Detection of EGFR Mutations Using Bronchial Washing-Derived Extracellular Vesicles in Patients with Non-Small-Cell Lung Carcinoma
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients
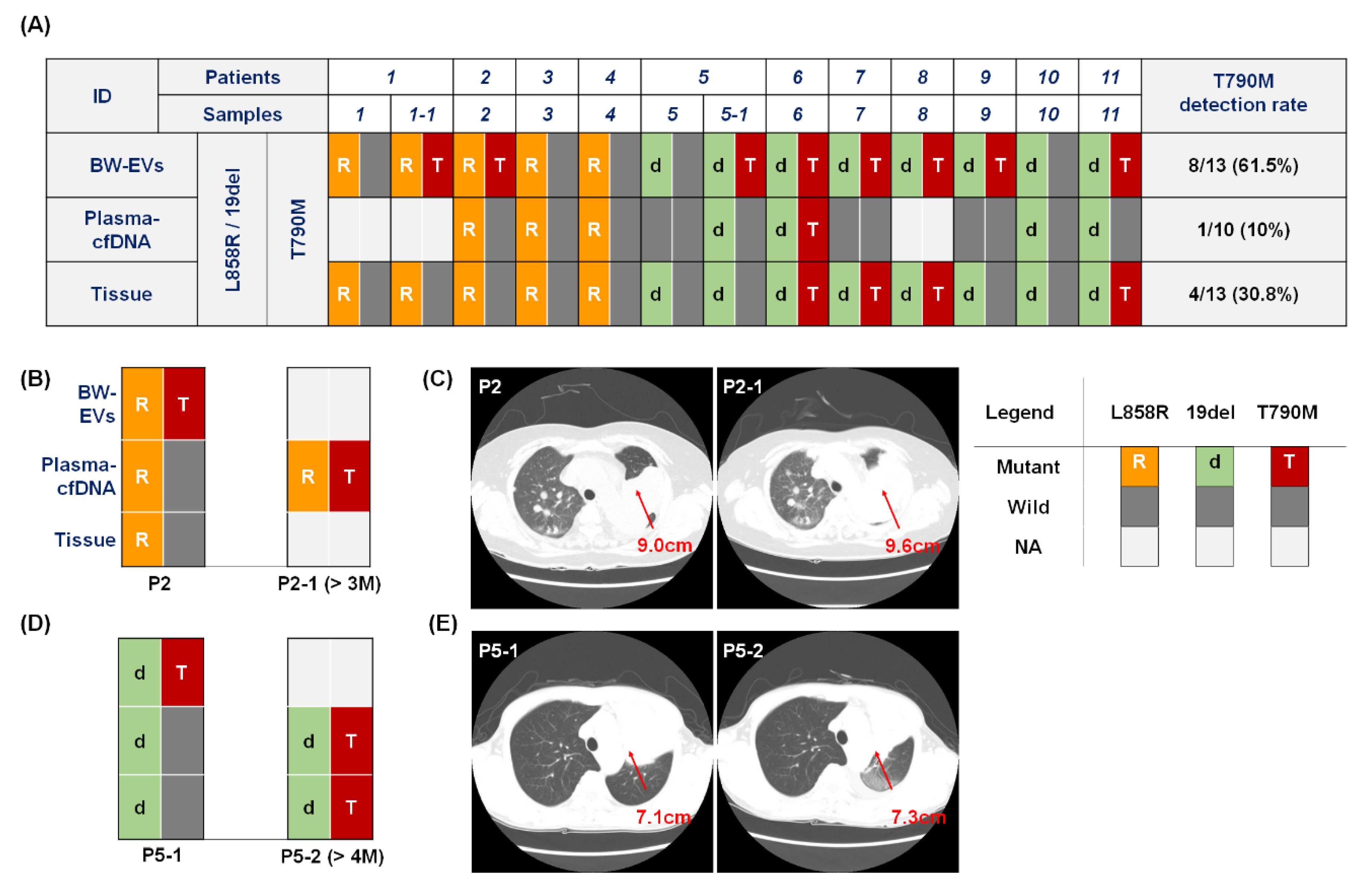
2.2. Detection of EGFR Mutation in Bronchial Washing-Derived Samples
2.3. EGFR T790M Mutation Detection in Bronchial Washing-Derived, Plasma, and Tissue Samples
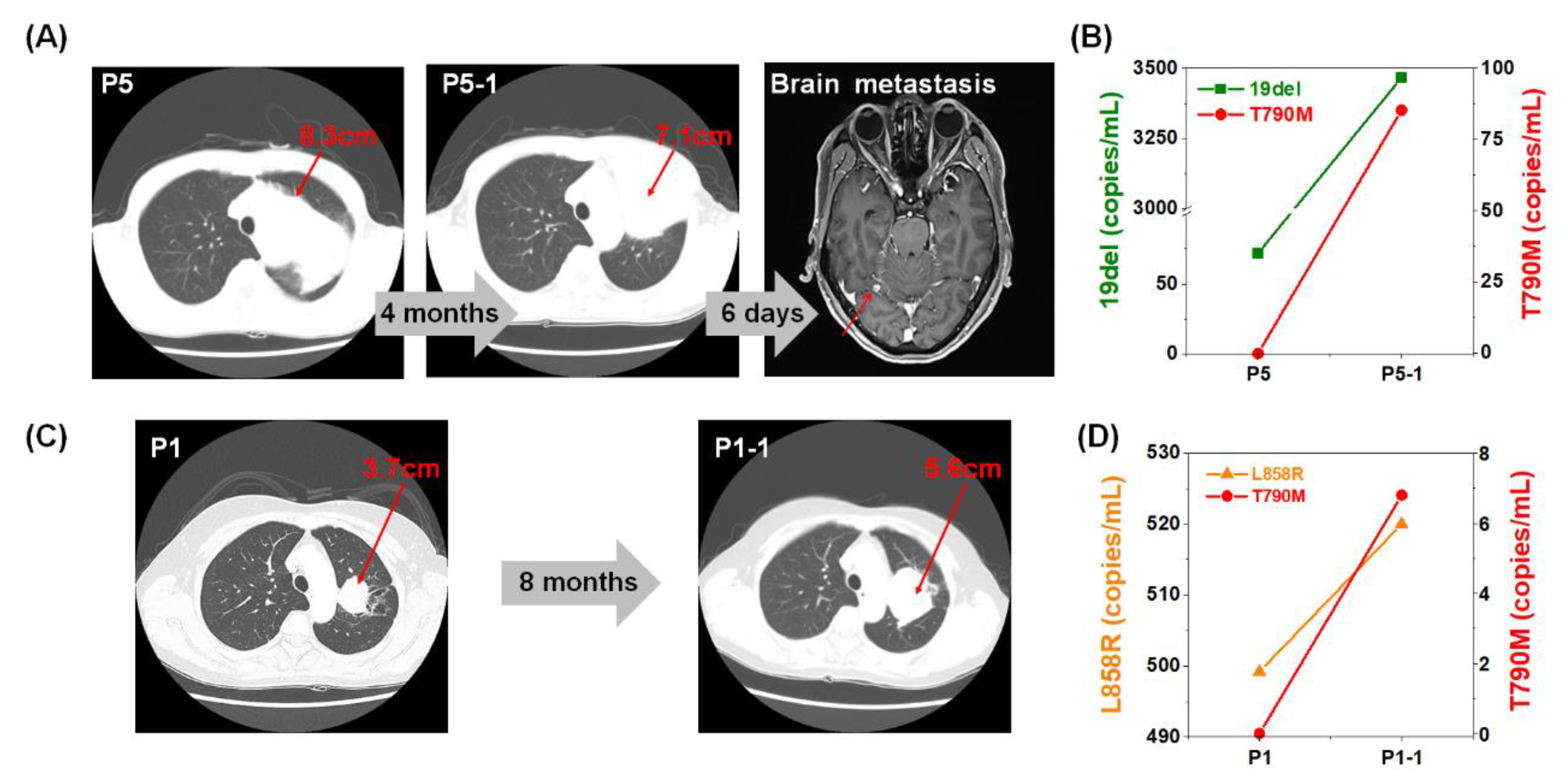
2.4. Monitoring of EGFR Mutation during Targeted Therapy
3. Discussion
4. Materials and Methods
4.1. Storage and Handling of Clinical Samples
4.2. Bronchoscopy and Process of Bronchial Washing
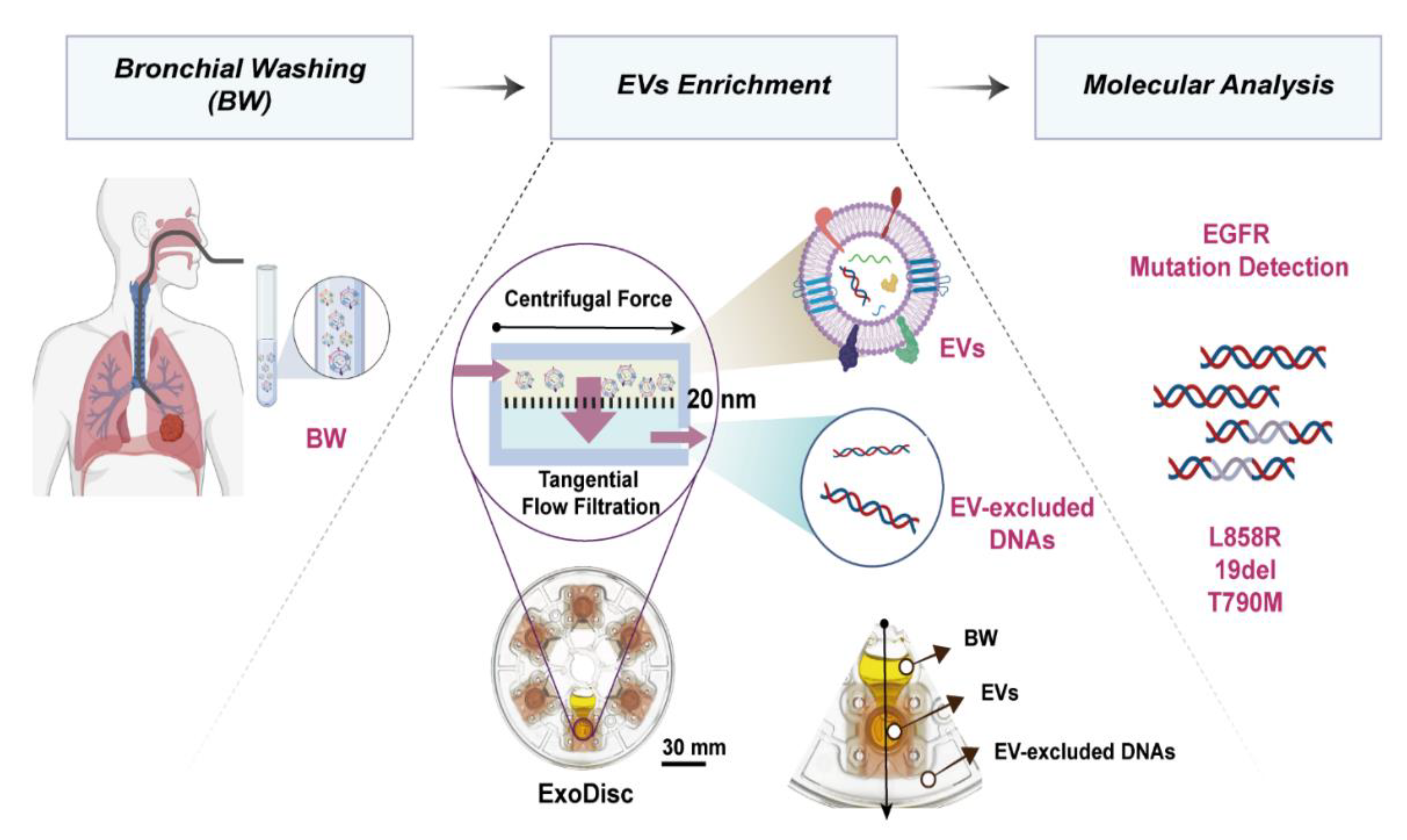
4.3. Isolation of Extracellular Vesicles
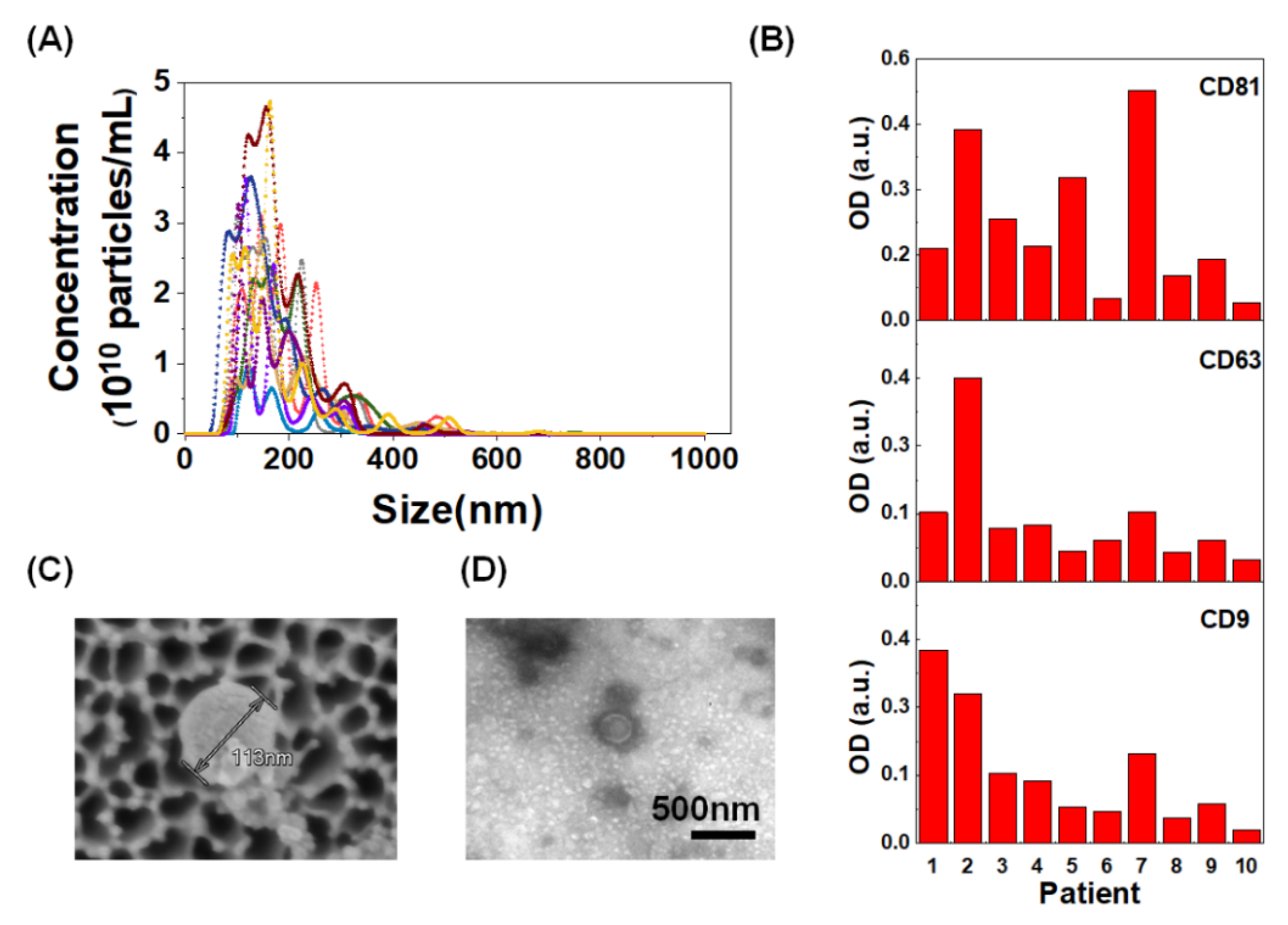
4.4. Nanoparticle Tracking Analysis
4.5. Confirmatory Indirect ELISA for Markers of Extracellular Vesicles
4.6. DNA Isolation from Bronchial Wash Sample
4.7. Cell-free DNA Isolation from Plasma
4.8. Real-Time Polymerase Chain Reaction
4.9. Droplet Digital PCR
4.10. Scanning Electron Microscope Imaging
4.11. Bio-Transmission Electron Microscope Imaging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Xu, Y.; Wang, X.; Liu, D.; Yang, F.; Zhu, X.; Lu, Y.; Xing, W. Rapid and efficient isolation and detection of extracellular vesicles from plasma for lung cancer diagnosis. Lab Chip 2019, 19, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, A.; Fukumitsu, C.; Taira, T.; Abe, H.; Takase, Y.; Murata, K.; Yamaguchi, T.; Azuma, K.; Ishii, H.; Takamori, S.; et al. Epidermal growth factor receptor mutation status in cell-free DNA supernatant of bronchial washings and brushings. Cancer Cytopathol. 