Radiotherapy-Mediated Immunomodulation and Anti-Tumor Abscopal Effect Combining Immune Checkpoint Blockade
Abstract
Simple Summary
Abstract
1. Introduction
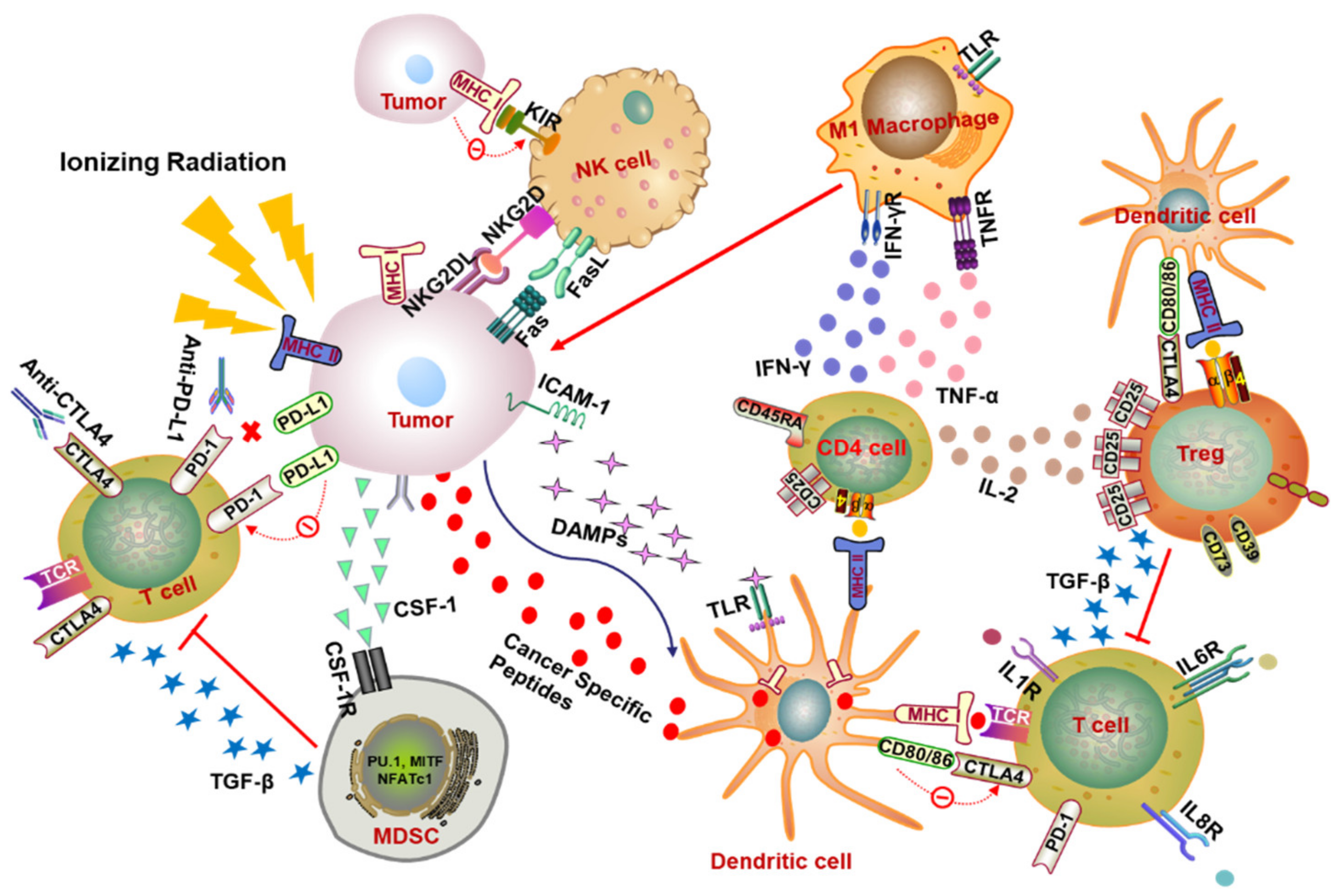
2. Modulation of Radiotherapy on Immune Cell Population in Tumor Microenvironment
2.1. T Lymphocytes
2.2. Nature Killer Cells
2.3. Macrophage
2.4. Myeloid-Derived Suppressor Cells
3. Impact of Radiotherapy Combined with Immune Checkpoint Blockade on Abscopal Effect
3.1. PD-1/PD-L1 Blockade
3.2. CTLA-4 Blockade
4. Potential Mechanism of Radiation-Induced Abscopal Effects
4.1. Radiation Increases the Antigen Presentation
4.2. Radiation-Induced Activation of the cGAS-STING Signaling
4.3. Modulation of Immune Checkpoints by Radiation-Induced Signaling
5. Clinical Outcome on Combined Therapy
6. Conclusions and Perspectives
Funding
Conflicts of Interest
Abbreviations
| RT | radiotherapy |
| IR | ionizing radiation |
| ICB | immune checkpoint blockade |
| MHC | major histocompatibility complex |
| APC | antigens of antigen presenting cell |
| DCs | dendritic cells |
| CTLs | cytotoxic T lymphocytes |
| Treg | regulatory cells |
| MDSCs | myeloid-derived suppressor cells |
| PD-1 | programmed cell death protein 1 |
| CTLA-4 | cytotoxic T-lymphocyte-associated antigen 4 |
| ICD | immunogenic cell death |
| DAMPs | damage-associated molecular patterns |
| HMGB1 | high-mobility group box 1 protein |
| ATP | adenosine triphosphate |
| TLR4 | toll-like receptor 4 |
| ICAM-1 | intercellular adhesion molecule 1 |
| Foxp3 | fork-head box protein 3 |
| NK cells | nature killer cells |
| MMPs | matrix metalloproteinases |
| TAMs | tumor-associated macrophages |
| IFN-γ | interferon-γ |
| TNF-α | tumor necrosis factor-α |
| LPS | lipopolysaccharide |
| NF-kB | nuclear factor-kB |
| CCL15 | chemokines C-C motif ligand 15 |
| CXCL10 | C-X-C motif ligand 10 |
| CCR7 | C-C motif chemokine receptor type 7 |
| CSF1 | colony stimulating factor 1 |
| VEGF | vascular endothelial growth factor |
| LDI | low-dose irradiation |
| SBRT | stereotactic body radiotherapy |
| TILs | tumor infiltrating lymphocytes |
| NKG2D | natural killer cell group 2D |
| NSCLC | non-small cell lung cancer |
| RCC | renal cell carcinoma |
| TCR | T cell receptor |
| cGAS | cyclic GMP-AMP synthase |
| STING | stimulator of interferon genes |
| IFN-I | type I interferon |
| ICIs | immune checkpoint inhibitors |
| GMC-SF | granulocyte-macrophage colony-stimulating factor |
| RAE-1 | retinoic acid early inducible protein–1 |
| Flt3-L | Fms-like tyrosine kinase receptor 3 ligand |
| Arg-1 | arginase-1 |
| JAK/STAT/IRF | Janus kinase/signal transducer and activator of transcription/interferon regulatory factor |
| DSB | double strand break |
| RIAE | radiation induced abscopal effect |
References
- Formenti, S.C.; Demaria, S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef]
- Herrera, F.G.; Bourhis, J.; Coukos, G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J. Clin. 2017, 67, 65–85. [Google Scholar] [CrossRef]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-pd-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, J.M.; Shinohara, E.; Summers, J.B.; Niermann, K.J.; Morimoto, A.; Brousal, J. The controversial abscopal effect. Cancer Treat. Rev. 2005, 31, 159–172. [Google Scholar] [CrossRef]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor microenvironment as a "game changer" in cancer radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, Z.S.; Wynne, J.; Nasti, T.H.; Zhu, S.; Mourad, W.F.; Yan, W.; Gupta, S.; Khleif, S.N.; Khan, M.K. Radiation, immune checkpoint blockade and the abscopal effect: A critical review on timing, dose and fractionation. Front. Oncol. 2018, 8, 612. [Google Scholar] [CrossRef]
