcinoma and an early gastric cancer. J. Nippon Med. Sch. 2008, 75, 41–45. [Google Scholar] [CrossRef]
- Schauer, M.; Vogelsang, H.; Siewert, J.R. Pancreatic resection for metastatic renal cell carcinoma: A single center experience and review of the literature. Anticancer Res. 2008, 28, 361–366. [Google Scholar]
- Tuech, J.J.; Lefebure, R.; Bridoux, V.; Albouy, B.; Lermite, E.; Le Pessot, F.; Le Blanc-Louvry, I.; Michot, F. Combined resection of the pancreas and inferior vena cava for pancreatic metastasis from renal cell carcinoma. J. Gastrointest. Surg. 2008, 12, 612–615. [Google Scholar] [CrossRef]
- Zerbi, A.; Ortolano, E.; Balzano, G.; Borri, A.; Beneduce, A.; Di Carlo, V. Pancreatic metastasis from renal cell carcinoma: Which patients benefit from surgical resection? Ann. Surg. Oncol. 2008, 15, 1161–1168. [Google Scholar] [CrossRef]
- Deguchi, Y.; Shimada, K.; Nara, S.; Esaki, M.; Sakamoto, Y.; Kosuge, T.; Hiraoka, N. Pancreaticojejunostomy with invagination of the punched pancreatic remnant after medial pancreatectomy and enucleation for multiple metastases of renal cell carcinoma: Report of a case. Surg. Today 2009, 39, 1086–1090. [Google Scholar] [CrossRef]
- Machado, N.O.; Chopra, P. Pancreatic metastasis from renal carcinoma managed by Whipple resection. A case report and literature review of metastatic pattern, surgical management and outcome. J. Pancreas 2009, 10, 13–18. [Google Scholar]
- Tanis, P.J.; van der Gaag, N.A.; Busch, O.R.; van Gulik, T.M.; Gouma, D.J. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br. J. Surg. 2009, 96, 579–592. [Google Scholar] [CrossRef]
- Volk, A.; Kersting, S.; Konopke, R.; Dobowolski, F.; Franzen, S.; Ockert, D.; Grützmann, R.; Saeger, H.D.; Bergert, H. Surgical therapy of intrapancreatic metastasis from renal cell carcinoma. Pancreatology 2009, 9, 392–397. [Google Scholar] [CrossRef]
- Hijioka, S.; Hifumi, M.; Mekky, M.; Takekuma, Y.; Kawaguchi, T.; Yokomizo, H.; Sato, T. Total pancreatectomy for metastatic renal carcinoma with marked extension into the main pancreatic duct. Inter. Med. 2010, 49, 557–562. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Dursun, A.; Zheng, H.; Wargo, J.; Thayer, S.P.; Fernandez-del Castillo, C.; Warshaw, A.L.; Ferrone, C.R. Metastatic tumors in the pancreas in the modern era. J. Am. Coll. Surg. 2010, 211, 749–753. [Google Scholar] [CrossRef]
- Mourra, N.; Arrive, L.; Balladur, P.; Flejou, J.F.; Tiret, E.; Paye, F. Isolated metastatic tumors to the pancreas: Hôpital St-Antoine experience. Pancreas 2010, 39, 577–580. [Google Scholar] [CrossRef]
- Ballarin, R.; Spaggiari, M.; Cautero, N.; De Ruvo, N.; Montalti, R.; Longo, C.; Pecchi, A.; Giacobazzi, P.; De Marco, G.; D’Amico, G.; et al. Pancreatic metastases from renal cell carcinoma: The state of the art. World J. Gastroenterol. 2011, 17, 4747–4756. [Google Scholar] [CrossRef]
- D’Ambra, M.; Ricci, C.; Casadei, R.; Minni, F. Pancreatic metastasis from renal cell carcinoma. Urologia 2011, 78, 5–8. [Google Scholar] [CrossRef]
- Miyao, M.; Naito, S.; Ozono, S.; Shinohara, N.; Masumori, N.; Igarashi, T.; Nakao, M.; Tsushima, T.; Senga, Y.; Horie, S.; et al. Late recurrence of renal cell carcinoma: Retrospective and collaborative study of the japanese society of renal cancer. Urology 2011, 77, 379–384. [Google Scholar] [CrossRef]
- Thadani, A.; Pais, S.; Savino, J. Metastasis of renal cell carcinoma to the pancreas 13 years postnephrectomy. Gastroenterol. Hepatol. 2011, 7, 697–699. [Google Scholar]
- You, D.; Choi, D.; Choi, S.; Heo, J.; Kim, W.; Ho, C.; Lee, H. Surgical resection of metastasis to the pancreas. J. Korean Surg. 2011, 80, 278–282. [Google Scholar] [CrossRef]
- Alzahrani, M.A.; Schmulewitz, N.; Grewal, S.; Lucas, F.V.; Turner, K.O.; McKenzie, J.T.; Sussman, J.J.; Ahmad, S.A. Metastases to the pancreas: The experience of a high volume center and a review of the literature. J. Surg. Oncol. 2012, 105, 156–161. [Google Scholar] [CrossRef]