2015, 123, 620–628. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Lao, X.Q.; Ho, K.F.; Goggins, W.B.; Tse, S.L.A. Incidence and mortality of lung cancer: Global trends and association with socioeconomic status. Sci. Rep. 2017, 7, 14300. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Quake, S.R.; McCabe, E.R.B.; Chng, W.J.; Chow, E.K.; Ding, X.; Gelb, B.D.; Ginsburg, G.S.; Hassenstab, J.; Ho, C.-M.; et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020, 38, 497–518. [Google Scholar] [CrossRef]
- Rossi, G.; Ignatiadis, M. Promises and Pitfalls of Using Liquid Biopsy for Precision Medicine. Cancer Res. 2019, 79, 2798–2804. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Bode, A.M.; Dong, Z. Addressing the challenges of applying precision oncology. NPJ Precis. Oncol. 2017, 1, 28. [Google Scholar] [CrossRef]
- Yu, H.A.; Perez, L.; Chang, Q.; Gao, S.P.; Kris, M.G.; Riely, G.J.; Bromberg, J. A Phase 1/2 Trial of Ruxolitinib and Erlotinib in Patients with EGFR-Mutant Lung Adenocarcinomas with Acquired Resistance to Erlotinib. J. Thorac. Oncol. 2017, 12, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.E.; Choi, K.; Lanman, R.B.; Licitra, E.J.; Skrzypczak, S.M.; Benito, R.P.; Wu, T.; Arunajadai, S.; Kaur, S.; Harper, H.; et al. Genomic Profiling of Advanced Non-Small Cell Lung Cancer in Community Settings: Gaps and Opportunities. Clin. Lung Cancer 2017, 18, 651–659. [Google Scholar] [CrossRef]
