Modulation of Rat Cancer-Induced Bone Pain is Independent of Spinal Microglia Activity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
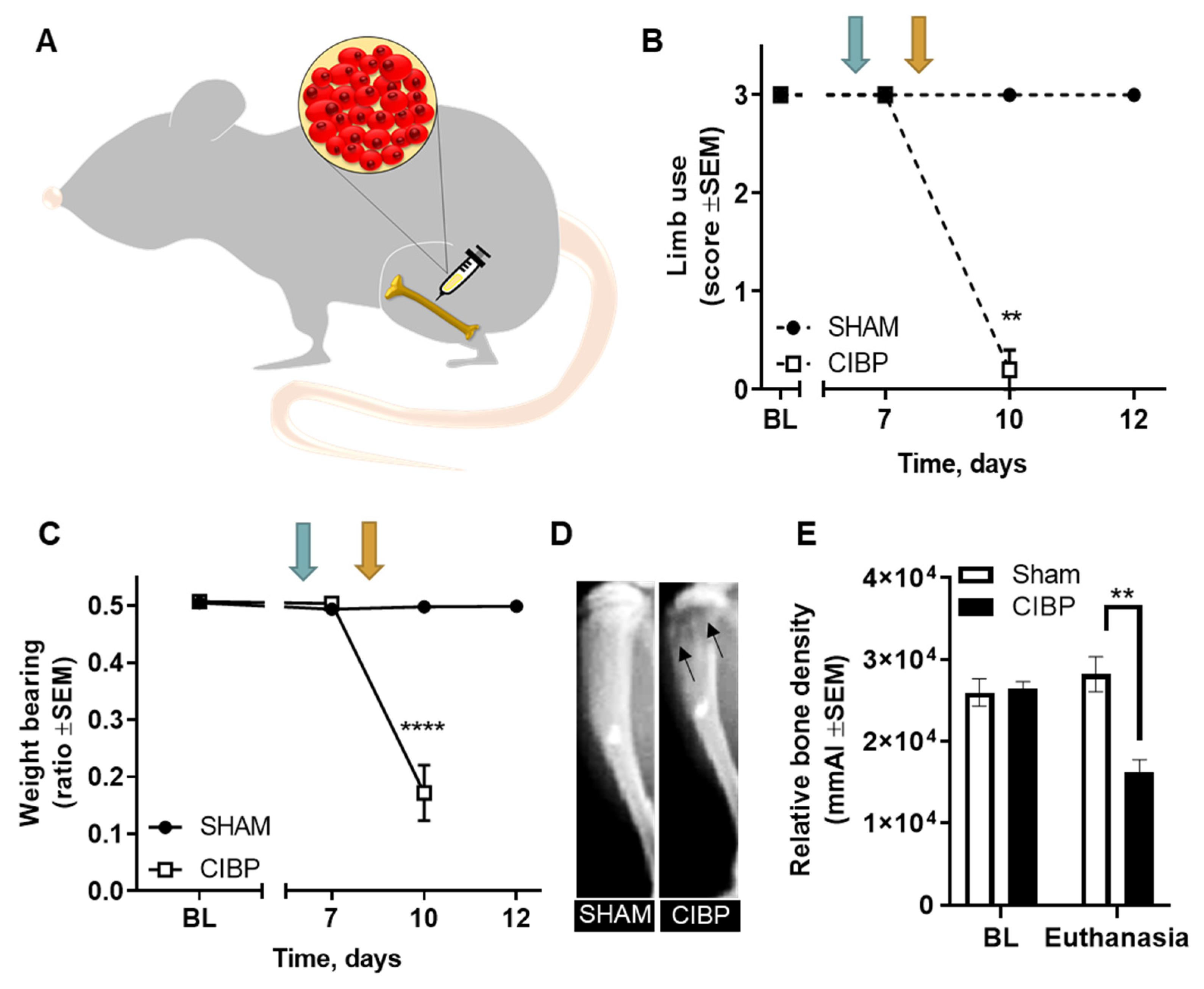
2.1. Intratibial Inoculation of Walker 256 Cancer Cells Causes Cancer-Induced Bone Pain in Female Rats
2.2. Absence of Microglial Reaction in the Ipsilateral Dorsal Horn of Female Rats Inoculated with Walker 256 Cancer Cells
2.3. Absence of Microglial Reaction Is Independent of Cancer Cell Line Inoculated
2.4. Absence of Microglial Reaction in Male Rats Inoculated with Walker 256 Cancer Cells
2.5. Microglial Reaction Is Observed in the Spinal Cord of Rats with Spared Nerve Injury
2.6. Pharmacological Inhibition of Spinal Microglia Does not Ameliorate Nociception in the CIBP Rat Model
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Animals
4.3. CIBP Surgery
4.4. Spared Nerve Injury (SNI) Surgery
4.5. Drug Interventions
4.6. Behavioral Tests
4.6.1. Limb Use
4.6.2. Weight Bearing
4.6.3. Von Frey
4.7. X-ray Imaging
4.8. Tissue Extraction
4.9. Immunohistochemistry
4.10. Image Analyses
4.11. Statistical Analyses
4.12. Data Sharing Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Breivik, H.; Cherny, N.; Collett, B.; De Conno, F.; Filbet, M.; Foubert, A.J.; Cohen, R.; Dow, L. Cancer-related pain: A pan-European survey of prevalence, treatment, and patient attitudes. Ann. Oncol. 2009, 20, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Grond, S.; Zech, D.; Diefenbach, C.; Radbruch, L.; Lehmann, K.A. Assessment of cancer pain: A prospective evaluation in 2266 cancer patients referred to a pain service. Pain 1996, 64, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S. Malignant bone pain: Pathophysiology and treatment. Pain 1997, 69, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Banning, A.; Sjøgren, P.; Henriksen, H. Treatment outcome in a multidisciplinary cancer pain clinic. Pain 1991, 47, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, A.; Brunelli, C.; Zecca, E.; Portenoy, R.K. Breakthrough pain characteristics and syndromes in patients with cancer pain. An international survey. Palliat. Med. 2004, 18, 177–183. [Google Scholar] [CrossRef]
- Laird, B.J.; Walley, J.D.; Murray, G.; Clausen, E.; Colvin, L.A.; Fallon, M. Characterization of cancer-induced bone pain: An exploratory study. Support. Care Cancer 2010, 19, 1393–1401. [Google Scholar] [CrossRef]
- Kurita, G.P.; Tange, U.B.; Farholt, H.; Sonne, N.M.; Strömgren, A.S.; Ankersen, L.; Kristensen, L.; Bendixen, L.; Groenvold, M.; Petersen, M.A.; et al. Pain characteristics and management of inpatients admitted to a comprehensive cancer centre: A cross-sectional study. Acta Anaesthesiol. Scand. 2013, 57, 518–525. [Google Scholar] [CrossRef]