- Thangamathesvaran, L.; Shah, R.; Verma, R.; Mahmoud, O. Immune checkpoint inhibitors and radiotherapy-concept and review of current literature. Ann. Transl. Med. 2018, 6, 155. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 104. [Google Scholar] [CrossRef]
- Gadducci, A.; Guerrieri, M.E. Immune checkpoint inhibitors in gynecological cancers: Update of literature and perspectives of clinical research. Anticancer Res. 2017, 37, 5955–5965. [Google Scholar] [CrossRef]
- Takeshima, T.; Chamoto, K.; Wakita, D.; Ohkuri, T.; Togashi, Y.; Shirato, H.; Kitamura, H.; Nishimura, T. Local radiation therapy inhibits tumor growth through the generation of tumor-specific ctl: Its potentiation by combination with th1 cell therapy. Cancer Res. 2010, 70, 2697–2706. [Google Scholar] [CrossRef]
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Wang, Y.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; et al. Therapeutic effects of ablative radiation on local tumor require cd8+ t cells: Changing strategies for cancer treatment. Blood 2009, 114, 589–595. [Google Scholar] [CrossRef]
- Lippitz, B.E.; Harris, R.A. A translational concept of immuno-radiobiology. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 140, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Mikhak, Z.; Strassner, J.P.; Luster, A.D. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor ccr4. J. Exp. Med. 2013, 210, 1855–1869. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, Y.; Matsubara, H.; Urashima, T.; Komatsu, A.; Sakata, H.; Nishimori, T.; Yoneyama, Y.; Hoshino, I.; Murakami, K.; Usui, A.; et al. Combination of direct intratumoral administration of dendritic cells and irradiation induces strong systemic antitumor effect mediated by grp94/gp96 against squamous cell carcinoma in mice. Int. J. Oncol. 2007, 31, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Chargari, C.; Marabelle, A.; Perfettini, J.L.; Magne, N.; Deutsch, E. Can immunostimulatory agents enhance the abscopal effect of radiotherapy? Eur. J. Cancer 2016, 62, 36–45. [Google Scholar] [CrossRef]
- Ozpiskin, O.M.; Zhang, L.; Li, J.J. Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics 2019, 9, 1215–1231. [Google Scholar] [CrossRef]
- Hu, Z.I.; McArthur, H.L.; Ho, A.Y. The abscopal effect of radiation therapy: What is it and how can we use it in breast cancer? Curr. Breast Cancer Rep. 2017, 9, 45–51. [Google Scholar] [CrossRef]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef]
- Matsumura, S.; Demaria, S. Up-regulation of the pro-inflammatory chemokine cxcl16 is a common response of tumor cells to ionizing radiation. Radiat. Res. 2010, 173, 418–425. [Google Scholar] [CrossRef]
- Chakraborty, M.; Abrams, S.I.; Camphausen, K.; Liu, K.; Scott, T.; Coleman, C.N.; Hodge, J.W. Irradiation of tumor cells up-regulates fas and enhances ctl lytic activity and ctl adoptive immunotherapy. J. Immunol. (Baltim. Md. 1950) 2003, 170, 6338–6347. [Google Scholar] [CrossRef]
- Garnett, C.T.; Palena, C.; Chakraborty, M.; Tsang, K.Y.; Schlom, J.; Hodge, J.W. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic t lymphocytes. Cancer Res. 2004, 64, 7985–7994. [Google Scholar] [CrossRef] [PubMed]
- Hallahan, D.; Kuchibhotla, J.; Wyble, C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996, 56, 5150–5155. [Google Scholar] [PubMed]