- Firek, P.; Richter, S.; Jaekel, J.; Brehmer, B.; Heidenreich, A. Metastasectomy in renal cell cancer after neoadjuvant therapy with multi-tyrosine kinase inhibitors. Urologe 2012, 51, 398–402. [Google Scholar] [CrossRef]
- Hung, J.; Wang, S.; Shyr, Y.; Su, C.; Chen, T.; Wu, C. Resection for secondary malignancy of the pancreas. Pancreas 2012, 41, 121–129. [Google Scholar] [CrossRef]
- Katsourakis, A.; Noussios, G.; Hadjis, I.; Alatsakis, M.; Chatzitheoklitos, E. Late solitary pancreatic metastasis from renal cell carcinoma: A case report. Case Rep. Med. 2012, 2012, 464808. [Google Scholar] [CrossRef]
- Yazbek, T.; Gayet, B. The place of enucleation and enucleo-resection in the treatment of pancreatic metastasis of renal cell carcinoma. JOP 2012, 13, 433–438. [Google Scholar]
- Hata, T.; Sakata, N.; Aoki, T.; Yoshida, H.; Kanno, A.; Fujishima, F.; Motoi, F.; Masamune, A.; Shimosegawa, T.; Unno, M. Repeated pancreatectomy for metachronous duodenal and pancreatic metastases of renal cell carcinoma. Case Rep. Gastroenterol. 2013, 7, 442–448. [Google Scholar] [CrossRef]
- Mqirage, M.; Zabala Egurrola, J.; Rodríguez, J.G.; Peña, C.P. Metachronous pancreatic metastasis of renal cell carcinoma: A case report. Can. Urol. Assoc. J. 2013, 7, e460–e461. [Google Scholar] [CrossRef]
- Niess, H.; Conrad, C.; Kleespies, A.; Haas, F.; Bao, Q.; Jauch, K.W.; Graeb, C.; Bruns, C. Surgery for metastasis to the pancreas: Is it safe and effective? J. Surg. Oncol. 2013, 107, 859–864. [Google Scholar] [CrossRef]
- Espinoza, E.; Hassani, A.; Vaishampayan, U.; Shi, D.; Pontes, J.E.; Weaver, D.W. Surgical excision of duodenal/pancreatic metastatic renal cell carcinoma. Front. Oncol. 2014, 4, 218. [Google Scholar] [CrossRef]
- Kimura, Y.; Keira, Y.; Imamura, M.; Ito, T.; Nobuoka, T.; Mizuguchi, T.; Masumori, N.; Hasegawa, T.; Hirata, K. Histopathological aspects of pancreatic metastases in renal cell carcinoma: Does the mode of invasion permit limited resections? Pancreat. Disord. Ther. 2014, 4, 2. [Google Scholar]
- Lauro, S.; Onesti, E.C.; Righini, R.; Carbonetti, F.; Cremona, A.; Marchetti, P. A synchronous pancreatic metastasis from renal clear cell carcinoma, with unusual CT characteristics, completely regressed after therapy with sunitinib. Case Rep. Med. 2014, 2014, 473431. [Google Scholar] [CrossRef]
- Minni, F.; Casadei, R.; Perence, B.; Greco, V.; Marrano, N.; Margiotta, A.; Marrano, D. Pancreatic metastasis: Observations of three cases and review of the literature. Pancreatology 2014, 4, 509–520. [Google Scholar] [CrossRef]
- Moletta, L.; Milanetto, A.C.; Vincenzi, V.; Alaggio, R.; Pedrazzoli, S.; Pasquali, C. Pancreatic secondary lesions from renal cell carcinoma. World J. Surg. 2014, 38, 3002–3006. [Google Scholar] [CrossRef]
- Schwarz, L.; Sauvanet, A.; Regenet, N.; Mabrut, J.Y.; Gigot, J.F.; Housseau, E.; Millat, B.; Ouaissi, M.; Gayet, B.; Fuks, D.; et al. Long-term survival after pancreatic resection for renal cell carcinoma metastasis. Ann. Surg. Oncol. 2014, 21, 4007–4013. [Google Scholar] [CrossRef]
- Takeshi, A.; Mitsuhiro, I.; Hiromitsu, A.; Naoyuki, Y.; Taiichiro, S.; Hiroki, S.; Takeaki, K.; Tatsuya, S.; Futoshi, O.; Hiroharu, S.; et al. Middle segment-preserving pancreatectomy for recurrent metastasis of renal cell carcinoma after pancreatoduodenectomy: A case report. Case Rep. Surg. 2014, 2014, 3. [Google Scholar] [CrossRef]
- Tosoian, J.J.; Cameron, J.L.; Allaf, M.E.; Hruban, R.H.; Nahime, C.B.; Pawlik, T.M.; Pierorazio, P.M.; Reddy, S.; Wolfgang, C.L. Resection of isolated renal cell carcinoma metastases of the pancreas: Outcomes from the Johns Hopkins Hospital. J. Gastrointest. Surg. 2014, 18, 542–548. [Google Scholar] [CrossRef]