- Van den Beuken-van Everdingen, M.H.J.; Hochstenbach, L.M.; Joosten, E.A.; Tjan-Heijnen, V.C.; Janssen, D.J.; Joosten, B.E. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090.e9. [Google Scholar] [CrossRef]
- Jimenez-Andrade, J.M.; Mantyh, W.G.; Bloom, A.P.; Ferng, A.S.; Geffre, C.P.; Mantyh, P.W. Bone cancer pain. Ann. N. Y. Acad. Sci. 2010, 1198, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, T.; Mercatali, L.; Amadori, D. Bone and cancer: The osteoncology. Clin. Cases Miner. Bone Metab. 2013, 10, 121–123. [Google Scholar]
- Yekkirala, A.S.; Roberson, D.P.; Bean, B.P.; Woolf, C.J. Breaking barriers to novel analgesic drug development. Nat. Rev. Drug Discov. 2017, 16, 545–564. [Google Scholar] [CrossRef] [Green Version]
- Hansen, R.B.; Frost, C.Ø.; Sonne, N.M.; Johnsen, A.T.; Heegaard, A.-M. Exploring the Patients’ Perception of Background and Breakthrough Pain: A McGill Pain Questionnaire Inquiry in Patients with Bone Cancer Pain. J. Palliat. Med. 2019, 22, 881–883. [Google Scholar] [CrossRef]
- Whiteside, G.; Adedoyin, A.; Leventhal, L. Predictive validity of animal pain models? A comparison of the pharmacokinetic–pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology 2008, 54, 767–775. [Google Scholar] [CrossRef]
- King, T.; Porreca, F. Preclinical Assessment of Pain: Improving Models in Discovery Research. In Neuroinflammation and Schizophrenia; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2014; Volume 20, pp. 101–120. [Google Scholar]
- Schwei, M.J.; Honore, P.; Rogers, S.D.; Salak-Johnson, J.L.; Finke, M.P.; Ramnaraine, M.L.; Clohisy, D.R.; Mantyh, P.W. Neurochemical and Cellular Reorganization of the Spinal Cord in a Murine Model of Bone Cancer Pain. J. Neurosci. 1999, 19, 10886–10897. [Google Scholar] [CrossRef] [Green Version]
- Mao-Ying, Q.-L.; Zhao, J.; Dong, Z.-Q.; Wang, J.; Yu, J.; Yan, M.-F.; Zhang, Y.-Q.; Wu, G.-C.; Wang, Y.-Q. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochem. Biophys. Res. Commun. 2006, 345, 1292–1298. [Google Scholar] [CrossRef]
- Medhurst, S.J.; Walker, K.; Bowes, M.; Kidd, B.L.; Glatt, M.; Muller, M.; Hattenberger, M.; Vaxelaire, J.; O’reilly, T.; Wotherspoon, G.; et al. A rat model of bone cancer pain. Pain 2002, 96, 129–140. [Google Scholar] [CrossRef]
- Falk, S.; Dickenson, A.H. Pain and Nociception: Mechanisms of Cancer-Induced Bone Pain. J. Clin. Oncol. 2014, 32, 1647–1654. [Google Scholar] [CrossRef] [Green Version]
- Honore, P.; Mantyh, P.W. Bone Cancer Pain: From Mechanism to Model to Therapy. Pain Med. 2000, 1, 303–309. [Google Scholar] [CrossRef]
- Honore, P.; Rogers, S.D.; Schwei, M.J.; Salak-Johnson, J.L.; Luger, N.M.; Sabino, M.C.; Clohisy, D.R.; Mantyh, P.W. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 2000, 98, 585–598. [Google Scholar] [CrossRef]