- Gao, H.; Dong, Z.; Gong, X.; Dong, J.; Zhang, Y.; Wei, W.; Wang, R.; Jin, S. Effects of various radiation doses on induced t-helper cell differentiation and related cytokine secretion. J. Radiat. Res. 2018, 59, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Adair, P.; Kim, Y.C.; Pratt, K.P.; Scott, D.W. Avidity of human t cell receptor engineered cd4(+) t cells drives t-helper differentiation fate. Cell. Immunol. 2016, 299, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Agnello, D.; Lankford, C.S.; Bream, J.; Morinobu, A.; Gadina, M.; O’Shea, J.J.; Frucht, D.M. Cytokines and transcription factors that regulate t helper cell differentiation: New players and new insights. J. Clin. Immunol. 2003, 23, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Brivio, F.; Fumagalli, L.; Messina, G.; Meregalli, S.; Porro, G.; Rovelli, F.; Vigorè, L.; Tisi, E.; D’Amico, G. Effects of the conventional antitumor therapies surgery, chemotherapy, radiotherapy and immunotherapy on regulatory t lymphocytes in cancer patients. Anticancer Res. 2009, 29, 1847–1852. [Google Scholar]
- Bos, P.D.; Plitas, G.; Rudra, D.; Lee, S.Y.; Rudensky, A.Y. Transient regulatory t cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J. Exp. Med. 2013, 210, 2435–2466. [Google Scholar] [CrossRef]
- Komatsu, N.; Hori, S. Full restoration of peripheral foxp3+ regulatory t cell pool by radioresistant host cells in scurfy bone marrow chimeras. Proc. Natl. Acad. Sci. USA 2007, 104, 8959–8964. [Google Scholar] [CrossRef]
- Muroyama, Y.; Nirschl, T.R.; Kochel, C.M.; Lopez-Bujanda, Z.; Theodros, D.; Mao, W.; Carrera-Haro, M.A.; Ghasemzadeh, A.; Marciscano, A.E.; Velarde, E.; et al. Stereotactic radiotherapy increases functionally suppressive regulatory t cells in the tumor microenvironment. Cancer Immunol. Res. 2017, 5, 992–1004. [Google Scholar] [CrossRef]
- Sharabi, A.B.; Nirschl, C.J.; Kochel, C.M.; Nirschl, T.R.; Francica, B.J.; Velarde, E.; Deweese, T.L.; Drake, C.G. Stereotactic radiation therapy augments antigen-specific pd-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol. Res. 2015, 3, 345–355. [Google Scholar] [CrossRef]
- Jones, E.; Dahm-Vicker, M.; Simon, A.K.; Green, A.; Powrie, F.; Cerundolo, V.; Gallimore, A. Depletion of cd25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002, 2, 1. [Google Scholar] [PubMed]
- Li, J.; Hu, P.; Khawli, L.A.; Epstein, A.L. Complete regression of experimental solid tumors by combination lec/chtnt-3 immunotherapy and cd25(+) t-cell depletion. Cancer Res. 2003, 63, 8384–8392. [Google Scholar] [PubMed]
- Wirsdörfer, F.; Cappuccini, F.; Niazman, M.; de Leve, S.; Westendorf, A.M.; Lüdemann, L.; Stuschke, M.; Jendrossek, V. Thorax irradiation triggers a local and systemic accumulation of immunosuppressive cd4+ foxp3+ regulatory t cells. Radiat. Oncol. 2014, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Makidono, R. A mechanism involved in augmentation of anti-tumor immunity by irradiation to the tumor: Tumor irradiation promoted generation of cd4+helper t cells against a murine immunogenic tumor. Nihon Igaku Hoshasen Gakkai Zasshi. Nippon Acta Radiol. 1996, 56, 575–578. [Google Scholar]
- Liu, C.; Hu, Q.; Xu, B.; Hu, X.; Su, H.; Li, Q.; Zhang, X.; Yue, J.; Yu, J. Peripheral memory and naïve t cells in non-small cell lung cancer patients with lung metastases undergoing stereotactic body radiotherapy: Predictors of early tumor response. Cancer Cell Int. 2019, 19, 121. [Google Scholar] [CrossRef]
- Wani, S.Q.; Dar, I.A.; Khan, T.; Lone, M.M.; Afroz, F. Radiation therapy and its effects beyond the primary target: An abscopal effect. Cureus 2019, 11, e4100. [Google Scholar] [CrossRef]
- Hodgins, J.J.; Khan, S.T.; Park, M.M.; Auer, R.C.; Ardolino, M. Killers 2.0: Nk cell therapies at the forefront of cancer control. J. Clin. Investig. 2019, 129, 3499–3510. [Google Scholar] [CrossRef]
- Wennerberg, E.; Vanpouille-Box, C.; Bornstein, S.; Yamazaki, T.; Demaria, S.; Galluzzi, L. Immune recognition of irradiated cancer cells. Immunol. Rev. 2017, 280, 220–230. [Google Scholar] [CrossRef]