- Benhaim, R.; Oussoultzoglou, E.; Saeedi, Y.; Mouracade, P.; Bachellier, P.; Lang, H. Pancreatic metastasis from clear cell renal cell carcinoma: Outcome of an aggressive approach. Urology 2015, 85, 135–140. [Google Scholar] [CrossRef]
- Chang, Y.H.; Liaw, C.; Chuang, C.K. The role of surgery in renal cell carcinoma with pancreatic metastasis. Biomed. J. 2015, 38, 173–176. [Google Scholar]
- Gajendra, S.; Sachdew, R.; Mohapatra, I.; Goel, R.; Goel, S. Metastatic renal cell carcinoma: An unusual cause of bleeding pancreatic mass. J. Clin. Diagn. Res. 2015, 9, 15–17. [Google Scholar] [CrossRef]
- Santoni, M.; Conti, A.; Partelli, S.; Porta, C.; Sternberg, C.N.; Procopio, G.; Bracarda, S.; Basso, U.; De Giorgi, U.; Derosa, L.; et al. Surgical resection does not improve survival in patients with renal metastases to the pancreas in the era of tyrosine kinase inhibitors. Ann. Surg. Oncol. 2015, 22, 2094–2100. [Google Scholar] [CrossRef]
- Wiltberger, G.; Bucher, J.N.; Krenzien, F.; Benzing, C.; Atanasov, G.; Schmelzle, M.; Hau, H.M.; Bartels, M. Extended resection in pancreatic metastases: Feasibility, frequency, and long-term outcome: A retrospective analysis. BMC Surg. 2015, 15, 126. [Google Scholar] [CrossRef]
- Yuasa, T.; Inoshita, N.; Saiura, A.; Yamamoto, S.; Urakami, S.; Masuda, H.; Fujii, Y.; Fukui, I.; Ishikawa, Y.; Yonese, J. Clinical outcome of patients with pancreatic metastases from renal cell cancer. BMC Cancer 2015, 15, 46. [Google Scholar] [CrossRef]
- Boussios, S.; Zerdes, J.; Batsi, O.; Papakostas, V.P.; Seraj, E.; Pentheroudakis, G.; Glantzounis, G. Pancreatic resection for renal cell carcinoma metastasis: An exceptionally rare coexistence. Int. J. Surg. Case Rep. 2016, 27, 198–201. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Cong, L.; Zhang, T.P.; Zhao, Y.P. Pancreatic metastasis of renal cell carcinoma. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 30–38. [Google Scholar] [CrossRef]
- Fikatas, P.; Klein, F.; Andreou, A.; Schmuck, R.B.; Pratschke, J.; Bahra, M. Long-term survival after surgical treatment of renal cell carcinoma metastasis within the pancreas. Anticancer Res. 2016, 36, 4273–4278. [Google Scholar]
- Miura, T.; Nakamura, N.; Ogawa, K.; Watanabe, Y.; Yonekura, K.; Sanada, T.; Kuwabara, H.; Goseki, N. Resection of pancreatic metastasis from renal cell carcinoma 21 years after nephrectomy. Cancer Chemother. 2016, 43, 2187–2189. [Google Scholar]
- Nihei, K.; Sakamoto, K.; Suzuki, S.; Mishina, T.; Otaki, M. A case of pancreatic metastasis of renal cell carcinoma. Cancer Chemother. 2016, 43, 2274–2276. [Google Scholar]
- Rückert, F.; Distler, M.; Ollmann, D.; Lietzmann, A.; Birgin, E.; Teoule, P.; Grützmann, R.; Wilhelm, T.J. Retrospective analysis of survival after resection of pancreatic renal cell carcinoma metastases. Int. J. Surg. 2016, 26, 64–68. [Google Scholar] [CrossRef]
- Chatzizacharias, N.A.; Rosich-Medina, A.; Dajani, K.; Harper, S.; Huguet, E.; Liau, S.S.; Praseedom, R.K.; Jah, A. Surgical management of hepato-pancreatic metastasis from renal cell carcinoma. World J. Gastrontest. Oncol. 2017, 15, 70–77. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, J.H.; Lee, C.R.; Han, W.K.; Kang, C.M.; Lee, W.J. Laparoscopic total pancreatectomy for multiple metastasis of renal cell carcinoma of the pancreas: A case report and literature review. Ann. Hepatobiliary Pancreat. Surg. 2017, 21, 96–100. [Google Scholar] [CrossRef]
- Ko, S.; Yun, S.; Kim, S.; Kim, T.N.; Seo, H. Pancreatic resection for renal cell carcinoma metastasis: A case review. Ann. Hepatobiliary Pancreat. Surg. 2017, 21, 176–179. [Google Scholar] [CrossRef]
- Zianne, M.; Takahashi, N.; Tsujibata, A.; Miwa, K.; Goto, Y.; Matano, Y. Asymptomatic pancreatic metastasis from renal cell carcinoma diagnosed 21 years after nephrectomy. Case Rep. Gastrointest. Med. 2017, 2017, 8765264. [Google Scholar] [CrossRef]