- Alifrangis, C.; Carter, P.; Cereser, B.; Chandrasinghe, P.; Belluz, L.D.B.; Lim, E.; Moderau, N.; Poyia, F.; Tabassum, N.; Zhang, H.; et al. Investigating the benefits of molecular profiling of advanced non-small cell lung cancer tumors to guide treatments. Oncotarget 2018, 9, 12805–12811. [Google Scholar] [CrossRef]
- Ellison, G.; Zhu, G.; Moulis, A.; Dearden, S.; Speake, G.; McCormack, R. EGFR mutation testing in lung cancer: A review of available methods and their use for analysis of tumour tissue and cytology samples. J. Clin. Pathol. 2013, 66, 79–89. [Google Scholar] [CrossRef]
- Moreira-Leite, F.F.; Harrison, L.R.; Mironov, A.; Roberts, R.A.; Dive, C. Inducible EGFR T790M-Mediated Gefitinib Resistance in Non-small Cell Lung Cancer Cells Does Not Modulate Sensitivity to PI103 Provoked Autophagy. J. Thorac. Oncol. 2010, 5, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Savic, S.; Tapia, C.; Grilli, B.; Rufle, A.; Bihl, M.P.; de Vito Barascud, A.; Herzog, M.; Terracciano, L.; Baty, F.; Bubendorf, L. Comprehensive epidermal growth factor receptor gene analysis from cytological specimens of non-small-cell lung cancers. Br. J. Cancer 2008, 98, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, R.; Digumarthy, S.; Turke, A.B.; Fidias, P.; Bergethon, K.; Shaw, A.T.; Gettinger, S.; Cosper, A.K.; et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci. Trans. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef]
- Denis, M.G.; Vallee, A.; Theoleyre, S. EGFR T790M resistance mutation in non small-cell lung carcinoma. Clin. Chim. Acta 2015, 444, 81–85. [Google Scholar] [CrossRef]