- Heo, W.; Lee, Y.S.; Son, C.H.; Yang, K.; Park, Y.S.; Bae, J. Radiation-induced matrix metalloproteinases limit natural killer cell-mediated anticancer immunity in nci-h23 lung cancer cells. Mol. Med. Rep. 2015, 11, 1800–1806. [Google Scholar] [CrossRef][Green Version]
- Walton, E.L. Radiotherapy and the tumor microenvironment: The "macro" picture. Biomed. J. 2017, 40, 185–188. [Google Scholar] [CrossRef]
- Jones, K.I.; Tiersma, J.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Buzzelli, J.; Im, J.H.; Muschel, R.J. Radiation combined with macrophage depletion promotes adaptive immunity and potentiates checkpoint blockade. EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Allouch, A.; Martins, I.; Modjtahedi, N.; Deutsch, E.; Perfettini, J.L. Macrophage biology plays a central role during ionizing radiation-elicited tumor response. Biomed. J. 2017, 40, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Shiao, S.L. The role of macrophage phenotype in regulating the response to radiation therapy. Transl. Res. J. Lab. Clin. Med. 2018, 191, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The m1 and m2 paradigm of macrophage activation: Time for reassessment. F1000prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Dinh, H.; Cook, A.D.; Hertzog, P.J.; Hamilton, J.A. Gm-csf- and m-csf-dependent macrophage phenotypes display differential dependence on type i interferon signaling. J. Leukoc. Biol. 2009, 86, 411–421. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-dose irradiation programs macrophage differentiation to an inos+/m1 phenotype that orchestrates effective t cell immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef]
- Okubo, M.; Kioi, M.; Nakashima, H.; Sugiura, K.; Mitsudo, K.; Aoki, I.; Taniguchi, H.; Tohnai, I. M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci. Rep. 2016, 6, 27548. [Google Scholar] [CrossRef]
- Chiang, C.S.; Fu, S.Y.; Wang, S.C.; Yu, C.F.; Chen, F.H.; Lin, C.M.; Hong, J.H. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front. Oncol. 2012, 2, 89. [Google Scholar] [CrossRef]
- Gough, M.J.; Young, K.; Crittenden, M. The impact of the myeloid response to radiation therapy. Clin. Dev. Immunol. 2013, 2013, 281958. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, M.R.; Cottam, B.; Savage, T.; Nguyen, C.; Newell, P.; Gough, M.J. Expression of nf-κb p50 in tumor stroma limits the control of tumors by radiation therapy. PLoS ONE 2012, 7, e39295. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.O.; Tseng, D.; Liao, C.H.; Dorie, M.J.; Czechowicz, A.; Brown, J.M. Inhibition of mac-1 (cd11b/cd18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc. Natl. Acad. Sci. USA 2010, 107, 8363–8368. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Li, C. Myeloid-derived suppressor cells: Roles in the tumor microenvironment and tumor radiotherapy. Int. J. Cancer 2019, 144, 933–946. [Google Scholar] [CrossRef]
- Brooks-Kaiser, J.C.; Bourque, L.A.; Hoskin, D.W. Heterogeneity of splenic natural suppressor cells induced in mice by treatment with cyclophosphamide. Immunopharmacology 1993, 25, 117–129. [Google Scholar] [CrossRef]
- Vatner, R.E.; Formenti, S.C. Myeloid-derived cells in tumors: Effects of radiation. Semin. Radiat. Oncol. 2015, 25, 18–27. [Google Scholar] [CrossRef]
- Xu, J.; Escamilla, J.; Mok, S.; David, J.; Priceman, S.; West, B.; Bollag, G.; McBride, W.; Wu, L. Csf1r signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013, 73, 2782–2794. [Google Scholar] [CrossRef]