- Boni, A.; Cochetti, G.; Ascani, S.; Del Zingaro, M.; Quadrini, F.; Paladini, A.; Diego Cocca, D.; Mearini, E. Robotic treatment of oligometastatic kidney tumor with synchronous pancreatic metastasis: Case report and review of the literature. BMC Surg. 2018, 18, 40. [Google Scholar] [CrossRef]
- Limaiem, F.; Bouraoui, S. Metastasis of renal cell carcinoma to the pancreas 11 years post nephrectomy. Pan Afr. Med. J. 2018, 30, 53. [Google Scholar] [CrossRef]
- Madkhali, A.A.; Shin, S.; Song, K.B.; Lee, J.H.; Hwang, D.W.; Park, K.M.; Lee, Y.J.; Kim, S.C. Pancreatectomy for secondary metastasis of the pancreas. Medicine 2018, 97, e12653. [Google Scholar] [CrossRef]
- Nogueira, M.; Dias, S.C.; Silva, A.C.; Pinto, J.; Machado, J. Solitary pancreatic renal cell carcinoma metastasis. Autopsy Case Rep. 2018, 8, e2018023. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, H.; Toyama, H.; Terai, S.; Mukubou, H.; Shirakawa, S.; Ishida, J.; Asakura, Y.; Shimizu, T.; Lee, D.; Tanaka, M.; et al. A patient with multiple pancreatic metastases undergoing total pancreatectomy 18 years after renal cell carcinoma resection. Cancer Chemother. 2018, 45, 2214–2216. [Google Scholar]
- Delahunt, B.; Cheville, J.C.; Martignoni, G.; Humphrey, P.A.; Magi-Galluzzi, C.; McKenney, J.; Egevad, L.; Algaba, F.; Moch, H.; Grignon, D.J.; et al. The international society of urological pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am. J. Surg. Pathol. 2013, 37, 1490–1504. [Google Scholar] [CrossRef]
- Moch, H. WHO classification 2016 and first S3 guidelines on renal cell cancer: What is important in practice? Pathologe 2016, 37, 127–133. [Google Scholar] [CrossRef]
- Fuhrman, S.; Lasky, L.; Limas, C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am. J. Surg. Pathol. 1982, 6, 655–663. [Google Scholar] [CrossRef]
- Brozzetti, S.; Sterpetti, A.V. Unexpected prolonged survival after extended and emergent resection of pancreatic metastases from renal cell carcinoma. J. Gastrointest. Cancer 2019. [Google Scholar] [CrossRef]
- Loré, J.M.; Madden, J.L.; Gerold, F.P. Pre-existing portocaval shunts. A hypothesis for the bizarre metastases of some carcinomas. Cancer 1958, 11, 24–27. [Google Scholar] [CrossRef]
- Saitoh, H.; Yoshida, K.; Uchijima, Y.; Kobayashi, N.; Suwata, J.; Nakame, Y. Possible metastatic routes via portocaval shunts in renal adenocarcinoma with liver metastasis. Urology 1991, 37, 598–601. [Google Scholar] [CrossRef]
- Allen-Mersh, T. Significance of the site of origin of pancreatic exocrine adenocarcinoma. J. Clin. Pathol. 1982, 35, 544–546. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Oudard, S.; Negrier, S.; Szczylik, C.; Pili, R.; Bjarnason, G.A.; et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cancer. J. Clin. Oncol. 2009, 27, 3584–3590. [Google Scholar] [CrossRef]
- Escudier, B.; Bellmunt, J.; Négrier, S.; Bajetta, E.; Melichar, B.; Bracarda, S.; Ravaud, A.; Golding, S.; Jethwa, S.; Sneller, V. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): Final analysis of overall survival. J. Clin. Oncol. 2010, 28, 2144–2150. [Google Scholar] [CrossRef]
- Beck, J.; Bellmunt, J.; Escudier, B. Long-term stable disease in metastatic renal cell carcinoma: Sorafenib sequenced to sunitinib and everolimus: A case study. Med. Oncol. 2011, 28, 1379–1383. [Google Scholar] [CrossRef]
- Rini, B.; Escudier, B.; Tomczak, P.; Kaprin, A.; Szczyloik, C.; Hutson, T.E.; Michaelson, M.D.; Gorbunova, V.; Gore, M.E.; Rusakov, I.G.; et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell caarcinoma (AXIS): A randomized phase III trial. Lancet 2012, 378, 1931–1939. [Google Scholar] [CrossRef]