- Gilmore, S.A. Proliferation of non-neuronal cells in spinal cords of irradiated, immature rats following transection of the sciatic nerve. Anat. Rec. 1975, 181, 799–811. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Chen, Q.; Eusman, M.A.; Pal, A.; Eguchi, S.; Wu, L.; Ma, D. The role of microglia in the pathobiology of neuropathic pain development: What do we know? Br. J. Anaesth. 2017, 118, 504–516. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Yu-Qiu, Z.; Qadri, Y.; Serhan, C.N.; Ji, R.-R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-N.; Yao, M.; Yang, J.-P.; Peng, J.; Peng, Y.; Li, C.-F.; Zhang, Y.-B.; Ji, F.; Cheng, H.; Xu, Q.-N.; et al. Cancer-induced bone pain sequentially activates the ERK/MAPK pathway in different cell types in the rat spinal cord. Mol. Pain 2011, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Sorge, R.E.; Mapplebeck, J.C.S.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.-S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Gao, Y.-J.; Wang, J.; Strichartz, G. Shifts in Cell-type Expression Accompany a Diminishing Role of Spinal P38-Mapkinase Activation over Time during Prolonged Postoperative Pain. Anesthesiology 2011, 115, 1281–1290. [Google Scholar] [CrossRef] [Green Version]
- Mapplebeck, J.C.S.; Beggs, S.; Salter, M.W. Sex differences in pain. Pain 2016, 157, S2–S6. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H.; Li, T.-T.; Luo, H.; Gu, X.-Y.; Lu, N.; Ji, R.-R.; Yu-Qiu, Z. Delayed Activation of Spinal Microglia Contributes to the Maintenance of Bone Cancer Pain in Female Wistar Rats via P2X7 Receptor and IL-18. J. Neurosci. 2015, 35, 7950–7963. [Google Scholar] [CrossRef] [Green Version]
- Lan, L.S.; Yang, J.-P.; Na, W.L.; Miao, J.; Qiu, Q.-C.; Ma, Z.; Lei, L.; Li, C.-F.; Ren, C.; Jin, Z.; et al. Down-regulation of Toll-like receptor 4 gene expression by short interfering RNA attenuates bone cancer pain in a rat model. Mol. Pain 2010, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Liu, Y.; Sun, B.; Sun, Y.; Hou, B.; Zhang, Y.; Ma, Z.; Gu, X. Intrathecal Injection of JWH-015 Attenuates Bone Cancer Pain Via Time-Dependent Modification of Pro-inflammatory Cytokines Expression and Astrocytes Activity in Spinal Cord. Inflammation 2015, 38, 1880–1890. [Google Scholar] [CrossRef]
- Cecchini, M.G.; Wetterwald, A.; Van Der Pluijm, G.; Thalmann, G.N. Molecular and Biological Mechanisms of Bone Metastasis. EAU Updat. Ser. 2005, 3, 214–226. [Google Scholar] [CrossRef]
- Hald, A.; Nedergaard, S.; Hansen, R.R.; Ding, M.; Heegaard, A.-M. Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur. J. Pain 2009, 13, 138–145. [Google Scholar] [CrossRef]
- Ducourneau, V.R.; Dolique, T.; Hachem-Delaunay, S.; Miraucourt, L.S.; Amadio, A.; Blaszczyk, L.; Jacquot, F.; Ly, J.; Devoize, L.; Oliet, S.H.; et al. Cancer pain is not necessarily correlated with spinal overexpression of reactive glia markers. Pain 2014, 155, 275–291. [Google Scholar] [CrossRef]