- Chen, H.M.; Ma, G.; Gildener-Leapman, N.; Eisenstein, S.; Coakley, B.A.; Ozao, J.; Mandeli, J.; Divino, C.; Schwartz, M.; Sung, M.; et al. Myeloid-derived suppressor cells as an immune parameter in patients with concurrent sunitinib and stereotactic body radiotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 4073–4085. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.X. Irradiation and anti-pd-l1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Xu, H.; Liu, Q.; Zhao, W.; Han, X.; Wu, K. Biomarkers for predicting efficacy of pd-1/pd-l1 inhibitors. Mol. Cancer 2018, 17, 129. [Google Scholar] [CrossRef]
- Mayoux, M.; Roller, A.; Pulko, V.; Sammicheli, S. Dendritic cells dictate responses to pd-l1 blockade cancer immunotherapy. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G.; Lee, Y.C.; Luh, F.; Kuo, C.N.; Chang, W.C.; Yen, Y. Development and clinical applications of cancer immunotherapy against pd-1 signaling pathway. J. Biomical Sci. 2019, 26, 96. [Google Scholar] [CrossRef]
- Sunshine, J.; Taube, J.M. Pd-1/pd-l1 inhibitors. Curr. Opin. Pharmacol. 2015, 23, 32–38. [Google Scholar] [CrossRef]
- Park, S.S.; Dong, H.; Liu, X.; Harrington, S.M.; Krco, C.J.; Grams, M.P.; Mansfield, A.S.; Furutani, K.M.; Olivier, K.R.; Kwon, E.D. Pd-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol. Res. 2015, 3, 610–619. [Google Scholar] [CrossRef]
- Pfannenstiel, L.W.; McNeilly, C.; Xiang, C.; Kang, K.; Diaz-Montero, C.M.; Yu, J.S.; Gastman, B.R. Combination pd-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma. Oncoimmunology 2019, 8, e1507669. [Google Scholar] [CrossRef]
- Theelen, W.S.; de Jong, M.C.; Baas, P. Synergizing systemic responses by combining immunotherapy with radiotherapy in metastatic non-small cell lung cancer: The potential of the abscopal effect. Lung Cancer 2020, 142, 106–113. [Google Scholar] [CrossRef]
- Ene, C.I.; Kreuser, S.A.; Jung, M.; Zhang, H.; Arora, S.; White Moyes, K.; Szulzewsky, F.; Barber, J.; Cimino, P.J.; Wirsching, H.G.; et al. Anti-pd-l1 antibody direct activation of macrophages contributes to a radiation-induced abscopal response in glioblastoma. Neuro-Oncology 2020, 22, 639–651. [Google Scholar] [CrossRef]
- Ye, J.C.; Formenti, S.C. Integration of radiation and immunotherapy in breast cancer—Treatment implications. Breast 2018, 38, 66–74. [Google Scholar] [CrossRef]
- Dudzinski, S.O.; Cameron, B.D.; Wang, J.; Rathmell, J.C.; Giorgio, T.D.; Kirschner, A.N. Combination immunotherapy and radiotherapy causes an abscopal treatment response in a mouse model of castration resistant prostate cancer. J. Immunother. Cancer 2019, 7, 218. [Google Scholar] [CrossRef]
- Xia, L.; Wu, H.; Qian, W. Irradiation enhanced the effects of pd-1 blockade in brain metastatic osteosarcoma. J. Bone Oncol. 2018, 12, 61–64. [Google Scholar] [CrossRef]
- Trommer, M.; Yeo, S.Y.; Persigehl, T.; Bunck, A.; Grüll, H.; Schlaak, M.; Theurich, S.; von Bergwelt-Baildon, M.; Morgenthaler, J.; Herter, J.M.; et al. Abscopal effects in radio-immunotherapy-response analysis of metastatic cancer patients with progressive disease under anti-pd-1 immune checkpoint inhibition. Front. Pharmacol. 2019, 10, 511. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent pd-l1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Cheadle, E.J.; Popple, A.L.; Poon, E.; Morrow, M.; Stewart, R.; Yusko, E.C.; Sanders, C.M.; Vignali, M.; Emerson, R.O.; et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal t-cell populations when combined with pd-1 blockade. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 5514–5526. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Chikuma, S. Ctla-4, an essential immune-checkpoint for t-cell activation. Curr. Top. Microbiol. Immunol. 2017, 410, 99–126. [Google Scholar] [CrossRef]
