1. Introduction
Urothelial bladder cancer (BCa) accounts for approximately 3% of global cancer diagnoses. It was recently reported to be the 10th most commonly diagnosed cancer and the 13th leading cause of cancer-related death worldwide [
1]. Approximately 25% of BCas are categorized as muscle-invasive BCa (MIBC) and 75% as non-muscle invasive BCa (NMIBC) [
2]. NMIBC treatment comprises transurethral resection of the bladder (TURB) and, depending on the risk of progression, instillation with bacillus Calmette-Guerin (BCG) or mitomycin [
3,
4,
5]. However, high-risk NMIBC remains a challenge because 30% to 60% of patients with stage pT1 NMIBC develop local recurrence, and up to 20% experience disease progression to MIBC [
6,
7,
8]. There is heterogeneity in stage pT1 NMIBC, and its risk stratification is based only on clinicopathological parameters that necessitate lifelong follow-up [
9]. Altogether, bladder cancers, including NMIBC, impose the highest costs on society among cancers per patient from diagnosis to death [
10]. However, bladder tumor markers cannot yet definitively replace cystoscopy in surveillance regimens [
10]. Therefore, the continued search for biomarkers in bladder cancer is necessary.
The tumor biology of BCa, including NMIBC, is related to cell lineage and cell proliferation [
11,
12,
13]. Therefore, we included an analysis of the mRNA of keratin 5 (
KRT5; basal-like lineage), keratin 20 (
KRT20; luminal-like lineage) and marker of proliferation KI67 (
MKI67,
KI67) in this study. Furthermore, studies conducted by other groups, as well as our own previous studies, showed that gene expression can differentiate NMIBCs into subsets that possess different risk profiles, and may impact treatment decisions in the future [
14,
15].
In the current study, we investigated the expression of genes associated with tumor immune status and their association with prognosis in stage pT1 NMIBC. Recently, we reported that a cytotoxic T-cell-related gene expression signature containing three genes (
CXCL9,
CD3 Z,
CD8) correlates with immune cell infiltration, and predicts improved survival in MIBC patients after radical cystectomy and adjuvant chemotherapy [
16]. All three immune signature genes were strongly associated with each other, which is why we chose only
CXCL9 for the current analysis. Additionally, we chose programmed cell death 1 gene (
PD1/PDCD1) and programmed cell death ligand 1 (
PD-L1/CD274/B7-H1) since they are also very prominent in the immune response of MIBC, and represent therapeutic targets for MIBC [
16,
17,
18].
CXCL9 (
SCYB9/MIG) and
CXCL10 (
SCYB10) genes are located in chromosome band 4 q21 [
19], and belong to the CXC family of chemokines [
20].
CXCL9 encodes a T-cell chemoattractant that is significantly induced by interferon gamma, which mediates a T-cell-driven antitumoral immune response [
21].
CXCL9 has not been previously studied in NMIBC. The
PD1 gene has been mapped to the chromosome region 2 q37.3 by the Honyo group [
22]. It encodes a cell surface receptor on T-cells and tumor-associated macrophages (TAMs), and is a member of the B7 superfamily involved in immunomodulation. PD1 acts as an inhibitory molecule on T-cells/TAMs after interacting with its ligand PD-L1 [
23,
24]. The
PD-L1 gene is located on chromosome 9 p24.1 and codes for a costimulatory molecule that negatively regulates cell-mediated immune responses [
23,
25]. PD-L1 is expressed by both tumor cells and tumor-associated antigen-presenting cells [
26]. Le Goux et al. [
27] did not find an association between
PD1 or
PD-L1 gene expression and prognosis (RFS and progression-free survival) in NMIBC. We recently demonstrated in an NMIBC cohort that increased
PD-L1 mRNA was an independent prognostic indicator for both RFS and DSS [
28]. However, in that study,
PD1 mRNA was not associated with prognosis [
28].
In this study, we analyzed a new independent cohort of NMIBC patients with extended follow-up periods to reassess the long-term association of PD-L1 mRNA with disease prognosis, and to determine whether the two immune markers CXCL9 and PD1 are associated with survival.
