Prognostic Role of Serum Cytokeratin-19 Fragment (CYFRA 21-1) in Patients with Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
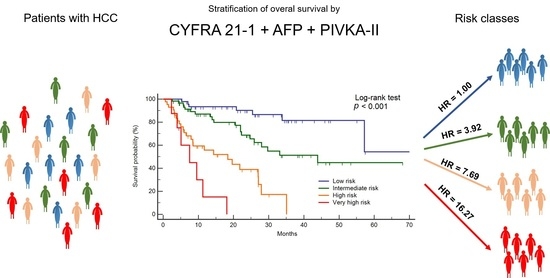
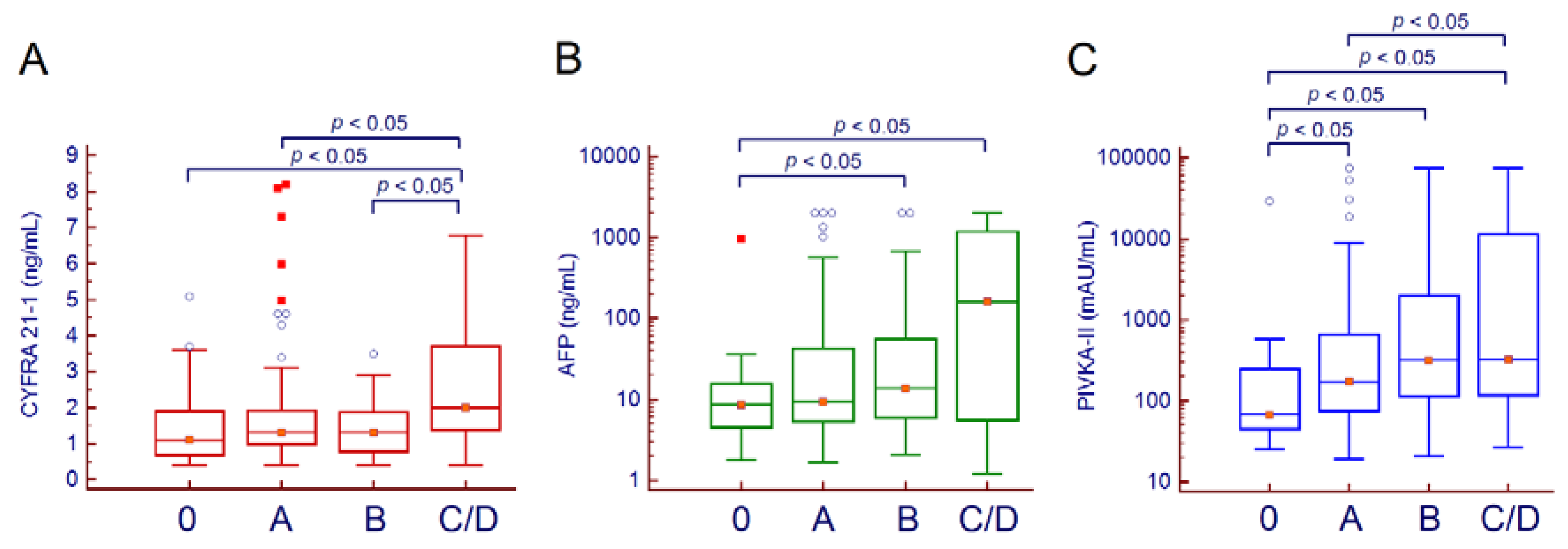
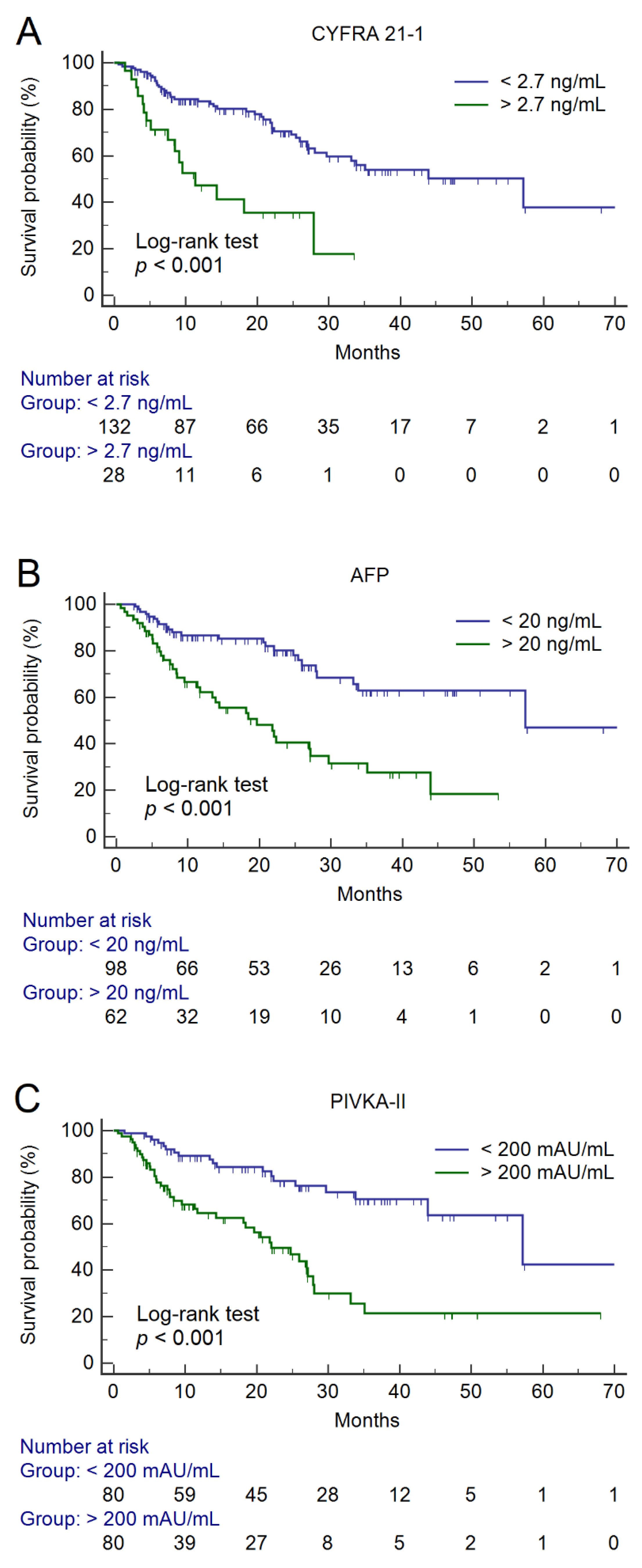
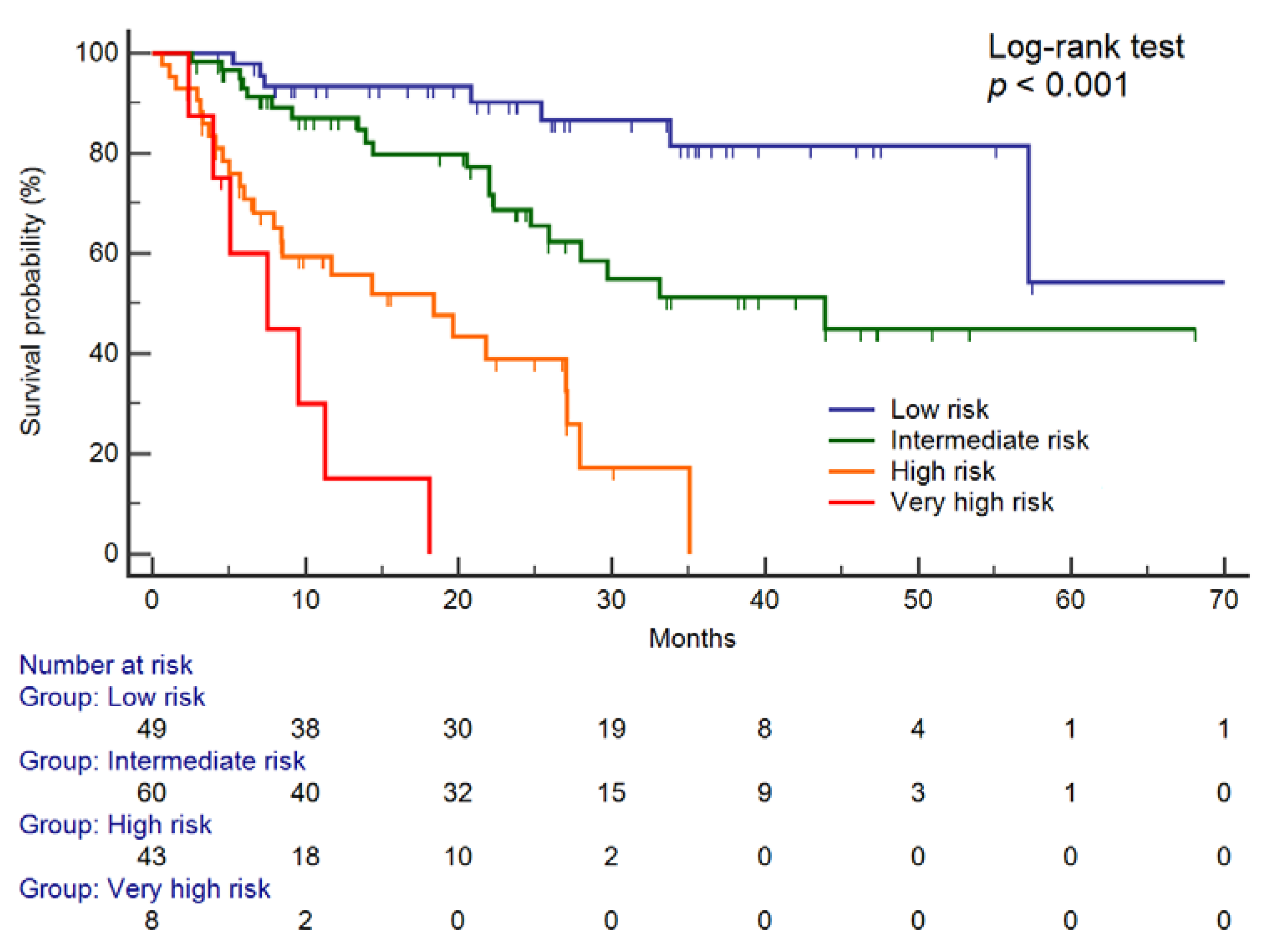
2. Results
Prediction of Overall Survival
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Measurement of Serum CYFRA 21-1, AFP, and PIVKA-II
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Global Cancer Observatory-IARC. Available online: https://gco.iarc.fr/today (accessed on 11 June 2019).
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [PubMed]
- Caviglia, G.P.; Rosso, C.; Fagoonee, S.; Saracco, G.M.; Pellicano, R. Liver fibrosis: The 2017 state of art. Panminerva Med. 2017, 59, 320–331. [Google Scholar] [PubMed]
- Sangiovanni, A.; Prati, G.M.; Fasani, P.; Ronchi, G.; Romeo, R.; Manini, M.; Del Ninno, E.; Morabito, A.; Colombo, M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology 2006, 43, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 model for analysis of competing risks of death following liver transplantation for hepatocellular carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Chon, C.Y.; Han, K.H.; Seong, J. Clinical usefulness of double biomarkers AFP and PIVKA-II for subdividing prognostic groups in locally advanced hepatocellular carcinoma. Liver Int. 2014, 34, 313–321. [Google Scholar] [CrossRef]
- Kawai, T.; Yasuchika, K.; Ishii, T.; Katayama, H.; Yoshitoshi, E.Y.; Ogiso, S.; Kita, S.; Yasuda, K.; Fukumitsu, K.; Mizumoto, M.; et al. Keratin 19, a Cancer Stem Cell Marker in Human Hepatocellular Carcinoma. Clin. Cancer Res. 2015, 21, 3081–3091. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Yasuchika, K.; Ishii, T.; Katayama, H.; Yoshitoshi, E.Y.; Ogiso, S.; Minami, T.; Miyauchi, Y.; Kojima, H.; Yamaoka, R.; et al. Identification of keratin 19-positive cancer stem cells associating human hepatocellular carcinoma using CYFRA 21-1. Cancer Med. 2017, 6, 2531–2540. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Xu, L.; Qiu, M.; Wang, J.; Zhou, Q.; Xu, L.; Wang, J.; Yin, R. Prognostic value of serum cytokeratin 19 fragments (Cyfra 21-1) in patients with non-small cell lung cancer. Sci. Rep. 2015, 5, 9444. [Google Scholar] [CrossRef]
- Ding, S.J.; Li, Y.; Tan, Y.X.; Jiang, M.R.; Tian, B.; Liu, Y.K.; Shao, X.X.; Ye, S.L.; Wu, J.R.; Zeng, R.; et al. From proteomic analysis to clinical significance: Overexpression of cytokeratin 19 correlates with hepatocellular carcinoma metastasis. Mol. Cell. Proteom. 2004, 3, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Toh, H.C.; Chen, P.J.; Carr, B.I.; Knox, J.J.; Gill, S.; Ansell, P.; McKeegan, E.M.; Dowell, B.; Pedersen, M.; Qin, Q.; et al. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer 2013, 119, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Galle, P.R.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Zhang, X.; Liu, J. Prognostic value of des-γ-carboxy prothrombin in patients with hepatocellular carcinoma treated with transarterial chemotherapy: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0225170. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.S.; Zhang, C.; Chen, P.; Jin, S.J.; Jiang, G.Q. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci. Rep. 2017, 7, 12870. [Google Scholar] [CrossRef]
- Payancé, A.; Dioguardi Burgio, M.; Peoc’h, K.; Achahboun, M.; Albuquerque, M.; Devictor, J.; Chor, H.; Manceau, H.; Soubrane, O.; Durand, F.; et al. Biological response under treatment and prognostic value of protein induced by vitamin K absence or antagonist-II in a French cohort of patients with hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2019. [Google Scholar] [CrossRef]