- Rowshanravan, B.; Halliday, N. Ctla-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Collins, A.V.; Brodie, D.W.; Gilbert, R.J.; Iaboni, A.; Manso-Sancho, R.; Walse, B.; Stuart, D.I.; van der Merwe, P.A.; Davis, S.J. The interaction properties of costimulatory molecules revisited. Immunity 2002, 17, 201–210. [Google Scholar] [CrossRef]
- Demaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.L.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-mediated inhibition of metastases after treatment with local radiation and ctla-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 728–734. [Google Scholar]
- Matsumura, S.; Wang, B.; Kawashima, N.; Braunstein, S.; Badura, M.; Cameron, T.O.; Babb, J.S.; Schneider, R.J.; Formenti, S.C.; Dustin, M.L.; et al. Radiation-induced cxcl16 release by breast cancer cells attracts effector t cells. J. Immunol. (Baltim. Md. 1950) 2008, 181, 3099–3107. [Google Scholar] [CrossRef]
- Golden, E.B.; Demaria, S.; Schiff, P.B.; Chachoua, A.; Formenti, S.C. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol. Res. 2013, 1, 365–372. [Google Scholar] [CrossRef]
- Ruocco, M.G.; Pilones, K.A.; Kawashima, N.; Cammer, M.; Huang, J.; Babb, J.S.; Liu, M.; Formenti, S.C.; Dustin, M.L.; Demaria, S. Suppressing T cell motility induced by anti-ctla-4 monotherapy improves antitumor effects. J. Clin. Investig. 2012, 122, 3718–3730. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Terabe, M.; Mamura, M.; Kang, M.J.; Chae, H.; Stuelten, C.; Kohn, E.; Tang, B.; Sabzevari, H.; Anver, M.R.; et al. An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008, 68, 3835–3843. [Google Scholar] [CrossRef] [PubMed]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, K.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef]
- Mouw, K.W.; Goldberg, M.S.; Konstantinopoulos, P.A.; D’Andrea, A.D. DNA damage and repair biomarkers of immunotherapy response. Cancer Discov. 2017, 7, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N. Mismatch repair deficiency predicts response of solid tumors to pd-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; de Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances mhc class i expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.G.; Yuan, H.; Deng, W.; Li, J.; Huang, Y. The reciprocity between radiotherapy and cancer immunotherapy. Clin. Cancer Res. 2019, 25, 1709–1717. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Formenti, S.C.; Demaria, S. Toward precision radiotherapy for use with immune checkpoint blockers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 259–265. [Google Scholar] [CrossRef]
- Sato, H.; Okonogi, N.; Nakano, T. Rationale of combination of anti-pd-1/pd-l1 antibody therapy and radiotherapy for cancer treatment. Int. J. Clin. Oncol. 2020, 25, 801–809. [Google Scholar] [CrossRef]
- Samstein, R.M.; Riaz, N. The DNA damage response in immunotherapy and radiation. Adv. Radiat. Oncol. 2018, 3, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. Sting-dependent cytosolic DNA sensing promotes radiation-induced type i interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yi, M.; Qin, S.; Song, Y.; Chu, Q.; Wu, K. Activating cgas-sting pathway for the optimal effect of cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 35. [Google Scholar] [CrossRef]
- McKelvey, K.J.; Hudson, A.L. Radiation, inflammation and the immune response in cancer. Mamm. Genome 2018, 29, 843–865. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, S.; Chen, X.; Shi, H.; Chen, C.; Sun, L.; Chen, Z.J. Cgas is essential for the antitumor effect of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2017, 114, 1637–1642. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J. DNA exonuclease trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon receptor signaling pathways regulating pd-l1 and pd-l2 expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef]