- Mohrmann, L.; Huang, H.J.; Hong, D.S.; Tsimberidou, A.M.; Fu, S.; Piha-Paul, S.A.; Subbiah, V.; Karp, D.D.; Naing, A.; Krug, A.; et al. Liquid Biopsies Using Plasma Exosomal Nucleic Acids and Plasma Cell-Free DNA Compared with Clinical Outcomes of Patients with Advanced Cancers. Clin. Cancer Res. 2018, 24, 181–188. [Google Scholar] [CrossRef]
- Lee, J.S.; Hur, J.Y.; Kim, I.A.; Kim, H.J.; Choi, C.M.; Lee, J.C.; Kim, W.S.; Lee, K.Y. Liquid biopsy using the supernatant of a pleural effusion for EGFR genotyping in pulmonary adenocarcinoma patients: A comparison between cell-free DNA and extracellular vesicle-derived DNA. BMC Cancer 2018, 18, 1236. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, Y.; Cho, E.; Kim, G.B.; Kim, I.S. Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. J. Extracell. Vesicles 2018, 7, 1440131. [Google Scholar] [CrossRef]
- Nana-Sinkam, S.P.; Acunzo, M.; Croce, C.M.; Wang, K. Extracellular Vesicle Biology in the Pathogenesis of Lung Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 1510–1518. [Google Scholar] [CrossRef]
- Lane, R.E.; Korbie, D.; Hill, M.M.; Trau, M. Extracellular vesicles as circulating cancer biomarkers: Opportunities and challenges. Clin. Transl. Med. 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef]
- De Rubis, G.; Rajeev Krishnan, S.; Bebawy, M. Liquid Biopsies in Cancer Diagnosis, Monitoring, and Prognosis. Trends Pharmacol. Sci. 2019, 40, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Pradines, A.; Favre, G.; Mazieres, J. Current and future applications of liquid biopsy in nonsmall cell lung cancer from early to advanced stages. Eur. Respir. Rev. 2020, 29, 190052. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Park, J.; Lowe, A.C.; Jeong, H.O.; Lee, S.; Park, H.C.; Lee, K.; Kim, G.H.; Kim, M.H.; Cho, Y.K. A lab-on-a-disc platform enables serial monitoring of individual CTCs associated with tumor progression during EGFR-targeted therapy for patients with NSCLC. Theranostics 2020, 10, 5181–5194. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Camussi, G.; Valadi, H.; Nazarenko, I.; Ekström, K.; Wang, X.; Principe, S.; Shah, N.; Ashraf, N.M.; Fatima, F. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat. Rev. Urol. 2014, 11, 688–701. [Google Scholar] [CrossRef]
- Brock, G.; Castellanos-Rizaldos, E.; Hu, L.; Coticchia, C.; Skog, J. Liquid biopsy for cancer screening, patient stratification and monitoring. Transl. Cancer Res. 2015, 4, 280–290. [Google Scholar]
- Babayan, A.; Pantel, K. Advances in liquid biopsy approaches for early detection and monitoring of cancer. Genome Med. 2018, 10, 21. [Google Scholar] [CrossRef]
- Conigliaro, A.; Cicchini, C. Exosome-Mediated Signaling in Epithelial to Mesenchymal Transition and Tumor Progression. J. Clin. Med. 2018, 8, 26. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Rijavec, E.; Coco, S.; Genova, C.; Rossi, G.; Longo, L.; Grossi, F. Liquid Biopsy in Non-Small Cell Lung Cancer: Highlights and Challenges. Cancers 2020, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Rizaldos, E.; Grimm, D.G.; Tadigotla, V.; Hurley, J.; Healy, J.; Neal, P.L.; Sher, M.; Venkatesan, R.; Karlovich, C.; Raponi, M.; et al. Exosome-Based Detection of EGFR T790M in Plasma from Non-Small Cell Lung Cancer Patients. Clin. Cancer Res. 2018, 24, 2944–2950. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.Y.; Kim, H.J.; Lee, J.S.; Choi, C.M.; Lee, J.C.; Jung, M.K.; Pack, C.G.; Lee, K.Y. Extracellular vesicle-derived DNA for performing EGFR genotyping of NSCLC patients. Mol. Cancer 2018, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, Y.; Hirotsu, Y.; Tsutsui, T.; Higashi, S.; Sogami, Y.; Kakizaki, Y.; Goto, T.; Amemiya, K.; Oyama, T.; Omata, M. Analysis of significantly mutated genes as a clinical tool for the diagnosis in a case of lung cancer. Respir. Med. Case Rep. 2017, 20, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-P.; Mehta, A.C.; Turner, J.F., Jr. Flexible Bronchoscopy, 3rd ed.; Wiley-Blackwell New Jersey: Hoboken, NJ, USA, 2012. [Google Scholar]
- Rao, S.; Rao, S.; Lal, A.; Barathi, G.; Dhanasekar, T.; Duvuru, P. Bronchial wash cytology: A study on morphology and morphometry. J. Cytol. 2014, 31, 63–67. [Google Scholar] [CrossRef]
- Ryu, J.S.; Lim, J.H.; Lee, M.K.; Lee, S.J.; Kim, H.J.; Kim, M.J.; Park, M.H.; Kim, J.S.; Nam, H.S.; Park, N.; et al. Feasibility of Bronchial Washing Fluid-Based Approach to Early-Stage Lung Cancer Diagnosis. Oncologist 2019, 24, e603–e606. [Google Scholar] [CrossRef]
- Shields, M.D.; Riedler, J. Bronchoalveolar Lavage and Tracheal Aspirate for Assessing Airway Inflammation in Children. Am. J. Respir. Crit. Care Med. 2000, 162, S15–S17. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef]