- Ryu, T.; Takami, Y.; Wada, Y.; Tateishi, M.; Matsushima, H.; Mikagi, K.; Saitsu, H. Double- and Triple-Positive Tumor Markers Predict Early Recurrence and Poor Survival in Patients with Hepatocellular Carcinoma within the Milan Criteria and Child-Pugh Class, A.J. Gastrointest. Surg. 2017, 21, 957–966. [Google Scholar] [CrossRef]
- Nitta, H.; Nakagawa, S.; Kaida, T.; Arima, K.; Higashi, T.; Taki, K.; Okabe, H.; Hayashi, H.; Hashimoto, D.; Chikamoto, A.; et al. Pre-treatment double- or triple-positive tumor markers are predictive of a poor outcome for patients undergoing radiofrequency ablation for hepatocellular carcinoma. Surg. Today 2017, 47, 375–384. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Touscoz, G.A.; Smedile, A.; Pellicano, R. Noninvasive assessment of liver fibrosis: Key messages for clinicians. Pol. Arch. Med. Wewn. 2014, 124, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Gaia, S.; Campion, D.; Evangelista, A.; Spandre, M.; Cosso, L.; Brunello, F.; Ciccone, G.; Bugianesi, E.; Rizzetto, M. Non-invasive score system for fibrosis in chronic hepatitis: Proposal for a model based on biochemical, FibroScan and ultrasound data. Liver Int. 2015, 35, 2027–2035. [Google Scholar] [CrossRef]
- Matsui, O.; Kobayashi, S.; Sanada, J.; Kouda, W.; Ryu, Y.; Kozaka, K.; Kitao, A.; Nakamura, K.; Gabata, T. Hepatocellular nodules in liver cirrhosis: Hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom. Imaging 2011, 36, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caviglia, G.P.; Abate, M.L.; Gaia, S.; Petrini, E.; Bosco, C.; Olivero, A.; Rosso, C.; Ciancio, A.; Pellicano, R.; Saracco, G.M.; et al. Risk of hepatocellular carcinoma in HBV cirrhotic patients assessed by the combination of miR-122, AFP and PIVKA-II. Panminerva Med. 2017, 59, 283–289. [Google Scholar] [PubMed]