- Sato, H.; Niimi, A.; Yasuhara, T. DNA double-strand break repair pathway regulates pd-l1 expression in cancer cells. Nat. Commun. 2017, 8, 1751. [Google Scholar] [CrossRef]
- Iijima, M.; Okonogi, N. Significance of pd-l1 expression in carbon-ion radiotherapy for uterine cervical adeno/adenosquamous carcinoma. J. Gynecol. Oncol. 2020, 31, e19. [Google Scholar] [CrossRef]
- Zhang, N.; Zeng, Y.; Du, W.; Zhu, J.; Shen, D.; Liu, Z.; Huang, J.A. The egfr pathway is involved in the regulation of pd-l1 expression via the il-6/jak/stat3 signaling pathway in egfr-mutated non-small cell lung cancer. Int. J. Oncol. 2016, 49, 1360–1368. [Google Scholar] [CrossRef]
- Tsui, J.M.; Mihalcioiu, C.; Cury, F.L. Abscopal effect in a stage iv melanoma patient who progressed on pembrolizumab. Cureus 2018, 10, e2238. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, T.; Rordorf, T.; Ikenberg, K.; Huber, G.F.; Guckenberger, M.; Garcia Schueler, H.I. Radiotherapy-induced anti-tumor immune response and immune-related adverse events in a case of recurrent nasopharyngeal carcinoma undergoing anti-pd-1 immunotherapy. BMC Cancer 2018, 18, 395. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Ishiwata, Y.; Nakagawa, K.; Yokochi, S.; Taruki, C.; Akuta, T.; Ohtomo, K.; Matsushima, K.; Tamatani, T.; Kanegasaki, S. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by eci301, an active variant of macrophage inflammatory protein-1alpha. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Kusmartsev, S.; Gabrilovich, D.I. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol. Immunother. 2002, 51, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr. Opin. Immunol. 2010, 22, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, A.A.; Watkins, S.K. Immune suppression in the tumor microenvironment: A role for dendritic cell-mediated tolerization of t cells. Cancer Immunol. Immunother. 2012, 61, 289–293. [Google Scholar] [CrossRef]
- Michelle Xu, M.; Pu, Y.; Weichselbaum, R.R.; Fu, Y.X. Integrating conventional and antibody-based targeted anticancer treatment into immunotherapy. Oncogene 2017, 36, 585–592. [Google Scholar] [CrossRef][Green Version]
- Marshall, H.T.; Djamgoz, M.B.A. Immuno-oncology: Emerging targets and combination therapies. Front. Oncol. 2018, 8, 315. [Google Scholar] [CrossRef]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of lag-3. Cell 2019, 176, 334–347.e312. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J. Blockade of the checkpoint receptor tigit prevents nk cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Yang, M.; Turner, A.; Xu, C.; Ferris, R.L.; Huang, J.; Kane, L.P.; Lu, B. Tim-3 as a target for cancer immunotherapy and mechanisms of action. Int. J. Mol. Sci. 2017, 18, 645. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Brunetti, O. Strategies to improve cancer immune checkpoint inhibitors efficacy, other than abscopal effect: A systematic review. Cancers 2019, 11, 539. [Google Scholar] [CrossRef] [PubMed]

| Inhibited Checkpoint | Tumor Type | RT Location | Treatment | Systemic Effects + Key Mediator |
|---|---|---|---|---|
| Preclinical Mouse Models | ||||
| PD-1 | Renal carcinoma, metastatic mammary carcinoma (4T1) | right hindlimb | SABR (15 Gy) of primary tumor + anti-PD-1 mAb, i.p. | Size reduction of primary renal tumor and distal renal tumor, but not distal breast tumor, ↑CD8 + CTLs [64] |