- Ishikara, H.; Kondo, T.; Yoshida, K.; Omae, K.; Takagi, T.; Iizuka, H.; Kobayashi, H.; Tanabe, K. Evaluation of tumor burden after sequential molecular-targeted therapy in patients with metastatic renal cancer. Jpn. J. Clin. Oncol. 2017, 47, 226–232. [Google Scholar] [CrossRef]
- Iacovelli, R.; Lanoy, E.; Albiges, L.; Escudier, B. Tumour burden is an independent prognosic factor in metastatic renal cell carcinoma. BJU Int. 2012, 110, 1747–1754. [Google Scholar] [CrossRef]
- Basappa, N.S.; Elson, P.; Golshayan, A.R.; Wood, L.; Garcia, J.A.; Dreicer, R.; Rini, B.I. The impact of tumor burden characteristics in patients with metastatic renal cell carcinoma treated with sunitinib. Cancer 2011, 117, 1183–1189. [Google Scholar] [CrossRef]
- Stein, W.D.; Huang, H.; Menefee, M.; Edgerly, M.; Kotz, H.; Dwyer, A.; Yang, J.; Bates, S.E. Other paradigms: Growth rate constants and tumor burden determinated using computed tomography data correlate strongly with the overall survival of patients with renal cell carcinoma. Cancer J. 2009, 15, 441–447. [Google Scholar] [CrossRef]
- Jonasch, E.; Gao, J.; Rathmell, K.W. Renal cell carcoinoma. BMJ 2014, 349, g4797. [Google Scholar] [CrossRef]
- McNichols, D.W.; Segura, J.W.; DeWeerd, J.H. Renal cell carcinoma: Long-term survival and late recurrence. J. Urol. 1981, 126, 17–23. [Google Scholar] [CrossRef]
- Dekernion, J.B.; Ramming, K.P.; Smith, R.B. The natural history of metastatic renal cell carcinoma—A computer analysis. J. Urol. 1978, 120, 148–152. [Google Scholar] [CrossRef]
- Chara, L.; Rodriguez, B.; Holgado, E.; Ramirez, N.; Fernández-Rañada, I.; Mohedano, N.; Arcediano, A.; García, I.; Cassinello, J. An unusual metastatic renal cell carcinoma with maintained complete response to sunitinib treatment. Case Rep. Oncol. 2011, 4, 583–586. [Google Scholar] [CrossRef]
- Mazza, C.; Escudier, B.; Albiges, L. Nivolumab in renal cell carcinoma: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2017, 9, 171–181. [Google Scholar] [CrossRef]
- Flippot, R.; Escuduer, B.; Albiges, L. Immune checkpoint inhibitors: Toward new paradigms in renal cell carcinoma. Drugs 2018, 78, 1443–1457. [Google Scholar] [CrossRef]
- Shatveryan, G.A.; Chardarov, N.K.; Bagmet, N.N.; Ratnikova, N.P.; Bedzhanyan, A.L.; Petrenko, K.N.; Polishchuk, L.O.; Karagyozyan, G.A. Isolated pancreatic metastases of renal cell carcinoma. Khirurgiia 2017, 12, 36–40. [Google Scholar] [CrossRef]
- Sbitti, Y.; Debbagh, A.; Slimani, K.; Mahi, M.; Errihani, H.; Ichou, M. When tyrosine kinase inhibitor sunitinib can be discontinued in metastatic renal cell carcinoma to pancreas: A case report. J. Med. Case Rep. 2018, 20, 80. [Google Scholar] [CrossRef]
- Medioni, J.; Choueiri, T.K.; Zinzindohoue, F.; Cho, D.; Fournier, L.; Oudard, S. Response of renal cell carcinoma pancreatic metastasis to Sunitinib treatment: A retrospective analysis. J. Urol. 2009, 181, 2470–2475. [Google Scholar] [CrossRef]
- Kalra, S.; Atkinson, B.J.; Matrana, M.R.; Matin, S.F.; Wood, C.G.; Karam, J.A.; Tamboli, P.; Sircar, K.; Rao, P.; Corn, P.G.; et al. Prognosis of patients with metastatic cell carcinoma and pancreatic metastases. BJU Int. 2016, 117, 761–765. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Jiang, C.; Qian, C.; Liu, Z.; Luo, D. Factors involved in cancer metastasis: A better understanding to “seed and soil” hypothesis. Mol. Cancer 2017, 16, 176. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Zhang, Y.; Yang, J.; Zhu, D.; Li, D.; Zhou, J. Clinical diagnosis and detection of genetic mutations of pancreatic metastases: A report of four cases and review of the literature. Oncol. Lett. 2017, 14, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- De Velasco, G.; Wankowicz, S.A.; Madison, R.; Ali, S.M.; Norton, C.; Duquette, A.; Ross, J.S.; Bosśe, D.; Lalani, A.A.; Miller, V.A.; et al. Targeted genomic landscape of metastases compasred to primary tumours in clear cell metastatic renal carcinoma. Br. J. Cancer 2018, 118, 1238–1242. [Google Scholar] [CrossRef]