- Woo, H.K.; Park, J.; Ku, J.Y.; Lee, C.H.; Sunkara, V.; Ha, H.K.; Cho, Y.K. Urine-based liquid biopsy: Non-invasive and sensitive AR-V7 detection in urinary EVs from patients with prostate cancer. Lab Chip 2018, 19, 87–97. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, B.; Lei, H.; Zhang, B.; Wang, Y.; Huang, H.; Chen, S.; Feng, Y.; Zhu, L.; Gu, Y.; et al. Nanoscale extracellular vesicle-derived DNA is superior to circulating cell-free DNA for mutation detection in early-stage non-small-cell lung cancer. Ann. Oncol. 2018, 29, 2379–2383. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, V.; Kim, C.J.; Park, J.; Woo, H.K.; Kim, D.; Ha, H.K.; Kim, M.H.; Son, Y.; Kim, J.R.; Cho, Y.K. Fully Automated, Label-Free Isolation of Extracellular Vesicles from Whole Blood for Cancer Diagnosis and Monitoring. Theranostics 2019, 9, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.K.; Sunkara, V.; Park, J.; Kim, T.H.; Han, J.R.; Kim, C.J.; Choi, H.I.; Kim, Y.K.; Cho, Y.K. Exodisc for Rapid, Size-Selective, and Efficient Isolation and Analysis of Nanoscale Extracellular Vesicles from Biological Samples. ACS Nano 2017, 11, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
| Characteristic (n = 52) | Mutant Type (%) n = 26 | Wild Type (%) n = 26 |
|---|---|---|
| Median age (range) | 66 (39–89) | 68 (50–81) |
| Sex | ||
| Male | 10 (38.5) | 24 (92.3) |
| Female | 16 (61.5) | 2 (7.7) |
| Histology | ||
| Adenocarcinoma | 23 (88.5) | 10 (38.5) |
| Squamous cell carcinoma | 2 (7.7) | 16 (61.5) |
| Non-small-cell lung carcinoma, NOS 1 | 1 (3.8) | 0 (0) |