| Melanoma (D4M) | head | RT (2 Gy × 4) of primary tumor + anti-PD-1 mAb, i.p. | Growth-inhibition of irradiated and non-irradiated tumor, ↑CD8+ CTLs, ↓CD4 + Treg [65] | |
| CTLA-4 | Metastatic mammary carcinoma (4T1) | right flank | RT (12 Gy × 2) of primary tumor + anti-CTLA-4 mAb, i.p (3×) | Inhibition of lung metastases, increased survival, ↑CD8+ CTLs [78] |
| right flank | RT (12 Gy × 2) of primary tumor + anti-CTLA-4 mAb, i.p. (3×) | Regression of primary tumor and metastases, ↑CD8 + CTLs [79] | ||
| PD-L1 and CTLA-4 | Melanoma (B16) | right flank | RT (20 Gy) of primary tumor + anti-CTLA-4 mAb + anti-PD-L1 mAb, i.p. (3×) | Regression of primary tumor and metastases and diversification of the T cell receptor (TCR) of TILs from unirradiated tumors, ↑CD8+ CTLs, ↓CD4+ Treg [83] |
| Breast cancer (TSA breast cancer cell) | right flank | RT (8 Gy × 3) of primary tumor + anti-CTLA-4 mAb + anti-PD-L1 mAb, i.p. (3×) | ||
| Pancreatic cancer (PDA.4662) | right flank | RT (20 Gy) of primary tumor + anti-CTLA-4 mAb + anti-PD-L1 mAb, i.p. (3×) | ||
| Clinical Studies | ||||
| PD-1 | Melanoma, NSCLC, RCC, head and neck cancer (n = 24) | primary tumor | RT + pembrolizumab or pembrolizumab | 29% (n = 7) patients showing the abscopal effect, 43% in melanoma, 43% in NSCLC, 14% in renal cell carcinoma [71] |
| CTLA-4 | NSCLC (n = 1) | hepatic metastases | RT (30 Gy in 5 fractions) + 3 cycles of ipilimumab (once in 3 weeks) | Liver, lung, bone, lymph nodes, and other non-radiated metastatic lesions subsided, improved survival [80] |
| Case | Sex/Age | Primary Tumor | First Treatment | Systemic Effects | Second Treatment | Systemic Effects |
|---|---|---|---|---|---|---|
| 1 | Female, 65 | Metastatic melanoma | IMRT (50 Gy in 20 fractions) in right buccal mucosa | Metastatic melanoma found in neck and lungs after 7 moths | IMRT (24 Gy in 3 fractions) in neck + 4 cycles of pembrolizumab (once in 3 weeks) (3 mg/kg) | The neck tumor shrank and the number of lung metastases decreased [101] |
| 2 | Female, 33 | Metastatic melanoma | 4 cycles of ipilimumab (once in 3 weeks) (10 mg/kg) | Pleural-based paraspinal mass and new splenic lesions gradually increased | RT (28.5 Gy in 3 fractions) in paraspinal + regular ipilimumab administration for 2 months | The lesions in the target irradiation field and outside of the irradiation site (right hilar lymphadenopathy and splenic lesions) regressed [102] |
| 3 | Male, 46 | Advanced non-keratotic nasopharyngeal carcinoma | IMRT (70 Gy in 2 fractions) in cavernous sinus and 54 Gy in metastatic lymph nodes of the neck + chemotherapy (cisplatin and fluorouracil) | Bone metastasis occurred | RT (45 Gy in 25 fractions) to bony metastasis + continuing pembrolizumab | All tumorous lesions regressed significantly, inflammation symptoms resolved, and there was local control without metastasis [103] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Shao, C. Radiotherapy-Mediated Immunomodulation and Anti-Tumor Abscopal Effect Combining Immune Checkpoint Blockade. Cancers 2020, 12, 2762. https://doi.org/10.3390/cancers12102762
Zhao X, Shao C. Radiotherapy-Mediated Immunomodulation and Anti-Tumor Abscopal Effect Combining Immune Checkpoint Blockade. Cancers. 2020; 12(10):2762. https://doi.org/10.3390/cancers12102762
Chicago/Turabian StyleZhao, Xinrui, and Chunlin Shao. 2020. "Radiotherapy-Mediated Immunomodulation and Anti-Tumor Abscopal Effect Combining Immune Checkpoint Blockade" Cancers 12, no. 10: 2762. https://doi.org/10.3390/cancers12102762
APA StyleZhao, X., & Shao, C. (2020). Radiotherapy-Mediated Immunomodulation and Anti-Tumor Abscopal Effect Combining Immune Checkpoint Blockade. Cancers, 12(10), 2762. https://doi.org/10.3390/cancers12102762