- Heinzelmann, J.; Unrein, A.; Wickmann, U.; Baumgart, S.; Stapf, M.; Szendroi, A.; Grimm, M.O.; Gajda, M.; Wunderlich, H.; Junker, K. MicroRNAs with prognostic potential for metastasis in clear cell renal cell carcinoma: A comparison of primary tumors and distant metastasis. Ann. Surg. Oncol. 2014, 21, 1046–1054. [Google Scholar] [CrossRef]
- Wu, X.; Weng, L.; Li, X.; Guo, C.; Pal, S.K.; Jin, J.M.; Li, Y.; Nelson, R.A.; Mu, B.; Onami, S.H.; et al. Identification of a 4-microRNA signature for clear cell renal carcinoma metastasis and prognosis. PLoS ONE 2012, 7, e35661. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, L.; Wang, K.; Peng, Z.; Ma, Y.; Zheng, Z.; Shang, D.; Xu, W.; Zheng, J. New mechanistic insights of clear cell renal carcinoma from integrated miRNA and mRNA expression profiling studies. Biomed. Pharmacother. 2019, 111, 821–834. [Google Scholar] [CrossRef]
- Kowalik, C.G.; Palmer, D.A.; Sullivan, T.B.; Teebagy, P.A.; Dugan, J.M.; Libertino, J.A.; Burks, E.J.; Canes, D.; Rieger-Christ, K.M. Profiling microRNA from nephrectomy and biopsy specimens: Predictors of progression and survival in clear cell renal cell carcinoma. BJU Int. 2017, 128, 428–440. [Google Scholar] [CrossRef]
- Heinzelmann, J.; Arndt, M.; Pleyers, R.; Fehlmann, T.; Hoelters, S.; Zeuschner, P.; Vogt, A.; Pryalukhin, A.; Schaeffeler, E.; Bohle, R.M.; et al. 4-miRNA score predicts the individual metastatic risk of renal cell carcinoma patients. Ann. Surg. Oncol. 2019. [Google Scholar] [CrossRef]
- Yu, L.; Xiang, L.; Feng, J.; Li, B.; Zhou, Z.; Li, J.; Lin, Y.; Lv, Y.; Zou, D.; Lei, Z.; et al. miRNA-21 and miRNA-223 expression singnature as a predictor for lymph node metastasis, distant metastasis and survival in kidney renal clear cell carcinoma. J. Cancer 2018, 9, 3651–3659. [Google Scholar] [CrossRef]
- Mlcochova, H.; Machakova, T.; Rabien, A.; Radova, L.; Fabian, P.; Iliev, R.; Slaba, K.; Poprach, A.; Kilic, E.; Stanik, M.; et al. Epithelial-mesenchymal transition associated microRNA/mRNA signature is linked to metastasis and prognosis in clear-cell renal cell carcinoma. Sci. Rep. 2016, 6, 31852. [Google Scholar] [CrossRef]
- Shiomi, E.; Sugai, T.; Ishida, K.; Osakabe, M.; Tsuyukubo, T.; Kato, Y.; Takata, R.; Obara, W. Analysis of expression patterns of microRNAs that are closely associated with renal carcinogenesis. Front. Oncol. 2019, 9, 431. [Google Scholar] [CrossRef]
- Yuan, J.; Dong, R.; Liu, F.; Zhan, L.; Liu, Y.; Wei, J.; Wang, N. The miR-183/182/96 cluster functions as a potential carcinogenic factor and prognostic factor in kidney renal clear cell carcinoma. Exp. Ther. Med. 2019, 17, 2457–2464. [Google Scholar] [CrossRef]
- Dasgupta, P.; Kulkarni, P.; Majid, S.; Varahram, S.; Hashimoto, Y.; Bhat, N.S.; Shiina, M.; Deng, G.; Saini, S.; Tabatabai, Z.L.; et al. MicroRNA-203 inhibits long noncoding RNA HOTAIR and regulates tumorigenesis through epithelial-to-mesenchymal transition pathway in renal cell carcinoma. Mol. Cancer Ther. 2018, 17, 1061–1069. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; Su, Z.; Li, Y.; Liu, J.; Jin, L.; Shi, M.; Jiang, Z.; Qi, Z.; Gui, Y.; et al. MicroRNA-106b functions as an oncogene in renal cell carcinoma by affecting cell proliferation, migration and apoptosis. Mol. Med. Rep. 2016, 13, 1420–1426. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, X.; Wang, T.; Xing, J. Mir-223-3p promotes cell proliferation and metastasis by downregulating SLC4A4 in clear cell renal cell carcinoma. Aging 2019, 11, 615–631. [Google Scholar] [CrossRef]
- Su, Z.; Chen, D.; Zhang, E.; Li, Y.; Yu, Z.; Shi, M.; Jiang, Z.; Ni, L.; Yang, S.; Gui, Y.; et al. MicroRNA-509-3p inhibits cancer cell proliferation and migration by targeting the mitogen- activated protein kinase kinase kinase 8 oncogene in renal cell carcinoma. Mol. Med. Rep. 2015, 12, 1535–1543. [Google Scholar] [CrossRef]