- Dore-Savard, L.; Otis, V.; Belleville, K.; Lemire, M.; Archambault, M.; Tremblay, L.; Beaudoin, J.-F.; Beaudet, N.; LeComte, R.; Lepage, M.; et al. Behavioral, Medical Imaging and Histopathological Features of a New Rat Model of Bone Cancer Pain. PLoS ONE 2010, 5, e13774. [Google Scholar] [CrossRef] [Green Version]
- Cao, F.; Gao, F.; Xu, A.-J.; Chen, Z.-J.; Chen, S.-S.; Yang, H.; Yu, H.-H.; Mei, W.; Liu, X.-J.; Xiao, X.-P.; et al. Regulation of spinal neuroimmune responses by prolonged morphine treatment in a rat model of cancer induced bone pain. Brain Res. 2010, 1326, 162–173. [Google Scholar] [CrossRef]
- Svensson, C.I.; Marsala, M.; Westerlund, A.; Calcutt, N.A.; Campana, W.M.; Freshwater, J.D.; Catalano, R.; Feng, Y.; Protter, A.A.; Scott, B.; et al. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J. Neurochem. 2003, 86, 1534–1544. [Google Scholar] [CrossRef]
- Jin, S.-X.; Zhuang, Z.-Y.; Woolf, C.J.; Ji, R.-R. p38 Mitogen-Activated Protein Kinase Is Activated after a Spinal Nerve Ligation in Spinal Cord Microglia and Dorsal Root Ganglion Neurons and Contributes to the Generation of Neuropathic Pain. J. Neurosci. 2003, 23, 4017–4022. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, M.; Mizokoshi, A.; Shigemoto-Mogami, Y.; Koizumi, S.; Inoue, K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia 2003, 45, 89–95. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, W.; Chabot, J.-G.; Quirion, R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009, 23, 2576–2586. [Google Scholar] [CrossRef]
- Clark, A.K.; Staniland, A.A.; Marchand, F.; Kaan, T.K.Y.; McMahon, S.B.; Malcangio, M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J. Neurosci. 2010, 30, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.-R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154, S10–S28. [Google Scholar] [CrossRef]
- Song, H.; Han, Y.; Pan, C.; Deng, X.; Dai, W.; Hu, L.; Jiang, C.; Yang, Y.; Cheng, Z.; Li, F.; et al. Activation of Adenosine Monophosphate–activated Protein Kinase Suppresses Neuroinflammation and Ameliorates Bone Cancer Pain. Anesthesiology 2015, 123, 1170–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.-Y.; Wen, Y.R.; Zhang, D.-R.; Borsello, T.; Bonny, C.; Strichartz, G.R.; Decosterd, I.; Ji, R.-R. A Peptide c-Jun N-Terminal Kinase (JNK) Inhibitor Blocks Mechanical Allodynia after Spinal Nerve Ligation: Respective Roles of JNK Activation in Primary Sensory Neurons and Spinal Astrocytes for Neuropathic Pain Development and Maintenance. J. Neurosci. 2006, 26, 3551–3560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao-Ying, Q.-L.; Wang, X.-W.; Yang, C.-J.; Li, X.; Mi, W.-L.; Wu, G.-C.; Wang, Y.-Q. Robust spinal neuroinflammation mediates mechanical allodynia in Walker 256 induced bone cancer rats. Mol. Brain 2012, 5, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.-B.; Zhang, T.; Sun, K.; Song, S.-P.; Cao, S.-B.; Zhang, H.; Shen, W. Corticotropin-releasing factor mediates bone cancer induced pain through neuronal activation in rat spinal cord. Tumor Biol. 2015, 36, 9559–9565. [Google Scholar] [CrossRef]