| EGFR mutation type | ||
| L858R | 10 (38.5) | 0 (0) |
| 19del | 16 (61.5) | 0 (0) |
| Stage, n (%) | ||
| I | 0 (0) | 3 (11.5) |
| II | 2 (7.7) | 4 (15.4) |
| III | 2 (7.7) | 6 (23.1) |
| IV | 22 (84.6) | 11 (42.3) |
| Unknown | 0 (0) | 2 (7.7) |
| EGFR Mutation | Tissue | BW (n = 55) | |
|---|---|---|---|
| EV-DNA | EV-X-cfDNA | ||
| Mutant | 29 | 26 | 9 |
| Wild | 26 | 26 | 26 |
| Sensitivity | 89.7% | 31.0% | |
| Specificity | 100% | 100% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, C.; Eom, J.S.; Kim, M.-H.; Cho, Y.-K. Detection of EGFR Mutations Using Bronchial Washing-Derived Extracellular Vesicles in Patients with Non-Small-Cell Lung Carcinoma. Cancers 2020, 12, 2822. https://doi.org/10.3390/cancers12102822
Park J, Lee C, Eom JS, Kim M-H, Cho Y-K. Detection of EGFR Mutations Using Bronchial Washing-Derived Extracellular Vesicles in Patients with Non-Small-Cell Lung Carcinoma. Cancers. 2020; 12(10):2822. https://doi.org/10.3390/cancers12102822
Chicago/Turabian StylePark, Juhee, Chaeeun Lee, Jung Seop Eom, Mi-Hyun Kim, and Yoon-Kyoung Cho. 2020. "Detection of EGFR Mutations Using Bronchial Washing-Derived Extracellular Vesicles in Patients with Non-Small-Cell Lung Carcinoma" Cancers 12, no. 10: 2822. https://doi.org/10.3390/cancers12102822
APA StylePark, J., Lee, C., Eom, J. S., Kim, M.-H., & Cho, Y.-K. (2020). Detection of EGFR Mutations Using Bronchial Washing-Derived Extracellular Vesicles in Patients with Non-Small-Cell Lung Carcinoma. Cancers, 12(10), 2822. https://doi.org/10.3390/cancers12102822