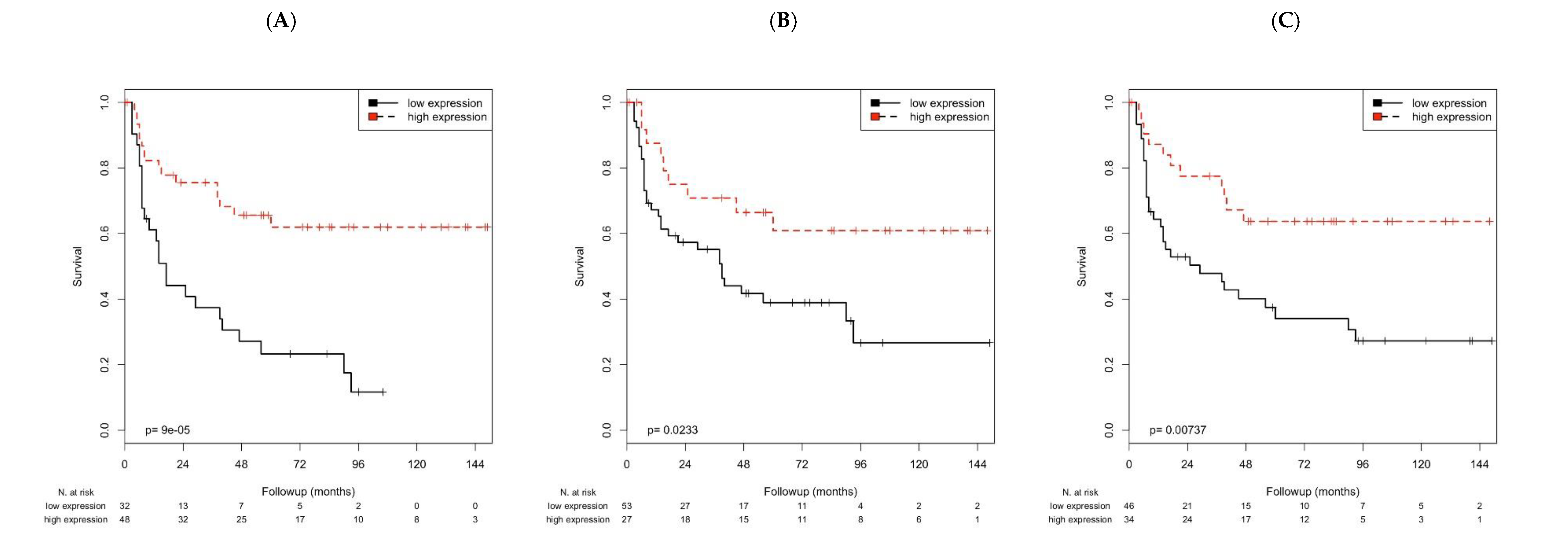
3. Discussion
In this study, we investigated the mRNA of the immune markers CXCL9, PD1 and PD-L1. First, we correlated mRNA data with clinicopathological data and with each other. We observed that CXCL9 mRNA was positively correlated with transcript levels of PD1 and PD-L1, but negatively correlated with incidence of recurrence, as well as KRT5 and KRT20 mRNA. In addition, PD1 was positively correlated with PD-L1 mRNA and time to RFS, while being negatively correlated with KRT20 mRNA. PD-L1 mRNA was additionally negatively correlated with KRT20 mRNA.
Similar to Huang et al. we showed a correlation between the mRNA of
PD-L1 and
C-C chemokines (
CCL2, CCL3, CCL8 and
CCL18) [
30,
31]. A correlation between
PD1 and
PD-L1 mRNA was previously shown by both Huang et al. [
31] and by us [
28]. These correlations can all be explained by the common expression of these factors by immune cells, i.e., leukocytes such as T-cells and macrophages.
In this study, multivariate Cox’s regression analyses revealed that high
CXCL9 mRNA was associated with longer OS and DSS, and high
PD-L1 mRNA was correlated with longer DSS. In addition, the high mRNA of
CXCL9 or
PD-L1 was significantly associated with longer RFS. Huang and colleagues found that elevated
PD-L1 mRNA was associated with reduced patient survival (OS, DSS), but they studied a mixed cohort of NMIBC and MIBC where the association could have been influenced by MIBC patients, and further, they did not examine RFS [
31]. We previously found that increased
PD-L1 mRNA expression was associated with longer DSS and RFS in pT1 NMIBC [
28]. In this study, we confirmed the association of high
PD-L1 mRNA with DSS and RFS. However, the impact of
PD-L1 on OS, DSS and RFS need to be evaluated further in prospective studies.
PD1 was previously not described to be associated with RFS [
28], but in this study, we observed an association between increased
PD1 mRNA and longer RFS. Although both studies were performed in consecutive patients, in this study, observation time was longer (62 vs. 42 months), and the numbers of recurrences (51.3% vs. 33.4%) were higher than in the previous study, which may explain the differential results.