- Silva, J.P.; Gorman, R.A.; Berger, N.G.; Tsai, S.; Christians, K.K.; Clarke, C.N.; Mogal, H.; Gamblin, T.C. The prognostic utility of baseline alpha-fetoprotein for hepatocellular carcinoma patients. J. Surg. Oncol. 2017, 116, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Gurakar, A.; Ma, M.; Garonzik-Wang, J.; Kim, A.; Anders, R.A.; Oshima, K.; Georgiades, A.; Gurakar, M.; Ottmann, S.; Cameron, A.M.; et al. Clinicopathological distinction of low-AFP-secreting vs. High-AFP-secreting hepatocellular carcinoma. Ann. Hepatol. 2018, 17, 1052–1066. [Google Scholar] [CrossRef]
- Kim, J.M.; Hyuck, C.; Kwon, D.; Joh, J.W.; Lee, J.H.; Paik, S.W.; Park, C.K. Protein induced by vitamin K antagonist-II (PIVKA-II) is a reliable prognostic factor in small hepatocellular carcinoma. World J. Surg. 2013, 37, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Tan, Z.; Xiang, X.; Dan, Y.; Deng, G. Effectiveness of PIVKA-II in the detection of hepatocellular carcinoma based on real-world clinical data. BMC Cancer 2017, 17, 608. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Patients (n = 160) |
|---|---|
| Age, years (median and range) | 62 (44–86) |
| Gender, M/F | 132/28 |
| BMI, Kg/m2 (median, 95% CI) | 25.9 (25.3–26.5) |
| Smoking (n, %) | |
| Current | 52 (32.5%) |
| Ex | 60 (37.5%) |
| No | 48 (30.0%) |
| Alcohol consumption *(n, %) | 25 (15.6%) |
| Etiology (n, %) | |
| HCV | 98 (61.3%) |
| HBV | 20 (12.5%) |
| Non-viral | 42 (26.2%) |
| Child-Pugh Score (n, %) | |
| A | 119 (74.4%) |
| B | 37 (23.1%) |
| C | 4 (2.5%) |
| Esophageal Varices (n, %) | |