- Hu, J.-H.; Yang, J.; Liu, L.; Li, C.-F.; Wang, L.-N.; Ji, F.-H.; Cheng, H. Involvement of CX3CR1 in bone cancer pain through the activation of microglia p38 MAPK pathway in the spinal cord. Brain Res. 2012, 1465, 1–9. [Google Scholar] [CrossRef]
- Jin, X.-H.; Wang, L.-N.; Zuo, J.-L.; Yang, J.-P.; Liu, S.-L. P2X4 receptor in the dorsal horn partially contributes to brain-derived neurotrophic factor oversecretion and toll-like receptor-4 receptor activation associated with bone cancer pain. J. Neurosci. Res. 2014, 92, 1690–1702. [Google Scholar] [CrossRef]
- Wang, L.-N.; Yang, J.-P.; Zhan, Y.; Ji, F.-H.; Wang, X.-Y.; Zuo, J.-L.; Xu, Q.-N. Minocycline-induced reduction of brain-derived neurotrophic factor expression in relation to cancer-induced bone pain in rats. J. Neurosci. Res. 2011, 90, 672–681. [Google Scholar] [CrossRef]
- Zhang, R.-X.; Liu, B.; Wang, L.; Ren, K.; Qiao, J.-T.; Berman, B.M.; Lao, L. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain 2005, 118, 125–136. [Google Scholar] [CrossRef]
- Lewis, K.; Harford-Wright, E.; Vink, R.; Ghabriel, M.N. Characterisation of Walker 256 breast carcinoma cells from two tumour cell banks as assessed using two models of secondary brain tumours. Cancer Cell Int. 2013, 13, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, P.; Heegaard, A.-M.; Hestehave, S.; Jeggo, R.; Bjerrum, O.J.; Munro, G. Vendor-derived differences in injury-induced pain phenotype and pharmacology of Sprague-Dawley rats: Does it matter? Eur. J. Pain 2016, 21, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Blackburn-Munro, G. Pain-like behaviours in animals—How human are they? Trends Pharmacol. Sci. 2004, 25, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.L.; Delaney, A.; Bennett, M.I.; Dickenson, A.H.; Egan, K.J.; Vesterinen, H.M.; Sena, E.S.; MacLeod, M.; Colvin, L.A.; Fallon, M. Animal models of bone cancer pain: Systematic review and meta-analyses. Pain 2013, 154, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Delaney, A.; Fleetwood-Walker, S.M.; Colvin, L.A.; Fallon, M. Translational medicine: Cancer pain mechanisms and management. Br. J. Anaesth. 2008, 101, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Wei, J.; Wang, D.; Zhu, X.; Zhou, Y.; Wang, S.; Xu, G.-Y.; Jiang, G. Upregulation of Spinal Voltage-Dependent Anion Channel 1 Contributes to Bone Cancer Pain Hypersensitivity in Rats. Neurosci. Bull. 2017, 33, 711–721. [Google Scholar] [CrossRef]