- Tong, Z.; Meng, X.; Wang, J.; Wang, L. MicroRNA-212 inhibits the proliferation and invasion of human renal cell carcinoma by targeting FOXA1. Mol. Med. Rep. 2018, 17, 1361–1367. [Google Scholar] [CrossRef]
- Gu, C.; Wang, Z.; Jin, Z.; Li, G.; Kou, Y.; Jia, Z.; Yang, J.; Tian, F. MicroRNA-212 inhibits the proliferation, migration ad invasion of renal cell carcinoma by targeting X-linked inhibitor of apoptosis protein (XIAP). Oncotarget 2017, 8, 92119–92133. [Google Scholar] [CrossRef]
- He, C.; Chen, Z.Y.; Li, Y.; Yang, Z.Q.; Zeng, F.; Cui, Y.; He, Y.; Chen, J.B.; Chen, H.Q. miR-10b suppresses cell invasion and metastasis through targeting HOXA3 regulated by FAK/YAP signalling pathway in clear-cell renal cell carcinoma. BMC Nephrol. 2019, 20, 127. [Google Scholar] [CrossRef]
- Yamasaki, T.; Seki, N.; Yoshino, H.; Itesako, T.; Hidaka, H.; Yamada, Y.; Tatarano, S.; Yonezawa, T.; Kinoshita, T.; Nakagawa, M.; et al. MicroRNA-218 inhibits cell migration and invasion in renal cell carcinoma through targeting caveolin-2 involved in focal adhesion pathway. J. Urol. 2013, 190, 1059–1068. [Google Scholar] [CrossRef]
- Ji, S.; Su, X.; Zhang, X.; Han, Z.; Zhao, Y.; Liu, Q. MicroRNA-372 functions as a tumor suppressor in cell invasion, migration and epithelial-mesenchymal transition by targeting ATAD2 in renal cell carcinoma. Oncol. Lett. 2019, 17, 2400–2408. [Google Scholar] [CrossRef]
- Li, Y.; Guan, B.; Liu, J.; Zhang, Z.; He, S.; Zhan, Y.; Su, B.; Han, H.; Zhang, X.; Wang, B.; et al. MicroRNA-200b is downregulated and suppresses metastasis by targeting LAMA4 in renal cell carcinoma. EBioMedicine 2019, 44, 439–451. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Tang, K.; Liu, H.; Zeng, J.; Li, H.; Yan, L.; Hu, J.; Guan, W.; Chen, K.; Xu, H.; et al. Regulatory network of two tumor-suppressive noncoding RNAs interferes with the growth and metastasis of renal cell carcinoma. Mol. Ther. Nucleic Acids 2019, 16, 554–565. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Y.; Xu, J.; Lu, J.; Wang, K.; Yang, D.R.; Yang, G.; Li, G.; Chang, C. Preclinical studies using miR-32-5p to suppress clear cell renal carcinoma metastasis via altering the miR-32-5p/TR4/HGF/Met signaling. Int. J. Cancer 2018, 143, 100–112. [Google Scholar] [CrossRef]
- Ding, X. MicroRNAs: Regulators of cancer metastasis and epithelial-mesenchymal transition (EMT). Chin. J. Cancer 2014, 33, 140–146. [Google Scholar] [CrossRef]
- Wei, R.; Ye, X.; Zhao, Y.; Jia, N.; Liu, T.; Lian, W.; Wei, H.; Zhang, G.; Song, L. MicroRNA-218 inhibits the cell proliferation and migration in clear cell renal cell carcinoma through targeting cancerous inhibitor of protein phosphatase 2A. Oncol. Lett. 2019, 17, 3211–3218. [Google Scholar] [CrossRef]
- Chen, X. Expression of microRNA-3133 correlates with the prognosis in patients with clear cell renal cell carcinoma. Medicine 2019, 98, e16008. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear renal cell carcinoma. Nature 2013, 499, 43–49. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef]
- Sceneay, J.; Smyth, M.J.; Möller, A. The pre-metastatic niche: Finding common ground. Cancer Metastasis Rev. 2013, 32, 449–464. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X. Characteristics and significance of the pre-metastatic niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, G.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Grange, C.; Brossa, A.; Bussolati, B. Extracellular vesicles and carried miRNAs in the progression of renal cell carcinoma. Int. J. Mol. Sci. 2019, 20, 1832. [Google Scholar] [CrossRef]