| No | 99 (61.9%) |
| F1 | 24 (15.0%) |
| F2 | 33 (20.6%) |
| F3 | 4 (2.5%) |
| Ascites (n, %) | 37 (23.1%) |
| ALT, IU/L (median, 95% CI) | 43 (37–49) |
| AST, IU/L (median, 95% CI) | 54 (45–59) |
| Haemoglobin, g/dL (median, 95% CI) | 13.7 (13.2–13.9) |
| Platelet count, ×109/L (median, 95% CI) | 115 (106–127) |
| Albumin, g/dL (median, 95% CI) | 4.0 (3.8–4.1) |
| INR (median, 95% CI) | 1.15 (1.12–1.16) |
| Total Bilirubin, mg/dL (median 95% CI) | 1.0 (0.9–1.1) |
| Creatinin, mg/dL (median 95% CI) | 0.82 (0.77–0.87) |
| BCLC staging (n, %) | |
| 0 | 18 (11.3%) |
| A | 77 (48.1%) |
| B | 39 (24.4%) |
| C | 23 (14.4%) |
| D | 3 (1.9%) |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR, 95% CI | p Value | HR, 95% CI | p Value | |
| Age, years | 1.01, 0.99–1.05 | 0.246 | // | // |
| Gender, M | 1.52, 0.75–3.10 | 0.248 | // | // |
| BMI, Kg/m2 | 1.06, 0.99–1.14 | 0.092 | // | // |
| Current smoking | 1.38, 0.80–2.41 | 0.250 | // | // |
| Alcohol consumption * | 1.03, 0.55–1.95 | 0.923 | // | // |
| Etiology, non-viral | 0.98, 0.56–1.74 | 0.956 | // | // |
| Child-Pugh Score, A | 0.48, 0.27–0.84 | 0.010 | 0.96, 0.56–1.64 | 0.888 |
| Esophageal Varices, absent | 0.62, 0.36–1.06 | 0.081 | // | // |
| ALT, IU/L | 1.00, 0.99–1.01 | 0.594 | // | // |
| AST, IU/L | 1.01, 1.00–1.01 | 0.012 | 1.00, 0.99–1.01 | 0.247 |
| Haemoglobin, g/dL | 0.98, 0.79–1.01 | 0.061 | // | // |
| Platelet count, ×109/L | 0.99, 0.99–1.00 | 0.600 | // | // |
| Creatinin, mg/dL | 1.13, 0.71–1.80 | 0.598 | // | // |
| BCLC staging | 2.10, 1.57–2.81 | <0.001 | 1.60, 1.16–2.22 | 0.004 |
| Radiological response | 0.11, 0.04–0.26 | <0.001 | 0.16, 0.06–0.45 | 0.001 |
| CYFRA 21-1 > 2.7 ng/mL | 3.27, 1.81–5.93 | <0.001 | 3.39, 1.76–6.52 | <0.001 |
| AFP > 20 ng/mL | 3.17, 1.87–5.38 | <0.001 | 2.27, 1.25–4.13 | 0.007 |