CXCL9 mRNA level has not been previously described in NMIBC to be associated with OS, DSS or RFS. The effect of an immune intravesical therapy with bacillus Calmette-Guérin (BCG) on
CXCL9 mRNA was controversially discussed. BCG therapy upregulates the mRNA of different chemokines, including
CXCL9, in an in vivo mouse model [
32]. Interestingly, using an in vitro approach in established human BCa cell lines, Özcan et al. demonstrated that BCG treatment reduced
CXCL9 mRNA [
33]. This supports the assumption that the tumor microenvironment is responsible for the chemokine reaction following BCG therapy. A recent review reports that the CXCL9/CXCL10/CXCL11/CXCR3 axis is responsible for angiogenesis inhibition, and the activation and migration of immune cells such as cytotoxic lymphocytes and natural killer cells into the tumor microenvironment, to prevent tumor progression in BCa [
34].
Next, we were interested in whether the association of
CXCL9, PD1 and
PD-L1 mRNA with RFS could be further stratified by clinicopathological parameter (age) or other parameters applied for lineage differentiation, such as
KRT5 or
KRT20 mRNA, proliferation activity (
KI67), or therapeutic application (instillation therapy). Interestingly, after separating patients by their median age (≤71 vs. >71 years), only in the younger age group (≤71 years) was higher
CXCL9 or higher
PD1 mRNA associated with longer RFS. This finding could be simply related to the fact that the immune system is more active in younger than in older persons, in whom immunosenescence has been reported [
35]. Increasing multi morbidity affecting health status in elderly patients may also play a role in shorter RFS, although time to recurrence was not significantly different between the age groups (data not shown).
KRT5 and
KRT20 are considered intrinsic markers for basal and luminal subtypes of muscle-invasive bladder cancer, respectively [
11,
36,
37]. Interestingly, high
PD-L1 mRNA was associated with longer RFS in both high
KRT5 and high
KRT20 groups, but not in the low
KRT5 or low
KRT20 groups. This finding suggests that high
PD-L1 mRNA is favorable for longer RFS in both basal and luminal subtypes of NMIBC. We previously showed that high
KRT20 mRNA was associated with shorter RFS [
38]. In this context,
PD-L1 mRNA further distinguishes the unfavorable RFS group (high
KRT20) in patients with longer RFS (
PD-L1 high) or shorter RFS (
PD-L1 low).
High KI67 expression has been described as a prognostic factor for poor OS, DSS, RFS and PFS in a meta-analysis of NMIBC patients [
12]. In the high
KI67 group, high
CXCL9 and high
PD-L1 mRNA were associated with longer RFS, but this association was not observed in the low
KI67 group. In this way, within the unfavorable high
KI67 group, patients with longer RFS (high
CXCL9 or high
PD-L1) and with shorter RFS (low
CXCL9 or low
PD-L1) could be distinguished.
Intravesical therapy with either BCG or cytostatic drugs, like mitomycin, is mostly standard therapy for intermediate or high risk NMIBC, but its application differs between several guidelines [
3,
5]. Interestingly, only in the no instillation group was high
CXCL9, high
PD1 or high
PD-L1 associated with longer RFS compared to the instillation group. One explanation for this finding could be that BCG therapy affects the immune response of patients, and
CXCL9, PD1 and
PD-L1 reflect intrinsic immune status. In this way, both the expression of the immune markers and the intravesical therapy may influence each other. As mentioned above, the BCG exposure of established BCa cell lines devoid of any tumor microenvironment reduced
CXCL9 mRNA in vitro [
33]. Furthermore, increases in
PD-L1 protein levels, which are considered a negative prognostic marker, have been reported after BCG therapy compared to before BCG treatment [
39].
5. Conclusions
Altogether, we confirmed that high PD-L1 mRNA is associated with increased DSS and RFS. Furthermore, we demonstrated for the first time that CXCL9 mRNA is associated with a longer OS, DSS and RFS. Associations with RFS were also identified or further pinpointed to special groups, including the younger age group (CXCL9, PD1), the high KRT5 or high KRT20 group (CXCL9, PD-L1), the high KI67 group (CXCL9, PD-L1) or the no instillation group (CXCL9, PD-L1).
An increased mRNA for PD1, PD-L1 and CXCL9 being associated with a better prognosis may mirror the host–tumor interaction. In this way, we suggest that the increased mRNA levels of all three genes may reflect the immune response of the host.
Our finding of associations between these immune markers and prognosis may aid in future therapeutic options and decisions.