- Wang, L.; Yang, G.; Zhao, D.; Wang, J.; Bai, Y.; Peng, Q.; Wang, H.; Fang, R.; Chen, G.; Wang, Z.; et al. CD103-positive CSC exosomes promotes EMT of clear cell renal carcinoma: Role of remote MiR-19b-3p. Mol. Cancer 2019, 18, 86. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Guo, N.; Wang, S. MDSCs: Key criminals of tumor pre-metastatic niche formation. Front. Immunol. 2019, 10, 172. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef]
- Gai, C.; Pomatto, M.A.; Grange, C.; Deregibus, M.C.; Camussi, G. Extracellular vesicles in onco-nephrology. Exp. Mol. Med. 2019, 51, 29. [Google Scholar] [CrossRef]
- Walenkamp, A.M.; Lapa, C.; Herrmann, K.; Wester, H.J. CXCR4 ligands: The next big hit? J. Nucl. Med. 2017, 58, 77S–82S. [Google Scholar] [CrossRef]
- Münst, S.; Läubli, H.; Soysal, S.D.; Zippelius, A.; Tzankov, A.; Hoeller, S. The immune system and cancer evasion strategies: Therapeutic concepts. J. Intern. Med. 2016, 279, 541–562. [Google Scholar] [CrossRef]
| Variable | Data | % |
|---|---|---|
| Age (years; N = 349) | 63.1 (9.7) | |
| Sex (m: f) | 371: 318 | 54: 46 |
| Synchronous: Metachronous | 25: 334 | 7: 93 |
| Time to Onset (years; N = 334) | 10.1 (6.3) | |
| Multiple (N = 456) | 174 | 38.1 |
| Localization (head, body, tail) | 99: 46: 61 | 48: 22: 30 |
| Size (mm; N = 174) | 37.0 (21.4) | |
| Radical Surgery (N = 477) | 256 | 54 |
| Grading 1, 2, 3, 4 (N = 137) | 22: 88: 27: 0 | 16: 64: 20: 0 |
| Actuarial 3-year Survival (N = 307) | 80 | |
| Actuarial 5-year Survival (N = 307) | 72 |
| Variable | Solitary | Multiple | Significance |
|---|---|---|---|
| Age (years; N = 180/110) | 63.7 (9.3) | 62.7 (8.8) | n.s. p = 0.706 |
| Synchronous | 18 (11%) | 6 (6%) | n.s. p = 0.432 |
| Metachronous | 153 (89%) | 98 (94%) | |
| Time to Onset (years; N = 143/95) | 10.1 (6.6) | 10.0 (6.4) | n.s. p = 0.432 |
| Grading (N = 27/23) 1 | 7 (24%) | 4 (26%) | n.s. p = 0.670 |
| 2 | 11 (48%) | 12 (41%) | |
| 3 | 9 (28%) | 7 (33%) | |
| 4 | 0 | 0 |
| Side Affected by Metasta | n | % |
|---|---|---|
| Right Side | 100 | 47.6 |
| Left Side | 110 | 52.4 |
| Site of Pancreatic Metastasis Site | Side Affected by Renal Cell Carcinoma | ||
|---|---|---|---|
| Left | Right | Bilateral | |
| Head | 36 | 28 | 1 |
| Body | 13 | 15 | 1 |
| Tail | 15 | 10 | 1 |
| Total | 64 | 53 | 3 |
| Author, Year | N | 5-yr Survival (%) |
|---|---|---|
| Madkhali [193], 2018 | 17 | 47 |
| Chatzizacharias [187], 2017 | 7 | 71 |
| Fikatas [183], 2016 | 19 | 71 |
| Yuasa [180], 2015 | 20 | 79 |
| Tosoian [174], 2014 | 42 | 52 |
| Schwarz [172], 2014 | 62 | 63 |
| Kimura [168], 2014 | 13 | 77 |
| Konstandinidis [152], 2010 | 20 | 61 |
| Zerbi [146], 2008 | 23 | 88 |
| Bahra [140], 2008 | 9 | 100 |
| Crippa [132], 2006 | 5 | 80 |
| Wente [131], 2005 | 12 | 53 |
| Law [124], 2003 | 14 | 75 |
| Bassi [121], 2003 | 22 | 53 |
| Sohn [111], 2001 | 10 | 75 |
| Thomson [104], 2000 | 21 | 43 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellner, F. Observations on Solitary Versus Multiple Isolated Pancreatic Metastases of Renal Cell Carcinoma: Another Indication of a Seed and Soil Mechanism? Cancers 2019, 11, 1379. https://doi.org/10.3390/cancers11091379
Sellner F. Observations on Solitary Versus Multiple Isolated Pancreatic Metastases of Renal Cell Carcinoma: Another Indication of a Seed and Soil Mechanism? Cancers. 2019; 11(9):1379. https://doi.org/10.3390/cancers11091379
Chicago/Turabian StyleSellner, Franz. 2019. "Observations on Solitary Versus Multiple Isolated Pancreatic Metastases of Renal Cell Carcinoma: Another Indication of a Seed and Soil Mechanism?" Cancers 11, no. 9: 1379. https://doi.org/10.3390/cancers11091379
APA StyleSellner, F. (2019). Observations on Solitary Versus Multiple Isolated Pancreatic Metastases of Renal Cell Carcinoma: Another Indication of a Seed and Soil Mechanism? Cancers, 11(9), 1379. https://doi.org/10.3390/cancers11091379