| PIVKA-II > 200 mAU/mL | 3.40, 1.95–5.91 | <0.001 | 2.17, 1.13–4.17 | 0.020 |
| Risk Category | Events | Cumulative | HR, 95% CI * | p Value |
|---|---|---|---|---|
| Low | 8/49 | 16.3% | 1.00 | reference |
| Intermediate | 20/60 | 33.3% | 3.92, 1.54–9.99 | 0.004 |
| High | 25/43 | 58.1% | 7.69, 3.09–19.12 | <0.001 |
| Very high | 7/8 | 87.5% | 16.27, 5.14–51.50 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caviglia, G.P.; Ciruolo, M.; Olivero, A.; Carucci, P.; Rolle, E.; Rosso, C.; Abate, M.L.; Risso, A.; Ribaldone, D.G.; Tandoi, F.; et al. Prognostic Role of Serum Cytokeratin-19 Fragment (CYFRA 21-1) in Patients with Hepatocellular Carcinoma. Cancers 2020, 12, 2776. https://doi.org/10.3390/cancers12102776
Caviglia GP, Ciruolo M, Olivero A, Carucci P, Rolle E, Rosso C, Abate ML, Risso A, Ribaldone DG, Tandoi F, et al. Prognostic Role of Serum Cytokeratin-19 Fragment (CYFRA 21-1) in Patients with Hepatocellular Carcinoma. Cancers. 2020; 12(10):2776. https://doi.org/10.3390/cancers12102776
Chicago/Turabian StyleCaviglia, Gian Paolo, Michela Ciruolo, Antonella Olivero, Patrizia Carucci, Emanuela Rolle, Chiara Rosso, Maria Lorena Abate, Alessandra Risso, Davide Giuseppe Ribaldone, Francesco Tandoi, and et al. 2020. "Prognostic Role of Serum Cytokeratin-19 Fragment (CYFRA 21-1) in Patients with Hepatocellular Carcinoma" Cancers 12, no. 10: 2776. https://doi.org/10.3390/cancers12102776
APA StyleCaviglia, G. P., Ciruolo, M., Olivero, A., Carucci, P., Rolle, E., Rosso, C., Abate, M. L., Risso, A., Ribaldone, D. G., Tandoi, F., Saracco, G. M., Bugianesi, E., & Gaia, S. (2020). Prognostic Role of Serum Cytokeratin-19 Fragment (CYFRA 21-1) in Patients with Hepatocellular Carcinoma. Cancers, 12(10), 2776. https://doi.org/10.3390/cancers12102776