- Martinez, V.; Szekely, B.; Lemarie, J.; Martin, F.; Gentili, M.; Ben Ammar, S.; Lepeintre, J.F.; De Loubresse, C.G.; Chauvin, M.; Bouhassira, D.; et al. The efficacy of a glial inhibitor, minocycline, for preventing persistent pain after lumbar discectomy: A randomized, double-blind, controlled study. Pain 2013, 154, 1197–1203. [Google Scholar] [CrossRef]
- Vanelderen, P.; Van Zundert, J.; Kozicz, T.; Puylaert, M.; De Vooght, P.; Mestrum, R.; Heylen, R.; Roubos, E.; Vissers, K. Effect of Minocycline on Lumbar Radicular Neuropathic Pain. Anesthesiology 2015, 122, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Curtin, C.M.; Kenney, D.; Suarez, P.; Hentz, V.R.; Hernandez-Boussard, T.; Mackey, S.; Carroll, I.R. A Double-Blind Placebo Randomized Controlled Trial of Minocycline to Reduce Pain After Carpal Tunnel and Trigger Finger Release. J. Hand Surg. 2017, 42, 166–174. [Google Scholar] [CrossRef]
- Sumitani, M.; Ueda, H.; Hozumi, J.; Inoue, R.; Kogure, T.; Ogata, T.; Yamada, Y. Minocycline Does Not Decrease Intensity of Neuropathic Pain, but Improves Its Affective Dimension. J. Pain Palliat. Care Pharmacother. 2015, 30. [Google Scholar] [CrossRef]
- Falk, S.; Al-Dihaissy, T.; Mezzanotte, L.; Heegaard, A.-M. Effect of sex in the MRMT-1 model of cancer-induced bone pain. F1000Research 2015, 4, 445. [Google Scholar] [CrossRef] [PubMed]
- Díaz-DelCastillo, M.; Christiansen, S.H.; Appel, C.K.; Falk, S.; Woldbye, D.P.; Heegaard, A.-M. Neuropeptide Y is Up-regulated and Induces Antinociception in Cancer-induced Bone Pain. Neuroscience 2018, 384, 111–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, W.J. Efficient Analysis of Experimental Observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-delCastillo, M.; Hansen, R.B.; Appel, C.K.; Nielsen, L.; Nielsen, S.N.; Karyniotakis, K.; Dahl, L.M.; Andreasen, R.B.; Heegaard, A.-M. Modulation of Rat Cancer-Induced Bone Pain is Independent of Spinal Microglia Activity. Cancers 2020, 12, 2740. https://doi.org/10.3390/cancers12102740
Diaz-delCastillo M, Hansen RB, Appel CK, Nielsen L, Nielsen SN, Karyniotakis K, Dahl LM, Andreasen RB, Heegaard A-M. Modulation of Rat Cancer-Induced Bone Pain is Independent of Spinal Microglia Activity. Cancers. 2020; 12(10):2740. https://doi.org/10.3390/cancers12102740
Chicago/Turabian StyleDiaz-delCastillo, Marta, Rie Bager Hansen, Camilla Kristine Appel, Lykke Nielsen, Sascha Nolsøe Nielsen, Konstantinos Karyniotakis, Louise M. Dahl, Rikke B. Andreasen, and Anne-Marie Heegaard. 2020. "Modulation of Rat Cancer-Induced Bone Pain is Independent of Spinal Microglia Activity" Cancers 12, no. 10: 2740. https://doi.org/10.3390/cancers12102740
APA StyleDiaz-delCastillo, M., Hansen, R. B., Appel, C. K., Nielsen, L., Nielsen, S. N., Karyniotakis, K., Dahl, L. M., Andreasen, R. B., & Heegaard, A.-M. (2020). Modulation of Rat Cancer-Induced Bone Pain is Independent of Spinal Microglia Activity. Cancers, 12(10), 2740. https://doi.org/10.3390/cancers12102740




